- 1Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Department of Microbiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Introduction: Vaccination seems to be a good solution for preventing and controlling coronavirus disease (COVID-19) pandemic, but still there are some challenges in COVID-19 vaccination. Investigating new therapeutic options for COVID-19 is necessary. The current study aimed to evaluate the safety and efficacy of stem cells in treating patients with COVID-19.
Methods: We reviewed the relevant scientific literature published up to April 1, 2021. The pooled risk ratio (RR) with 95% CI was assessed using a fixed or random-effect model. We considered P < 0.05 as statistically significant for publication bias. Data were analyzed by Comprehensive Meta-Analysis software, Version 2.0 (Biostat, Englewood, NJ).
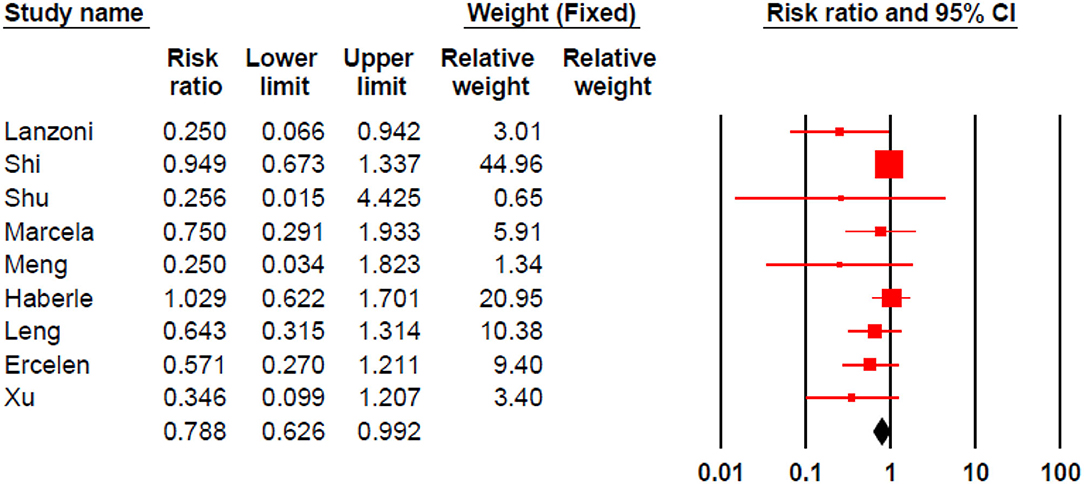
Results: After reviewing 1,262 records, we identified 10 studies that met the inclusion criteria. The analysis showed that stem cell therapy could significantly reduce the mortality rate (RR 0.471, 95% CI: 0.270–0.821) and morbidity (RR 0.788, 95% CI: 0.626–0.992) in patients with COVID-19; compared with the control group.
Conclusions: The present study suggests that stem cell therapy has a remarkable effect on reducing mortality and morbidity of patients with COVID-19. Further large-scale studies are needed to approve these results. Defining a protocol for stem cell therapy in patients with COVID-19 can lead to achieving the best clinical outcomes.
Introduction
Since the primary detection of coronavirus disease (COVID-19) in December 2019 in Wuhan, China, it has caused more than 175 million infected cases and 3.7 million deaths up to June 10, 2021, all over the world (1). COVID-19 pandemic has been putting a massive mental, health, social, and economic burden on individuals and societies (2, 3).
Vaccination has been shown to be a good solution for this problem. However, vaccines have some limitations, including, they are for prevention and not for treatment. Their protection is not 100%, and their distribution worldwide is unfair (4, 5).
A broad spectrum of treatments was introduced for COVID-19, such as remdesivir, favipiravir, corticosteroids, tocilizumab, hydroxychloroquine, and convalescent plasma therapy (4, 5). Furthermore, another therapeutic option is stem cell therapy (6, 7). Stem cells are used in the treatment of a wide variety of diseases, from autoimmune diseases (8) to heredity (9) and infectious diseases (10). Mechanisms, which include immunomodulation, regenerative ability, clearance of alveolar fluid, and preventing thrombotic events, drive the researchers to use these cells to treat COVID-19 (11). Mesenchymal stem cells (MSCs) are shown to be effective in reducing inflammation by releasing chemokines (CCL5, CXCL9,10,11) and other factors [transforming growth factor-beta (TGF-β), nitric oxide (NO)/indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2)] in their secretomes. The immunomodulation mechanisms include: (a) inhibiting B cells, T cells, and natural killer (NK) cells proliferation and activity; (b) inhibiting maturation and antigen-presenting of dendritic cells; (c) enhancing M2 macrophage activation; and (d) restraining cytokine storm (12–14). This anti-inflammatory property has such importance that MSCs are used to prevent graft vs. host disease (GvHD) in many organ transplantations (15). Some studies reported that stem cells could reduce mortality rate and improve pulmonary function and disease remission in patients with COVID-19. However, a comprehensive analysis on this issue has not yet been performed. The current study aimed to evaluate the safety and efficacy of stem cells in treating patients with COVID-19.
Methods
This study was conducted and reported according to the PRISMA statement (16).
Search Strategy
We searched Pubmed/Medline, Embase, Scopus, Clinicaltrials.gov, and gray literature (i.e., google scholar and L·OVE) for studies reporting the efficacy/effectiveness of stem cells in patients with COVID-19, published up to April 1, 2021. The search terms were the following: stem cell, progenitor cell, mesenchymal stem cell, cell therapy, COVID-19, SARS-CoV-2. Only studies written in English were selected.
Study Selection
The records found through database searching were merged, and the duplicates were removed using EndNote X7 (Thomson Reuters, New York, NY, USA). Two reviewers independently screened the records by title/abstract and full texts to exclude those unrelated to the study topic. The studies included met the following inclusion criteria: (i) patients were diagnosed with COVID-19 based on the WHO criteria; (ii) patients were treated with stem cells; and (iii) treatment outcomes were recorded. Conference abstracts, editorials, reviews, and experimental studies on animal models were excluded.
Data Extraction
Two reviewers designed a data extraction form. These reviewers extracted data from all eligible studies, and differences were resolved by consensus. The following data were extracted: first author name; year of publication; type of epidemiological study, country/ies where the research was conducted; treatment protocols, demographics, adverse effects, and outcomes.
Quality Assessment
The checklists provided by the Joanna Briggs Institute (JBI) for randomized controlled trials (RCTs) were used to perform the quality assessment (17).
