
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 01 November 2021
Sec. Dermatology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.737813
This article is part of the Research TopicInsights in Dermatology: 2021View all 15 articles
Lichen planus (LP) is a T cell-mediated disease affecting the stratified squamous epithelia of the skin and/or mucus membrane. Histologically, the disease is characterized by a lichenoid inflammatory infiltrate and vacuolar degeneration of the basal layer of the epidermis. LP has three major subtypes: Cutaneous, mucosal and appendageal LP. Rarely, it may affect the nails in the absence of skin and/or mucosal changes. LP may also be induced by several drugs, typically anti-hypertensive medication or be associated with infections, particularly viral hepatitis. The diagnosis is based on the clinical presentation and characteristic histological findings. Although the disease is often self-limiting, the intractable pruritus and painful mucosal erosions result in significant morbidity. The current first-line treatment are topical and/or systemic corticosteroids. In addition, immunosuppressants may be used as corticosteroid-sparing agents. These, however are often not sufficient to control disease. Janus kinase inhibitors and biologics (anti-IL-12/23, anti-IL17) have emerged as novel future treatment options. Thus, one may expect a dramatic change of the treatment landscape of LP in the near future.
The term lichen planus (LP) stems from the Greek word “leichen,” which means “tree moss,” and the Latin word “planus,” which means “flat,” which aptly describes the surface of the cutaneous lesion (1). LP is a group of chronic inflammatory diseases affecting stratified squamous epithelia. Recently, LP is perceived as a T cell-mediated autoimmune disease, in which cytotoxic CD8+ T-cells are recruited into the skin and subsequently lead to an interface dermatitis (2–8). Viruses, drugs and contact allergens have all been reported to be possibly associated with development of LP (9–19). Clinically, LP is hallmarked by characteristic lesions, affecting the skin, hair, nails and/or mucous membranes. The classical skin changes are pruritic, purple, polygonal, flat-topped (planar) papules crossed by fine white lines, while erosions are seen on the mucous membranes (Figure 1). The latter may be associated with pain and/oral burning sensation (1). An overview of clinical subtypes and rare variants are listed in Table 1. LP preferentially affects middle-aged adults, with no known gender pre-disposition (1, 14). Whilst the clinical features are relatively characteristic, histological confirmation of the diagnosis is recommended to exclude potential differential diagnoses. The typical band-like lymphocytic infiltrate and interface dermatitis are the characteristic findings—irrespective of skin location or disease subtype. In addition to routine histology, direct immunofluorescence (IF) microscopy may demonstrate C3 and/or IgG at the dermal-epidermal junction and deposition of IgM as so-called colloid bodies (20). The overall goal of treatment is symptom control and resolution of the skin lesions. Selection of treatment should be based on the severity of the disease, the extent of the subjective symptoms, as well as taking into account relevant co-morbidities (14). Cutaneous LP is usually self-limiting and resolves within 6 months in over 50% of patients and within 18 months in up to 85% of patients (14, 21). By contrast, mucosal LP is often chronic and may be refractory to treatment (22, 23). LP with hypertrophic cutaneous lesions and isolated nail or scalp involvement is also often chronic in nature. Persistent cutaneous and mucosal lesions are considered as a premalignant condition. Thus, patients should be followed up regularly for both, adjustment of treatment, and screening for the development of malignancies.
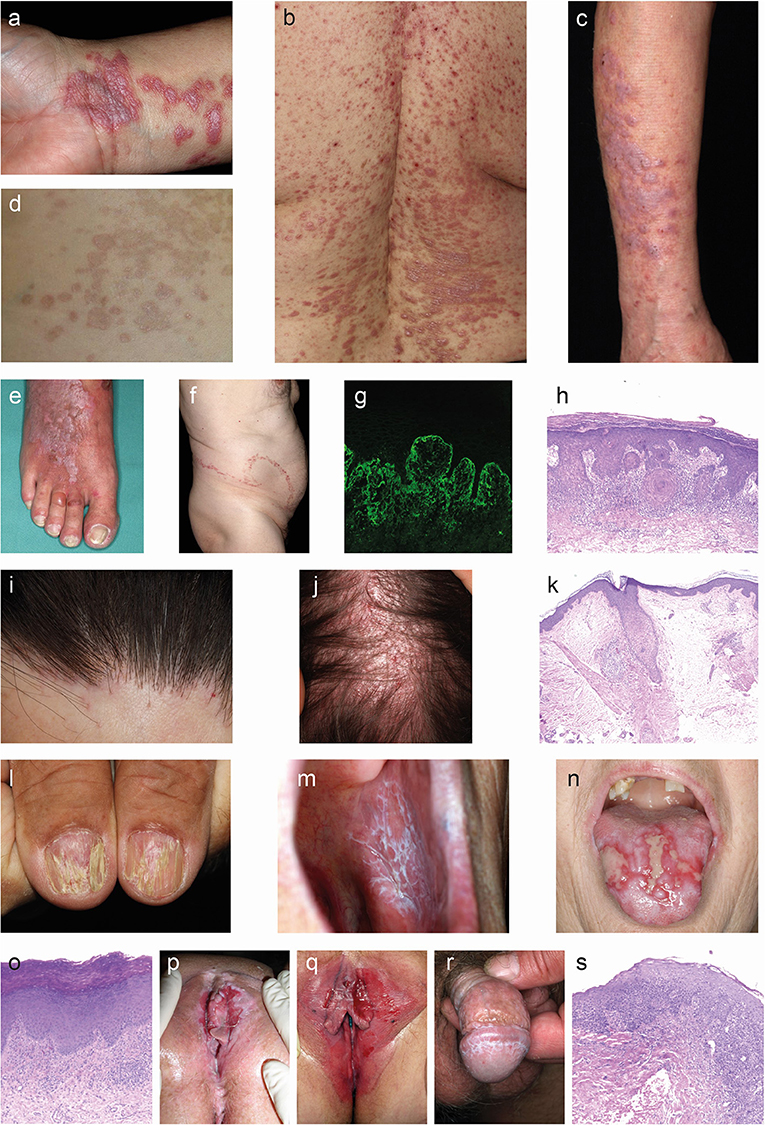
Figure 1. Clinical and histological hallmarks of lichen planus. (a–f) Cutaneous lichen planus (LP). (a) Polygonal, flat-topped, violaceous confuting plaques with fine white scales on the inner wrist in a patient with localized LP. (b) Symmetric red plaques on the back of a patient with generalized cutaneous LP. (c) Thick reddish-brown plaques on the arms of a patient with hypertrophic LP. (e) Blister on the 3rd toe along with widespread red plaques with whit streaks in a patient with lichen planus pemphigoides. (f) Linear lichen planus. (g) Direct immunofluorescence microcopy staining with fibrin deposition in the epidermis (400×). (h) The histology from a skin biopsy from a lichen planus lesion characteristically shows an irregularly epidermis with saw-toothed rete ridges, hypergranulosis, liquefaction degeneration of the dermal-epidermal junction and a lichenoid (band-like) lymphocytic infiltrate (H&E staining, 200×). (i–l) Appendageal LP. (i) Scaring alopecia and inflammation around hair follicles along the frontal scalp hair margin in a patient with frontal fibrosing alopecia. (j) Image from a patent with lichen planopilaris. (k) Lichenoid interface dermatitis of the hair infundibulum and apoptotic keratinocytes (Civatte bodies) and fibrous tracts in a biopsy from a patient with frontal fibrosing alopecia. (l) Grooved and ridged nails in a patient with nail LP. (m–s) Mucosal LP. (m) Wickham striae in the oral mucosa of a patient with oral LP. (n) Severe ulcera of the tounge in a patient with erosive oral LP. (o) Parakeratosis, acanthosis. band of inflammatory cells just beneath the epidermis, plasma cells in infiltrate in an oral biopsy from a patient with oral LP. (p) Severe vulval ulcerations in a patient with vulval LP. (q) Erythema and erosions in a patient with vulval LP. (r) Wickham striae on the glans penis in a patient with penile LP. (s) Occasional parakeratosis, irregularly thickened epidermis, apoptotic basal keratinocytes, lymphohistiocytic infiltrate in a biopsy from a patient with genital LP.
The prevalence of LP is 0.89% in the general population and 0.98% in patients seeking dermatological care according to a recent meta-analysis of 46 studies (24). The prevalence of cutaneous LP was reported to range between 0.2 and 1.0% of the adult population, and it is outnumbered by oral LP in most study populations (1, 9). The incidence of LP is less well-characterized and displays considerable geographical heterogeneity as it ranges between 14 and 250 cases/100,000 person-years (25–29). This variability more likely mirrors methodological differences in the sampled populations rather than the existence of an ethnic pre-disposition. Moreover, the aforementioned studies adopted various eligibility criteria and pooled patients with oral and cutaneous LP together. While oral LP affects females more frequently than males (24, 30), cutaneous LP does not demonstrate a prominent sex predilection (21). Cutaneous LP tends to manifest during the fifth and sixth decades of life, with almost two-thirds of patients presenting with the disease between the ages of 30 and 60 years (9, 31, 32). Oral LP tends to develop 10 years later than cutaneous LP (33). While no ethnic predilection is renowned in LP, a recent meta-analysis revealed that the pooled prevalence of oral LP was lower among patients of Asian ancestry (24). The epidemiology of LP remains to be fully delineated as the current knowledge stems mainly from scattered small-scale retrospective studies. Given that the care of patients with LP spreads across different medical specialties, in both primary and specialized healthcare, precise estimation of its incidence and prevalence is methodologically challenging.
The observation of familial LP (34), the occurrence of LP in monozygotic twins (35) and HLA-based susceptibility association studies all point toward a genetic pre-disposition for LP. Several HLA alleles are associated with LP, for example between HLA-B27, HLA-B51, HLA-Bw57 (oral LP in English patients), HLA DR1 (cutaneous/oral LP), HLA-DR9 (oral LP in Japanese and Chinese patients), HLA DR6 (HCV-associated oral LP), and HLA DRB1*11 and DQB1*03 alleles (lichen planopilaris) (17, 36–40). So far, only one genome-wide association study (GWAS) has been published in LP. In total, 261 patients with hepatitis C infection with (n = 71) or without (n = 190) LP were genotyped. The findings were validated in a small group of patients (n = 45), of which only 7 were affected by LP. In addition to the association with the HLA, single-nucleotide polymorphisms (SNP) in loci encoding for NRP2 and IGFBP4 that increase or reduce risk of LP association, respectively, were found (37). Recently, a phenome-wide association study confirmed the HLA association in LP and additionally found two additional SNPs to be associated with LP. These SNP encode for three genes: TSBP1, HCG23, and BTNL2 (41). Further gene associations had been described for several cytokines (IFN-γ, TNF, TNFαR, IL-4, IL-6, IL-18) and others (NFκB, PGE2, Prothrombin) (40).
Several environmental factors have been implemented to trigger LP. Systemic viral infection, such as hepatitis C, may modify self-antigens on the surface of basal keratinocytes, or alter the immune balance, promoting a lichenoid inflammation (15–18, 42). The association between LP and hepatitis C has recently been substantiated in a large cohort study. Here, the prevalence of chronic inflammatory skin disease, including LP, was contrasted in over 23,000 patients with hepatitis C and a 3-fold greater number of non-hepatitis controls. In this study, the adjusted hazard ratio (HR) for subjects with hepatitis C to develop LP was 13.14 (95% CI: 7.10–24.31), indicating a significantly higher risk to develop LP for patients with hepatitis C. Among all evaluated chronic inflammatory skin diseases, the HR to develop LP for patients with hepatitis C was the second highest (43). Other viruses that are associated with triggering LP are members of the human herpesvirus (HHV) family, specifically (HHV)-6 and HHV-7 (44). However, studies relating to HHV-6 were not validated in other studies (45). Moreover, localized skin disease due to herpes simplex, varicella zoster, or human papilloma virus 16 (46–52) may cause LP. There are also reports that vaccine administration, including influenza and hepatitis B virus vaccines, may be associated with the development of LP (53). Additional environmental factors have been implicated in the development of oral LP. These include changes in the oral microbiome (e.g., Candida sp., various other bacterial infections) and dental metals precipitating allergic contact reaction (54–57). In line with this observation, the diversity of the skin bacterial communities may be involved in LP pathogenesis. Under steady-state conditions, a low diversity of bacterial communities on the skin are associated with an increased expression of proinflammatory cytokines (TNFα and CXCL1) and CD11c, pointing toward an increased infiltration with macrophages (58). These cytokines, as well as macrophages are also found in lesional LP skin (59–61). Thus, a low diversity of cutaneous bacterial communities may generate a pro-inflammatory state, even under steady-state conditions, that shares features of LP (Figure 2); thereby, potentially lowering the threshold for LP to develop. Among metals that may be associated with oral LP include amalgam (mercury), copper, and gold. Drugs may also elicit lichenoid-like reactions, which may be both clinically and histologically indistinguishable from classic LP. The most commonly implicated drugs (Table 2) are angiotensin-converting enzyme inhibitors, thiazide diuretics, antimalarials, anti-inflammatory drugs, antimicrobials, antihypertensives, psychiatric drugs, antidiabetics, PD-1-inhibitors, quinidine, penicillamine, and metals (62–65). Another peculiar potential environmental trigger for LP is UV-filters in sunscreens and hair-care products that have been noted to be associated with frontal fibrosing alopecia and lichen planopilaris (66, 67).
