
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 27 September 2021
Sec. Rheumatology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.733599
 Yimin Li1†
Yimin Li1† Yuhui Li1†
Yuhui Li1† Yuguang Wang2
Yuguang Wang2 Lianjie Shi3
Lianjie Shi3 Fuan Lin4
Fuan Lin4 Zongxue Zhang2
Zongxue Zhang2 Jingli Zhang5
Jingli Zhang5 Yanying Liu1
Yanying Liu1 Xu Liu1
Xu Liu1 Fangjingwei Xu6*
Fangjingwei Xu6* Xiaolin Sun1*
Xiaolin Sun1*Background: Rapidly progressive interstitial lung disease (RP-ILD) is a fatal complication of dermatomyositis (DM) and clinically amyopathic DM (CADM). The objective of this study was to evaluate risk markers associated with RP-ILD incidence in patients with DM/CADM and to develop a RP-ILD risk prediction (RRP) model.
Methods: The clinical records of 229 patients with DM/CADM from Peking University People's Hospital, and 97 patients from four other independent clinical centers were retrospectively reviewed. Univariate and multivariate logistic regression analyses were performed to identify independent risk factors associated with later RP-ILD incidence to build a risk score model. The concordance index (C-index) and calibration curve were calculated to evaluate the predictive accuracy of the RRP model.
Results: A multiparametric RRP model was established based on weighted clinical features, including fever (yes, 5; no, 0), periungual erythema (yes, 6; no, 0), elevated CRP (yes, 5; no, 0), anti-MDA5 antibody (positive, 8; negative, 0), and anti-Ro-52 antibody (positive, 6; negative, 0). Patients were divided into three risk groups according to the RRP total score: low, 0–9; medium, 10–19; high, 20–30. The C-index and calibration curve of the RRP model showed a promising predictive accuracy on the incidence of RP-ILD.
Conclusion: The RRP model might promisingly predict the incidence of RP-ILD in DM/CADM patients to guide early individual treatment and further improve the prognosis of DM/CADM patients.
Dermatomyositis is an autoimmune disease characterized by skin and muscle damage caused by muscular involvement and frequent extramuscular symptoms such as Raynaud's phenomenon, arthritis, and interstitial lung disease (ILD) (1). Clinically amyopathic dermatomyositis (CADM) is a combination of hypomyopathic DM (HDM) and amyopathic DM (ADM), with characteristic skin-predominant lesions (2–5). ILD is one of the most severe complications of DM/CADM. Despite aggressive treatments, respiratory failure following rapidly progressive interstitial lung disease (RP-ILD) remains the main cause of death in more than 50% of DM/CADM patients (6–10). Consequently, the prediction and timely identification of RP-ILD symptom onset is vital for effective treatment during early disease development stages and might lead to improved prognosis with significantly reduced mortality rates in patients with DM/CADM (11–13).
A variety of studies have explored baseline parameters associated with RP-ILD in patients with DM/CADM but few practical quantitative methods were established and validated in clinical practice to predict the incidence of RP-ILD. Anti-melanoma differentiation-associated gene 5 (MDA5) antibody, lymphocytes in peripheral blood, C-reactive protein (CRP), skin ulceration, and ferritin were reported as predictive factors for disease onset and poor prognosis of RP-ILD (7, 8, 14, 15). However, previous studies were generally supported by limited cohort sizes or scattered case reports. Moreover, it seems reductive and inefficient to use a single clinical factor to predict the risk of an extremely heterogeneous disease as RP-ILD. In contrast, a holistic approach, based on multiple factors comprehensively evaluating personalized clinical characteristics, might provide a better predictive model for RP-ILD. Combining clinical and immunological factors might be valuable to evaluate disease severity, predict outcomes, and guide individualized treatment. Therefore, it is clinically significant to explore the RP-ILD-associated parameters at the onset of DM/CADM to establish a reliable early-stage RP-ILD risk prediction (RRP) model.
In this study, a practical score model to predict the incidence of RP-ILD was established through the identification and quantification of specific clinical parameters associated with later RP-ILD incidence in a patient cohort with DM/CADM. Moreover, this model was validated in different cohorts, aiming to guide personalized treatment during early RP-ILD development stages.