Statistical Analysis
The pooled risk ratios (RRs) with 95% CI were assessed using random or fixed-effect models. The random-effects model was used because of the estimated heterogeneity of the true effect sizes. The between-study heterogeneity was assessed by Cochran's Q and the I2 statistic. Publication bias was evaluated statistically by using Egger's and Begg's tests as well as the funnel plot (p < 0.05 was considered indicative of statistically significant publication bias; funnel plot asymmetry also suggests bias). All analyses were performed using “Comprehensive Meta-Analysis” software, Version 2.0 (Biostat, Englewood, NJ).
Results
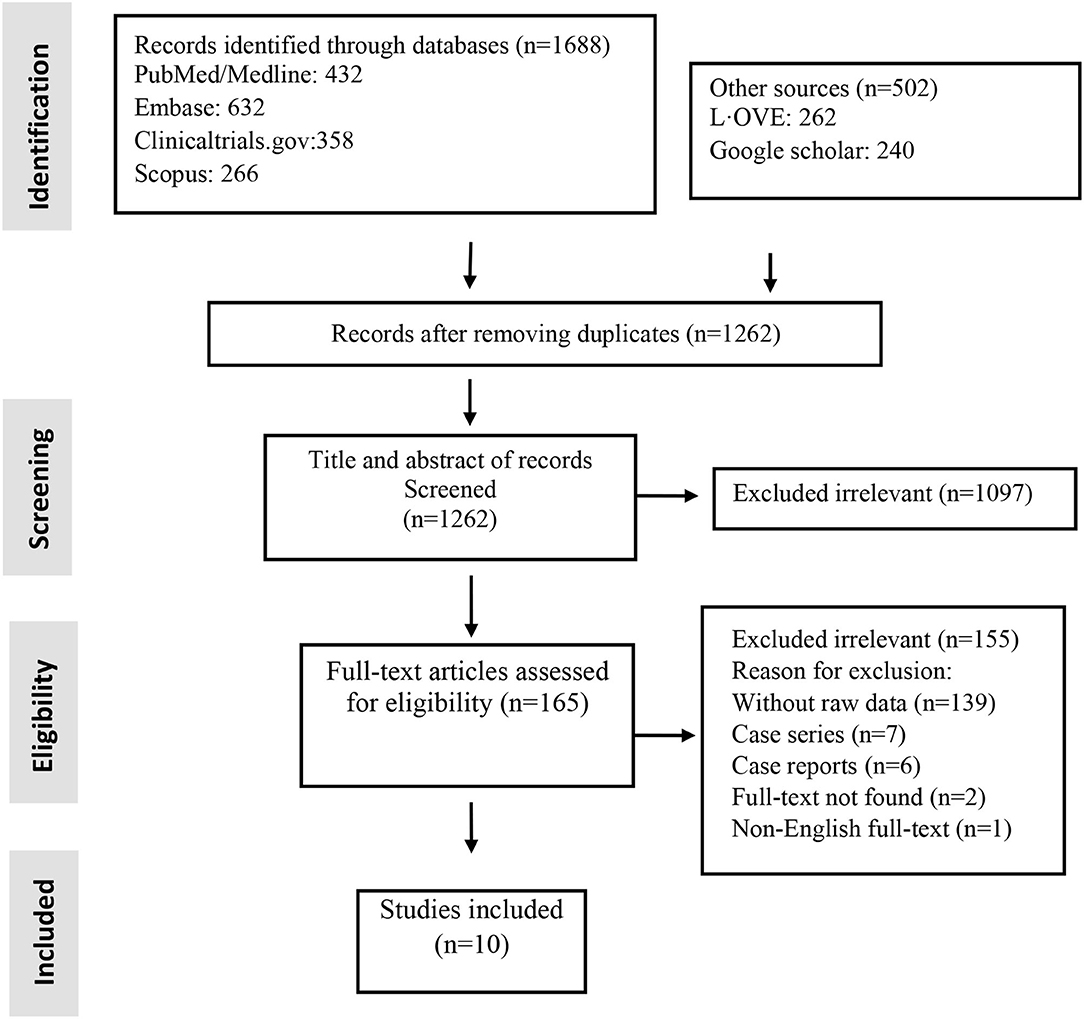
Studies included and excluded through the review process are summarized in Figure 1. A total of 1,262 records were found in the initial search; after removing duplicate articles, and full-text review, 10 were chosen (Figure 1). Of the included studies, there were five non-randomized clinical trials and five RCTs (Table 1). Totally, 184 patients underwent stem cell therapy, while 161 patients took part as controls. Most studies assessed mortality, morbidity, adverse event (AE) and severe adverse event (SAE), pulmonary function, imaging changes, systematic symptoms, and inflammatory markers.
Quality of Included Studies
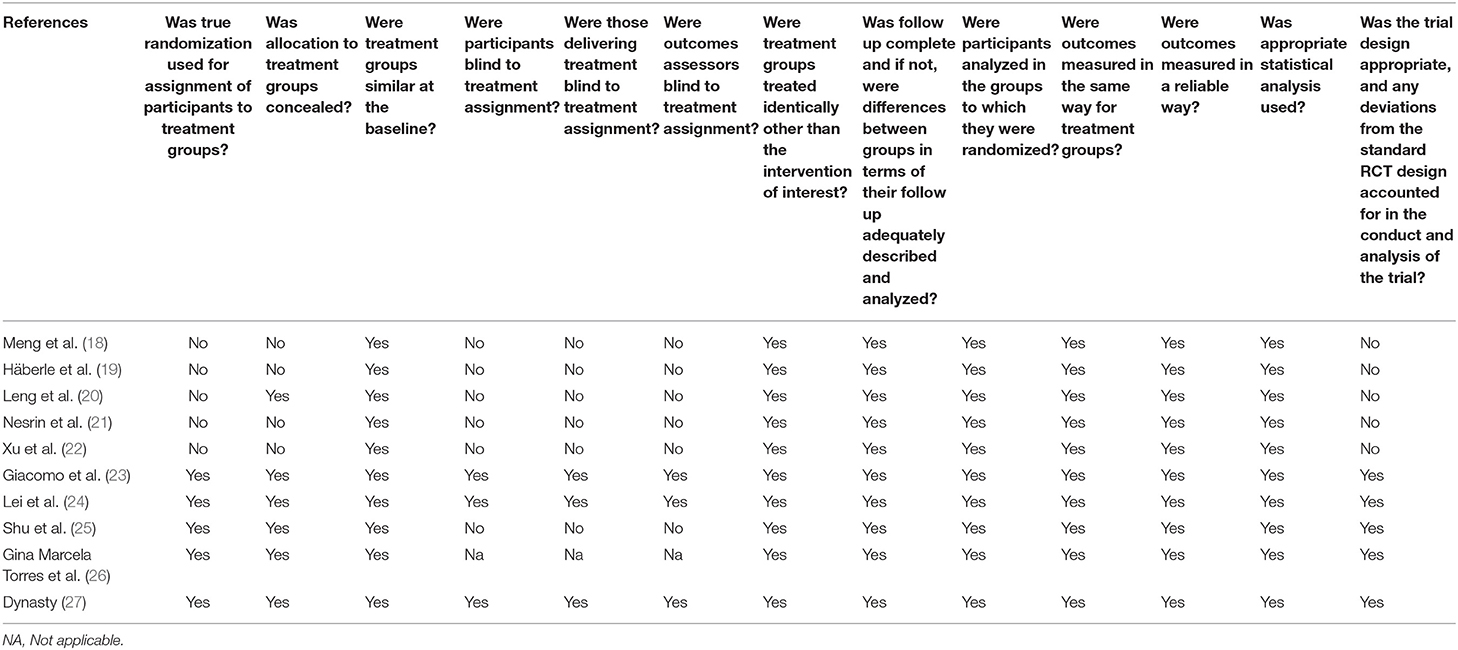
Based on the JBI checklist for experimental studies, the included studies had a low risk of bias (Table 2).
