- 1Critical Care Research Group, Faculty of Medicine, The Prince Charles Hospital, University of Queensland, Brisbane, QLD, Australia
- 2Department of Emergency and Critical Care Medicine, Hitachi General Hospital, Hitachi, Japan
- 3Japanese Society for Early Mobilization, Tokyo, Japan
- 4Nursing Research, Department of Anesthesiology and Intensive Care Medicine, University Hospital of Schleswig-Holstein, Kiel, Germany
- 5Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center, Vanderbilt University School of Medicine, Nashville, TN, United States
- 6Department of Veterans Affairs Medical Center, Geriatric Research Education and Clinical Center (GRECC), Tennessee Valley Healthcare System, Nashville, TN, United States
- 7Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 8Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 9Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 10Department of Biostatistics, M&D Data Science Center, Tokyo Medical and Dental University, Tokyo, Japan
- 11Faculty of Medicine, University of Tripoli, Tripoli, Libya
- 12Department of Critical Care Medicine, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS), Lucknow, India
- 13Department of Rehabilitation Medicine, Sarawak General Hospital, Kuching, Malaysia
- 14Department of Critical Care Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea
- 15Rehabilitation Department, Woodlands Health Campus, Yishun, Singapore
- 16Emergency and Critical Care Center, Kobe University Hospital, Kobe, Japan
- 17Department of Disaster and Emergency Medicine, Graduate School of Medicine, Kobe University, Kobe, Japan
- 18Department of Surgery, Jichi Medical University, Tochigi, Japan
- 19Department of Anesthesiology and Critical Care Medicine, Fujita Health University School of Medicine, Toyoake, Japan
Background: Data regarding delivery of evidence-based care to critically ill patients in Intensive Care Units (ICU) during the COVID-19 pandemic is crucial but lacking. This study aimed to evaluate the implementation rate of the ABCDEF bundle, which is a collection of six evidence-based ICU care initiatives which are strongly recommended to be incorporated into clinical practice, and ICU diaries for patients with and without COVID-19 infections in ICUs, and to analyze the impact of COVID-19 on implementation of each element of the bundle and independent associated factors.
Methods: A world-wide 1-day point prevalence study investigated the delivery of the ABCDEF bundle and ICU diary to patients without or with COVID-19 infections on 27 January 2021 via an online questionnaire. Multivariable logistic regression analysis with adjustment for patient demographics evaluated the impact of COVID-19 and identified factors in ICU administrative structures and policies independently associated with delivery.
Results: From 54 countries and 135 ICUs, 1,229 patients were eligible, and 607 (49%) had COVID-19 infections. Implementation rates were: entire bundle (without COVID-19: 0% and with COVID-19: 1%), Element A (regular pain assessment: 64 and 55%), Element B (both spontaneous awakening and breathing trials: 17 and 10%), Element C (regular sedation assessment: 45 and 61%), Element D (regular delirium assessment: 39 and 35%), Element E (exercise: 22 and 25%), Element F (family engagement/empowerment: 16 and 30%), and ICU diary (17 and 21%). The presence of COVID-19 was not associated with failure to implement individual elements. Independently associated factors for each element in common between the two groups included presence of a specific written protocol, application of a target/goal, and tele-ICU management. A lower income status country and a 3:1 nurse-patient ratio were significantly associated with non-implementation of elements A, C, and D, while a lower income status country was also associated with implementation of element F.
Conclusions: Regardless of COVID-19 infection status, implementation rates for the ABCDEF bundle, for each element individually and an ICU diary were extremely low for patients without and with COVID-19 infections during the pandemic. Strategies to facilitate implementation of and adherence to the complete ABCDEF bundle should be optimized and addressed based on unit-specific barriers and facilitators.
Introduction
For patients in the intensive care unit (ICU), evidence-based treatment such as the ABCDEF bundle (1–4) and ICU diary (5), should be established as part of routine clinical practice because they are strongly linked not only to short-term outcomes of ICU patients (6, 7) but also their long-term function and quality of life (QOL) (8). Recent studies confirmed that the beneficial effects of the ABCDEF bundle are maximized when provided as a combination of elements or as the entire bundle (9, 10).
However, drastic changes in practice related to the COVID-19 pandemic, including unbalanced resources, overwhelmed facility capacity, and strict infectious regulations, occurred world-wide and prevented ICU staff from performing evidence-based approaches to patient care in ICUs (11). Our recent survey demonstrated low implementation rates of each element and the entire ABCDEDF bundle and other supportive ICU care for patients with COVID-19 infections in the ICU (12). We could not assess the total impact of COVID-19 in the ICU because of a lack of data on patients without COVID-19 infections. To overcome low implementation rates which result in poor outcomes of ICU patients (13), a number of studies proposed efficient ways associated with the ICU administrative structure and environment to promote evidence-based ICU care before the pandemic (14–16). Nonetheless, clinical data on promoting factors and barriers during the pandemic are lacking and these factors could vary when treating patients without or with COVID-19 infections because the policy of less physical contact in a short time while wearing protective personal equipment was generally enforced only for patients with COVID-19 infections. Moreover, low- and middle-income countries are more vulnerable to these resource-dependent changes (17).
Therefore, we conducted a 1-day point prevalence study, to investigate the implementation rate of evidence-based ICU care for both patients without and with COVID-19 infections and the impact of COVID-19 infections on implementation on a world-wide scale to capture the current clinical practice situation. We sought to identify ICU-related factors associated with implementation in the ICU.
Materials and Methods
Study Design and Settings
This was an international 1-day point prevalence study conducted on 27 January 2021, with approval by the ethics committee of the Saiseikai Utsunomiya Hospital (2020-69) and pre-registration in UMIN (ID: 000040405). The study design and construction followed the STROBE cross-sectional guidelines.
The study committee recruited participants from January 8 to 26 by disseminating an invitation letter to members of the Indian Society of Critical Care Medicine, the Korean Society of Critical Care Medicine, and other local or national networks in collaboration with regional/national coordinators (Appendix 1). The invitation letter included a brief introduction of this study, a specific link to the web site explaining the study details (https://form.jsea2005.org/isiic-II-study/), ethical considerations, and the URL for registration created by Google Forms (Google Inc.). According to the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan (18), ethical approval at each participating institution was waived because of the anonymous nature of this study which will not collect specific data that could identify ICUs or individual patients. All ICUs which agreed to the study policies could register and there were no exclusion criteria. The name of one representative for each participating ICU, the name of the hospital, and its country were registered to confirm the reliability of data sources (Appendix 1).