Demographic, clinical, and laboratory test data from 229 patients with DM/CADM admitted in Peking University People's Hospital from 2010 to 2019 was collected, and a retrospective analysis was performed to establish a risk score model to predict the RP-ILD incidence in the early stage of patients with DM/CADM. External validation was based on retrospective demographic, clinical, and laboratory test data from 97 patients with DM/CADM admitted in four other independent clinical centers (People's Hospital of Jianyang City, Peking University International Hospital, Beijing Hospital of Traditional Chinese Medicine, and Hongqi Hospital of Mudanjiang Medical University) from 2010 to 2019. Patients with idiopathic inflammatory myositis (IIM) were reviewed in this study. IIM was diagnosed according to criteria proposed by Bohan and Peter or 2017 European League Against Rheumatism and American College of Rheumatology (EULAR/ACR) (16, 17). The inclusion criteria include all the patients who had a definite DM or CADM diagnosis. DM was diagnosed according to the criteria of 2017 EULAR/ACR (17) and CADM was diagnosed according to criteria proposed by Sontheimer (5). By using incidence data of RP-ILD in DM/CADM of Peking University People's Hospital (33.6%, 77/229) and the other four independent clinical centers (9.3%, 9/97), the sample size was calculated in Power and Sample Size Free Calculators (http://www.powerandsamplesize.com/). According to the calculation results, the study sample size should include 41 patients in each cohort, with a one-sided α of 5%, and a power of 80%. In this study, the sample size collected was larger than 41. To improve the accuracy of the model, it was ensured that each group of samples was larger than 41. Finally, grouped by the time period, there were 165 cases in the development cohort from 2010 to 2015, 64 cases in the internal validation cohort from 2016 to 2019, and 97 cases in the external validation cohort from 2010 to 2019. This study was approved by the ethics committee of Peking University People's Hospital according to the declaration of Helsinki. The waiver of consent was agreed upon by the institutional ethics committee due to the retrospective nature of the study.
Patient exclusion criteria included recent acute infection, pulmonary infarction, presence of heart failure, history of neoplasm, other connective tissue diseases concomitantly, or insufficient demographic, clinical, and laboratory test data. ILD was diagnosed by the findings of high-resolution computed tomography (HRCT), according to the International Consensus Statement of Idiopathic Pulmonary Fibrosis of the American Thoracic Society (18) and defined as previously described (19). Chest HRCT patterns were classified into non-specific interstitial pneumonia (NSIP), organizing pneumonia (OP), NSIP combined with OP pattern. Pulmonary function tests (PFT) and Bronchoalveolar lavage (BAL) examinations were performed to evaluate ILD% predicted forced vital capacity (FVC), percent predicted diffusing lung capacity for carbon monoxide (DLco), and total lung capacity (TLC) at the initial diagnosis. Based on the radiological assessment of the chest HRCT results, RP-ILD was defined as a progressive deterioration of ILD in 3 months combining with rapidly progressive severe dyspnea and hypoxemia, requiring oxygen therapy or ventilator care (20, 21).
Demographic and clinical information including age at onset, gender, and initial symptoms, including fever, proximal muscle weakness, Gottron's sign/papules, skin ulceration, periungual erythema, and ILD were assessed. Periungual erythema was defined as erythematous rashes in perionychium, and erythema with accompanying changes including ulceration or black eschar. Gottron's sign/papules were defined as erythematous to violaceous papules, plaques, or macules (sign) over extensor surfaces of joints, which are sometimes scaly. Laboratory data included serological creatine kinase (CK), aspartate aminotransferase (AST), alanine transaminase (ALT), erythrocyte sedimentation rate (ESR), C-reactive protein(CRP), and lactate dehydrogenase (LDH). Myositis-specific autoantibodies (antigen panel, included Jo-1, PL-7, PL-12, EJ, OJ, KS, MDA5, NXP2, SAE, Mi-2, and TIF-1γ), and myositis-associated autoantibodies (antigen panel, included Ro-52, PM-Scl, and Ku) were screened in all patients by immunoblotting according to the instructions of the manufacturer (Euroimmun, Germany). Antinuclear antibodies (ANA) and rheumatoid factor (RF) were also recorded. All data were collected before initiating diagnosis.
Demographic and clinical characteristics of the development cohort and validation cohort were compared. Categoric variables were reported as counts (%) and compared using the χ2 test. Continuous variables were presented as the mean ± SD and compared by the ANOVA or the Kruskal–Wallis test. The least significant difference (LSD) of ANOVA and the chi-square test were used in a pair-wise post-hoc analysis. Logistic regression analysis and forward elimination process selection were used to explore independent risk factors with multivariate analyses. Results of the regression models were shown as the odds ratio (OR) with 95% confidence interval (95% CI). The performance of the RRP model was measured by the Harrel concordance index (C-index) and calibration curve in R version 3.6.1 (http://www.r-project.org/). The degree of agreement between the predicted probabilities with the actual outcomes numerically was measured by calibration curve, with a larger C-index value denoting better predictive accuracy. A value of p < 0.05 was considered statistically significant.