Patient Characteristics
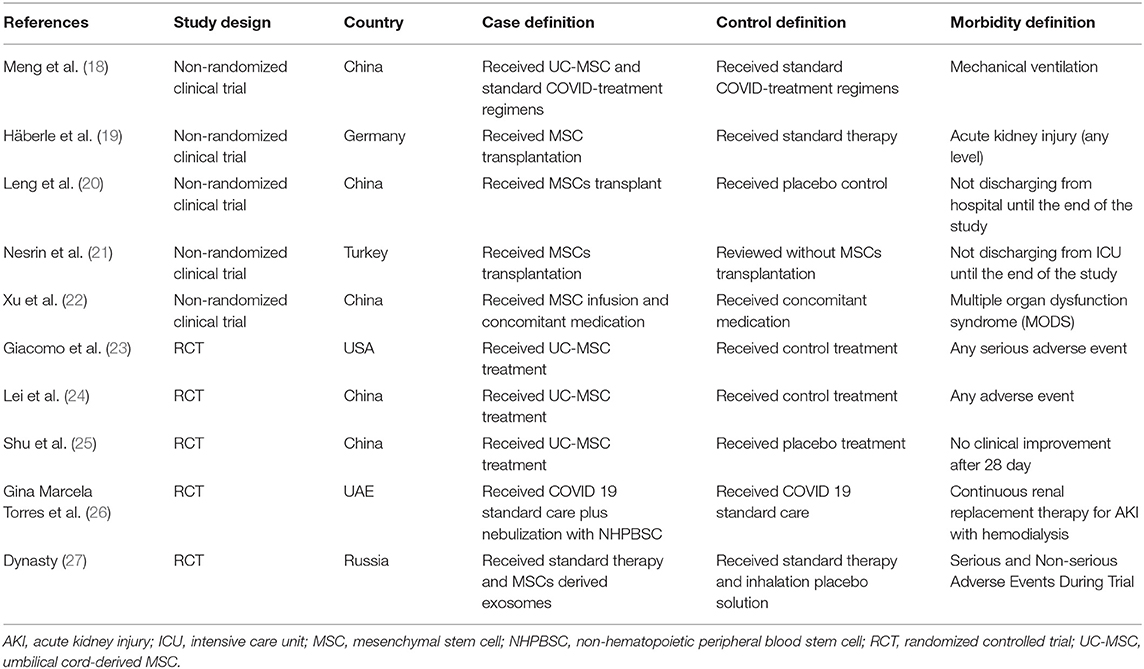
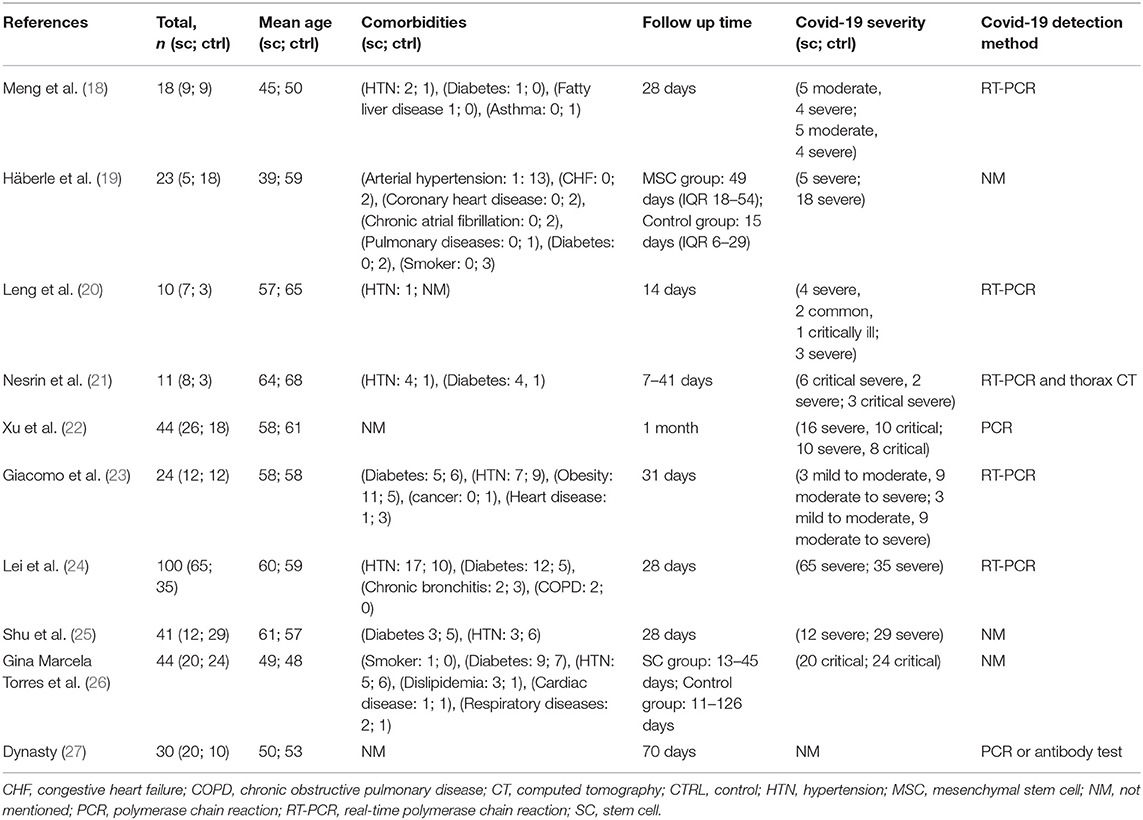
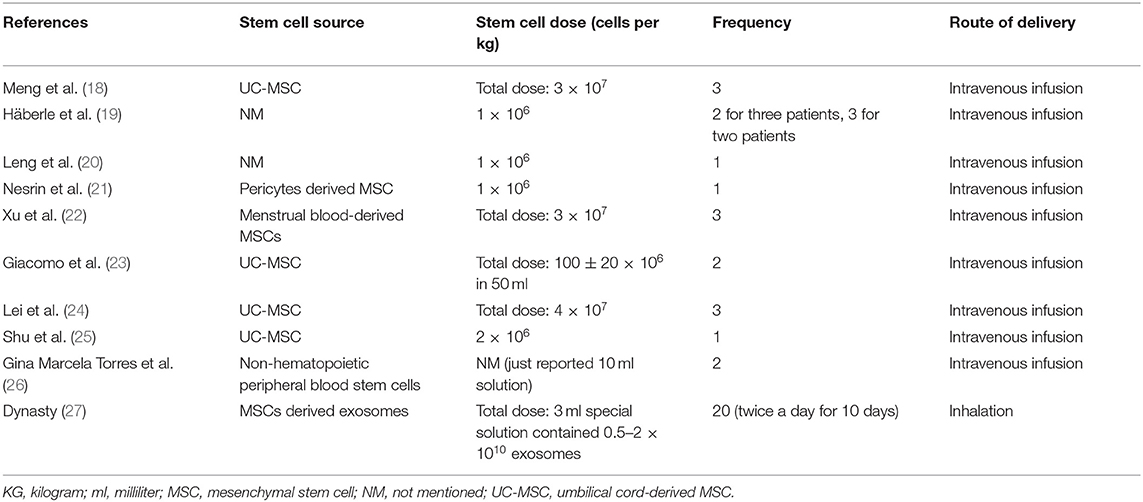
The origins of studies were six countries: China (n = 5) (18, 20, 22, 24, 25), United States (n = 1) (23), Germany (n = 1) (19), Turkey (n = 1) (21), UAE (n = 1) (26), and Russia (n = 1) (27). In these 10 studies, the age range for stem cell groups was 39–64 and 48–64 for control groups. Except for two studies (22, 27), which did not report comorbidities of the study population, diabetes mellitus (34 of 138 cases) and hypertension (40 of 138 cases) were the most common reported comorbidities (Table 3).
Intervention Characteristics
Among the 10 studies, four used umbilical cord-derived MSC (UC-MSC) (18, 23–25), one pericytes-derived MSC (21), one menstrual blood-derived MSC (22), one non-hematopoietic peripheral blood stem cells (NHPBSC) (26), and one used MSCs-derived exosomes (27). Two studies did not report the type of MSC (19, 20). In dosing, four studies administered 1–2 × 106 cells per kg of body weight (19–21, 25), four administered 30–120 million cells per infusion (18, 22–24), one used 0/5–2 × 1010 of MSC-derived exosomes per administration (27), and one did