Study Process
The study committee requested all registered representatives to provide background data for their hospitals and ICUs via a Google Form starting 20 January 2021, before the survey date. The questionnaire used to obtain background data (e.g., number of hospital beds, ICU beds, COVID-19 specific ICU beds, nurse-to-patient ratio) is shown in Appendix 2 (18 questions, 3 min). The income level was classified according to the World Bank Country Classification (https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups) according to the country where the participating ICUs are located, which was obtained as the background data. Each representative received a different Facility Registration Number automatically soon after the completion of the questionnaire. On the survey date, 27 January 2021, the URL for the survey of evidence-based and supportive ICU care (21 questions, 3–5 min, Google Form) were sent to all registered representatives. All representatives were asked to input the institution-specific Facility Registration Number at the first question, and only those who had it could continue to complete the survey (Appendix 3). The questions in the survey were pre-reviewed by the study co-authors and pre-tested by collaborative physicians and nurses listed in the acknowledgment. The URL for the survey was open from January 27 to 30.
Data Collection
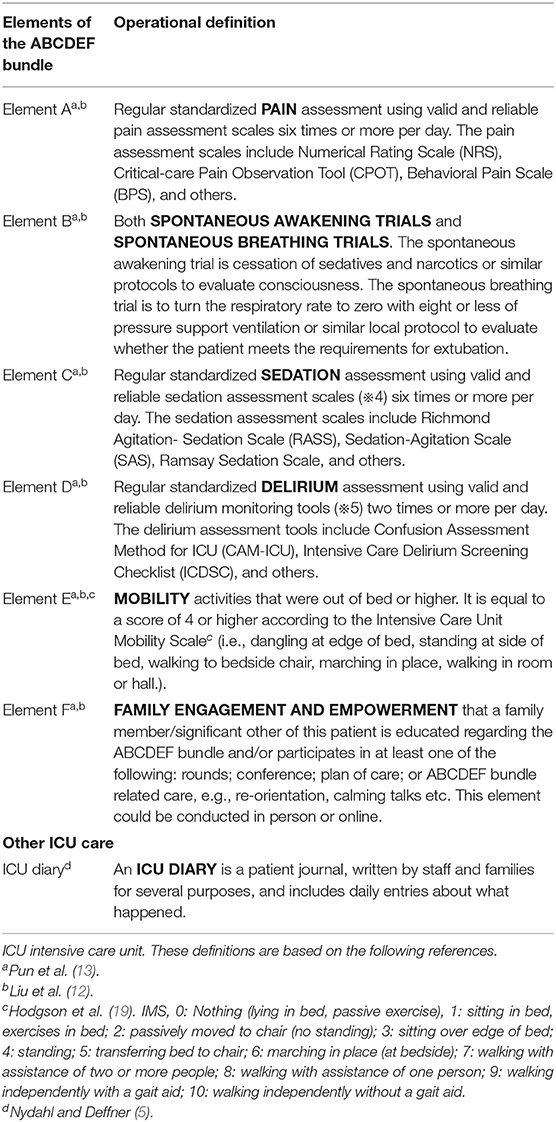
In the survey, patient demographics, such as age, gender, Body Mass Index (BMI), and ICU length of stay as of the survey date, use of medical devices, continuous use of neuromuscular blockade, vasoactive, analgesia, and sedation agents, prone positioning and its duration, the presence of a target/goal of each ICU care modality given to ICU patients on the survey date, and the implementation of each element of the ABCDEF bundle, ICU diary provided on the survey data were collected. The operational definitions of each element of the ABCDEF bundle and ICU diary (Table 1) were provided to respondents at the appropriate place in the survey. The representatives completed one questionnaire for each patient, except for patients who were terminally ill and receiving palliative care. For example, if there were three ICU patients in the ICU, the representative needed to complete the questionnaire for the survey of evidence-based and supportive ICU care three times. Data obtained from the survey were anonymous both for patients and institutions, the data of evidence-based and supportive ICU care was linked to data of the background data for their hospitals and ICUs by the facility-specific Facility Registration Number.
All the data were stored online (Google Drive, Google Inc.) and managed or exported by the authorized person out of the authors (Appendix 1).
Outcomes
The primary outcome was the implementation rate of the entire ABCDEF bundle. Secondary outcomes were the implementation rates for each element of the ABCDEF bundle, including element A (regular pain assessment), element B [both spontaneous awakening trials (SAT) and spontaneous breathing trials (SBT)], element C (regular sedation assessment), element D (regular delirium assessment), element E (early mobility and exercise), and element F (family engagement and empowerment), and an ICU diary.
The implementation of element E during mechanical ventilation, the implementation of element F performed online, and visitation policies for family members were also described. Independent factors associated with successful implementation of each element of the ABCDEF bundle were evaluated by multivariable logistic regression analysis.
Statistical Analysis
Non-normally distributed continuous data were reported as medians with interquartile range (IQR). Categorical data were described as numbers or percentages. Comparisons of patient demographics, implementation of the ABCDEF bundle, and the ICU diary between the groups of patients with out and with COVID-19 infections were made with the Mann-Whitney U-test for non-normally distributed continuous data and the chi-squared test and Fisher's exact test for categorical data appropriately. There was no missing data.
In multivariable logistic regression analysis with adjustment for patient demographics, the association between the implementation of each element of the ABCDEF bundle and the presence of COVID-19 infection or ICU administrative structures was investigated. Patient demographics included length of ICU stay, age, gender, body mass index, use of mechanical ventilation, extracorporeal membrane oxygenation including veno-venous and veno-arterial, renal replacement therapy, and left ventricular unloading device, continuous use of neuromuscular blockade, vasoactive drugs, analgesia agents and sedation agents, and prone positioning. The following variables were changed to factors and used in the multivariable logistic regression analysis: number of hospital beds, nurse-to-patient ratio, frequency of multidisciplinary rounds, number of visiting hours for a family, type of hospital and ICU, primary responsibility to make decisions to implement the ABCDEF bundle, age, body mass index, income level. As a sub-analysis, associated independent factors among ICU administrative structures for each group (non-COVID-19 and COVID-19) were evaluated through the stepwise method with Akaike information criterion and with adjustments of the same variables of patients demographics described above. The stepwise method was used to focus on significant factors. In the sub-analysis, the variables that the number of patients allocated to the category is too few (≤5 patients) to create a suitable model were excluded from multivariable logistic regression analysis.
The calculated sample size with 95% power and a two-sided alpha of 0.05 was 508 patients under the assumption of the implementation rate of the entire ABCDEF bundle for patients without and with COVID-19 infections (8 and 1%, respectively) based on previous surveys (12, 13). All statistical analyses were carried out using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (20) and R (R Project, Vienna, Austria). The p-value was reported as two-sided and p <0.05 was considered statistically significant.