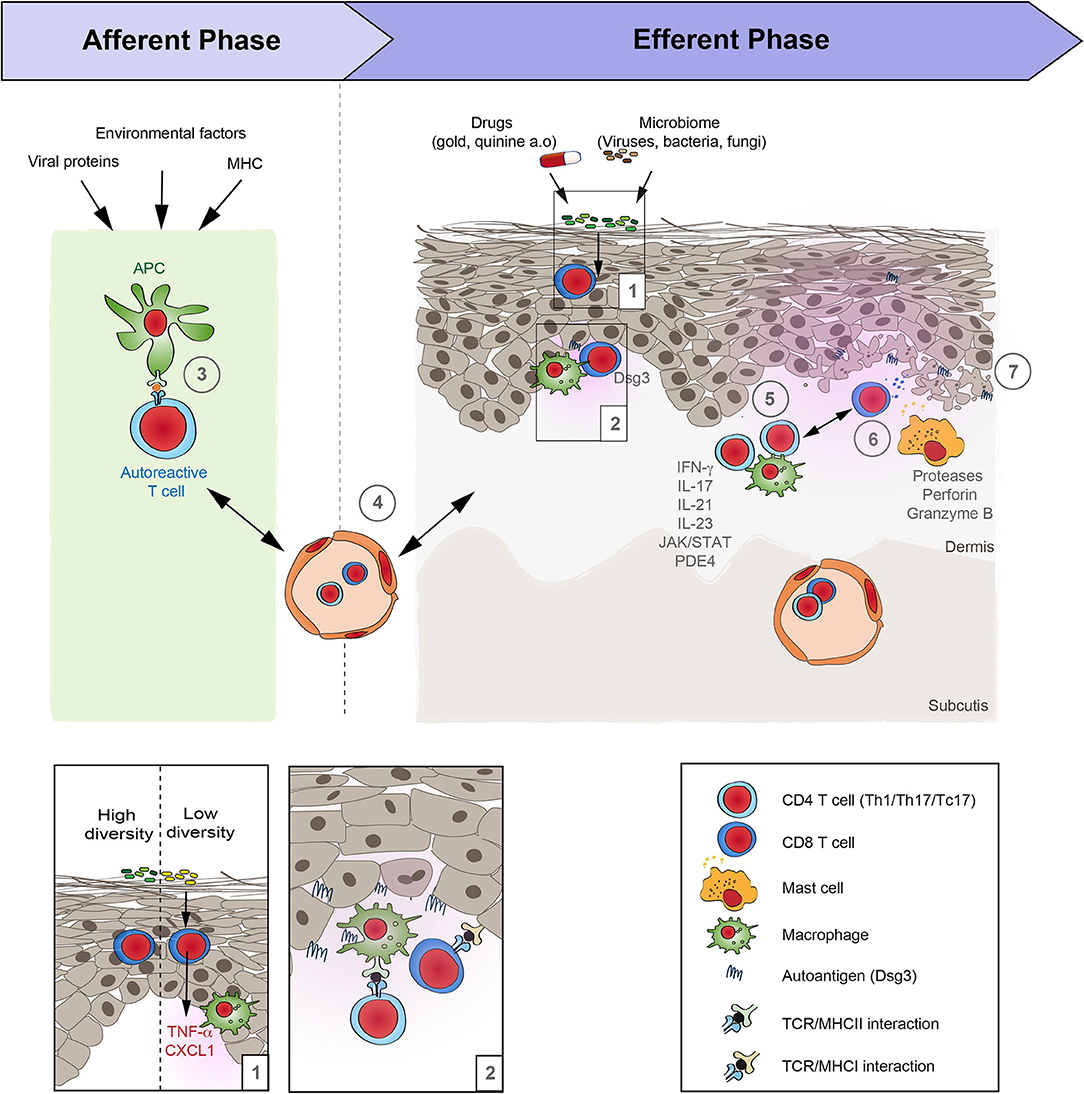
Figure 2. Schematic overview of lichen planus pathogenesis. Based on the increasing evidence of the autoimmune nature of lichen planus (LP), its pathogenesis may be divided in two distinct phases: Afferent phase, where tolerance to autoantigens is lost, and the efferent phase that is characterized by a T cell-driven skin inflammation. (1) Under steady state conditions the diversity of the cutaneous microbiome can alter the “inflammatory state” of the skin. If the diversity of the bacterial communities on the skin is low, this is associated with an increased expression of pro-inflammatory cytokines such as TNFα and CXCL1, as well as an increase of CD11c, suggesting an increased presence of macrophages. (2, 3) Following this presumed injury, the loss of tolerance in LP occurs in the context of an association with the MHC that may additionally be shaped by associated viral infections and other environmental factors. The site of the APC/T-cell interaction is so far unknown. It may occur locally and/or in skin draining lymph nodes. (4) Next, autoreactive T cells reach the skin by extravasation from the blood vessels. (5) Within the skin, T cells become activated by binding to the specific autoantigens. Effector functions of cytotoxic T cells is mediated by (6) proteases, such as granzyme B, as well as perforin. In addition, (7) mast cells become activated and may further aggravate inflammation in LP. However, the precise sequence of events and their interactions are only incompletely understood.
Most of the findings on LP pathogenesis are based on morphology. Only a limited number of studies also demonstrated a functional impact of cells and/or molecules on LP pathogenesis. A cell-mediated immune response is at the core of LP pathogenesis, with cytotoxic, CD8+ T-cells in the center (Figure 2). Yet, both CD4+ and CD8+ T-cells accumulate in the dermis and oral mucosa, whilst a CD8+ T-cell-dominant infiltrate is seen within the epidermis (3, 4). Other groups have reported that CD8+ and CD45RO+ T-cells are the major cell type in the inflammatory infiltrate and that the T cell receptor (TCR) αβ, and to a lesser extent TCR γδ, are expressed (68). The functional contribution of T-cells to LP pathogenesis is further supported by a recent study that showed granule exocytosis with the release of perforin and granzyme B. In this context, to a lesser extent, the Fas/Fas-ligand system appears to be involved, the main pathway of cytotoxicity by CD4+ and CD8+ T-cells in humans (5). In addition to T-cells, mast cells may contribute to LP pathogenesis given that they are often found in the inflammatory infiltrate and show signs of activation (69–72). Immunohistochemistry of oral LP also demonstrated the presence of dendritic cells (73). The fact that CD8+ T-cells and mast cells are detected in lesions of LP patients led to the conclusion that non-specific mechanisms like mast cell degranulation and protease activation are involved in the pathogenesis of LP. These mechanisms may combine to cause T-cell accumulation in lesions and induce keratinocyte apoptosis (74). In line, an increased protease expression has been described in LP lesions that potentially contributes to the disruption of the basement membrane gelatinases (e.g., MMP-2, MMP-7, and−9), chymase, tryptase, capthepsins and caspase-3 (74–79).
Several alterations in the expression of cytokines and chemokines in lesions or serum of patients with LP have been described. Serum levels of interleukin (IL)-5, IL-6, IL-8, IL-9, IL-10, IL-12 IL-17, IL-22, tumor necrosis factor-α, transforming growth factor-β, interferon (IFN)-γ, CXCR-3, CXCR-4, CXCL-10, CXCL-12, CCR1, CCR3, CCR4, CCL5-CCR5, and CCL17-CCR4) have been found elevated (80–89). In addition, an increased expression IFN-γ and IL-17 in the skin of LP lesions has been described (81, 90)—albeit some other studies refuted these observations (91). Case reports indicated that off-label treatment of LP with Janus kinase (JAK) inhibitors (JAKi), such as tofacitinib, led to marked improvement of the disease (92–94). As IFN-γ-induced signaling centers on the activation of JAK (95), IFN-γ and JAK are likely to be central to the pathogenesis of LP. Functional evidence for a pathogenic contribution of IL-17, including the IL-17 pathway, stems from the observation of increased IL-17 and IL-23 expression in LP (84), as well as the clinical improvement following off-label treatment of LP patients with the anti-IL-17 antibody secukinumab, or the IL-12/23-targeting ustekinumab or the IL-23 inhibitor guselkumab. Of note, clinical improvement of LP following IL-17 or IL-23 blockade was accompanied by a strong reduction of the Th1 and Th17/Tc17 cellular mucosal and cutaneous infiltrates (96). This supports the previously mentioned notion that these T-cell subsets may be key effector cells in LP.
The relative lack of functional insights into LP pathogenesis may be due to the limited number of pre-clinical model systems. So far, only one mouse model has been established that resembles aspects of LP pathogenesis. This model is based on the intradermal transfer of autoreactive CD4+ T-cells producing IFN-γ and TNF into syngeneic mice, inducing cellular infiltrates with epidermotropism with basal vacuolar degeneration and colloid bodies (2). Furthermore, desmoglein (Dsg) 3-specific T-cells are also capable of inducing histologically LP-like changes (97). Transfer of Dsg3-specific T-cells into immunodeficient mice induced an interface dermatitis (a distinct form of T-cell–mediated autoimmunity) in mice. The induction of the interface dermatitis depended on the specificity of the T-cell receptor as well as IFN-γ (97).
Besides systemic viral infection, several other diseases were shown to be associated with LP. A high prevalence of thyroid disease is found amongst patients with oral LP (98), whereas the association between LP and diabetes mellitus is less well-established (99). In addition to the association with chronic inflammatory diseases, patients with LP present a higher risk for dyslipidaemia, which could be explained by the cytokines involved in the pathogenesis of the disease, such as TNF-α, IL-6, IL-10, and IL-4 (100, 101). Autoimmune diseases such as alopecia areata, ulcerative colitis, vitiligo, morphea, lichen sclerosus and myasthenia gravis are over-represented in patients with LP (14).
Over 20 different clinical manifestations of LP are described (Table 1). Herein, we focus on the most common variants, as well as LP pemphigoides, lichenoid GVHD and lichenoid drug eruptions.
The hallmark of cutaneous LP are purple or violet, polygonal, shiny, flat-topped, firm, papules, and plaques with white streaks (Wickham striae) (40). Wickham striae are best visualized by dermoscopy (102, 103). The cutaneous lesions may vary in size from several millimeters to more than one centimeter. The lesions may be clustered or disseminated and whilst the typical locations are the wrists, lower back, and ankles, a distribution in photo-exposed areas is also well-recognized (Figures 1a–c). Skin conditions may also appear following the lines of trauma (isomorphic response, Figure 1f). The dominant subjective symptom is pruritus, which may be severe and refractory to standard anti-pruritic therapies.
The typical lesions of mucosal LP are painful and persistent erosions (erosive LP) or diffuse erythema and peeling of the mucosa (desquamative LP) (40). In addition, Wickham striae may be present in a lacy or fern-like pattern. Mucosal LP can be further subclassified into oral LP, affecting the buccal mucosa, the tongue, and to a lesser extend the gums and lips (Figures 1m,n) or genital LP, affecting the glans penis, labia majora, labia minora and vaginal introitus (Figures 1p–r). Chronic disease may result in scarring, with the formation of adhesions, resorption of labia minora and ultimately introital stenosis. Penile LP usually presents with papules around the glans penis, white streaks and erosions. In rare cases, mucosal LP may also affect the lacrimal glands, eyelids, external ear canal, esophagus, larynx, bladder, and anus.