A total of 400 cases were included in this study, including 271 DM/CADM patients in Peking University People's Hospital and 129 DM/CADM patients in the other four independent hospitals. After screening for exclusion criteria, 229 patients in Peking University People's Hospital were included in the study for model development and internal validation, and 97 patients from the other four independent hospitals were included for the external validation cohort. The development cohort includes patients in the Peking University People's Hospital from 2010 to 2015 (n = 165), and the internal validation cohort includes the remaining patients from 2016 to 2019 (n = 64). The flowchart of patient inclusion in the Peking University People's Hospital is shown in Supplementary Figure 1, and the flowchart of external validation set is shown in Supplementary Figure 2.
Demographic, clinical, and laboratory characteristics of the development cohort and the internal validation cohort, the external validation cohort are summarized in Supplementary Table 1. The demographic, clinical, and laboratory manifestations of the three cohorts were compared with pair-wise post-hoc analyses in Supplementary Table 2. All patients underwent CT scans and none was diagnosed as RP-ILD at the baseline. At the outset, 84.8% (140/165) of the patients in the development cohort showed ILD onset on CT, but they were unable to be diagnosed as RP-ILD at that time. In the clinical practice, RP-ILD was diagnosed later, with the occurrence and development of the disease during 2 to 4 weeks of hospitalization, which was defined as a progressive deterioration of ILD in 3 months combining with rapidly progressive severe dyspnea and hypoxemia, requiring oxygen therapy or ventilator care (20, 21). Among 326 patients with DM/CADM, RP-ILD was found in 35.8% (59/165) of the developed cohort, 28.1% (18/64) of the internal validation cohort, 9.3% (9/97) of the external validation cohort, respectively. The incidence of RP-ILD did not differ among the three groups (p = 0.273, Supplementary Table 1). Based on the results of Supplementary Tables 1 and 2, it could be known that there was no difference between developed cohort and internal validation cohort in demographic, clinical, and laboratory factors, which was not like in pairs of internal validation cohort vs. external validation cohort and developed cohort vs. external validation cohort. Different features including Gottron's sign/papules, heliotrope rash, V sign, shawl sign, skin ulceration, fever, arthralgia, anti-aminoacyl-tRNA synthetase (ARS) antibodies, ANA, FVC% predicted, TLC% predicted, AST, ESR, and CRP, indicating that characteristics of patients were various in different hospitals. Detailed results were shown in Supplementary Tables 1, 2.
Univariate and multivariable logistic regression analyses were used to explore independent risk factors for RP-ILD incidence in DM/CADM patients. To accurately screen for risk factors, factors with p < 0.05 in the univariate analysis were included in multivariate analysis as covariates. Analyzed factors included mechanic's hands (yes vs. no), skin ulceration (yes vs. no), periungual erythema (yes vs. no), fever (yes vs. no), anti-MDA5 antibody (positive vs. negative), anti-Ro-52 antibody (positive vs. negative), and elevated CRP (yes vs. no) (Table 1). Multivariable logistic regression analysis indicated that fever, periungual erythema, anti-MDA5 antibody, anti-Ro-52 antibody, and elevated CRP were independent risk factors for RP-ILD in DM/CADM patients [fever: odds ratio (OR) = 3.407, 95% CI = 1.462-7.942, p = 0.005; periungual erythema: OR = 5.911, 95% CI = 1.886–18.531, p = 0.002; anti-MDA5 antibody: OR = 8.721, 95% CI = 3.147–24.166, p = 0.000; anti-Ro-52 antibody: OR = 6.244, 95% CI = 2.565–15.200, p = 0.000; elevated CRP: OR = 4.039, 95% CI = 1.679–9.716, p = 0.002; Table 1].
The above five independent risk factors were then combined to establish an RRP model. Scores were based on five independent risk predictors weighted by regression coefficients and rounded to the nearest whole number (Table 2). Total risk scores were calculated by adding the weighted values of all prognostic variables. According to the RRP scores, patients were categorized into three risk groups: low-risk group, with RRP scores ranging from 0 to 9; medium-risk group, with RRP scores ranging from 10 to 19; high-risk group, with RRP scores ranging from 20 to 30.