Results
Background of Hospitals and ICUs
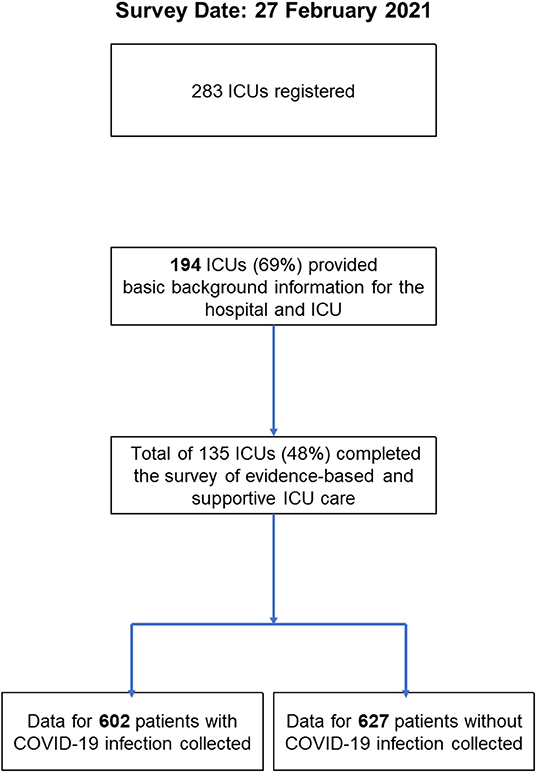
Of 283 registered ICUs, 135 ICUs completed the survey (response rate 48%) (Figures 1, 2). Respondents included 78% physicians. The most common size of participating hospitals was 800 beds or more, with a median of 14 ICU beds and 4 beds allocated for patients with COVID-19 infections (Table 2). The nurse: patient ratio was 1:2 in 53% of ICUs. Multidisciplinary rounds were conducted significantly less frequently for patients with COVID-19 infections (p = 0.004). Compared to before the pandemic, family visiting hours to patients both without and with COVID-19 infection were reduced (<0.001 and p = 0.004, respectively), and more stringent restrictions imposed on families of patients with COVID-19 infections (p <0.001). A specific protocol for each element of the ABCDEF bundle was in place in <50% of ICUs except for a protocol for pain management (51%).
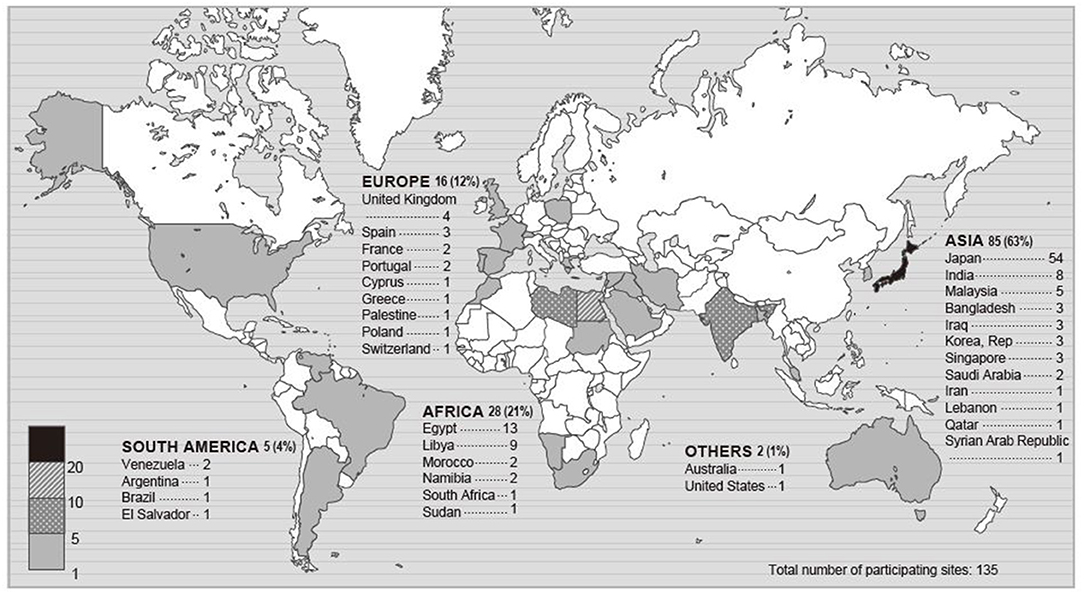
Figure 1. Map of participating sites. The numbers in this figure indicate the number of participating ICUs in each country or a percentage. Percentage indicates the proportion of the total participating ICUs.
The details of the types of hospitals and ICUs participating, professionals dedicated to the ICU, and the personnel with primary responsibility for implementing the ABCDEF bundle are shown in Supplementary Table 1.
Patient Demographics
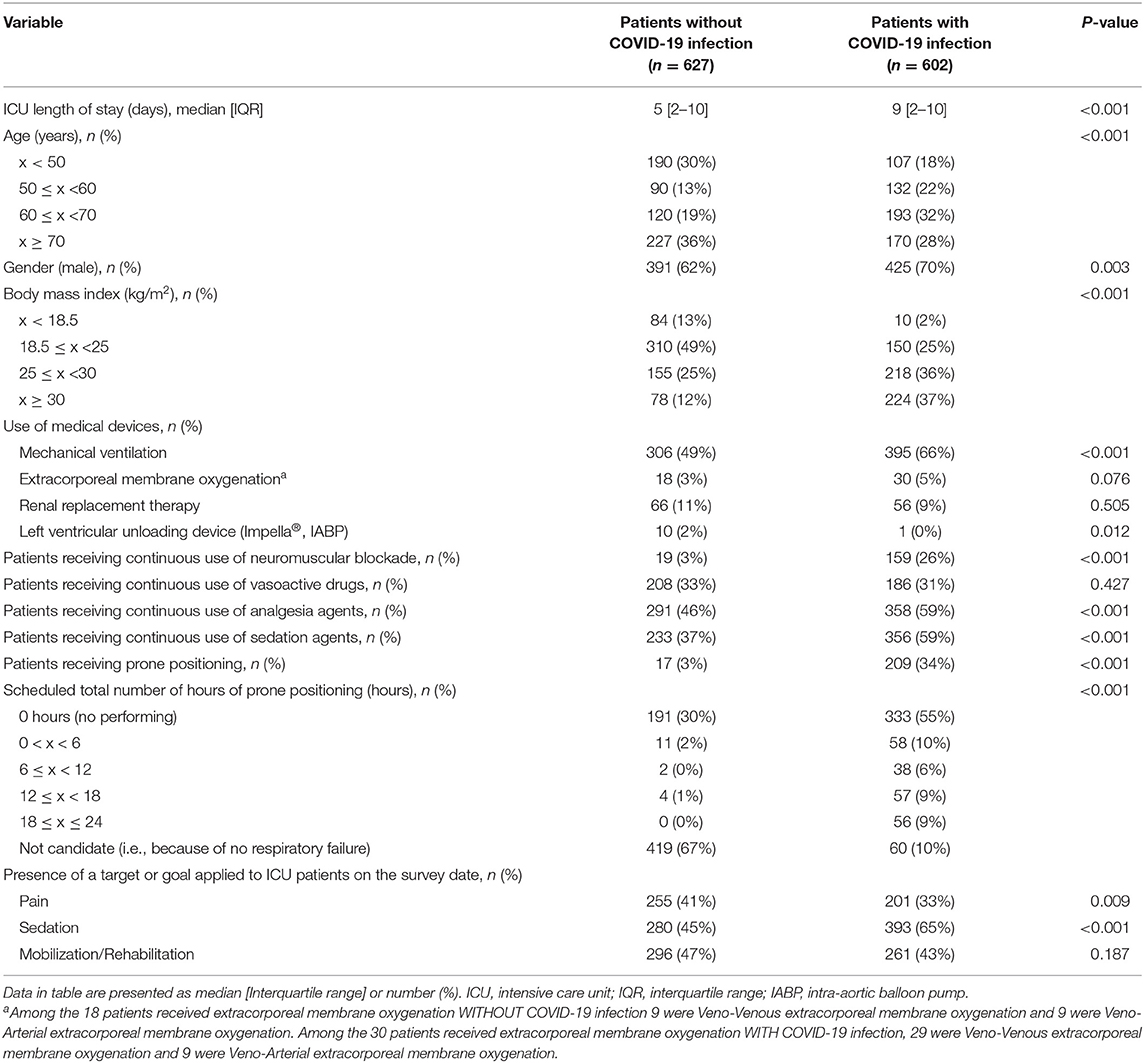
There were significant differences in the demographics of the two groups for ICU length of stay, age, BMI, gender, use of mechanical ventilation (49 vs. 66%) and left-ventricular unloading device, continuous use of neuromuscular blockade, analgesia and sedation agents, prone positioning, and its duration (Table 3). The two groups were not significantly different regarding the use of extracorporeal membrane oxygenation and renal replacement therapy and continuous use of vasoactive drugs (Table 3). The target/goal for pain control was less frequently applied to patients with COVID-19 infections and more sedation was given to them, while no difference was seen for early mobilization and rehabilitation.