Lichen planopilaris (LPP) presents as tiny red spiny follicular papules and extending smooth areas on the scalp or less often, elsewhere on the hair-bearing regions body areas (104, 105). Destruction of the hair follicles leads to permanently bald patches characterized by sparse “lonely hairs” (Figures 1i,j). Frontal fibrosing alopecia is a variant of LPP that affects the anterior scalp, forehead and eyebrows. Another subtype of LPP is Graham-Little-Piccardi-Lasseur Syndrome with the following characteristics: multifocal, patchy, cicatricial alopecia present on the scalp, non-cicatricial alopecia of the axillae, non-cicatricial alopecia of the perineum, and follicular hyperkeratosis of the trunk and extremities (106).
LP may affect one or more nails (Figure 1l), sometimes in the absence of skin involvement. LP thins the nail plate, which may become grooved and ridged. The nail may darken, thicken or lift off the nail bed (onycholysis). Sometimes, the cuticle is destroyed and forms a scar (pterygium). The nails may shed or stop growing altogether, and they may rarely, completely disappear (anonychia). An important clinical feature of nail LP is the occurrence of a dorsal pterygium.
LP pemphigoides is clinically characterized by the simultaneous occurrence of lichenoid and bullous skin lesions. By some, LP pemphigoides is considered as an autoimmune dermatosis with autoimmunity toward type XVII collagen (COL17). By contrast, others consider LP pemphigoides the co-occurrence of 2 independent skin diseases, or as a variant of LP (14, 107, 108).
Graft-vs.-host disease (GVHD) is the primary complication of allogeneic bone marrow transplantation and the skin is the most commonly involved organ. The clinical picture varies and often is similar to autoimmune or inflammatory diseases. Cutaneous GVDH can imitate classical lichen planus with purple, polygonal, pruritic papules (but without Wickham striae) or lichen planus pigmentosus (109, 110). GVHD refers to the inflammatory manifestations, when immunocompetent T-cells from a donor recognize and react against “foreign” tissue antigens in an immunocompromised host, this autoreactive pre-condition leads to a Th2 immune response induced interface dermatitis (109).
Lichenoid drug eruptions often mimic idiopathic lichen planus although there can be features that may help to distinguish them, which may include: symmetrical rash on the trunk and limbs, predominantly in sun-exposed areas. Skin features do normally not show Wickham striae, nail and mucous membrane involvement is missing. Medications reported to trigger a lichenoid drug eruptions are, exemplary (14): ACE inhibitors, beta-blockers, nifedipine, methyldopa, hydrochlorothiazide, frusemide, spironolactone, non-steroidal anti-inflammatory drugs (NSAIDs), carbamazepine, phenytoin, ketoconazole, 5-fluorouracil, imatinib, hydroxychloroquine, sulfonylurea, dapsone, mesalazine, sulfasalazine, allopurinol, iodides and radiocontrast media, interferon-α, omeprazole, penicillamine, tetracycline, infliximab, etanercept, adalimumab, imatinib, misoprostol, sildenafil, and herpes zoster/influenza vaccines.
Contact allergies also may mimic lichen planus: Oral lichenoid lesions may be associated to type-IV-sensitization to mercury or dental amalgam (111, 112); lichenoid skin lesions usually result from contact with rubber, chemicals used in clothing dyes or chemicals in wine industries (113).
A skin/mucosal biopsy is recommended to confirm the diagnosis of LP. The typical histological findings are acanthosis and hyperkeratosis, wedge-shaped hypergranulosis, vacuolic degeneration of the basal layer, alteration or loss of rete ridges resulting in a sawtooth appearance and a dense, band-like lymphocytic infiltrate in the upper dermis along the dermal-epidermal junction (Figures 1h,k,o,s). Apoptotic keratinocytes are often seen near the basal layer and are termed colloid bodies. For LP affecting the scalp, for example LPP, shows beside the penitent LP features often the destruction of hair follicle root sheaths and follicular plugging as well as the loss of sebaceous glands as well (114) (Figure 1k).
Additionally, a lesional biopsy for direct IF microscopy can be a useful, especially when trying to differentiate between LP and other autoimmune diseases, such as pemphigus vulgaris, mucous membrane pemphigoid, or lupus erythematosus (LE) (115, 116). In LP, direct IF microscopy (Figure 1g) may reveal globular deposits of IgA, IgM, IgG, C3, or fibrinogen mixed with apoptotic keratinocytes (117, 118).
The differential diagnosis of cutaneous LP is broad and includes graft-vs. host-disease, psoriasis vulgaris, guttate psoriasis, secondary syphilis, pityriasis lichenoides, pityriasis rosea, lichen nitidus, lichen simplex chronicus, lichen sclerosus, lichen striatus, linear epidermal naevus, eczema, prurigo nodularis, erythema dyschromicum perstans, eczematid-like purpura, drug eruption, granuloma annulare, lichen amyloidosus, Kaposi sarcoma and lupus erythematosus. In most cases, histology permits a reasonable differentiation between these diseases and inflammatory disorders.
Similarly, an extensive list of differential diagnoses should be considered when diagnosing LP of the oral cavity including pemphigus vulgaris, mucous membrane pemphigoid, lupus erythematosus, secondary syphilis, traumatic patches, and candidiasis. Vulval/penile LP can be difficult to distinguish from lichen sclerosis, mucous membrane pemphigoid, psoriasis, intraepithelial neoplasia, graft-vs.-host disease, erosive dermatitis, and intertrigo. Histology and direct IF microscopy should allow a definite diagnosis of LP and the exclusion of other diseases.
Various diseases may appear similar to LPP, especially when the destruction of the hair follicles leads to permanently bald or even scarring patches without inflammation or tiny red spiny follicular papules, such as patchy alopecia in systemic LE, alopecia areata, diffuse alopecia due to secondary syphilis or severe folliculitis. Brunsting-Perry cicatricial pemphigoid is rare variant of mucous membrane pemphigoid associated with scarring on the head and neck region. Differentiating them can be difficult, besides punch biopsy trichometric analysis, fungal culture, blood tests are recommended to find the underlying medical condition.
Nail LP can be challenging to differentiate from psoriasis, atopical dermatitis, alopecia areata and onychomycosis. For the later, appropriate laboratory testing for presence of fungi is recommended.
The ultimate aim of treatment is the resolution of the skin lesions and their associated symptoms. This is particularly important in oral LP where painful erosions can result in significant malnutrition and weight loss. Drug-induced LP should always be considered and excluded prior to commencing immunosuppressive therapy (14) and the responsible drug discontinued or substituted. An LP-associated diseases should be checked in each patient. Hypertrophic and mucosal LP lesions are potentially premalignant and regular follow-up and biopsies should be considered to exclude malignant transformation (Table 3).
The first-line treatments for limited LP are (super)potent topical steroids, with intralesional steroid injection reserved for hypertrophic and/or unresponsive lesions (14, 119, 120). For disseminated disease, systemic corticosteroids can be considered, either as oral therapy or intravenous “pulse” therapy, to achieve disease control. Thereafter, the oral dose can be tapered or the interval between intravenous administrations extended (14, 121, 122). Other first-line therapies include systemic retinoids (acitretin/isotretinoin) or cyclosporine (14, 123–126). If diffuse cutaneous LP remains unresponsive, second-line therapy should be considered. These include sulphasalazine, and phototherapy such as broadband/narrowband UVB or psoralen and UVA (PUVA), and the combination of UV/PUVA with retinoids (14, 121, 127–130). For topical treatment of limited and diffuse cutaneous LP calcineurin inhibitors can be used to reduce side-effects of topical steroids (14, 131). Third-line treatments include hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or biologics targeting IL-12/23 (14, 121, 130, 132–136). Based on the fact that in LP proinflammatory signaling pathways result in T-cell-dependent immune response, oral JAKi may represent a future treatment option (137). Oral antihistamines may be helpful to minimize the itch (14). Topical antipruritic agents such as menthol, camphor, or polidocanol can be prescribed as an adjuvant to the main treatment (14). The majority of patients with cutaneous lesions spontaneously clear within 12–24 months (21); however, relapses are common. Healing may also be complicated by the development of post-inflammatory hyperpigmentation (1, 9).
Mucosal LP is often difficult to treat, particularly when extensive erosions are present. Long-term follow-up is necessary to monitor disease activity and to exclude malignant transformation of erosive lesions (14). The mainstay of treatment of mucosal LP are topical corticosteroids (14, 138). Superpotent steroids can be applied topically (in the form of an adhesive paste) twice daily for 1–2 months, and then administered as required (14). Intralesional steroid injections are worth considering when lesions are particularly painful and fail to respond to topical therapy (14, 138, 139). Systemic corticosteroids are reserved for patients with severe erosive mucosal LP (recalcitrant, multi-site, ulcers) and to more rapidly induce a remission (14). Further systemic first-line treatments are retinoids (acitretin/isotretinoin) and cyclosporine (14). Second-line treatments include sulphasalasine, azathioprine, hydroxychloroquine, methotrexate, mycophenolate mofetil, and/or use of topical calcineurin inhibitors (14, 133, 140–147). Third-line treatment may include cyclophosphamide, thalidomide, metronidazole, trimethoprim–sulphomethoxazole, antibiotic treatment, itraconazole, griseofulvin, dapsone and extracorporeal photochemotherapy (ECP) (14, 148–156).
In the management of oral LP, lidocain solution as mouthwash may be helpful to reduce pain. Amphotericin B solution as mouthwash several times daily (after food consumption) may prevent secondary candida infection. Patch tests may be recommended for patients with oral lichen planus affecting the gums and who have fillings with amalgam, to assess for contact allergy to thiomersal, a mercurial compound (14). Mucosal LP may clear spontaneously within 5 years, but typically it is a chronic disease with a remitting and relapsing course (22, 23).
The general principles of the management of genital LP are similar to those of LP confined to the oral mucosa (14). Most cases of papulosquamous genital LP are self-limited, and treatment with emollients and mid-potency steroids for a few weeks leads to complete remission. First-line treatment for erosive LP of the vulval or penile mucosa are superpotent topical corticosteroids (14, 157), which can be gradually tapered (14). Calcineurin inhibitors (tacrolimus/pimecrolimus) are a further topical treatment option (14). The aim for the treatment of erosive genital lesions is the prevention or limitation of scarring. In women, synechia formation with vaginal stenosis may be prevented by the use of vaginal dilators and application of intra-vaginal steroids to treat mucosal inflammation (14). In uncircumcised men, circumcision is usually recommended to avoid phimosis (14). Local anesthetic gel, low-dose tricyclic antidepressants or anticonvulsants may relieve itch and ease discomfort and nystatin cream can prevent secondary fungal infections (14).
The aim of the treatment is disease control to prevent permanent hair loss due to scarring (14). Furthermore, treatment can reduce itching and burning of the scalp. Topical steroids are treatment of first choice (97, 158–160). Intralesional steroid injections may improve response rates (161). Topical calcineurin inhibitors may be used as monotherapy or as an adjuvant to systemic therapy proved effective (162). Systemic steroids are the mainstay of treatment for rapidly progressive disease to prevent scarring (163), while introducing cyclosporine, methotrexate, or hydroxychloroquine as steroid sparing agents (160, 164–168). Suggested second-line options are retinoids (acitretin/isotretinoin), tetracycline/doxycycline, mycophenolate mofetil, adalimumab, pioglitazone, thalidomide, or rituximab (160, 162, 165, 167, 169–175).
LP of the nails is generally difficult to treat and the prognosis is poor (14). LP affecting the nails frequently leads to permanent destruction of the nail matrix and bed with functional limitations. Therefore, early treatment is essential, even in mild cases of nail LP (176). Potent topical steroids under occlusive dressings are the preferred, first-line topical treatment (14). Due to the poor short-term efficacy of topical steroids and long-term side effects, triamcinolone acetonide injections (intralesional) should be considered as further first-line therapies (176, 177). Oral prednisone 0.5 mg/kg for 3 weeks demonstrated a marked improvement and is useful when multiple nails are affected (14). Oral retinoids are second-line choices (178–180), and immunosuppressive agents may also be considered (14, 181, 182). In a case series, topical tacrolimus ointment 0.1% was successfully used in treatment of nail LP (183).