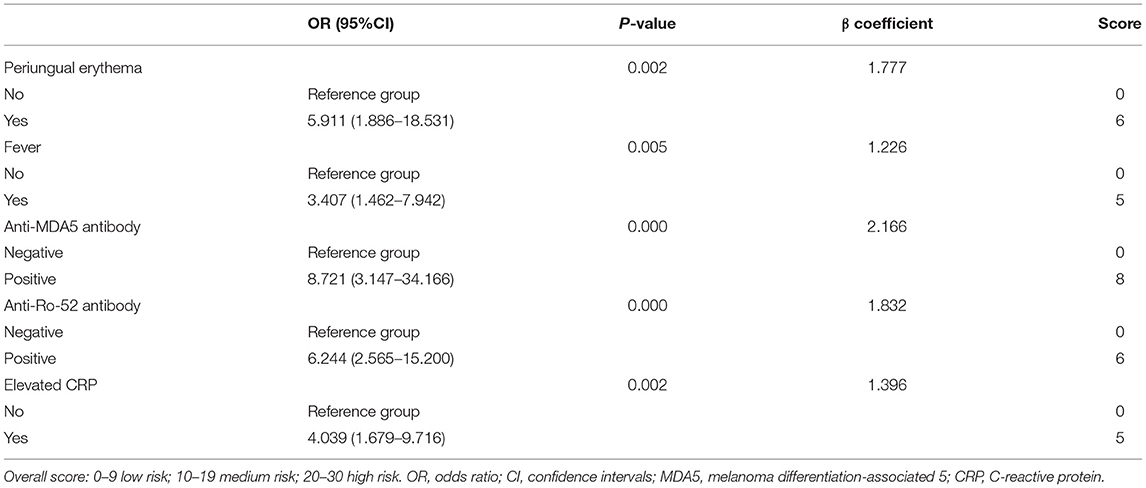
Table 2. Calculation of the score for risk stratification in the development and validation cohorts.
After classifying the development cohort according to the RRP scores, 40.6% of the patients were assigned to the low-risk group, 49.70% to the medium-risk group, and 9.69% to the high-risk group. The results of the internal validation cohort were similar: 50% of the patients were in the low-risk group, 40.63% in the medium-risk group, and 9.37% in the high-risk group. In the external validation cohort, 56.70% of the patients were assigned to the low-risk group, 37.11% to the medium-risk group, and 6.19% to the high-risk group (Table 3). The C-index for differences in RP-ILD incidence among risk groups from the development cohort was 0.849, with 95% CI 0.791 to 0.907. The mean predicted RP-ILD rates of the low-risk, medium-risk and high-risk groups were 8.61%, 46.41%, and 94.84%, respectively, while the observed rates were 8.96%, 45.12%, and 100%, respectively (Table 3, Figure 1A). Similarly, the C-index for differences in RP-ILD incidence rates among risk groups from the internal validation cohort was 0.928, with 95% CI 0.866 to 0.990 and the mean predicted RP-ILD rates of the low-risk, medium-risk, and high-risk groups were 2.7%, 42.87%, and 99.82%, with observed rates were 3.13%, 42.31%, and 100%, respectively (Table 3, Figure 1B). Finally, the C-index for differences in RP-ILD incidence rates among different risk groups from the external validation cohort was 0.948, with 95% CI 0.862 to 1.000 with mean predicted RP-ILD rates of the low-risk, medium-risk, and high-risk groups at 0.38%, 25.07%, and 96.14%, and observed rates were 1.82%, 22.22%, and 100%, respectively (Table 3, Figure 1C).