not report stem cell dose (26). Of the 184 patients in the stem cell group, 27 received a single dose, 35 received two doses, 102 received three doses of therapy, and 20 received therapy for 20 doses. Route of delivery was intravenous (IV) in nine studies and inhalation in one study (27) (Table 4).
Primary Outcomes: Safety
Severe Adverse Events
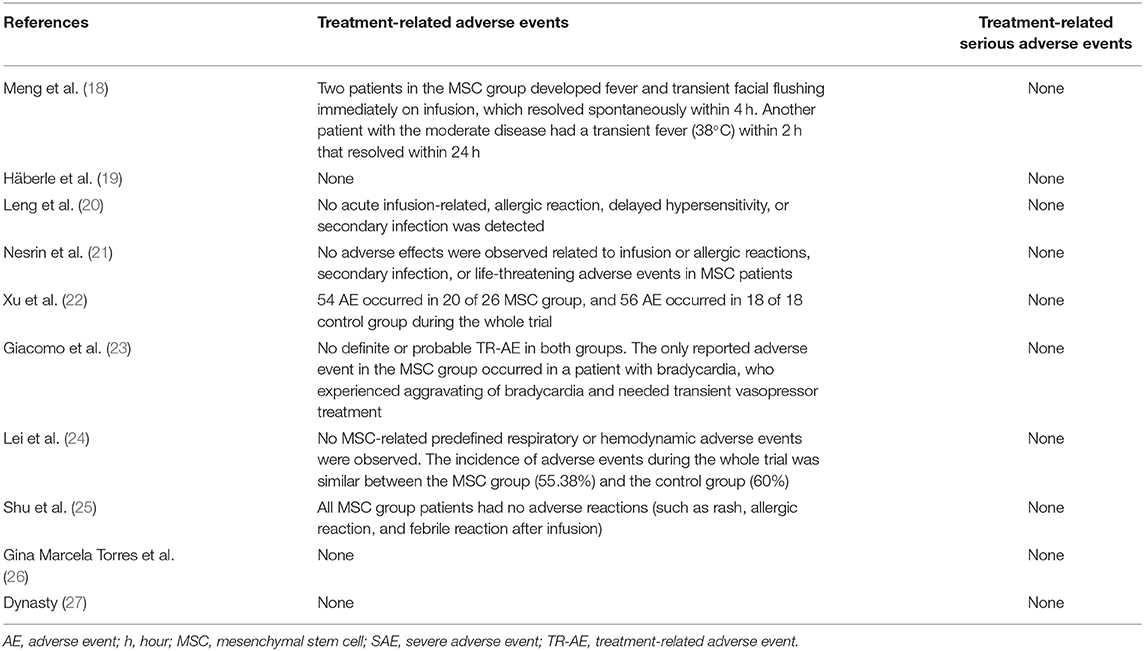
None of the 10 studies reported treatment-related SAEs. Furthermore, none of the 13 deaths in stem cell groups were related to cell infusion (Table 5).
Adverse Events
Two studies reported minimal infusion-related AE in the stem cell group. Meng et al. (18) reported two cases of transient facial flushing and fever immediately on infusion and one case of transient fever within 2 h; all of these AEs resolved without intervention. Giacomo et al. (23) reported only one AE in the MSC group, who needed an increase in vasopressor dose because of exacerbating of bradycardia. Lei et al. and Xu et al. (22, 24) had assessed AEs during the trial for COVID-19 (not for infusion). In both the studies, the incidence of AE in the MSC group was less than in the control group. All other seven studies reported no cell infusion-related AE (Table 5).