Corticosteroids are the backbone the treatment of cutaneous lichenoid GVHD, but ~30% need additional immunosuppressant such as cyclosporine. cyclophosphamide, methotrexate, azathioprine, mycophenolate mofetil, pentostatin, or high-dose thalidomide and hydroxychloroquine (184). Another option for skin involvement might be phototherapy, while extracorporeal photochemotherapy may improve cutaneous as well as systemic involvement (184).
The triggering agent should be stopped (14); improvement of the skin lesions can take weeks to months. Commonly flat pigmented freckles persist and fade more slowly. Steroids (topical/systemically) may be supportive to give relief or rapid resolution.
In LP, new therapeutic options currently stem from case reports and/or case report series. These have set the rationale for the planning of current clinical trials in LP (Table 4). The molecular targets currently persued for LP can be categorized into biologics targeting cytokines and small molecules blocking intracellular signaling. In addition, photodynamic therapy has consistently been reported to have favorable outcomes in LP patients (185).
Currently licensed (for other indications than LP) biologics targeting IL-17 or the IL-17R are secukinumab, ixekizumab and brodalumab. In 2017, occurrence of oral LP was noted in a psoriasis patient treated with secukinumab. As concurrently oral candidiasis, a relatively common adverse event under anti-IL-17 treatment, was present, the causality of IL-17 inhibition and induction of oral LP remained ambiguous (186). In addition to this case, 3 more cases of cutaneous/oral lichenoid eruptions associated with IL-17 inhibition were noted, albeit (oral) LP was not formally diagnosed (187–189). By contrast, response to IL-17 inhibition has been reported in a total of 5 LP patients (96, 190, 191). Grounded on the latter observations, as well as the increased serum and tissue IL-17 expression in both oral and cutaneous LP (83, 192, 193), two clinical studies currently evaluate the impact of IL-17 inhibition using secukinumab or ixekizumab in patients with LP (NCT04300296, NCT05030415).
Biologics targeting either IL-12 and/or IL-23 (ustekinumab) or IL-23 alone (risankizumab, tildrakizumab and guselkumab) have so far not been associated with the induction of LP. However, one report noted a failure of LP to respond to ustekinumab (194). Later observations noted a response of IL-12/23 inhibition on one patient with LP pemphigoides (195), and three patients with LP (96, 196). Use of IL-12/23 was grounded on the observation of increased IL-23 expression in oral LP (84), as well as an increased serum concentration of IL-23 in LP patients (197). Of note, we are not aware of any study addressing the impact of IL-12/23 inhibition in LP.
By contrast to IL-17 and IL-23, blockade of TNF-α has not emerged as a promising therapeutic target in LP. There are several reports on lichenoid drug eruptions following TNF-α inhibition (198, 199), and only one report on a successful treatment of lichen planus with the anti-TNF-α antibody adalimumab (135). On the expression level, increased TNF-α expression has been noted in the skin and serum of LP patients (60, 90). In line, a study evaluating the impact of the TNF-α inhibitor etanercept of LP was terminated due to slow recruitment in 2018 (NCT00285779).
Recent work showed that the inflammation in LP is dominated by an IFN-γ and an IL-21 signature, along with an increased expression of phospho-STAT1 in the dermal infiltrate (200). In another T-cell mediated inflammatory skin disease, namely alopecia areata, the identification of an IFN gene signature in affected skin identified JAK inhibitors as potential new treatments for alopecia areata, which showed efficacy in phase 2 clinical trials (201, 202). Based on these morphological observations and considerations, the authors concluded that use of JAK inhibitors may be beneficial in LP (200). Successful treatment of a treatment-refractory LP patient with the JAK1/3-selective JAKi tofacitinib supports this notion. Furthermore, in 2 independent case series, tofacitinib used as either monotherapy or adjunctive therapy led to clinical improvement in 11/13 patients (95, 96). In line with these observations, a clinical trial currently investigated the impact of topical ruxolitinib in LP patients. The trial was completed in 2020. Results are shown at clinicaltrial.gov: 12 patients were enrolled, 3 were lost to follow-up, most likely related to the Covid-19 pandemic, and no serious adverse events occurred (NCT03697460).
Grounded on the broad anti-inflammatory activity of the PDE4 inhibitor apremilast, the safety and efficacy of the drug in LP patient's refractory to topical corticosteroid treatment was evaluated in an investigator-initiated, single-center, non-randomized, open-label, pilot study in 2013. Patients were treated with 2 × 20 mg apremilast per day for 12 weeks. The primary endpoint was achieving a 2-grade or more in Physician Global Assessment (PGA) at 12 weeks. While all patients demonstrated a significant clinical improvement, 3/10 met the primary endpoint (137). Subsequently, a total of 5 LP patients, mostly with treatment-refractory disease, were reported to improve when treated with apremilast (203–205). Based on these reports, apremilast is currently evaluated in a randomized placebo-controlled clinical trial in women with genital erosive lichen planus (206). Currently, patients are recruited to this study (NCT03656666).
Other clinical trials are evaluating the impact of topical mechlorethamine, a topical chemotherapy used for the treatment of cutaneous T cell lymphoma (207), in LP. The study was completed in 2019, but so far results have not been published (NCT03417141). A study treating LP patients with the opiate opioid receptor antagonist naltrexone was recently completed (NCT04409041). Again, results are pending to be published. In this line, a small case series reported on the beneficial outcome of naltrexone in LPP (208).
Most of non-pharmacological interventions for LP that are currently evaluated in clinical trails focus on the use of photodynamic therapy (PDT) (NCT04976673, NCT01282515). Both studies are completed, while the results have not been published so far. Up to date, a total of 5 controlled studies has addressed the impact of PDT in oral LP (209–213). In most studies, topical steroids were used as an active comparator. In 3/5 studies, no difference between the 2 treatment modalities (that both led to a reduced severity of LP) was observed (209, 211, 212), whilst in 2/5 studies, a superior effect of PDT was noted (211, 213). In an open study using PDT in oral LP, a significant change of molecular disease markers (reduced numbers of CD4+ and CD8+ T-cells in the lesions, reduced numbers of activated T cells in the circulation) were observed in parallel to the clinical improvement (214).
In addition, a recent retrospective investigation and review of the impact of narrowband UVB phototherapy and psoralen plus UVA (PUVA) photochemotherapy as second-line treatment of LP showed a relatively good response (complete responses in a little over 70% for both narrow-bad UVB and PUVA), whereby adverse events were only observed in patients treated with oral PUVA (215).
Previous reports also indicated a good response of recalcitrant LP to extracorporeal photochemotherapy (ECP) (155, 216, 217). In a larger case series, 9/12 patients showed complete remission and 3/12 a partial response. In follow-up, relapse occurred frequently when ECP sessions were less frequent or stopped (216). Since 2010, no more reports on the use of ECP in LP were published. Hence, ECP may be used in LP cases refractory of several previous therapies, and additional treatments should be administrated to maintain the (presumed) initial good response to ECP.
Overall, LP is an under-recognized dermatosis, whose epidemiology and pathogenesis is only partially understood, the disease is associated with significant morbidity, and current treatment options are limited in their success. Given the lack of double-blind randomized control trials, treatment is often based on clinical experience and the results of retrospective meta-analyses (121, 218). Biological treatments (93) and JAKi (96) hold significant promise as future therapeutic options. The lack of animal models underscores the importance of a comprehensive understanding of the pathogenesis of LP elucidating human phenotype-genotype correlations facilitating renewed efforts to unravel the cellular and molecular changes underlying the disease (219). Still, with the emergence of biological treatment options and of JAKi that both derived from careful clinical observations, the treatment landscape of LP will hopefully improve in the near future.
KBo, EL, KK, RL, and KBi wrote the manuscript. All authors read, commented, and approved the final version of the manuscript.
This work was supported by structural funding from the Deutsche Forschungsgemeinschaft through Excellence Cluster 2167/1 Precision Medicine in Chronic Inflammation and the Schleswig-Holstein Excellence Chair Program from the State of Schleswig-Holstein. KK received support from the Alexander von Humboldt Foundation (Humboldt Research Fellowship for Post-doctoral Researchers).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. (1991) 25:593–619. doi: 10.1016/0190-9622(91)70241-S
2. Shiohara T, Moriya N, Mochizuki T, Nagashima M. Lichenoid tissue reaction (LTR) induced by local transfer of Ia-reactive T-cell clones. II LTR by epidermal invasion of cytotoxic lymphokine-producing autoreactive T cells. J Investig Dermatol. (1987) 89:8–14. doi: 10.1111/1523-1747.ep12523539
3. Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. (2000) 142:449–56. doi: 10.1046/j.1365-2133.2000.03355.x
4. Shiohara T, Moriya N, Nagashima M. Induction and control of lichenoid tissue reactions. Springer Semin Immunopathol. (1992) 13:369–85. doi: 10.1007/BF00200535
5. Yasukawa M, Ohminami H, Arai J, Kasahara Y, Ishida Y, Fujita S. Granule exocytosis, and not the fas/fas ligand system, is the main pathway of cytotoxicity mediated by alloantigen-specific CD4(+) as well as CD8(+) cytotoxic T lymphocytes in humans. Blood. (2000) 95:2352–5. doi: 10.1182/blood.V95.7.2352
6. Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives. J Invest Dermatol. (2009) 129:1088–99. doi: 10.1038/jid.2009.42
7. Scheler M, Wenzel J, Tuting T, Takikawa O, Bieber T, von Bubnoff D. Indoleamine 2,3-dioxygenase (IDO): the antagonist of type I interferon-driven skin inflammation? Am J Pathol. (2007) 171:1936–43. doi: 10.2353/ajpath.2007.070281
8. Dutz JP. T-cell-mediated injury to keratinocytes: insights from animal models of the lichenoid tissue reaction. J Invest Dermatol. (2009) 129:309–14. doi: 10.1038/jid.2008.242
9. Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. (2012) 366:723–32. doi: 10.1056/NEJMcp1103641
10. Alaizari NA, Al-Maweri SA, Al-Shamiri HM, Tarakji B, Shugaa-Addin B. Hepatitis C virus infections in oral lichen planus: a systematic review and meta-analysis. Aust Dent J. (2016) 61:282–7. doi: 10.1111/adj.12382
11. Giannetti L, Dello Diago AM, Spinas E. Oral Lichen planus. J Biol Regul Homeost Agents. (2018) 32:391–5.
12. Halevy S, Shai A. Lichenoid drug eruptions. J Am Acad Dermatol. (1993) 29:249–55. doi: 10.1016/0190-9622(93)70176-T
13. Asarch A, Gottlieb AB, Lee J, Masterpol KS, Scheinman PL, Stadecker MJ, et al. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. J Am Acad Dermatol. (2009) 61:104–11. doi: 10.1016/j.jaad.2008.09.032
14. Ioannides D, Vakirlis E, Kemeny L, Marinovic B, Massone C, Murphy R, et al. European S1 guidelines on the management of lichen planus: a cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J Eur Acad Dermatol Venereol. (2020) 34:1403–14. doi: 10.1111/jdv.16464
15. Chuang TY, Stitle L, Brashear R, Lewis C. Hepatitis C virus and lichen planus: a case-control study of 340 patients. J Am Acad Dermatol. (1999) 41:787–9. doi: 10.1016/S0190-9622(99)70025-3
16. Nagao Y, Kameyama T, Sata M. Hepatitis C virus RNA detection in oral lichen planus tissue. Am J Gastroenterol. (1998) 93:850. doi: 10.1111/j.1572-0241.1998.850_a.x
17. Carrozzo M, Francia Di Celle P, Gandolfo S, Carbone M, Conrotto D, Fasano ME, et al. Increased frequency of HLA-DR6 allele in Italian patients with hepatitis C virus-associated oral lichen planus. Br J Dermatol. (2001) 144:803–8. doi: 10.1046/j.1365-2133.2001.04136.x
18. Pilli M, Penna A, Zerbini A, Vescovi P, Manfredi M, Negro F, et al. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. (2002) 36:1446–52. doi: 10.1002/hep.1840360622
19. Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet. (2015) 385:1124–35. doi: 10.1016/S0140-6736(14)62401-6
20. Jain S, Basavaraj V. Direct Immunofluorescence Studies in Lichen Planus. Turk Patoloji Derg. (2019) 35:193–7. doi: 10.5146/tjpath.2018.01455
21. Irvine C, Irvine F, Champion RH. Long-term follow-up of lichen planus. Acta Derm Venereol. (1991) 71:242–4.
22. Mignogna MD, Lo Muzio L, Lo Russo L, Fedele S, Ruoppo E, Bucci E. Oral lichen planus: different clinical features in HCV-positive and HCV-negative patients. Int J Dermatol. (2000) 39:134–9. doi: 10.1046/j.1365-4362.2000.00903.x
23. Boch K, Langan EA, Zillikens D, Ludwig RJ, Kridin K. Retrospective analysis of the clinical characteristics and patient-reported outcomes in vulval lichen planus: results from a single-center study. J Dermatol. (2021). doi: 10.1111/1346-8138.16191
24. Li C, Tang X, Zheng X, Ge S, Wen H, Lin X, et al. Global prevalence and incidence estimates of oral lichen planus: a systematic review and meta-analysis. JAMA Dermatol. (2020) 156:172–81. doi: 10.1001/jamadermatol.2019.3797
25. Bhonsle RB, Pindborg JJ, Gupta PC, Murti PR, Mehta FS. Incidence rate of oral lichen planus among Indian villagers. Acta Derm Venereol. (1979) 59:255–7.