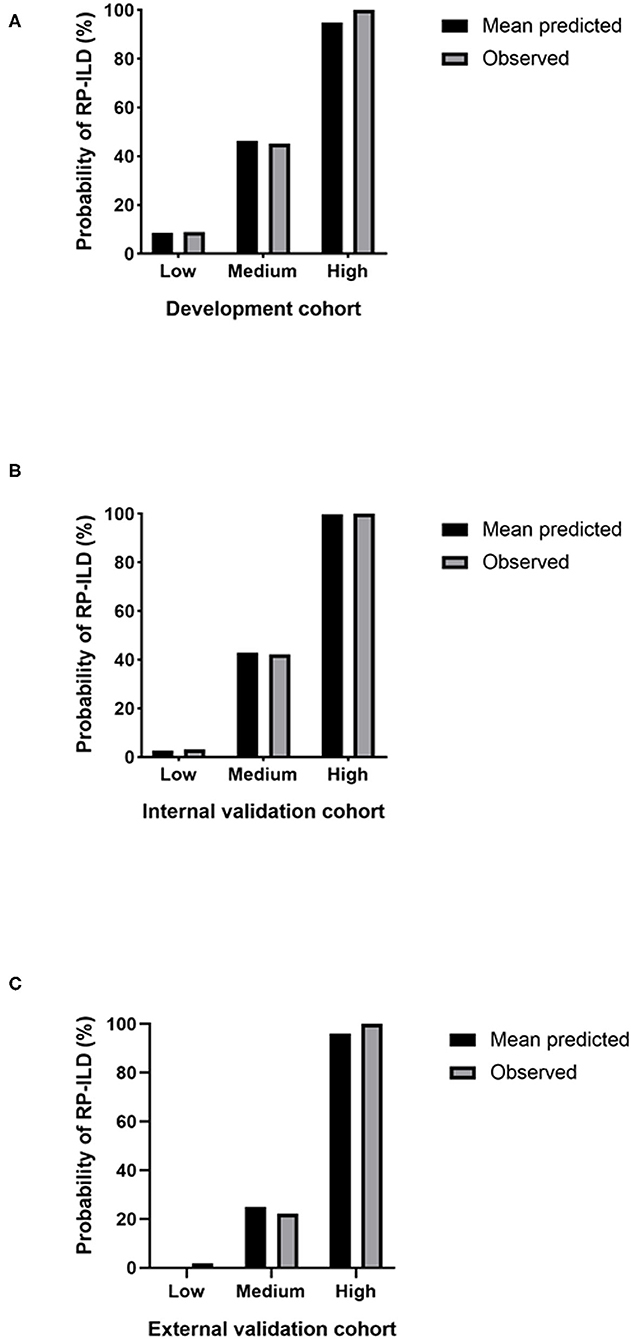
Figure 1. Predicted and observed rapidly progressive interstitial lung disease (RP-ILD) incidence in different risk groups. (A) The development cohort. (B) The internal validation cohort. (C) The external validation cohort. Low: low-risk; Medium: medium-risk; High: high-risk.
Moreover, the calibration plot based on bootstrap resampling validation demonstrated promising agreement between predicted and observed RP-ILD rates in the development cohort (Figure 2A), the internal validation cohort (Figure 2B), and the external validation cohort (Figure 2C).
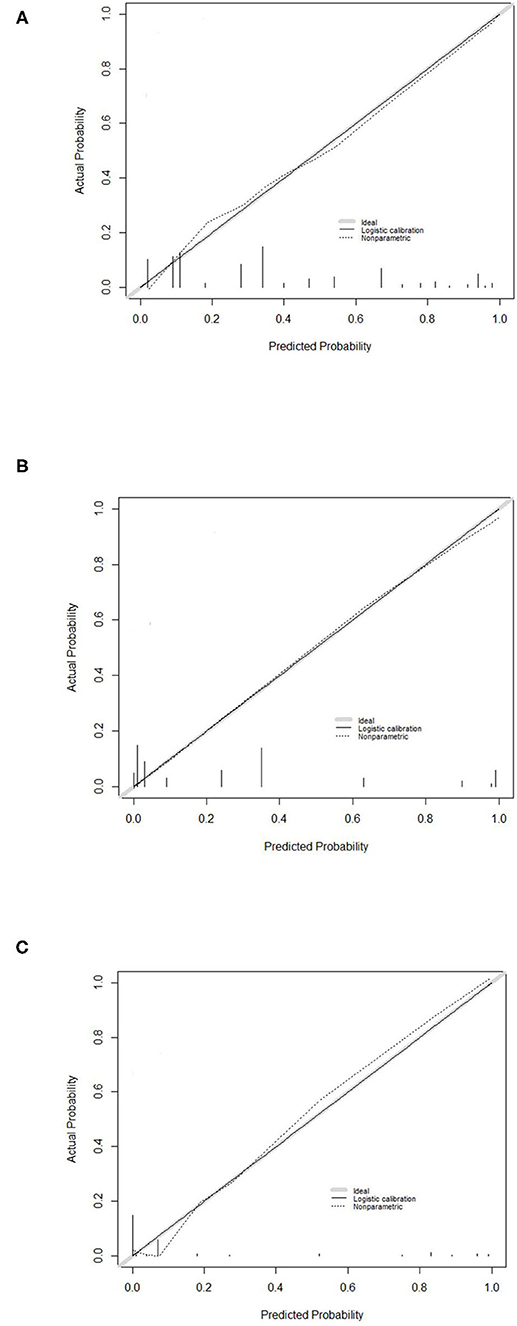
Figure 2. Calibration curve for the RP-ILD risk prediction (RRP) score system. Calibration curve for predicting RP-ILD in (A) the development cohort, (B) the internal validation cohort, and (C) the external validation cohort. RRP: RP-ILD risk prediction.