Implementation of Evidence-Based ICU Care
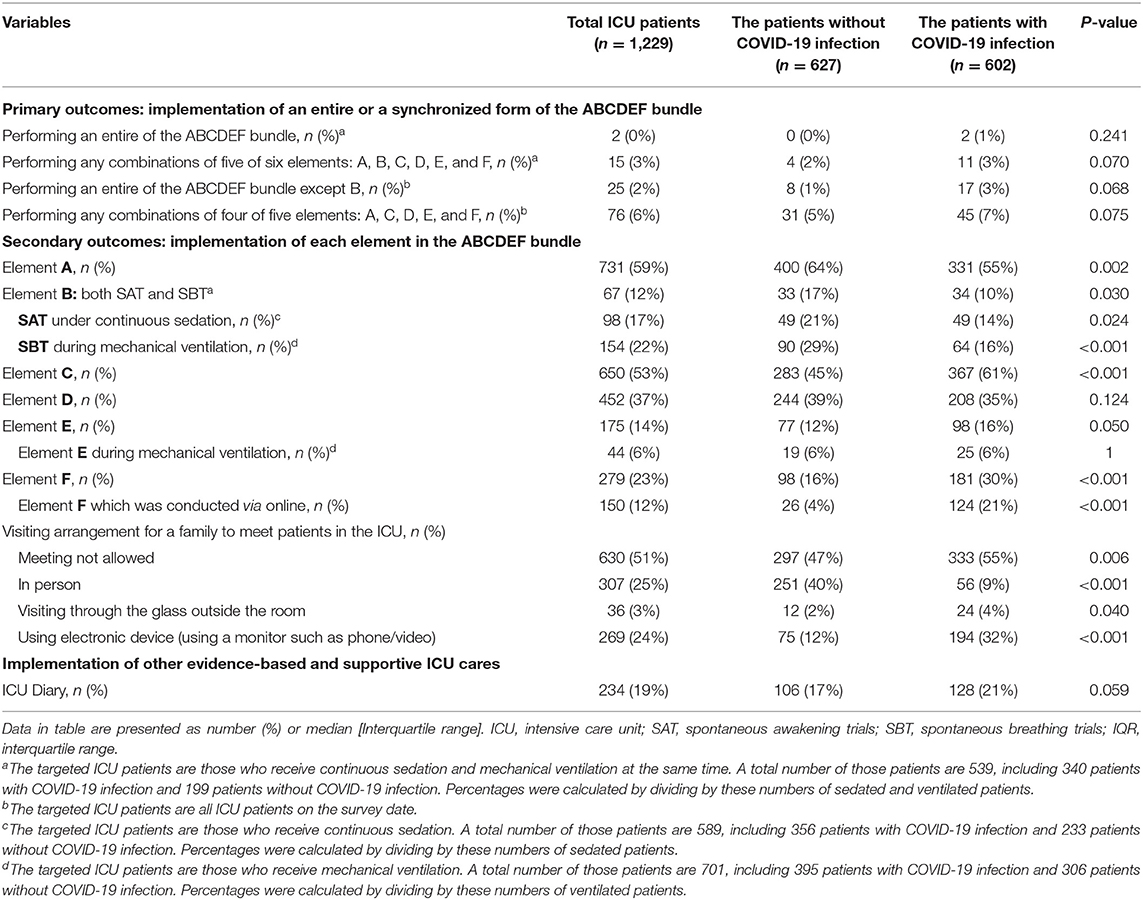
The implementation of the entire ABCDEF bundle, including elements A, B, C, D, E, and F which targets patients undergoing mechanical ventilation and continuous sedation, was rarely performed for patients both without and with COVID-19 infections (without COVID-19: 0% vs. with COVID-19: 1%, p = 0.53) (Table 4). The rate was similar if one element of the six was excluded (2 vs. 3%, p = 0.59). Given elements A, C, D, E, and F which target all ICU patients, the implementation rate of all of these elements was low (1 vs. 3%. P = 0.07), even when one of the five was excluded (5 vs. 7%, p = 0.08).
Element A (64 vs. 55%), element B (17 vs. 10%), SAT (21 vs. 14%), and SBT (29vs. 16%) were implemented significantly less often for patients with COVID-19 infection, while element C (45 vs. 61%), element F (16 vs. 30%) and the online conduct of element F (4 vs. 21%), were performed significantly more frequently for patients with COVID-19 infections. There was no significant difference in the implementation of element D (39 vs. 35%), element E (22 vs. 25%), even while patients were undergoing mechanical ventilation (6 vs. 6%), and the ICU diary (17 vs. 21%). In-person visits were significantly less frequently allowed but online visits using electronic devices were more often used for the families of patients with COVID-19 infection.
Independent Factors Associated With Implementation of the ABCDEF Bundle
In multivariable regression analysis adjusted for baseline conditions, the presence of COVID-19 infection was not associated with non-implementation of individual elements of the bundle, but was significantly associated with implementation of elements D, E, and F (Figure 3).