26. Nagao T, Ikeda N, Fukano H, Hashimoto S, Shimozato K, Warnakulasuriya S. Incidence rates for oral leukoplakia and lichen planus in a Japanese population. J Oral Pathol Med. (2005) 34:532–9. doi: 10.1111/j.1600-0714.2005.00349.x
27. Pannell RS, Fleming DM, Cross KW. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect. (2005) 133:985–91. doi: 10.1017/S0950268805004425
28. Diop A, Ly F, Ndiaye MT, Seck B, El Omari A, Diouf A, et al. Epidemiology, clinical features, and associated factors in 78 cases of lichen planus on black skin. Int J Dermatol. (2020) 59:137–42. doi: 10.1111/ijd.14698
29. Halonen P, Jakobsson M, Heikinheimo O, Gissler M, Pukkala E. Incidence of Lichen planus and subsequent mortality in finnish women. Acta Dermato-Venereol. (2020) 100:adv00303. doi: 10.2340/00015555-3664
30. Axell T, Rundquist L. Oral lichen planus–a demographic study. Commun Dent Oral Epidemiol. (1987) 15:52–6. doi: 10.1111/j.1600-0528.1987.tb00480.x
31. Schwager Z, Stern M, Cohen J, Femia A. Clinical epidemiology and treatment of lichen planus: a retrospective review of 2 tertiary care centers. J Am Acad Dermatol. (2019) 81:1397–9. doi: 10.1016/j.jaad.2019.04.027
32. Wagner G, Rose C, Sachse MM. Clinical variants of lichen planus. J Dtsch Dermatol Ges. (2013) 11:309–19. doi: 10.1111/ddg.12031
33. Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, Bertolusso G, et al. Course of oral lichen planus: a retrospective study of 808 northern Italian patients. Oral Dis. (2009) 15:235–43. doi: 10.1111/j.1601-0825.2009.01516.x
34. Valsecchi R, Bontempelli M, di Landro A, Barcella A, Lainelli T. Familial lichen planus. Acta Derm Venereol. (1990) 70:272–3.
35. Mukhopadhyay AK, Dave JN, Shah S, Vora NS, Cardoso BJ, Ghosh A. Lichen planus in monozygotic twins. Indian J Dermatol Venereol Leprol. (1996) 62:252–3.
36. Pavlovsky L, Israeli M, Sagy E, Berg AL, David M, Shemer A, et al. Lichen planopilaris is associated with HLA DRB1*11 and DQB1*03 alleles. Acta Derm Venereol. (2015) 95:177–80. doi: 10.2340/00015555-1884
37. Nagao Y, Nishida N, Toyo-Oka L, Kawaguchi A, Amoroso A, Carrozzo M, et al. Genome-wide association study identifies risk variants for lichen planus in patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. (2017) 15:937–44.e5. doi: 10.1016/j.cgh.2016.12.029
38. Gao XH, Barnardo MC, Winsey S, Ahmad T, Cook J, Agudelo JD, et al. The association between HLA DR, DQ antigens, and vulval lichen sclerosus in the UK: HLA DRB112 and its associated DRB112/DQB10301/04/09/010 haplotype confers susceptibility to vulval lichen sclerosus, and HLA DRB10301/04 and its associated DRB10301/04/DQB10201/02/03 haplotype protects from vulval lichen sclerosus. J Invest Dermatol. (2005) 125:895–9. doi: 10.1111/j.0022-202X.2005.23905.x
39. Setterfield JF, Neill S, Shirlaw PJ, Theron J, Vaughan R, Escudier M, et al. The vulvovaginal gingival syndrome: a severe subgroup of lichen planus with characteristic clinical features and a novel association with the class II HLA DQB1*0201 allele. J Am Acad Dermatol. (2006) 55:98–113. doi: 10.1016/j.jaad.2005.12.006
40. Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci World J. (2014) 2014:742826. doi: 10.1155/2014/742826
41. Liu J, Ye Z, Mayer JG, Hoch BA, Green C, Rolak L, et al. Phenome-wide association study maps new diseases to the human major histocompatibility complex region. J Med Genet. (2016) 53:681–9. doi: 10.1136/jmedgenet-2016-103867
42. Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol. (2009) 145:1040–7. doi: 10.1001/archdermatol.2009.200
43. Ma SH, Tai YH, Dai YX, Chang YT, Chen TJ, Chen MH. Association between hepatitis C virus infection and subsequent chronic inflammatory skin disease. J Dermatol. (2021). doi: 10.1111/1346-8138.16129. [Epub ahead of print].
44. de Vries HJ, Teunissen MB, Zorgdrager F, Picavet D, Cornelissen M. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. (2007) 299:213–9. doi: 10.1007/s00403-007-0750-0
45. Balighi K, Daneshpazhooh M, Lajevardi V, Mahmoudi H, Azizzadeh-Roodpishi S, Tavakolpour S, et al. Association of human herpes virus 6 infection with lichen planopilaris. Mymensingh Med J. (2020) 29:977–82.
46. Requena L, Kutzner H, Escalonilla P, Ortiz S, Schaller J, Rohwedder A. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. (1998) 138:161–8. doi: 10.1046/j.1365-2133.1998.02045.x
47. Mizukawa Y, Horie C, Yamazaki Y, Shiohara T. Detection of varicella-zoster virus antigens in lesional skin of zosteriform lichen planus but not in that of linear lichen planus. Dermatology. (2012) 225:22–6. doi: 10.1159/000339771
48. Viguier M, Bachelez H, Poirier B, Kagan J, Battistella M, Aubin F, et al. Peripheral and local human papillomavirus 16-specific CD8+ T-cell expansions characterize erosive oral lichen planus. J Invest Dermatol. (2015) 135:418–24. doi: 10.1038/jid.2014.397
49. Shiohara T, Mizukawa Y, Takahashi R, Kano Y. Pathomechanisms of lichen planus autoimmunity elicited by cross-reactive T cells. Curr Dir Autoimmun. (2008) 10:206–26. doi: 10.1159/000131456
50. Casu C, Vigano L. Oral lichen planus and HPV lesions. Pan Afr Med J. (2018) 29:74. doi: 10.11604/pamj.2018.29.187.15110
51. Shang Q, Peng J, Zhou Y, Chen Q, Xu H. Association of human papillomavirus with oral lichen planus and oral leukoplakia: a meta-analysis. J Evid Based Dent Pract. (2020) 20:101485. doi: 10.1016/j.jebdp.2020.101485
52. Chovatiya R, Silverberg JI. Association of herpes zoster and chronic inflammatory skin disease in US inpatients. J Am Acad Dermatol. (2020). 17:S0190–9622(20)30065-7. doi: 10.1016/j.jaad.2019.12.073
53. Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol. (2001) 45:614–5. doi: 10.1067/mjd.2001.114590
54. Bombeccari GP, Gianni AB, Spadari F. Oral Candida colonization and oral lichen planus. Oral Dis. (2017) 23:1009–10. doi: 10.1111/odi.12681
55. Liu J, Geng F, Sun H, Wang X, Zhang H, Yang Q, et al. Candida albicans induces TLR2/MyD88/NF-kappaB signaling and inflammation in oral lichen planus-derived keratinocytes. J Infect Dev Ctries. (2018) 12:780–6. doi: 10.3855/jidc.8062
56. Yang XH Li HF, Xing FL, Tao MC, Cao Y. Correlation between the sap gene of Candida albicans and oral lichen planus. J Biol Regul Homeost Agents. (2019) 33:935–40.