Lung involvement is the most common reason for respiratory failure and death in DM/CADM. RP-ILD in DM patients is usually fatal within a few weeks or months (22). Therefore, predictive parameters for the onset of RP-ILD are important in the early treatment of DM/CADM patients. Previous studies mainly focused on short-term or small sample size studies to identify individual risk parameters of RP-ILD instead of exploring the multifactorial approach englobing the extensive clinical data from DM/CADM patients. Furthermore, none of the previous studies proposed a quantitative model to predict the risk of RP-ILD incidence in clinical practice. RP-ILD is an extremely heterogeneous disease. It is then likely, that multiple prognostic factors combined will better evaluate disease severity, predict outcomes, and guide individualized treatment than single-factor based models. Therefore, it was hypothesized that RP-ILD related risk parameters of DM/CADM could establish a dependable RRP model. In this study, an RRP model was built by combining independent predictive factors, including fever, periungual erythema, elevated CRP, anti-MDA5 antibody, and anti-Ro52 antibody. The early identification of RP-ILD progression is crucial for effective therapy, prognosis, and reduced mortality. In recent knowledge, this is the first score system available for clinical practice, which is able to predict the risk of RP-ILD incidence in DM/CADM patients during the early disease stages. Moreover, an independent external validation further confirmed the reliability of this model, with high accuracy in patients with a high risk of RP-ILD incidence.
Predictors of poor outcome during DM-ILD have been previously reported (23–25), however, fewer studies assessed the prognostic factors for myositis-associated RP-ILD. This study confirmed many single clinical and laboratory prognostic factors previously reported, such as periungual erythema, fever, and CRP, as risk factors for RP-ILD in myositis (8, 15, 26–28). However, the majority of predictive factors for ILD (8, 15, 26, 27, 29), e.g., skin ulceration and hands of a mechanic, which have been previously identified by univariate analysis, were not classified as independent risk factors by multivariate analysis in the study.
A dose-dependent relationship between anti-MDA5 antibody and RP-ILD has been recently reported, unveiling anti-MDA5 antibodies as a significant risk factor for poor prognosis (30). Similarly, patients with anti-Ro-52 antibodies during juvenile myositis are more likely to develop severe ILD with a poor prognosis (31). Consistent with these reports, this study also demonstrated that anti-MDA5 antibody and anti-Ro-52 antibody were both specific biomarkers for DM/CADM-associated RP-ILD (8, 32).
Based on the results in Supplementary Tables 1 and 2, it was found that the characteristics of patients in different hospitals were not the same. Therefore, there was a need to establish an evaluation system with a universal applicability in different hospitals. In the RRP score model of the study, patients with a score between 0 and 9 are considered as low-risk subgroups for the development of RP-ILD, while scores between 10 and 19 and 20 or more are at medium-risk and high-risk of RP-ILD development, respectively. The results of summarized calibration and discrimination for the RRP model in three cohorts all presented a high predictive accuracy for estimating the risk of RP-ILD supported in clinical settings. Therefore, researchers of the study thought that this model has general applicability, which still needs to be proved in the future.
This study presented several limitations. First, this study was a retrospective study, suboptimal compared to the prospective study and included hospitalized patients only. Therefore, it might have shown biases in patient selection. Second, the prediction model was only tested in a Chinese population. The generalizability of this model to other ethnicities needs to be validated in future studies. Third, the validation cohort has a limited sample size because of the rarity of the disease; this model needs to be further validated by more multi-center studies with enlarged patient cohorts. Fourth, this risk score was mainly based on clinical characteristics. Although several studies have reported that some serum biomarkers, including ferritin, KL-6, and IL-18, were related to RP-ILD and might be used as potential biomarkers for predicting disease severity and prognosis (10, 33); the researchers of the study were unable to include these markers in the model due to the lack of these data in the retrospective study, whether those markers could be used as a reliable alternative biomarker needs further validation.
The first RRP score model capable of predicting the risk of RP-ILD incidence during early disease stages was provided. It is expected to be a valuable clinical tool that could guide early personalized treatment, improve prognosis, and reduce morality for DM/CADM patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Ethic Committee of Peking University People's Hospital according to the declaration of Helsinki. Waiver of consent was agreed upon by the institutional ethics committee due to the retrospective nature of the study.
XS, YHL, and FX contributed to the conception or design of the study. YML contributed to the analysis of the data. YW, LS, FL, ZZ, JZ, YYL, and XL contributed to the collection of the data. All authors were involved in the interpretation of the data and reviewed and approved the letter's content before submission.
This work was supported by National Natural Science Foundation of China (81971520 and 81801617), Peking University People's Hospital Research and Development Funds (RDX2019-03, RDX2020-03).