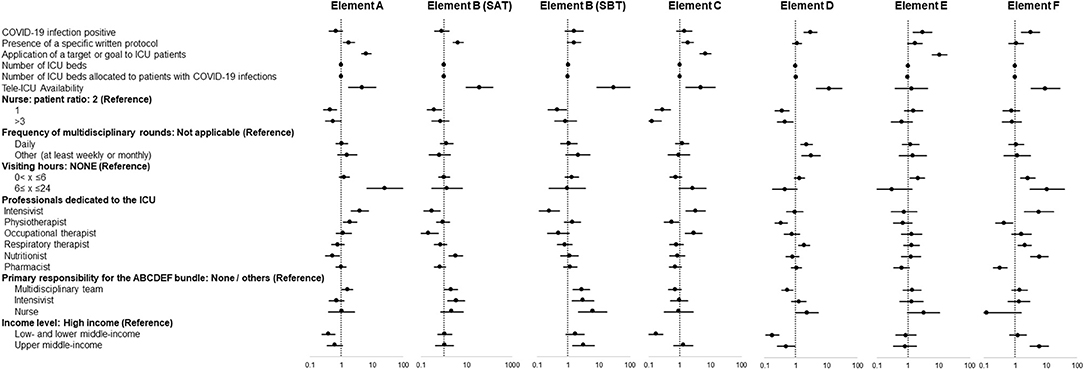
Figure 3. Independent factors associated with implementation of each element of the ABCDEF bundle. Data in figure are shown as adjusted odds ratio with 95% confidential interval.
Among ICU administrative structural elements, specific factors associated with implementation were identified for each element of the bundle. Element A: presence of a written protocol and a target/goal, management as a tele-ICU, the presence of dedicated intensivists, and responsibility by a multidisciplinary team. Element B (SAT): presence of a written protocol, management as a tele-ICU, and responsibility by a multidisciplinary team and intensivists. Element B (SBT): management as a tele-ICU, responsibility by a multidisciplinary team, intensivists, and nurses, and being in an upper-middle-income country. Element C: presence of a written protocol and a target/goal, management as a tele-ICU, and presence of dedicated intensivists. Element D: management as a tele-ICU, performing multidisciplinary rounds daily and at least once a week or month, presence of dedicated respiratory therapists, and responsibility by nurses. Element E: presence of a target/goal and visiting hours (0 < x < 6 h). Element F: management as a tele-ICU, visiting hours (0 < x ≤ 24 h), presence of dedicated Intensivists, respiratory therapist, and nutritionist, and being in an upper-middle-income country.
In the sub-analysis, a variety of different independent factors were identified for patients without and with COVID-19 infections (Figure 4). The presence of a specific written protocol, application of a target/goal, and tele-ICU management were associated with implementation of elements of the bundle in both groups. For patients without and with COVID-19 infections, a 1:1 nurse-patient ratio and daily multidisciplinary round were not significant independent factors, and being in lower- and lower-middle-income countries and a 3:1 nurse-patient ratio were significantly associated with a lower rate of implementation of elements C and D for both groups and element A for those without COVID-19 infections.
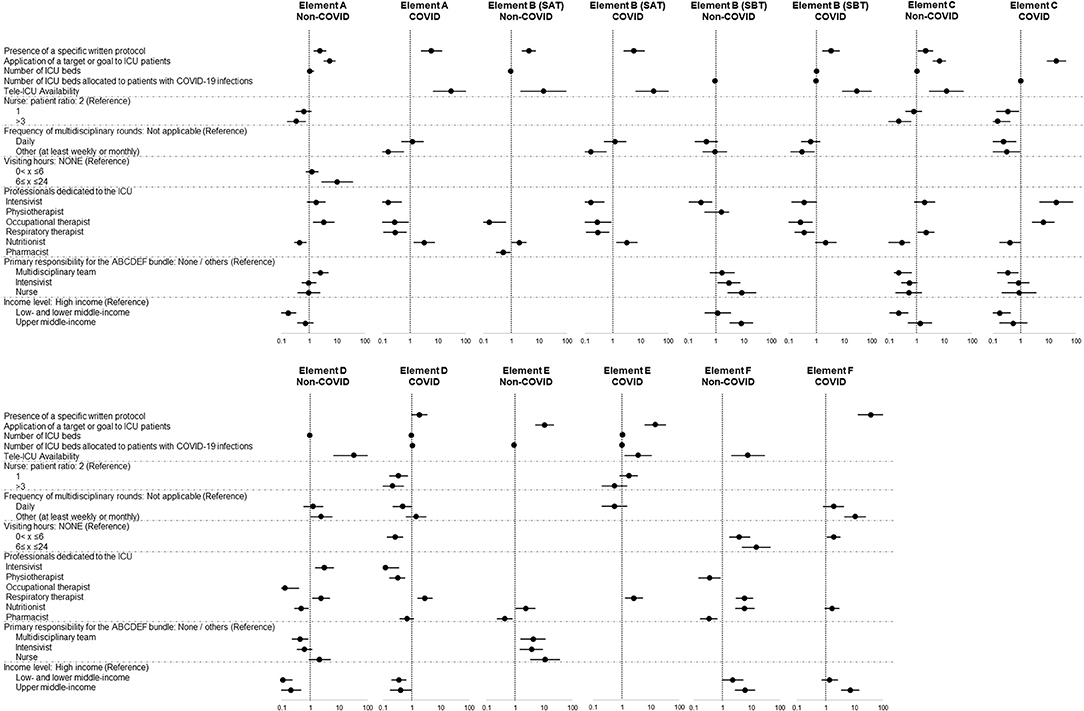
Figure 4. Differences in associated independent factors in patients without and with COVID-19 infections. Data in figure are shown as adjusted odds ratio with 95% confidential interval. The blank lines in the figure are the variables which are excluded from the multivariable logistic regression analysis because the number of patients allocated to the category is too small (≤5 patients) to create the suitable model or the variables which are removed through the stepwise method with Akaike information criterion.
Discussion
This world-wide 1-day prevalence study demonstrates that implementation of the entire ABCDEF bundle, or its individual elements and an ICU diary for patients without and with COVID-19 infections is extremely low even though the implementation rate of specific individual elements of the ABCDEF bundle was different for the two groups. The presence of COVID-19 infection was not a factor preventing implementation. A variety of ICU-related factors were identified as independently associated facilitators or barriers for the implementation of the ABCDEF bundle, and these were different for each element, comparing patients without and with COVID-19 infections.
Implementation of the ABCDEF bundle is much lower in this study compared with that reported by a survey before the pandemic (13, 21), but similar to a survey conducted in the early stages of the COVID-19 pandemic on 3 June and 1 July 2020 (12) (Table 5). These results suggest that COVID-19 affects the care of not only patients with COVID-19 infections but also patients without COVID-19 infections and this effect may have been present since the beginning of the pandemic. Numerous studies strongly show that each element of the ABCDEF bundle or an ICU diary itself has a beneficial effect on patient outcomes (7, 22–26), while low and incomplete implementation can result in adverse outcomes including increased time of ventilatory support, longer ICU and hospital lengths of stay, increased incidence of delirium, functional disability, and increased medical costs and mortality (13, 27). Efficient ways to incorporate evidence-based ICU care into clinical practice during the pandemic are urgently needed. To note, the studies included in Table 5 had various methods to collect data, hence the simple comparison might lead to the misleading. Further and continuous international surveys with same methodology are necessary to follow the implementation rate of the ABCDEF bundle in future. In addition, studies to investigate the effects of the ABCDEF bundle and other evidence-based ICU care are expected.