57. Choi YS, Kim Y, Yoon HJ, Baek KJ, Alam J, Park HK, et al. The presence of bacteria within tissue provides insights into the pathogenesis of oral lichen planus. Sci Rep. (2016) 6:29186. doi: 10.1038/srep29186
58. Ellebrecht CT, Srinivas G, Bieber K, Banczyk D, Kalies K, Künzel S, et al. Skin microbiota-associated inflammation precedes autoantibody induced tissue damage in experimental epidermolysis bullosa acquisita. J Autoimmun. (2016) 68:14–22. doi: 10.1016/j.jaut.2015.08.007
59. Adami GR, Yeung AC, Stucki G, Kolokythas A, Sroussi HY, Cabay RJ, et al. Gene expression based evidence of innate immune response activation in the epithelium with oral lichen planus. Arch Oral Biol. (2014) 59:354–61. doi: 10.1016/j.archoralbio.2013.12.010
60. Chen X, Liu Z, Yue Q. The expression of TNF-alpha and ICAM-1 in lesions of lichen planus and its implication. J Huazhong Univ Sci Technolog Med Sci. (2007) 27:739–41. doi: 10.1007/s11596-007-0632-x
61. Wang Y, Shang S, Sun Q, Chen J, Du G, Nie H, et al. Increased infiltration of CD11 c +/CD123 + dendritic cell subsets and upregulation of TLR/IFN-α signaling participate in pathogenesis of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol. (2018) 125:459–467.e2. doi: 10.1016/j.oooo.2017.12.003
62. Sasaki G, Yokozeki H, Katayama I, Nishioka K. Three cases of linear lichen planus caused by dental metal compounds. J Dermatol. (1996) 23:890–2. doi: 10.1111/j.1346-8138.1996.tb02720.x
63. Schlosser BJ. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol Ther. (2010) 23:251–67. doi: 10.1111/j.1529-8019.2010.01322.x
64. Tan SV, Berth-Jones J, Burns DA. Lichen planus and photo-onycholysis induced by quinine. Clin Exp Dermatol. (1989) 14:335. doi: 10.1111/j.1365-2230.1989.tb02006.x
66. Callander J, Frost J, Stone N. Ultraviolet filters in hair-care products: a possible link with frontal fibrosing alopecia and lichen planopilaris. Clin Exp Dermatol. (2018) 43:69–70. doi: 10.1111/ced.13273
67. Westphal DC, Caballero-Uribe N, Regnier A, Taguti P, Dutra Rezende H, Trüeb RM. Male frontal fibrosing alopecia: study of 35 cases and association with sunscreens, facial skin and hair care products. J Eur Acad Dermatol Venereol. (2021) 35:e587–9. doi: 10.1111/jdv.17317
68. Wenzel J, Tuting T. An IFN-associated cytotoxic cellular immune response against viral, self-, or tumor antigens is a common pathogenetic feature in “interface dermatitis”. J Invest Dermatol. (2008) 128:2392–402. doi: 10.1038/jid.2008.96
69. Ramalingam S, Malathi N, Thamizhchelvan H, Sangeetha N, Rajan ST. Role of mast cells in oral lichen planus and oral lichenoid reactions. Autoimmune Dis. (2018) 2018:7936564. doi: 10.1155/2018/7936564
70. Zhao ZZ, Sugerman PB, Zhou XJ, Walsh LJ, Savage NW. Mast cell degranulation and the role of T cell RANTES in oral lichen planus. Oral Dis. (2001) 7:246–51. doi: 10.1034/j.1601-0825.2001.70408.x
71. Zhao ZZ, Savage NW, Pujic Z, Walsh LJ. Immunohistochemical localization of mast cells and mast cell-nerve interactions in oral lichen planus. Oral Dis. (1997) 3:71–6. doi: 10.1111/j.1601-0825.1997.tb00015.x
72. Regauer S, Beham-Schmid C. Benign mast cell hyperplasia and atypical mast cell infiltrates in penile lichen planus in adult men. Histol Histopathol. (2014) 29:1017–25. doi: 10.14670/HH-29.1017
73. Santoro A, Majorana A, Roversi L, Gentili F, Marrelli S, Vermi W, et al. Recruitment of dendritic cells in oral lichen planus. J Pathol. (2005) 205:426–34. doi: 10.1002/path.1699
74. Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus–a review. J Oral Pathol Med. (2010) 39:729–34. doi: 10.1111/j.1600-0714.2010.00946.x
75. Wang H, Guan X, Luo Z, Liu Y, Ren Q, Zhao X. The association and potentially destructive role of Th9/IL-9 is synergistic with Th17 cells by elevating MMP9 production in local lesions of oral lichen planus. J Oral Pathol Med. (2018) 47:425–33. doi: 10.1111/jop.12690
76. Rubaci AH, Kazancioglu HO, Olgac V, Ak G. The roles of matrix metalloproteinases-2,−7,−10 and tissue inhibitor of metalloproteinase-1 in the pathogenesis of oral lichen planus. J Oral Pathol Med. (2012) 41:689–96. doi: 10.1111/j.1600-0714.2012.01160.x
77. Kitkhajornkiat A, Rungsiyanont S, Talungchit S, Jirawechwongsakul P, Taebunpakul P. The expression of Cathepsin L in oral lichen planus. J Oral Biol Craniofacial Res. (2020) 10:281–6. doi: 10.1016/j.jobcr.2020.06.003
78. Satelur KP, Bopaiah S, Bavle RM, Ramachandra P. Role of cathepsin B as a marker of malignant transformation in oral lichen planus: an immunohistochemical study. J Clin Diagn Res. (2017) 11:ZC29–32. doi: 10.7860/JCDR/2017/30740.10274
79. Siponen M, Bitu CC, Al-Samadi A, Nieminen P, Salo T. Cathepsin K expression is increased in oral lichen planus. J Oral Pathol Med. (2016) 45:758–65. doi: 10.1111/jop.12446
80. Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 122:72–80. doi: 10.1016/j.oooo.2016.03.011
81. Weber B, Schlapbach C, Stuck M, Simon HU, Borradori L, Beltraminelli H, et al. Distinct interferon-gamma and interleukin-9 expression in cutaneous and oral lichen planus. J Eur Acad Dermatol Venereol. (2017) 31:880–6. doi: 10.1111/jdv.13989
82. Chen J, Feng J, Chen X, Xu H, Zhou Z, Shen X, et al. Immunoexpression of interleukin-22 and interleukin-23 in oral and cutaneous lichen planus lesions: a preliminary study. Mediators Inflamm. (2013) 2013:801974. doi: 10.1155/2013/801974
83. Zychowska M, Batycka-Baran A, Baran W. Increased serum level and high tissue immunoexpression of interleukin 17 in cutaneous lichen planus: a novel therapeutic target for recalcitrant cases? Dis Markers. (2020) 2020:6521274. doi: 10.1155/2020/6521274
84. Lu R, Zeng X, Han Q, Lin M, Long L, Dan H, et al. Overexpression and selectively regulatory roles of IL-23/IL-17 axis in the lesions of oral lichen planus. Mediat Inflamm. (2014) 2014:701094. doi: 10.1155/2014/701094
85. Rivera C, Crisostomo MF, Pena C, Gonzalez-Diaz P, Gonzalez-Arriagada WA. Oral lichen planus interactome reveals CXCR4 and CXCL12 as candidate therapeutic targets. Sci Rep. (2020) 10:5454. doi: 10.1038/s41598-020-62258-7
86. Shan J, Li S, Wang C, Liu L, Wang X, Zhu D, et al. Expression and biological functions of the CCL5-CCR5 axis in oral lichen planus. Exp Dermatol. (2019) 28:816–21. doi: 10.1111/exd.13946
87. Fang J, Wang C, Shen C, Shan J, Wang X, Liu L, et al. The expression of CXCL10/CXCR3 and effect of the axis on the function of T lymphocyte involved in oral lichen planus. Inflammation. (2019) 42:799–810. doi: 10.1007/s10753-018-0934-0
88. Shan J, Shen C, Fang J, Li S, Fan Y. Potential roles of the CCL17-CCR4 axis in immunopathogenesis of oral lichen planus. J Oral Pathol Med. (2020) 49:328–34. doi: 10.1111/jop.12928
89. Iijima W, Ohtani H, Nakayama T, Sugawara Y, Sato E, Nagura H, et al. Infiltrating CD8+ T cells in oral lichen planus predominantly express CCR5 and CXCR3 and carry respective chemokine ligands RANTES/CCL5 and IP-10/CXCL10 in their cytolytic granules: a potential self-recruiting mechanism. Am J Pathol. (2003) 163:261–8. doi: 10.1016/S0002-9440(10)63649-8
90. Lu R, Zhang J, Sun W, Du G, Zhou G. Inflammation-related cytokines in oral lichen planus: an overview. J Oral Pathol Med. (2015) 44:1–14. doi: 10.1111/jop.12142
91. Pekiner FN, Demirel GY, Borahan MO, Ozbayrak S. Cytokine profiles in serum of patients with oral lichen planus. Cytokine. (2012) 60:701–6. doi: 10.1016/j.cyto.2012.08.007
92. Yang CC, Khanna T, Sallee B, Christiano AM, Bordone LA. Tofacitinib for the treatment of lichen planopilaris: a case series. Dermatol Ther. (2018) 31:e12656. doi: 10.1111/dth.12656
93. Damsky W, Wang A, Olamiju B, Peterson D, Galan A, King B. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J Allergy Clin Immunol. (2020) 145:1708–10 e2. doi: 10.1016/j.jaci.2020.01.031
94. Seiringer P, Lauffer F, Pilz AC, Boehmer D, Biedermann T, Eyerich K. Tofacitinib in Hypertrophic Lichen Planus. Acta Dermato-Venereol. (2020) 100:adv00220. doi: 10.2340/00015555-3585
95. Barrat FJ, Crow MK, Ivashkiv LB. Interferon target-gene expression and epigenomic signatures in health and disease. Nat Immunol. (2019) 20:1574–83. doi: 10.1038/s41590-019-0466-2
96. Solimani F, Pollmann R, Schmidt T, Schmidt A, Zheng X, Savai R, et al. Therapeutic Targeting of Th17/Tc17 Cells Leads to Clinical Improvement of Lichen Planus. Front Immunol. (2019) 10:1808. doi: 10.3389/fimmu.2019.01808
97. Takahashi H, Kouno M, Nagao K, Wada N, Hata T, Nishimoto S, et al. Desmoglein 3-specific CD4+ T cells induce pemphigus vulgaris and interface dermatitis in mice. J Clin Invest. (2011) 121:3677–88. doi: 10.1172/JCI57379
98. Li D, Li J, Li C, Chen Q, Hua H. The association of thyroid disease and oral lichen planus: a literature review and meta-analysis. Front Endocrinol. (2017) 8:310. doi: 10.3389/fendo.2017.00310
99. Otero Rey EM, Yanez-Busto A, Rosa Henriques IF, Lopez-Lopez J, Blanco-Carrion A. Lichen planus and diabetes mellitus: systematic review and meta-analysis. Oral Dis. (2019) 25:1253–64. doi: 10.1111/odi.12977
100. Arias-Santiago S, Buendia-Eisman A, Aneiros-Fernandez J, Giron-Prieto MS, Gutierrez-Salmeron MT, Garcia-Mellado V, et al. Lipid levels in patients with lichen planus: a case-control study. J Eur Acad Dermatol Venereol. (2011) 25:1398–401. doi: 10.1111/j.1468-3083.2011.03983.x
101. Arias-Santiago S, Buendia-Eisman A, Aneiros-Fernandez J, Giron-Prieto MS, Gutierrez-Salmeron MT, Mellado VG, et al. Cardiovascular risk factors in patients with lichen planus. Am J Med. (2011) 124:543–8. doi: 10.1016/j.amjmed.2010.12.025
102. Vazquez-Lopez F, Gomez-Diez S, Sanchez J, Perez-Oliva N. Dermoscopy of active lichen planus. Arch Dermatol. (2007) 143:1092. doi: 10.1001/archderm.143.8.1092
103. Lallas A, Kyrgidis A, Tzellos TG, Apalla Z, Karakyriou E, Karatolias A, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasis rosea. Br J Dermatol. (2012) 166:1198–205. doi: 10.1111/j.1365-2133.2012.10868.x
104. Soares VC, Mulinari-Brenner F, Souza TE. Lichen planopilaris epidemiology: a retrospective study of 80 cases. An Bras Dermatol. (2015) 90:666–70. doi: 10.1590/abd1806-4841.20153923
105. Errichetti E, Figini M, Croatto M, Stinco G. Therapeutic management of classic lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. (2018) 11:91–102. doi: 10.2147/CCID.S137870
106. Griffiths AAC, Sancho MI, Plaza AI. Piccardi-Lassueur-Graham-Little syndrome associated with frontal fibrosing alopecia. An Bras Dermatol. (2017) 92:867–9. doi: 10.1590/abd1806-4841.20176741
107. Hübner F, Langan EA, Recke A. Lichen planus pemphigoides: from lichenoid inflammation to autoantibody-mediated blistering. Front Immunol. (2019) 10:1389. doi: 10.3389/fimmu.2019.01389
108. Matos-Pires E, Campos S, Lencastre A, João A, Mendes-Bastos P. Lichen planus pemphigoides. J Dtsch Dermatol Ges. (2018) 16:335–7. doi: 10.1111/ddg.13434
109. Aractingi S, Chosidow O. Cutaneous graft-versus-host disease. Arch Dermatol. (1998) 134:602–12. doi: 10.1001/archderm.134.5.602
110. Tétu P, Jachiet M, de Masson A, Hurabielle C, Rybojad M, Michonneau D, et al. Chronic graft versus host disease presenting as lichen planus pigmentosus. Bone Marrow Transplant. (2018) 53:1048–50. doi: 10.1038/s41409-018-0110-z
111. Thanyavuthi A, Boonchai W, Kasemsarn P. Amalgam contact allergy in oral lichenoid lesions. Dermatitis. (2016) 27:215–21. doi: 10.1097/DER.0000000000000204
112. McParland H, Warnakulasuriya S. Oral lichenoid contact lesions to mercury and dental amalgam–a review. J Biomed Biotechnol. (2012) 2012:589569. doi: 10.1155/2012/589569
113. Sehgal VN, Srivastava G, Sharma S, Sehgal S, Verma P. Lichenoid tissue reaction/interface dermatitis: recognition, classification, etiology, and clinicopathological overtones. Indian J Dermatol Venereol Leprol. (2011) 77:418–29. doi: 10.4103/0378-6323.82389
114. Babahosseini H, Tavakolpour S, Mahmoudi H, Balighi K, Teimourpour A, Ghodsi SZ, et al. Lichen planopilaris: retrospective study on the characteristics and treatment of 291 patients. J Dermatolog Treat. (2019) 30:598–604. doi: 10.1080/09546634.2018.1542480
115. Orteu CH, Buchanan JA, Hutchison I, Leigh IM, Bull RH. Systemic lupus erythematosus presenting with oral mucosal lesions: easily missed? Br J Dermatol. (2001) 144:1219–23. doi: 10.1046/j.1365-2133.2001.04236.x
116. Lospinoso DJ, Fernelius C, Edhegard KD, Finger DR, Arora NS. Lupus erythematosus/lichen planus overlap syndrome: successful treatment with acitretin. Lupus. (2013) 22:851–4. doi: 10.1177/0961203313492243
117. Scully C. el-Kom M. Lichen planus: review and update on pathogenesis. J Oral Pathol. (1985) 14:431–58. doi: 10.1111/j.1600-0714.1985.tb00516.x
118. Van den Haute V, Antoine JL, Lachapelle JM. Histopathological discriminant criteria between lichenoid drug eruption and idiopathic lichen planus: retrospective study on selected samples. Dermatologica. (1989) 179:10–3. doi: 10.1159/000248091
119. Brewer JD, Ekdawi NS, Torgerson RR, Camilleri MJ, Bruce AJ, Rogers RS III, et al. Lichen planus and cicatricial conjunctivitis: disease course and response to therapy of 11 patients. J Eur Acad Dermatol Venereol. (2011) 25:100–4. doi: 10.1111/j.1468-3083.2010.03693.x
120. Volden G. Successful treatment of chronic skin diseases with clobetasol propionate and a hydrocolloid occlusive dressing. Acta Derm Venereol. (1992) 72:69–71.