FX was employed by the company China National Biotec Group.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.733599/full#supplementary-material
1. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. (2003) 362:971–82. doi: 10.1016/S0140-6736(03)14368-1
2. Bendewald MJ, Wetter DA Li X, Davis MD. Incidence of dermatomyositis and clinically amyopathic dermatomyositis: a population-based study in Olmsted County, Minnesota. Arch Dermatol. (2010) 146:26–30. doi: 10.1001/archdermatol.2009.328
3. Galimberti F, Li Y, Fernandez AP. Clinically amyopathic dermatomyositis: clinical features, response to medications and malignancy-associated risk factors in a specific tertiary-care-centre cohort. Br J Dermatol. (2016) 174:158–64. doi: 10.1111/bjd.14227
4. Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. (2002) 20:387–408. doi: 10.1016/S0733-8635(02)00021-9
5. Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol. (2002) 46:626–36. doi: 10.1067/mjd.2002.120621
6. Mukae H, Ishimoto H, Sakamoto N, Hara S, Kakugawa T, Nakayama S, et al. Clinical differences between interstitial lung disease associated with clinically amyopathic dermatomyositis and classic dermatomyositis. Chest. (2009) 136:1341–7. doi: 10.1378/chest.08-2740
7. Sato S, Hirakata M, Kuwana M, Suwa A, Inada S, Mimori T, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. (2005) 52:1571–6. doi: 10.1002/art.21023
8. Xu Y, Yang CS, Li YJ, Liu XD, Wang JN, Zhao Q, et al. Predictive factors of rapidly progressive-interstitial lung disease in patients with clinically amyopathic dermatomyositis. Clin Rheumatol. (2016) 35:113–6. doi: 10.1007/s10067-015-3139-z
9. Lega JC, Reynaud Q, Belot A, Fabien N, Durieu I, Cottin V. Idiopathic inflammatory myopathies and the lung. Eur Respir Rev. (2015) 24:216–38. doi: 10.1183/16000617.00002015
10. Kobayashi N, Takezaki S, Kobayashi I, Iwata N, Mori M, Nagai K, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology. (2015) 54:784–91 doi: 10.1093/rheumatology/keu385
11. Kotani T, Makino S, Takeuchi T, Kagitani M, Shoda T, Hata A, et al. Early intervention with corticosteroids and cyclosporin A and 2-hour postdose blood concentration monitoring improves the prognosis of acute/subacute interstitial pneumonia in dermatomyositis. J Rheumatol. (2008) 35:254–9. doi: 10.1007/BF00498814
12. Molina-Molina M, Aburto M, Acosta O, Ancochea J, Rodriguez-Portal JA, Sauleda J, et al. Importance of early diagnosis and treatment in idiopathic pulmonary fibrosis. Expert Rev Respir Med. (2018) 12:537–9. doi: 10.1080/17476348.2018.1472580
13. Go DJ, Park JK, Kang EH, Kwon HM, Lee YJ, Song YW, et al. Survival benefit associated with early cyclosporine treatment for dermatomyositis-associated interstitial lung disease. Rheumatol Int. (2016) 36:125–31. doi: 10.1007/s00296-015-3328-8
14. Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology. (2012) 51:1278–84. doi: 10.1093/rheumatology/ker518
15. Cao H, Pan M, Kang Y, Xia Q, Li X, Zhao X, et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res. (2012) 64:1602–10. doi: 10.1002/acr.21728
16. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. (1975) 292:344–7. doi: 10.1056/NEJM197502132920706
17. Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M, et al. 2017 European league against rheumatism/american college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. (2017) 76:1955–64. doi: 10.1136/annrheumdis-2017-211468
18. American Thoracic Society, European Respiratory Society. American thoracic society/european respiratory society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. this joint statement of the american thoracic society (ATS), and the european respiratory society (ERS) was adopted by the ats board of directors, june 2001 and by the ERS executive committee, June 2001. Am J Respir Crit Care Med. (2002) 165:277–304. doi: 10.1164/ajrccm.165.2.ats01
19. Suda T, Fujisawa T, Enomoto N, Nakamura Y, Inui N, Naito T, et al. Interstitial lung diseases associated with amyopathic dermatomyositis. Eur Respir J. (2006) 28:1005–12. doi: 10.1183/09031936.06.00038806