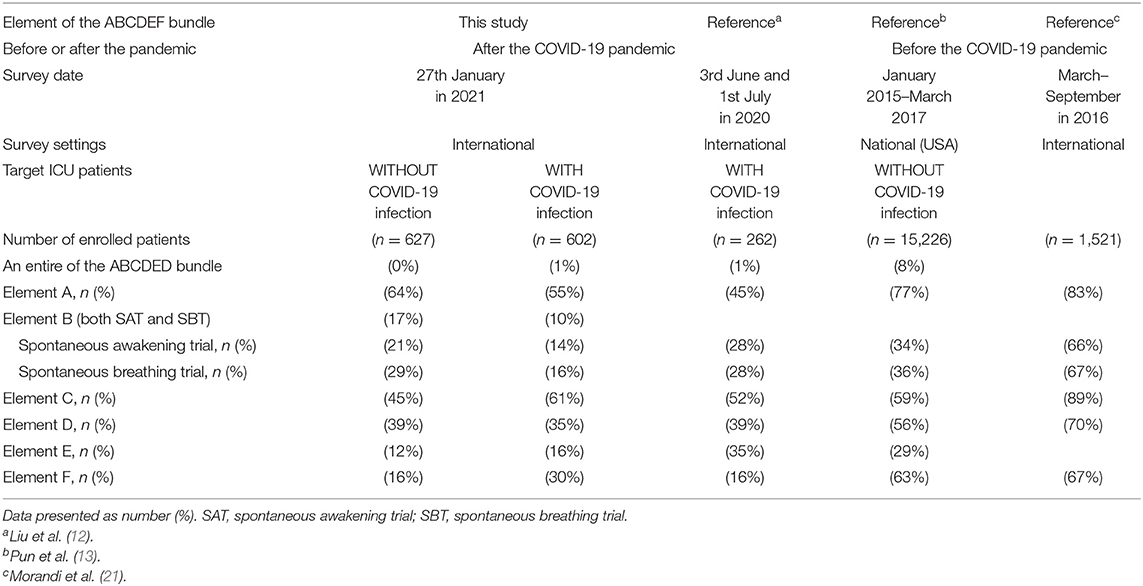
Table 5. Implementation of the ABCDEF bundle compared with previous studies before and after the COVID-19 pandemic.
Differences in the implementation of elements of the ABCDEF bundle might be caused by differences in underlying diseases and ICU length of stay between the two groups. Patients with COVID-19 infections, admitted to the ICU because of severe respiratory failure (28), need longer mechanical ventilation treatment with more sedation and require more intense sedation monitoring compared to patients without COVID-19 infections who have a variety of reasons for admission to the ICU but result in a shorter ICU length of stay. This might lead to more frequent implementation of element C for patients with COVID-19 infections. However, the deeply sedated state could result in non-implementation of element A as seen in patients with COVID-19 infections because few pain assessment tools can be used in heavily sedated patients. The strong respiratory drive and effort associated with COVID-19 infections could be a factor contributing to progression of lung injury (29–31) and could prevent conduct of SAT and SBT for patients with COVID-19 infection, to avoid further exacerbation of the lung injury. However, element F of the ABCDEF bundle was more frequently performed for patients with COVID-19 infections, especially using electronic devices (using a monitor such as phone/video). COVID-19 brought technological expansion in the fields of remote and tele-practice which have been applied to the COVID-19 situation (32). However, it might result in overlooking patients without COVID-19 infections who received less online benefits as shown in this survey, and also need involvement of their families. In the context of an increasing trend for implementation of element E (33, 34), COVID-19 brought it to a previous level, but into more resource-unbalanced and time-restricted settings. As the guideline suggests, it is important to note that evidence-based ICU care, such as the ABCDEF bundle and ICU diary, should be incorporated into clinical practice for all ICU patients regardless of their underlying diseases or the ICU length of stay (1–4, 16, 35).
After adjusting for the backgrounds of hospitals and ICUs and the baseline condition of patients, the presence of COVID-19 infection was not a barrier to the implementation of each element of the ABCDEF bundle. For elements D, E, and F, the presence of COVID-19 was significantly associated with their implementation. The warnings of high risk for and high incidence of delirium in the early stage of the pandemic may be a factor (36) or the use of online systems in patients with COVID-19 infections (32) might contribute to these results. In addition, this could be a strong message that the impact of the COVID-19 pandemic broadly affected patients without and with COVID-19 infections and special considerations are necessary to improve the quality of ICU care for both types of patients.
This study demonstrates the diversity of independent factors associated with the implementation of each element of the ABCDEF bundle in addition to variations comparing non-COVID-19 and COVID-19 settings. These results particularly show that a promising strategy to introduce or implement a specific element of the bundle in an ICU could vary and should be designed depending on the context and local situation in which it will be implemented. For many elements in the ABCDEF bundle, regardless of COVID-19 status, a specific protocol and presence of a target/goal for ICU care were consistently identified as facilitating independent factors. However, this study also showed the low frequency to equip the specific protocol in each ICU, or 50% or less, which could be considered as one of the major barriers to be managed regardless of the presence of COVID-19. As many studies successfully showed a pivotal role for implementation or introduction of ICU care, this simple, but not time- or resource-consuming approach could be a key stimulus and should be routine in the ICU to facilitate efficient implementation of evidence-based approaches to ICU care (12, 16, 37, 38). Tele-medicine, which is getting public interest and recommended in several elements such as elements E (39) and F (37, 40), could be also an alternative to promote implementation instead of strict regulations regarding infection control or family visits. This is a relatively novel field of intensive care. Therefore, the impact of tele-medicine on implementation of evidence-based ICU care and its effect on outcomes should be investigated in a large prospective cohort study or randomized controlled study. The professionals dedicated to the ICU and the individual with primary responsibility could be decided by a policy maker in the hospital or ICU director based on what is to be achieved (41–44). The income level, used as a resource barometer, might show that less resources prevent implementation of evidence-based approaches (17, 37, 45). Relatively resource-intense care, such as a 1:1 nurse-patient ratio and daily multidisciplinary rounds, were not independently associated with implementation of the ABCDEF bundle, consistent with a previous report (12). ICUs in lower income countries performed more element F in this survey. These countries might apply relatively flexible visiting hours, which was also detected as a facilitating factor for element F, for a family rather than being in a high-income country.