121. Atzmony L, Reiter O, Hodak E, Gdalevich M, Mimouni D. Treatments for cutaneous lichen planus: a systematic review and meta-analysis. Am J Clin Dermatol. (2016) 17:11–22. doi: 10.1007/s40257-015-0160-6
122. Malhotra AK, Khaitan BK, Sethuraman G. Sharma VK. Betamethasone oral mini-pulse therapy compared with topical triamcinolone acetonide (01%) paste in oral lichen planus: a randomized comparative study. J Am Acad Dermatol. (2008) 58:596–602. doi: 10.1016/j.jaad.2007.11.022
123. Laurberg G, Geiger JM, Hjorth N, Holm P, Hou-Jensen K, Jacobsen KU, et al. Treatment of lichen planus with acitretin. A double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. (1991) 24:434–7. doi: 10.1016/0190-9622(91)70067-C
124. Handler HL. Isotretinoin for oral lichen planus. J Am Acad Dermatol. (1984) 10:674. doi: 10.1016/S0190-9622(84)80285-6
125. Woo TY. Systemic isotretinoin treatment of oral and cutaneous lichen planus. Cutis. (1985) 35:385–6, 90–1, 93.
126. Ho VC, Gupta AK, Ellis CN, Nickoloff BJ, Voorhees JJ. Treatment of severe lichen planus with cyclosporine. J Am Acad Dermatol. (1990) 22:64–8. doi: 10.1016/0190-9622(90)70009-7
127. Iraji F, Faghihi G, Asilian A, Siadat AH, Larijani FT, Akbari M. Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: a randomized clinical trial. J Res Med Sci. (2011) 16:1578–82.
128. Alsenaid A, Alamri A, Prinz JC, Ruzicka T, Wolf R. Lichen planus of the lower limbs: successful treatment with psoralen cream plus ultraviolet A photochemotherapy. Dermatol Ther. (2016) 29:109–13. doi: 10.1111/dth.12321
129. Bauza A, Espana A, Gil P, Lloret P, Vazquez Doval FJ. Successful treatment of lichen planus with sulfasalazine in 20 patients. Int J Dermatol. (2005) 44:158–62. doi: 10.1111/j.1365-4632.2005.02070.x
130. Cribier B, Frances C, Chosidow O. Treatment of lichen planus. An evidence-based medicine analysis of efficacy. Arch Dermatol. (1998) 134:1521–30. doi: 10.1001/archderm.134.12.1521
131. Samycia M, Lin AN. Efficacy of topical calcineurin inhibitors in lichen planus. J Cutan Med Surg. (2012) 16:221–9. doi: 10.1177/120347541201600403
132. Manousaridis I, Manousaridis K, Peitsch WK, Schneider SW. Individualizing treatment and choice of medication in lichen planus: a step by step approach. J Dtsch Dermatol Ges. (2013) 11:981–91. doi: 10.1111/ddg.12141
133. Verma KK, Mittal R, Manchanda Y. Azathioprine for the treatment of severe erosive oral and generalized lichen planus. Acta Derm Venereol. (2001) 81:378–9. doi: 10.1080/000155501317140197
134. Turan H, Baskan EB, Tunali S, Yazici S, Saricaoglu H. Methotrexate for the treatment of generalized lichen planus. J Am Acad Dermatol. (2009) 60:164–6. doi: 10.1016/j.jaad.2008.09.054
135. Hollo P, Szakonyi J, Kiss D, Jokai H, Horvath A, Karpati S. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. (2012) 92:385–6. doi: 10.2340/00015555-1249
136. Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. (2017) 100:415–8.
137. Paul J, Foss CE, Hirano SA, Cunningham TD, Pariser DM. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. (2013) 68:255–61. doi: 10.1016/j.jaad.2012.07.014
138. Carbone M, Goss E, Carrozzo M, Castellano S, Conrotto D, Broccoletti R, et al. Systemic and topical corticosteroid treatment of oral lichen planus: a comparative study with long-term follow-up. J Oral Pathol Med. (2003) 32:323–9. doi: 10.1034/j.1600-0714.2003.00173.x
139. Silverman S Jr, Gorsky M, Lozada-Nur F, Giannotti K. A prospective study of findings and management in 214 patients with oral lichen planus. Oral Surg Oral Med Oral Pathol. (1991) 72:665–70. doi: 10.1016/0030-4220(91)90007-Y
140. Omidian M, Ayoobi A, Mapar MA, Feily A, Cheraghian B. Efficacy of sulfasalazine in the treatment of generalized lichen planus: randomized double-blinded clinical trial on 52 patients. J Eur Acad Dermatol Venereol. (2010) 24:1051–4. doi: 10.1111/j.1468-3083.2010.03583.x
141. Verma KK, Sirka CS, Khaitan BK. Generalized severe lichen planus treated with azathioprine. Acta Derm Venereol. (1999) 79:493. doi: 10.1080/000155599750010111
142. Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. (1993) 28:609–12. doi: 10.1016/0190-9622(93)70082-5
143. Torti DC, Jorizzo JL, McCarty MA. Oral lichen planus: a case series with emphasis on therapy. Arch Dermatol. (2007) 143:511–5. doi: 10.1001/archderm.143.4.511
144. Dalmau J, Puig L, Roe E, Peramiquel L, Campos M, Alomar A. Successful treatment of oral erosive lichen planus with mycophenolate mofetil. J Eur Acad Dermatol Venereol. (2007) 21:259–60. doi: 10.1111/j.1468-3083.2006.01832.x
145. Chang AL, Badger J, Rehmus W, Kimball AB. Alefacept for erosive lichen planus: a case series. J Drugs Dermatol. (2008) 7:379–83.
146. Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. (2009) 84:325–8.
147. O'Neill ID. Off-label use of biologicals in the management of inflammatory oral mucosal disease. J Oral Pathol Med. (2008) 37:575–81. doi: 10.1111/j.1600-0714.2008.00693.x
148. Paslin DA. Sustained remission of generalized lichen planus induced by cyclophosphamide. Arch Dermatol. (1985) 121:236–9. doi: 10.1001/archderm.121.2.236
149. Wu Y, Zhou G, Zeng H, Xiong CR, Lin M, Zhou HM, et al. randomized double-blind, positive-control trial of topical thalidomide in erosive oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2010) 110:188–95. doi: 10.1016/j.tripleo.2010.03.034
150. Rasi A, Behzadi AH, Davoudi S, Rafizadeh P, Honarbakhsh Y, Mehran M, et al. Efficacy of oral metronidazole in treatment of cutaneous and mucosal lichen planus. J Drugs Dermatol. (2010) 9:1186–90.
151. Hantash BM, Kanzler MH. The efficacy of tetracycline antibiotics for treatment of lichen planus: an open-label clinical trial. Br J Dermatol. (2007) 156:758–60. doi: 10.1111/j.1365-2133.2006.07733.x
152. Khandpur S, Sugandhan S, Sharma VK. Pulsed itraconazole therapy in eruptive lichen planus. J Eur Acad Dermatol Venereol. (2009) 23:98–101. doi: 10.1111/j.1468-3083.2008.02743.x
153. Naylor GD. Treating erosive lichen planus with griseofulvin: a report of four cases. Quintessence Int. (1990) 21:943–7.
154. Beck HI, Brandrup F. Treatment of erosive lichen planus with dapsone. Acta Derm Venereol. (1986) 66:366–7.
155. Becherel PA, Bussel A, Chosidow O, Rabian C, Piette JC, Frances C. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. (1998) 351:805. doi: 10.1016/S0140-6736(05)78932-7
156. Wackernagel A, Legat FJ, Hofer A, Quehenberger F, Kerl H, Wolf P. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. (2007) 23:15–9. doi: 10.1111/j.1600-0781.2007.00261.x
157. Cooper SM, Wojnarowska F. Influence of treatment of erosive lichen planus of the vulva on its prognosis. Arch Dermatol. (2006) 142:289–94. doi: 10.1001/archderm.142.3.289
158. Lajevardi V, Ghodsi SZ, Goodarzi A, Hejazi P, Azizpour A, Beygi S. Comparison of systemic mycophenolate mofetil with topical clobetasol in lichen planopilaris: a parallel-group, assessor- and analyst-blinded, randomized controlled trial. Am J Clin Dermatol. (2015) 16:303–11. doi: 10.1007/s40257-015-0122-z
159. Chieregato C, Zini A, Barba A, Magnanini M, Rosina P. Lichen planopilaris: report of 30 cases and review of the literature. Int J Dermatol. (2003) 42:342–5. doi: 10.1046/j.1365-4362.2003.01695.x
160. Lyakhovitsky A, Amichai B, Sizopoulou C, Barzilai A. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. (2015) 26:275–9. doi: 10.3109/09546634.2014.933165
161. Cevasco NC, Bergfeld WF, Remzi BK, de Knott HR. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. (2007) 57:47–53. doi: 10.1016/j.jaad.2007.01.011
162. Blazek C, Megahed M. [Lichen planopilaris. Successful treatment with tacrolimus]. Der Hautarzt Zeitschrift Dermatol Venerol verwandte Gebiete. (2008) 59:874–7. doi: 10.1007/s00105-008-1650-8
163. Mehregan DA, Van Hale HM, Muller SA. Lichen planopilaris: clinical and pathologic study of forty-five patients. J Am Acad Dermatol. (1992) 27:935–42. doi: 10.1016/0190-9622(92)70290-V
164. Racz E, Gho C, Moorman PW, Noordhoek Hegt V, Neumann HA. Treatment of frontal fibrosing alopecia and lichen planopilaris: a systematic review. J Eur Acad Dermatol Venereol. (2013) 27:1461–70. doi: 10.1111/jdv.12139
165. Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. (2009) 28:3–10. doi: 10.1016/j.sder.2008.12.006
166. Spencer LA, Hawryluk EB, English JC III. Lichen planopilaris: retrospective study and stepwise therapeutic approach. Arch Dermatol. (2009) 145:333–4. doi: 10.1001/archdermatol.2008.590
167. Chiang C, Sah D, Cho BK, Ochoa BE, Price VH. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. (2010) 62:387–92. doi: 10.1016/j.jaad.2009.08.054
168. Naeini FF, Saber M, Asilian A, Hosseini SM. Clinical efficacy and safety of methotrexate versus hydroxychloroquine in preventing lichen planopilaris progress: a randomized clinical trial. Int J Prev Med. (2017) 8:37. doi: 10.4103/ijpvm.IJPVM_156_17
169. Cho BK, Sah D, Chwalek J, Roseborough I, Ochoa B, Chiang C, et al. Efficacy and safety of mycophenolate mofetil for lichen planopilaris. J Am Acad Dermatol. (2010) 62:393–7. doi: 10.1016/j.jaad.2009.05.018
170. Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. (2014) 12:74–6. doi: 10.1111/ddg.12224_suppl
171. Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch Dermatol. (2009) 145:1363–6. doi: 10.1001/archdermatol.2009.283
172. Baibergenova A, Walsh S. Use of pioglitazone in patients with lichen planopilaris. J Cutan Med Surg. (2012) 16:97–100. doi: 10.2310/7750.2011.11008
173. George SJ, Hsu S. Lichen planopilaris treated with thalidomide. J Am Acad Dermatol. (2001) 45:965–6. doi: 10.1067/mjd.2001.119559
174. Jouanique C, Reygagne P, Bachelez H, Dubertret L. Thalidomide is ineffective in the treatment of lichen planopilaris. J Am Acad Dermatol. (2004) 51:480–1. doi: 10.1016/S0190-9622(03)00849-1