Secondary Outcome: Efficacy
Mortality
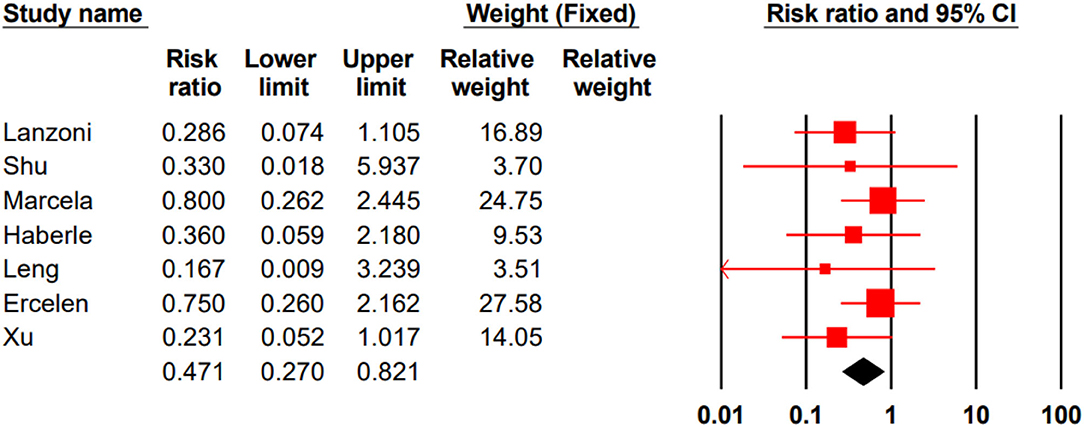
In three studies, all participated patients survived (18, 24, 27). In seven remained studies, the total mortality rate for the stem cell group was %14/4 (13 of 90) and %32/7 (35 of 107) for the control group. Our meta-analysis showed that stem cell therapy could significantly decrease the mortality rate in patients with COVID-19 (RR 0.471, 95% CI 0.270–0.821) (Figure 2).
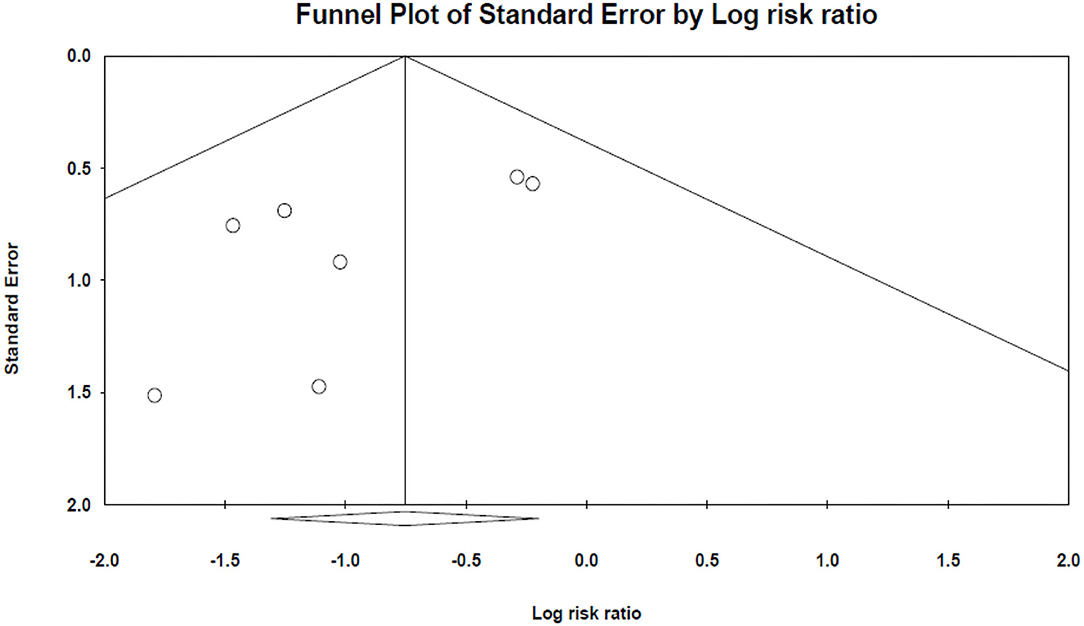
The results of Egger's and Begg's tests did not show any evidence of publication bias (The P-value of Egger's tests was 0.1, and Begg's tests was 0.5) (Figure 3).
Morbidity
Stem cell therapy could significantly decrease morbidities in patients with COVID-19 (RR 0.788, 95% CI 0.626–0.992) (Figure 4).
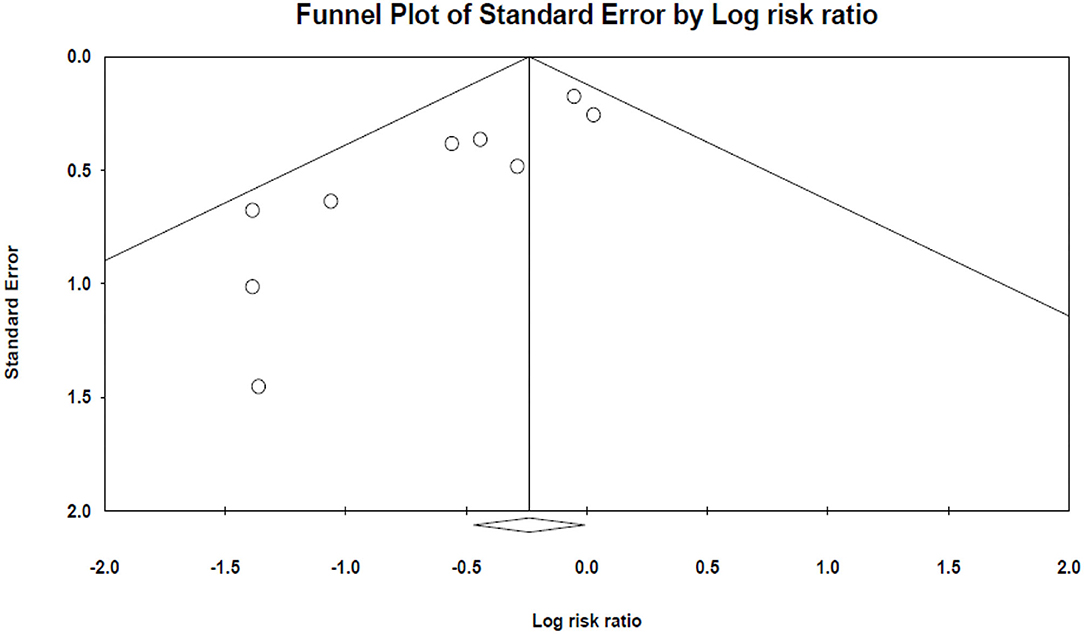
Egger's and Begg's tests indicated significant publication bias (The P-value of Egger's tests was 0.001, and Begg's tests was 0.047) (Figure 5).