This study has several acknowledged limitations. First, the limited number of patients and participating countries (Japan accounts for 40%) could lead to selection bias and limit generalizability to other ICUs and countries. These numbers might not be enough for the multivariable analysis with a number of covariates. Although the survey date captured a peak in the wave in Japan (46), the status of the pandemic in each area could affect the results. In addition, some COVID-19 hotspots, such as the USA, Brazil, and Russia, were under-represented. Second, the nature of a point prevalence study does not define a causal relationship and reflects the overwhelming situation at participating sites. This point prevalence study took place entirely on 1 day. Third, potential confounding factors associated with implementation, such as disease-related factors, were not investigated. Finally, an odds ratio with a relatively broad confidence interval may indicate an unstable model created by multivariate analysis. For example, Tele-ICU availability and Visiting hours might not be suitable to be incorporated into the multivariable analysis. Interpreting the results into the clinical world needs cautions regarding these statistical aspects. Further investigation and observations are necessary to validate these results.
Conclusions
Though having a COVID-19 infection was not associated with a failure to implement evidence-based ICU care, the implementation rates for the entire ABCDEF bundle, each of its elements and the ICU diary for patients without and with COVID-19 infections, were various, but extremely low on the whole regardless of the presence of COVID-19 infection. Since the impact of the COVID-19 pandemic on evidence-based ICU care varies depending on the conditions in each ICU, strategies to facilitate the implementation of each element of the ABCDEF bundle must be tailored to each institution.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Saiseikai Utsunomiya Hospital. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
KL, KN, HK, PN, EE, SK, KT, SI, and ON: study conception and design. KL, KN, HK, PN, EE, SK, and KT: statistical analysis or interpretation of data and drafting the manuscript. KN, HK, ME, PN, EE, SK, KT, MG, BL, CC, JB, SI, AL, and ON: critical review and revision of the manuscript for important intellectual insight. PN, EE, SK, KT, SI, AL, and ON: study supervision. KL, KN, HK, ME, PN, EE, SK, MG, BL, CC, JB, SI, and ON: recruitment the participating ICUs in overseas countries. KN confirmed that all authors meet authorship criteria according to ICMJE. All authors drafted the manuscript for important intellectual content, contributed to revision of the final version of the manuscript, approved the final version submitted, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
KL reports personal fees from MERA and receives a salary from TXP Medical completely outside the submitted work. KN reports personal fees from Abbott Laboratory, Nestle, TERUMO, GETINGE, Asahi Kasei Pharma, Ono Pharmaceutical, Japan Blood Products Organization, Nihon Pharmaceutical, Otsuka Pharmaceutical, Pfizer, Toray, and Baxter, and grants from Asahi Kasei Pharma outside the submitted work. HK receives a salary from the Japanese Society for Early Mobilization (non-profit society) as a chair (full time) outside the submitted work. EE reports grants from the VA/NIH; personal fees from Pfizer, Orion, and Lilly; personal fees from Masimo; and grants from Kohler outside the submitted work. SI reports personal fees from MERA, Abbott Laboratory, Teijin Pharma, Nestle, and Nihon Pharmaceutical. ON reports grants from Asahi Kasei Pharma, Ono Pharmaceutical, Baxter, Maruishi Pharmaceutical, Torii Pharmaceutical, Teijin Pharma, Shionogi Pharmaceutical, and Fuso Pharmaceutical outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank the Indian Society of Critical Care Medicine and the Korean Society of Critical Care Medicine. We also thank Hiromu Naraba, Ayako Kawasaki, Noriyo Suzuki, Sayaka Hosoi, and Akira Kawauchi who pre-tested the survey questionnaire. Finally, we thank all the investigators from the countries listed in Appendix 1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.735860/full#supplementary-material
References
1. Pandharipande P, Banerjee A, McGrane S, Ely EW. Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back-end of critical care. Crit Care. (2010) 14:157. doi: 10.1186/cc8999
2. Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness–crossing the quality chasm. Chest. (2010) 138:1224–33. doi: 10.1378/chest.10-0466
3. Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the 'ABCDE' approach. Curr Opin Crit Care. (2011) 17:43–9. doi: 10.1097/MCC.0b013e3283427243
4. Marra A, Ely EW, Pandharipande PP, Patel MB. The ABCDEF bundle in critical care. Crit Care Clin. (2017) 33:225–43. doi: 10.1016/j.ccc.2016.12.005
5. Nydahl P, Deffner T. Use of diaries in intensive care unit delirium patients: German nursing perspectives. Crit Care Nurs Clin North Am. (2021) 33:37–46. doi: 10.1016/j.cnc.2020.10.007
6. Balas MC, Vasilevskis EE, Olsen KM, Schmid KK, Shostrom V, Cohen MZ, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med. (2014) 42:1024–36. doi: 10.1097/CCM.0000000000000129
7. Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. (2009) 373:1874–82. doi: 10.1016/S0140-6736(09)60658-9
8. Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med. (2017) 45:321–30. doi: 10.1097/CCM.0000000000002175
9. Hsieh SJ, Otusanya O, Gershengorn HB, Hope AA, Dayton C, Levi D, et al. Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med. (2019) 47:885–93. doi: 10.1097/CCM.0000000000003765
10. Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. (2017) 45:171–8. doi: 10.1097/CCM.0000000000002149
11. Aziz S, Arabi YM, Alhazzani W, Evans L, Citerio G, Fischkoff K, et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. (2020) 46:1303–25. doi: 10.1007/s00134-020-06092-5
12. Liu K, Nakamura K, Katsukawa H, Elhadi M, Nydahl P, Ely EW, et al. ABCDEF bundle and supportive ICU practices for patients with coronavirus disease 2019 infection: an international point prevalence study. Crit Care Explor. (2021) 3:e0353. doi: 10.1097/CCE.0000000000000353
13. Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, et al. Caring for critically ill patients with the ABCDEF bundle: Results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. (2019) 47:3–14. doi: 10.1097/CCM.0000000000003482
14. Costa DK, White MR, Ginier E, Manojlovich M, Govindan S, Iwashyna TJ, et al. Identifying barriers to delivering the awakening and breathing coordination, delirium, and early exercise/mobility bundle to minimize adverse outcomes for mechanically ventilated patients: a systematic review. Chest. (2017) 152:304–11. doi: 10.1016/j.chest.2017.03.054
15. Moraes FDS, Marengo LL, Silva MT, Bergamaschi CC, Lopes LC, Moura MDG, et al. ABCDE and ABCDEF care bundles: a systematic review protocol of the implementation process in intensive care units. Medicine. (2019) 98:e14792. doi: 10.1097/MD.0000000000014792
16. Devlin JW, O'Neal HR Jr, Thomas C, Barnes Daly MA, Stollings JL, Janz DR, et al. Strategies to optimize ICU liberation (A to F) bundle performance in critically ill adults with coronavirus disease 2019. Crit Care Explor. (2020) 2:e0139. doi: 10.1097/CCE.0000000000000139
17. Bong CL, Brasher C, Chikumba E, McDougall R, Mellin-Olsen J, Enright A. The COVID-19 pandemic: effects on low- and middle-income countries. Anesth Analg. (2020) 131:86–92. doi: 10.1213/ANE.0000000000004846
18. Ministry of Health, Labour and Welfare, Japan. Ethical Guidelines for Medical and Health Research Involving Human Subjects. (2015). Available online at: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf (accessed January 20, 2021).
19. Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. (2014) 43:19–24. doi: 10.1016/j.hrtlng.2013.11.003
20. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
21. Morandi A, Piva S, Ely EW, Myatra SN, Salluh JIF, Amare D, et al. Worldwide ABCDEF (Assessing Pain Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium monitoring/management, Early exercise/mobility, and Family Empowerment). Crit Care Med. (2017) 45:e1111–22. doi: 10.1097/CCM.0000000000002640
22. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. (2000) 342:1471–7. doi: 10.1056/NEJM200005183422002
23. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. (2008) 371:126–34. doi: 10.1016/S0140-6736(08)60105-1
24. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
25. Menges D, Seiler B, Tomonaga Y, Schwenkglenks M, Puhan MA, Yebyo HG. Systematic early versus late mobilization or standard early mobilization in mechanically ventilated adult ICU patients: systematic review and meta-analysis. Crit Care. (2021) 25:16. doi: 10.1186/s13054-020-03446-9
26. Jabre P, Belpomme V, Azoulay E, Jacob L, Bertrand L, Lapostolle F, et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. (2013) 368:1008–18. doi: 10.1056/NEJMoa1203366
27. Liu K, Ogura T, Takahashi K, Nakamura M, Ohtake H, Fujiduka K, et al. A progressive early mobilization program is significantly associated with clinical and economic improvement: a single-center quality comparison study. Crit Care Med. (2019) 47:e744–52. doi: 10.1097/CCM.0000000000003850
28. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. (2020) 383:2451–60. doi: 10.1056/NEJMcp2009575
29. Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. (2020) 46:1099–102. doi: 10.1007/s00134-020-06033-2
30. Cruces P, Retamal J, Hurtado DE, Erranz B, Iturrieta P, González C, et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. (2020) 24:494. doi: 10.1186/s13054-020-03197-7
31. Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. (2020) 323:2329–30. doi: 10.1001/jama.2020.6825
32. Keesara S, Jonas A, Schulman K. Covid-19 and health care's digital revolution. N Engl J Med. (2020) 382:e82. doi: 10.1056/NEJMp2005835
33. Timenetsky KT, Neto AS, Assunção MSC, Taniguchi L, Eid RAC, Corrêa TD; e-MOTION group. Mobilization practices in the ICU: a nationwide 1-day point- prevalence study in Brazil. PLoS ONE. (2020) 15:e0230971. doi: 10.1371/journal.pone.0230971
34. Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr HJ, et al. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. (2014) 42:1178–86. doi: 10.1097/CCM.0000000000000149
35. Barnes-Daly MA, Pun BT, Harmon LA, Byrum DG, Kumar VK, Devlin JW, et al. Improving health care for critically ill patients using an evidence-based collaborative approach to ABCDEF bundle dissemination and implementation. Worldviews Evid Based Nurs. (2018) 15:206–16. doi: 10.1111/wvn.12290
36. Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. (2020) 24:176. doi: 10.1186/s13054-020-02882-x
37. Arabi YM, Azoulay E, Al-Dorzi HM, Phua J, Salluh J, Binnie A, et al. How the COVID-19 pandemic will change the future of critical care. Intensive Care Med. (2021) 47:282–91. doi: 10.1007/s00134-021-06352-y
38. Weiss CH. Why do we fail to deliver evidence-based practice in critical care medicine? Curr Opin Crit Care. (2017) 23:400–5. doi: 10.1097/MCC.0000000000000436
39. Taito S, Yamauchi K, Kataoka Y. Telerehabilitation in subjects with respiratory disease: a scoping review. Respir Care. (2021) 66:686–98. doi: 10.4187/respcare.08365
40. Azoulay E, Kentish-Barnes N. A 5-point strategy for improved connection with relatives of critically ill patients with COVID-19. Lancet Respir Med. (2020) 8:e52. doi: 10.1016/S2213-2600(20)30223-X
41. Liu K, Ogura T, Takahashi K, Nakamura M, Ohtake H, Fujiduka K, et al. The safety of a novel early mobilization protocol conducted by ICU physicians: a prospective observational study. J Intensive Care. (2018) 6:10. doi: 10.1186/s40560-018-0281-0
42. Borthwick M. The role of the pharmacist in the intensive care unit. J Intensive Care Soc. (2019) 20:161–4. doi: 10.1177/1751143718769043
43. Terblanche E. The role of dietitians in critical care. J Intensive Care Soc. (2019) 20:255–7. doi: 10.1177/1751143718774715
44. Li J, Zhan QY, Liang ZA, Tu ML, Sun B, Yao XL, et al. Respiratory care practices and requirements for respiratory therapists in Beijing intensive care units. Respir Care. (2012) 57:370–6. doi: 10.4187/respcare.01093
45. Kitano T, Kitano M, Krueger C, Jamal H, Al Rawahi H, Lee-Krueger R, et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: A systematic review of fatality and ICU admission in children worldwide. PLoS ONE. (2021) 16:e0246326. doi: 10.1371/journal.pone.0246326
46. Nonprofit organization ECMOnet, Japan. Survey of Critically ill COVID-19 patients in Japan, managed by the Japan ECMOnet for COVID-19. (2021). Available online at: https://crisis.ecmonet.jp/ (accessed June 9, 2021).
Keywords: ABCDEF bundle, COVID-19, ICU diary, ICU liberation bundle, pandemic (COVID-19)
Citation: Liu K, Nakamura K, Katsukawa H, Nydahl P, Ely EW, Kudchadkar SR, Takahashi K, Elhadi M, Gurjar M, Leong BK, Chung CR, Balachandran J, Inoue S, Lefor AK and Nishida O (2021) Implementation of the ABCDEF Bundle for Critically Ill ICU Patients During the COVID-19 Pandemic: A Multi-National 1-Day Point Prevalence Study. Front. Med. 8:735860. doi: 10.3389/fmed.2021.735860
Received: 03 July 2021; Accepted: 05 October 2021;
Published: 28 October 2021.
Edited by:
Björn Tampe, University Medical Center Göttingen, GermanyReviewed by:
Marija Vukoja, University of Novi Sad, SerbiaManabu Kinoshita, National Defense Medical College, Japan
Copyright © 2021 Liu, Nakamura, Katsukawa, Nydahl, Ely, Kudchadkar, Takahashi, Elhadi, Gurjar, Leong, Chung, Balachandran, Inoue, Lefor and Nishida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kensuke Nakamura, bWFtYXNob2NrcGFwYXNob2NrQHlhaG9vLmNvLmpw
†These authors have contributed equally to this work
 Keibun Liu1†
Keibun Liu1† Kensuke Nakamura
Kensuke Nakamura Sapna R. Kudchadkar
Sapna R. Kudchadkar Kunihiko Takahashi
Kunihiko Takahashi Muhammed Elhadi
Muhammed Elhadi Osamu Nishida
Osamu Nishida