175. Erras S, Mouna Z, Akhdari N, Belaabidia B, Essaadouni L. Rapid and complete resolution of lichen planopilaris in juvenile chronic arthritis treated with rituximab. Eur J Dermatol. (2011) 21:116–7. doi: 10.1684/ejd.2010.1176
176. Iorizzo M, Tosti A, Starace M, Baran R, Daniel CR III, Di Chiacchio N, et al. Isolated nail lichen planus: An expert consensus on treatment of the classical form. J Am Acad Dermatol. (2020) 83:1717–23. doi: 10.1016/j.jaad.2020.02.056
177. Brauns B, Stahl M, Schon MP, Zutt M. Intralesional steroid injection alleviates nail lichen planus. Int J Dermatol. (2011) 50:626–7. doi: 10.1111/j.1365-4632.2010.04786.x
178. Alsenaid A, Eder I, Ruzicka T, Braun-Falco M, Wolf R. Successful treatment of nail lichen planus with alitretinoin: report of 2 cases and review of the literature. Dermatology. (2014) 229:293–6. doi: 10.1159/000365655
179. Iorizzo M. Nail lichen planus - a possible new indication for oral alitretinoin. J Eur Acad Dermatol Venereol. (2016) 30:509–10. doi: 10.1111/jdv.12904
180. Alsenaid A, Lang A, Ruzicka T, Braun-Falco M, Wolf R. Lichen planus with associated myasthenia gravis-successful treatment with acitretin. Eur J Dermatol. (2013) 23:909–10. doi: 10.1684/ejd.2013.2162
181. Irla N, Schneiter T, Haneke E, Yawalkar N. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. (2010) 2:173–6. doi: 10.1159/000321419
182. Florian B, Angelika J, Ernst SR. Successful treatment of palmoplantar nail lichen planus with cyclosporine. J Dtsch Dermatol Ges. (2014) 12:724–5. doi: 10.1111/ddg.12325
183. Ujiie H, Shibaki A, Akiyama M, Shimizu H. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. (2010) 90:218–9. doi: 10.2340/00015555-0814
184. Ramakrishnaiah PK, Lakshman A, Aradhya SS, Veerabhadrappa NH. Lichenoid variant of chronic cutaneous graft versus host reaction post blood transfusion: a rare event post blood transfusion. Indian J Dermatol. (2015) 60:525. doi: 10.4103/0019-5154.159667
185. Hesse J, Schmalfuss A, Kvaal SI. Photodynamic therapy of oral lichen planus. Photochem Photobiol Sci. (2020) 19:1271–9. doi: 10.1039/D0PP00249F
186. Komori T, Honda T, Endo Y, Kaku Y, Otsuka A, Kabashima K. Oral lichen planus associated with candidiasis during secukinumab treatment. J Dermatol. (2017) 44:e60–1. doi: 10.1111/1346-8138.13637
187. Capusan TM, Herrero-Moyano M, Martínez-Mera CR, Freih-Fraih AW, Dauden E. Oral lichenoid reaction in a psoriatic patient treated with secukinumab: a drug-related rather than a class-related adverse event? JAAD Case Rep. (2018) 4:521–3. doi: 10.1016/j.jdcr.2018.04.015
188. Ghiam N, Ojong O, Vasile G, Romanelli P, Kerdel F. Lichenoid drug eruption after treatment with ixekizumab for plaque psoriasis. Dermatol Online J. (2020) 26:13030/qt3v13t2kt. doi: 10.5070/D32612051366
189. Thompson JM, Cohen LM, Yang CS, Kroumpouzos G. Severe, ulcerative, lichenoid mucositis associated with secukinumab. JAAD Case Rep. (2016) 2:384–6. doi: 10.1016/j.jdcr.2016.07.009
190. Maurelli M, Colato C, Tessari G, Girolomoni G. Lichen planopilaris coexisting with plaque psoriasis effectively treated with brodalumab. Dermatol Ther. (2020) 33:e13967. doi: 10.1111/dth.13967
191. Rezzag-Mahcene C, Cardot-Leccia N, Lacour JP, Montaudié H, Passeron T. Successful treatment of recalcitrant genital lichen planus with secukinumab. J Eur Acad Dermatol Venereol. (2021) 35:e321–3. doi: 10.1111/jdv.17068
192. Monteiro BV, Pereira Jdos S, Nonaka CF, Godoy GP, da SilveiraÉJ, Miguel MC. Immunoexpression of Th17-related cytokines in oral lichen planus. Appl Immunohistochem Mol Morphol. (2015) 23:409–15. doi: 10.1097/PAI.0000000000000096
193. Pouralibaba F, Babaloo Z, Pakdel F, Aghazadeh M. Serum level of interleukin 17 in patients with erosive and non erosive oral lichen planus. J Dent Res Dent Clin Dent Prospects. (2013) 7:91–4. doi: 10.5681/joddd.2013.016
194. Webster G. Failure of lichen planopilaris to respond to ustekinumab. Dermatol Online J. (2015) 21:13030/qt30z76472. doi: 10.5070/D32111029305
195. Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. (2017) 100:415–8.
196. Ismail FF, Sinclair R. Clinical healing of erosive oral lichen planus with tildrakizumab implicates the interleukin-23/interleukin-17 pathway in the pathogenesis of lichen planus. Australas J Dermatol. (2020) 61:e244–5. doi: 10.1111/ajd.13183
197. Mardani M, Mofidi H, Dastgheib L, Ranjbar S, Hamidizadeh N. Elevated serum interleukin-23 levels in patients with oral and cutaneous lichen planus. Mediators Inflamm. (2021) 2021:5578568. doi: 10.1155/2021/5578568
198. Inoue A, Sawada Y, Yamaguchi T, Ohmori S, Omoto D, Haruyama S, et al. Lichenoid drug eruption caused by adalimumab: a case report and literature review. Eur J Dermatol. (2017) 27:69–70. doi: 10.1684/ejd.2016.2898
199. Wendling D, Biver-Dalle C, Vidon C, Prati C, Aubin F. Lichen planus under anti TNF therapy for ankylosing spondylitis. Joint Bone Spine. (2013) 80:227–8. doi: 10.1016/j.jbspin.2012.07.017
200. Pietschke K, Holstein J, Meier K, Schäfer I, Müller-Hermelink E, Gonzalez-Menendez I, et al. The inflammation in cutaneous lichen planus is dominated by IFN-⋎ and IL-21-A basis for therapeutic JAK1 inhibition. Exp Dermatol. (2021) 30:262–70. doi: 10.1111/exd.14226
201. Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. (2014) 20:1043–9. doi: 10.1038/nm.3645
202. King B, Guttman-Yassky E, Peeva E, Banerjee A, Sinclair R, Pavel AB, et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J Am Acad Dermatol. (2021) 85:379–87. doi: 10.1016/j.jaad.2021.03.050
204. Hafner J, Gubler C, Kaufmann K, Nobbe S, Navarini AA. French LE. Apremilast is effective in lichen planus mucosae-associated stenotic esophagitis. Case Rep Dermatol. (2016) 8:224–6. doi: 10.1159/000447051
205. AbuHilal M, Walsh S, Shear N. Treatment of recalcitrant erosive oral lichen planus and desquamative gingivitis with oral apremilast. J Dermatol Case Rep. (2016) 10:56–7. doi: 10.3315/jdcr.2016.1232
206. Skullerud KH, Gjersvik P, Pripp AH, Qvigstad E, Helgesen ALO. Apremilast for genital erosive lichen planus in women (the AP-GELP Study): study protocol for a randomised placebo-controlled clinical trial. Trials. (2021) 22:469. doi: 10.1186/s13063-021-05428-w
207. Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 002%, gel in mycosis fungoides. JAMA Dermatol. (2013) 149:25–32. doi: 10.1001/2013.jamadermatol.541
208. Strazzulla LC, Avila L, Lo Sicco K, Shapiro J. Novel treatment using low-dose naltrexone for lichen planopilaris. J Drugs Dermatol. (2017) 16:1140–2.
209. Jajarm HH, Falaki F, Sanatkhani M, Ahmadzadeh M, Ahrari F, Shafaee H, et al. comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: a randomized clinical controlled trial. Lasers Med Sci. (2015) 30:1475–80. doi: 10.1007/s10103-014-1694-1
210. Helgesen AL, Warloe T, Pripp AH, Kirschner R, Peng Q, Tanbo T, et al. Vulvovaginal photodynamic therapy vs. topical corticosteroids in genital erosive lichen planus: a randomized controlled trial. Br J Dermatol. (2015) 173:1156–62. doi: 10.1111/bjd.14033
211. Mostafa D, Moussa E, Alnouaem M. Evaluation of photodynamic therapy in treatment of oral erosive lichen planus in comparison with topically applied corticosteroids. Photodiagnosis Photodyn Ther. (2017) 19:56–66. doi: 10.1016/j.pdpdt.2017.04.014
212. Bakhtiari S, Azari-Marhabi S, Mojahedi SM, Namdari M, Rankohi ZE, Jafari S. Comparing clinical effects of photodynamic therapy as a novel method with topical corticosteroid for treatment of Oral Lichen Planus. Photodiagnosis Photodyn Ther. (2017) 20:159–64. doi: 10.1016/j.pdpdt.2017.06.002
213. Mirza S, Rehman N, Alrahlah A, Alamri WR, Vohra F. Efficacy of photodynamic therapy or low level laser therapy against steroid therapy in the treatment of erosive-atrophic oral lichen planus. Photodiagnosis Photodyn Ther. (2018) 21:404–8. doi: 10.1016/j.pdpdt.2018.02.001
214. Cosgarea R, Pollmann R, Sharif J, Schmidt T, Stein R, Bodea A, et al. Photodynamic therapy in oral lichen planus: a prospective case-controlled pilot study. Sci Rep. (2020) 10:1667. doi: 10.1038/s41598-020-58548-9
215. Weber B, Marquart E, Radakovic S, Tanew A. Effectiveness of narrowband UVB phototherapy and psoralen plus UVA photochemotherapy in the treatment of generalized lichen planus: results from a large retrospective analysis and an update of the literature. Photodermatol Photoimmunol Photomed. (2021). doi: 10.1111/phpp.12723. [Epub ahead of print].
216. Guyot AD, Farhi D, Ingen-Housz-Oro S, Bussel A, Parquet N, Rabian C, et al. Treatment of refractory erosive oral lichen planus with extracorporeal photochemotherapy: 12 cases. Br J Dermatol. (2007) 156:553–6. doi: 10.1111/j.1365-2133.2006.07647.x
217. Zingoni A, Deboli T, Savoia P, Bernengo MG. Effectiveness of extracorporeal photochemotherapy in the treatment of a case of refractory erosive lichen planus. J Dermatolog Treat. (2010) 21:119–21. doi: 10.3109/09546630902991468
218. Lodi G, Manfredi M, Mercadante V, Murphy R, Carrozzo M. Interventions for treating oral lichen planus: corticosteroid therapies. Cochrane Datab Syst Rev. (2020) 2:CD001168. doi: 10.1002/14651858.CD001168.pub3
Keywords: lichen planus, skin disease, inflammation, T-cell mediated, treatment
Citation: Boch K, Langan EA, Kridin K, Zillikens D, Ludwig RJ and Bieber K (2021) Lichen Planus. Front. Med. 8:737813. doi: 10.3389/fmed.2021.737813
Received: 07 July 2021; Accepted: 11 October 2021;
Published: 01 November 2021.
Edited by:
Robert Gniadecki, University of Alberta, CanadaCopyright © 2021 Boch, Langan, Kridin, Zillikens, Ludwig and Bieber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Boch, a2F0aGFyaW5hLmJvY2hAdWtzaC5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.