Pulmonary Function and Imaging Changes
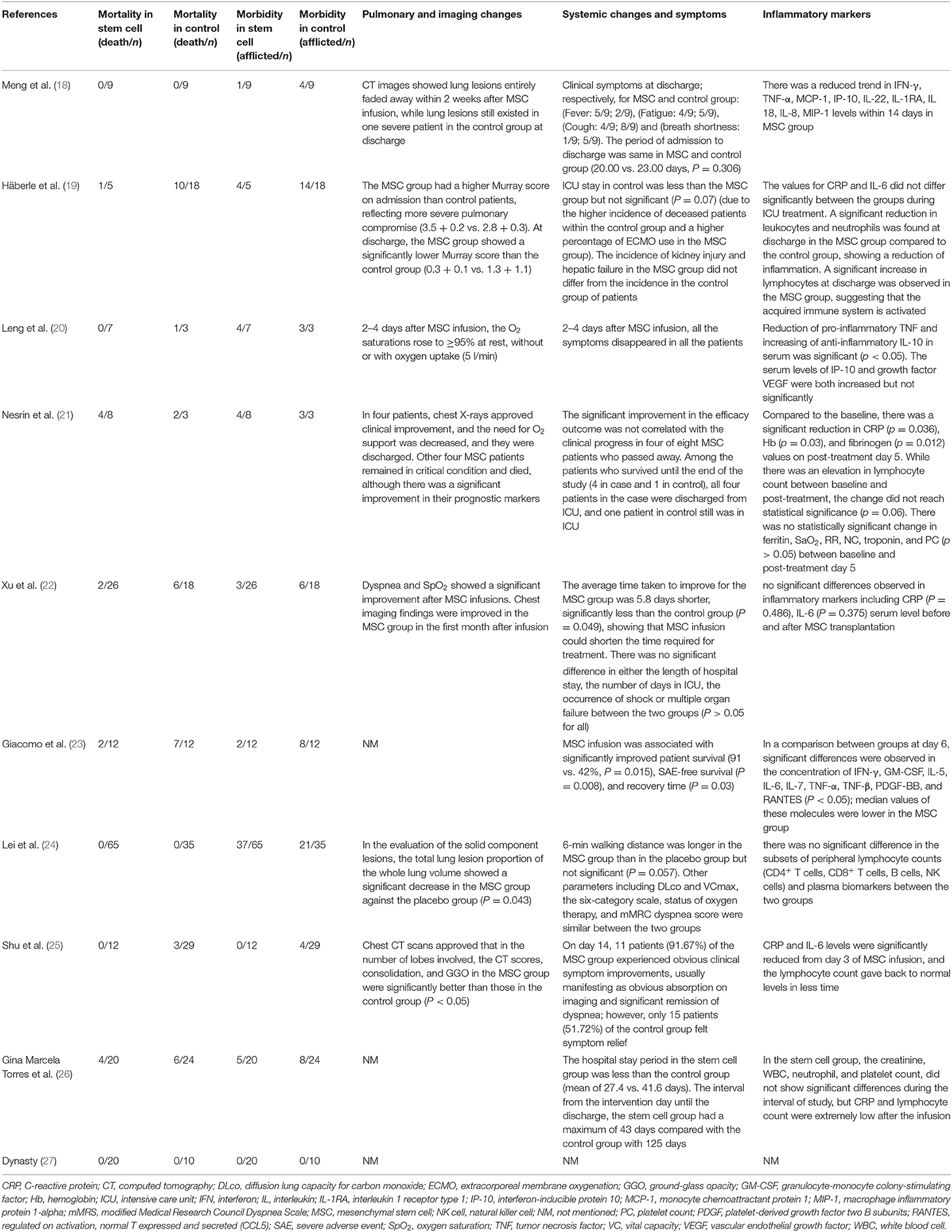
Pulmonary function and imaging changes have been assessed in seven studies (18–22, 24, 25). These studies showed that stem cell therapy could improve O2 saturation, Murray score, and lung lesions (Table 6).
Systematic Changes and Symptoms
Systematic changes were defined as any changes from the overall baseline status. Except for one study that had not reported these changes (27), these data are available for the other nine studies in Table 6.
Inflammatory Cells and Cytokines
Except for one study that had not reported the changes in inflammatory cells and cytokines (27), these changes are available for the other nine studies in Table 6.
Discussion
In the current study, we found that stem cells are safe and can significantly decrease the mortality and morbidity of patients with COVID-19. Stem cell infusion can also improve pulmonary function, ameliorate symptoms, and suppress inflammation.
Safety is the primary issue that should be considered for any therapy. In our included studies, none of the mortalities were related to cell infusion. Thompson et al. (28) showed that intravascular administration of MSCs is associated with an increased fever risk (RR = 2.48, 95% CI 1.27–4.86). One of the most common symptoms in patients with COVID-19 is fever. In our studies, just Meng et al. (18) reported that two patients with MSC developed fever immediately after infusion, which resolved without intervention within 4 h, and none of the other studies reported infusion-related fever. So it seems stem cell transplantation is safe in patients with COVID-19.
Our analysis showed that stem cell therapy could significantly reduce disease mortality and severity. Qu et al. (29) conducted a meta-analysis of human studies on acute respiratory distress syndrome (ARDS). ARDS is a significant cause of mortality in patients with COVID-19. They reported that MSC therapy could reduce mortality in patients with ARDS but was not statistically significant. This dissimilarity could be due to study design and baseline characteristics; we just included patients with COVID-19, but Qu et al. included patients with ARDS with various ARDS causes like COVID-19, influenza, sepsis, and aspiration.
Based on the evaluation of the pulmonary function, and inflammatory markers changes in the included studies, MSC therapy could reduce inflammation and enhance pulmonary function. Similarly, a systematic review by Mahendiratta et al. (30) suggests that MSCs are capable of reducing systemic inflammation and protecting patients against COVID-19 infection. It seems the mechanism of action is due to the immunomodulatory effect of MSCs; which involves: (a) inhibiting B cells, T cells, NK cells, and dendritic cells activation; (b) decreasing macrophage M1 and enhancing macrophage M2 activation; (c) inhibition of mast cell degranulation; (d) promoting T regulatory and Th2 cell (31).
Interleukin 6 (IL-6) is a prognostic marker in COVID-19 infection, and tocilizumab blocks its receptors, a drug of choice for COVID-19 infection (32). Our data show that MSCs can reduce IL-6 in serum, which means that MSCs can mimic tocilizumab by decreasing IL-6 activity. Das (33) proposes that the anti-inflammatory effect of MSCs is attributable to the ability to secreting lipoxin A4 (LXA4), PGE2, and their precursors, which prevent the production of pro-inflammatory cytokines like IL-6 and tumor necrosis factor-alpha (TNF-α); this feature could be engaged to confront “cytokine storm” that which is seen in COVID-19 infection.