20. Gono T, Kawaguchi Y, Satoh T, Kuwana M, Katsumata Y, Takagi K, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology. (2010) 49:1713–9. doi: 10.1093/rheumatology/keq149
21. Huh JW, Kim DS, Lee CK, Yoo B, Seo JB, Kitaichi M, et al. Two distinct clinical types of interstitial lung disease associated with polymyositis-dermatomyositis. Respir Med. (2007) 101:1761–9. doi: 10.1016/j.rmed.2007.02.017
22. Kameda H, Nagasawa H, Ogawa H, Sekiguchi N, Takei H, Tokuhira M, et al. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J Rheumatol. (2005) 32:1719–26. doi: 10.1097/01.bor.0000175460.75675.d3
23. Sato S, Masui K, Nishina N, Kawaguchi Y, Kawakami A, Tamura M, et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology. (2018) 57:1212–21. doi: 10.1093/rheumatology/key060
24. Marie I, Hatron PY, Dominique S, Cherin P, Mouthon L, Menard JF. Short-term and long-term outcomes of interstitial lung disease in polymyositis and dermatomyositis: a series of 107 patients. Arthritis Rheum. (2011) 63:3439–47. doi: 10.1002/art.30513
25. Fujisawa T, Hozumi H, Kono M, Enomoto N, Hashimoto D, Nakamura Y, et al. Prognostic factors for myositis-associated interstitial lung disease. PLoS ONE. (2014) 9:e98824. doi: 10.1371/journal.pone.0098824
26. Hozumi H, Fujisawa T, Nakashima R, Johkoh T, Sumikawa H, Murakami A, et al. Comprehensive assessment of myositis-specific autoantibodies in polymyositis/dermatomyositis-associated interstitial lung disease. Respir Med. (2016) 121:91–9. doi: 10.1016/j.rmed.2016.10.019
27. Fujiki Y, Kotani T, Isoda K, Ishida T, Shoda T, Yoshida S, et al. Evaluation of clinical prognostic factors for interstitial pneumonia in anti-MDA5 antibody-positive dermatomyositis patients. Mod Rheumatol. (2018) 28:133–40. doi: 10.1080/14397595.2017.1318468
28. Zhang L, Wu G, Gao D, Liu G, Pan L, Ni L, et al. Factors associated with interstitial lung disease in patients with polymyositis and dermatomyositis: a systematic review and meta-analysis. PLoS ONE. (2016) 11:e0155381. doi: 10.1371/journal.pone.0155381
29. Cao H, Xia Q, Pan M, Zhao X, Li X, Shi R, et al. Gottron papules and gottron sign with ulceration: a distinctive cutaneous feature in a subset of patients with classic dermatomyositis and clinically amyopathic dermatomyositis. J Rheumatol. (2016) 43:1735–42. doi: 10.3899/jrheum.160024
30. Lian X, Zou J, Guo Q, Chen S, Lu L, Wang R, et al. Mortality risk prediction in amyopathic dermatomyositis associated with interstitial lung disease: the FLAIR model. Chest. (2020) 158:1535–45. doi: 10.1016/j.chest.2020.04.057
31. Sabbagh S, Pinal-Fernandez I, Kishi T, Targoff IN, Miller FW, Rider LG, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis. (2019) 78:988–95. doi: 10.1136/annrheumdis-2018-215004
32. Kishaba T, McGill R, Nei Y, Ibuki S, Momose M, Nishiyama K, et al. Clinical characteristics of dermatomyosits/polymyositis associated interstitial lung disease according to the autoantibody. J Med Invest. (2018) 65:251–7. doi: 10.2152/jmi.65.251
33. Gono T, Sato S, Kawaguchi Y, Kuwana M, Hanaoka M, Katsumata Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology. (2012) 51:1563–70. doi: 10.1093/rheumatology/kes102
Keywords: dermatomyositis, rapidly progressive interstitial lung disease, risk prediction, risk marker, anti-MDA5 antibody
Citation: Li Y, Li Y, Wang Y, Shi L, Lin F, Zhang Z, Zhang J, Liu Y, Liu X, Xu F and Sun X (2021) A Clinical Risk Model to Predict Rapidly Progressive Interstitial Lung Disease Incidence in Dermatomyositis. Front. Med. 8:733599. doi: 10.3389/fmed.2021.733599
Received: 30 June 2021; Accepted: 24 August 2021;
Published: 27 September 2021.
Edited by:
Ashish Jacob Mathew, Christian Medical College & Hospital, IndiaReviewed by:
Latika Gupta, Sanjay Gandhi Postgraduate Institute of Medical Sciences, IndiaCopyright © 2021 Li, Li, Wang, Shi, Lin, Zhang, Zhang, Liu, Liu, Xu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangjingwei Xu, eHVmYW5namluZ3dlaUBzaW5vcGhhcm0uY29t; Xiaolin Sun, c3VueGlhb2xpbl9zeGxAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.