One complication of COVID-19 is excessive lung fluid production and pulmonary edema, which disturb proper pulmonary function. MSCs release keratinocyte growth factor (KGF), angiopoietin-1, and LXA4 in their exosomes. These factors activate the Na+-K+ pump, thus reducing the permeability of the alveolar epithelium to proteins and fluid and inhibiting fluid accumulation in lung tissue and edema. Another complication of COVID-19 is lung fibrosis. This complication should be taken seriously because it is irreversible if it occurs. MSCs prevent lung fibrosis by two mechanisms: (a) differentiating to alveolar type II cells; (b) paracrine signals (like KGF) that induce proliferation and inhibit apoptosis in type II alveolar cells (11, 34).
An important question is: Whether MSCs can get infected by COVID-19? Researches have shown that COVID-19 uses angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) as receptors to enter cells and infect them. Avanzini et al. (35) and Schäfer et al. (36) in their in vitro studies found that both fetal and adult MSCs have a deficient expression of ACE2 and TMPRSS2. Hernandez et al. (37) reported the same result in human UC-MSCs. However, Desterke et al. (38) reported that adult MSCs express ACE2 highly, while placenta-derived MSCs express ACE2 at a low level and only in initial passages of cultures. Anyhow, it seems MSCs are resistant to get infected by COVID-19 by low expression of ACE2 and TMPRSS2, which will cause them to dodge the infection and perform their immunomodulatory functions.
The COVID-19 mortality rate is higher among immunocompromised patients (39), and as we discussed before, MSCs modulate the immune response. So MSCs infusion for an immunocompromised patient may exacerbate the infection. There is still no large-scale clinical trial that evaluates the safety and efficacy of cell infusion for COVID-19 infection in immunocompromised patients. However, many patients in our studies received corticosteroids during the trial, which suppressed the immune system, and cell infusion was still safe and effective. Cui et al. (40), in an in vitro study, found that coculturing of human MSCs and NK cells of immunocompromised patients can improve the function of impaired NK cells and enhance the synthesis of interferon-gamma (IFN-γ) (which is a crucial part of the innate immune response during viral infection). Also, Lim et al. (41) found that human MSCs reduce lung injury in severe combined immunodeficiency (SCID) mice but not in immunocompetent mice.
Unfortunately, there is no protocol for stem cell therapy in patients with COVID-19. So trials are heterogeneous in terms of stem cell source, culture, dose, delivery route, and even the stage of COVID-19 infection that MSCs are administered. Despite these heterogeneities in reviewed studies, they all reported stem cell therapy as a safe and effective treatment for patients with COVID-19. Large-scale RCTs with accurate methodology will help to develop the best protocol in this field.
Some limitations of this study should be taken into consideration. First, although we started with a large number of articles, after several screenings, the number of eligible studies was relatively small. This could have reduced the power of the conclusion. Second, the potential influence of preexisting conditions, the severity of infection, and the COVID-19 variants could not be investigated because of the limited information obtained from the studied articles. Third, as with any systematic review, limitations associated with potential publication bias should be considered. Furthermore, studies' variability, different patients' characteristics, different morbidity definitions, and wide range of outcome metrics for pulmonary changes and inflammatory indicators were other limitations.
Conclusions
Our analysis demonstrated that stem cells are safe to use in patients with COVID-19 and could significantly reduce mortality and morbidity rate in infected patients. Due to the low number of included studies, a large-scale analysis is needed to measure the outcomes. Likewise, a protocol for stem cell therapy in COVID-19 infection should be defined to achieve the best possible clinical outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
The study was designed by MZ and MN. EA, SK, NT, and AA performed the search, study selection, and data extraction. Statistical analysis was performed by MN. The first draft of the manuscript was written by MZ, EA, SK, NT, and AA. MN and MZ revised the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AB declared a shared affiliation with the authors to the handling editor at the time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
1. Worldmeters. COVID Live Update. (2021). Available online at: https://www.worldometers.info/coronavirus/
2. Tisdell CA. Economic, social and political issues raised by the COVID-19 pandemic. Econ Anal Policy. (2020) 68:17–28. doi: 10.1016/j.eap.2020.08.002
3. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immunity. (2020) 89:531–42. doi: 10.1016/j.bbi.2020.05.048
4. Ali MJ, Hanif M, Haider MA, Ahmed MU, Sundas F, Hirani A, et al. Treatment options for COVID-19: a review. Front Med. (2020) 7:480. doi: 10.3389/fmed.2020.00480
5. Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID-19 diagnosis and management: a comprehensive review. J Internal Med. (2020) 288:192–206. doi: 10.1111/joim.13091
6. Lee WC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. (2018) 155:236–50. doi: 10.1016/j.biomaterials.2017.10.004
7. Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig. (2019) 6:1–11. doi: 10.21037/sci.2019.06.04
8. Munir H, McGettrick HM. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. (2015) 24:2091–100. doi: 10.1089/scd.2015.0008
9. Watson LM, Wong MM, Becker EB. Induced pluripotent stem cell technology for modelling and therapy of cerebellar ataxia. Open Biol. (2015) 5:150056. doi: 10.1098/rsob.150056
10. Mezey É, Nemeth K. Mesenchymal stem cells and infectious diseases: smarter than drugs. Immunol Lett. (2015) 168:208–14. doi: 10.1016/j.imlet.2015.05.020
11. Jamshidi E, Babajani A, Soltani P, Niknejad H. Proposed mechanisms of targeting COVID-19 by delivering mesenchymal stem cells and their exosomes to damaged organs. Stem Cell Rev Rep. (2021) 17:1–17. doi: 10.1007/s12015-020-10109-3
12. Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regeneration versus immunomodulatory challenges. Am J Stem Cells. (2013) 2:22.
13. Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. (2020) 41:653–664. doi: 10.1016/j.tips.2020.06.009
14. Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int. (2018) 2018:1–12. doi: 10.1155/2018/3057624
15. Weng J, Du X, Geng S, Peng Y, Wang Z, Lu Z, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. (2010) 45:1732–40. doi: 10.1038/bmt.2010.195
16. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
17. Borisov SE, Dheda K, Enwerem M, Leyet RR, D'Ambrosio L, Centis R, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR-and XDR-TB: a multicentre study. Euro Respir J. (2017) 49:3.
18. Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduction Targeted Ther. (2020) 5:1–7. doi: 10.1038/s41392-020-00286-5
19. Häberle H, Magunia H, Lang P, Gloeckner H, Körner A, Koeppen M, et al. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J Intensive Care Med. (2021) 36:681–8. doi: 10.1177/0885066621997365
20. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. (2020) 11:216. doi: 10.14336/AD.2020.0228
21. Nesrin OE, Beliz B, Berrin M, Fethi G, Gokay Rasit G, Nagihan A, et al. MSC transplantation in eight severe COVID-19 patients: can cytokine storm be reversed? J Stem Cell Res Ther. (2020) 10:460. doi: 10.35248/2157-7633/2020.10.460
22. Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. (2021) 11:e297. doi: 10.1002/ctm2.297
23. Giacomo L, Elina L, Diego C, Shari Messinger C, Antonio CM, Roger AA, et al. Umbilical cord mesenchymal stem cells for COVID-19 ARDS: a double blind, phase 1/2a, randomized controlled trial. SSRN. (2020) 10:660–73. doi: 10.1002/sctm:20-0472
24. Lei S, Hai H, Xuechun L, Xiaoyan Y, Xiaojing J, Ruonan X, et al. Treatment with human umbilical cord-derived mesenchymal stem cells for COVID-19 patients with lung damage: a randomised, double-blind, placebo controlled phase 2 trial. medRxiv. (2021) 6:58. doi: 10.1038/s41392-021-00488-5
25. Shu L, Niu C, Li R, Huang T, Wang Y, Ji N, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Res Square. (2020) 11:1–11. doi: 10.21203/rs.3.rs-23696/v1
26. Gina Marcela Torres Z, Carlos Alfonso Villegas V, Antonio Bencomo H, Lobna Abdel H, Rene Antonio R, Yendry Ventura C. Renal involvement in patients with covid-19 pneumonia and outcomes after stem cell nebulization. medRxiv. (2020).
27. Dynasty SRMC. Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. (2020). Available online at: https://ClinicalTrials.gov/show/NCT04491240
28. Thompson M, Mei SH, Wolfe D, Champagne J, Fergusson D, Stewart DJ, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine. (2020) 19:100249. doi: 10.1016/j.eclinm.2019.100249
29. Qu W, Wang Z, Hare JM, Bu G, Mallea JM, Pascual JM, et al. Cell-based therapy to reduce mortality from COVID-19: systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl Med. (2020) 9:1007–22. doi: 10.1002/sctm.20-0146
30. Mahendiratta S, Bansal S, Sarma P, Kumar H, Choudhary G, Kumar S, et al. Stem cell therapy in COVID-19: pooled evidence from SARS-CoV-2, SARS-CoV, MERS-CoV and ARDS: a systematic review. Biomed Pharmacother. (2021) 137:111300. doi: 10.1016/j.biopha.2021.111300
31. Jayaramayya K, Mahalaxmi I, Subramaniam MD, Raj N, Dayem AA, Lim KM, et al. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatmen. BMB Rep. (2020) 53:400. doi: 10.5483/BMBRep.2020.53.8.121
32. Perrone F, Piccirillo MC, Ascierto PA, Salvarani C, Parrella R, Marata AM, et al. Tocilizumab for patients with COVID-19 pneumonia. The TOCIVID-19 phase 2 trial. MedRxiv. (2020) 18:405. doi: 10.1101/2020.06.01.20119149
33. Das UN. Bioactive lipids as mediators of the beneficial action (s) of mesenchymal stem cells in COVID-19. Aging Dis. (2020) 11:746. doi: 10.14336/AD.2020.0521
34. Yadav P, Vats R, Bano A, Bhardwaj R. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics. Life Sci. (2020) 263:118588. doi: 10.1016/j.lfs.2020.118588
35. Avanzini MA, Mura M, Percivalle E, Bastaroli F, Croce S, Valsecchi C, et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl Med. (2021) 10:636–42. doi: 10.1002/sctm.20-0385x
36. Schäfer R, Spohn G, Bechtel M, Bojkova D, Baer PC, Kuçi S, et al. Human mesenchymal stromal cells are resistant to SARS-CoV-2 infection under steady-state, inflammatory conditions and in the presence of SARS-CoV-2-infected cells. Stem Cell Rep. (2021) 16:419–27. doi: 10.1016/j.stemcr.2020.09.003
37. Hernandez JJ, Beaty DE, Fruhwirth LL, Chaves APL, Riordan NH. Dodging COVID-19 infection: low expression and localization of ACE2 and TMPRSS2 in multiple donor-derived lines of human umbilical cord-derived mesenchymal stem cells. J Transl Med. (2021) 19:1–10. doi: 10.1186/s12967-021-02813-6
38. Desterke C, Griscelli F, Imeri J, Marcoux P, Lemonnier T, Latsis T, et al. Molecular investigation of adequate sources of mesenchymal stem cells for cell therapy of COVID-19-associated organ failure. Stem Cells Transl Med. (2020) 10:568–571. doi: 10.1002/sctm.20-0189
39. Belsky JA, Tullius BP, Lamb MG, Sayegh R, Stanek JR, Auletta JJ. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. (2021) 82:329–38. doi: 10.1016/j.jinf.2021.01.022
40. Cui R, Rekasi H, Hepner-Schefczyk M, Fessmann K, Petri RM, Bruderek K, et al. Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther. (2016) 7:1–13. doi: 10.1186/s13287-016-0353-9
Keywords: stem cell, mesenchymal stem cell, cell therapy, COVID-19, SARS-CoV-2, 2019 novel coronavirus
Citation: Arabpour E, Khoshdel S, Tabatabaie N, Akhgarzad A, Zangiabadian M and Nasiri MJ (2021) Stem Cells Therapy for COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 8:737590. doi: 10.3389/fmed.2021.737590
Received: 07 July 2021; Accepted: 22 October 2021;
Published: 29 November 2021.
Edited by:
Andrea Ballini, University of Bari Aldo Moro, ItalyReviewed by:
Sajad Yaghuby, Iranshahr University of Medical Sciences, IranAmirhesam Babajani, Shahid Beheshti University of Medical Sciences, Iran
Robert Chunhua Zhao, Peking Union Medical College Hospital (CAMS), China
Copyright © 2021 Arabpour, Khoshdel, Tabatabaie, Akhgarzad, Zangiabadian and Nasiri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moein Zangiabadian, emFuZ2lhYmFkaWFuMTk5OEBnbWFpbC5jb20=; Mohammad Javad Nasiri, bWoubmFzaXJpQGhvdG1haWwuY29t
 Erfan Arabpour
Erfan Arabpour Sina Khoshdel
Sina Khoshdel Negin Tabatabaie1
Negin Tabatabaie1 Moein Zangiabadian
Moein Zangiabadian Mohammad Javad Nasiri
Mohammad Javad Nasiri