Abstract
Purpose: To quantify the severity and location of corneal neovascularization (cNV) and its impact on the visual acuity and corneal sensitivity in a cohort of the patients referred to a specialist cornea clinic and also to describe the etiology of cNV in the cohort.
Methods: We retrospectively evaluated the charts of 13,493 subjects referred to the San Raffaele Cornea Unit between January 2004 and December 2018 to search for cNV diagnosis. The corneal neovascularization severity was measured in the quadrants (range: 1–4) and location was defined as superficial, deep, or both. Best spectacle corrected visual acuity (BSCVA) was measured in logMar. We used the multiple regression analysis to identify the independent predictors of logMAR, after adjusting for age, gender, keratoconus, herpes keratitis, penetrating keratoplasty, trauma, and cataract surgery.
Results: Corneal neovascularization was diagnosed in 10.4% of the patients analyzed. The most prevalent etiology of cNV in our population was non-infectious corneal dystrophies/degenerations followed by herpes simplex virus infection. cNV affected OD, OS, or both eyes in 35.6, 40.2, and 24.2 of cases, respectively. Mean BSCVA (SD) was 0.59 (0.76), 0.74 (0.94), and 1.24 (1.08) in cNV one, two, and three or four of the quadrant groups. Superficial, deep, or mixed cNV occurred in 1,029, 348, and 205 eyes. Severe cNV (three or four of the quadrants) was a significant predictor of low visual acuity (p < 0.001) and reduced corneal sensitivity (p < 0.05). cNV location and its severity were associated (p < 0.05). In addition, corneal anesthesia was associated with lower BSCVA (p < 0.001).
Conclusion: Severe and deep cNV are associated with the reduced visual acuity and corneal sensitivity. Our data strongly support the relevance of appropriate follow-up as cNV is a major risk factor for graft rejection.
Introduction
The incidence of corneal neovascularization (cNV) in the patients with ocular surface and its impact on visual acuity are not clear, although there is a general consensus that arresting cNV progression is beneficial (1).
A well-known immune-privileged site, the normal cornea is avascular (2, 3). A number of ocular diseases, generally associated with acute or persistent inflammation, results in an “angiogenic shift” and the development of cNV (4). The second cause of blindness worldwide, cNV, is a sight-threatening condition (5). Colby et al. reported a prevalence of 4.14% in a cohort of the patients visiting a general eye service (6).
The etiopathology of cNV varies across the world. In Western countries, herpes simplex keratitis is the most common infectious cause of cNV. In the US alone, 500,000 cases of ocular herpes simplex virus are reported every year (7). Contact lenses and specifically extended-wear soft contact lenses are the most frequent non-infectious cause of cNV in the US affecting 11–30% of the contact lens wearers (8). In addition, cNV invariably follows the corneal chemical burns with an incidence of 37,000 people/year in the US. Other diseases frequently associated with cNV include pterygium, Stevens–Johnson syndrome, Lyell syndrome, and limbal stem cell deficiency (9, 10). Of note, cNV is universally considered as a significant risk factor for corneal transplant rejection as the extent of cNV is directly related to the risk of rejection (11). This is a relevant clinical problem, since nearly 20% of the corneal buttons excised during corneal transplantation exhibit histological evidence of cNV (12).
Literature suggests that the diseases commonly associated with cNV affect visual acuity, the exact impact of cNV and its location on vision are unknown. At the same time, experimental evidence in the animal models of cNV shows impairment of the corneal nerves, but it is not clear if this phenomenon is replicated in human subjects.
In this study, we aimed to quantify the impact of cNV on the visual acuity and corneal sensitivity. Furthermore, we provided an estimate of cNV prevalence in the patients affected with ocular surface diseases.
Materials and Methods
Study Design
This retrospective analysis was conducted at the Cornea and Ocular Surface Unit of the San Raffaele Scientific Institute, Milan, Italy. The study was carried out in accordance with the guidelines established by the Declaration of Helsinki and the Institutional Review Board/Ethics Committee (Comitato EticoIstituto Scientifico Ospedale San Raffaele) approval was obtained.
All the data used in this study were extracted from our electronic medical record system (OCULI, Bedigital SrL, Verona, Italy), which includes all the patients evaluated at the Cornea and Ocular Surface Unit of the San Raffaele Scientific Institute, Milan, Italy, between January 1, 2004, and December 31, 2018. Specific database search included “history of systemic and/or ocular illness,” “best spectacle corrected visual acuity (BSCVA),” “slit-lamp biomicroscopy of the anterior segment” (including the extension of the cNVs in one to four quadrants), “corneal sensitivity” (quantified as present or absent), and “suspect/definitive diagnosis.” In this study, the following keyword combination was searched: “corneal neovascularization,” which retrieved all the patients affected with cNV in one or both eyes in at least one visit. If cNV was documented in both the eyes, they were both considered in the analysis. In total, the charts of 1,406 subjects were analyzed retrospectively.
Clinical Parameters
Best spectacle corrected visual acuity was recorded in the Snellen equivalents and converted to logMAR scale. “Counting finger” (CF) and “hand motion” (HM) BSCVA were converted to 2.0 and 3.0 logMAR values, respectively; “light perception” (LP) and “no light perception” (NLP) BSCVA were not converted to any logMAR value (13) and were not considered in this study. Therefore, visual acuity data from the patients with BSCVA less than HM were not included in the analysis. Corneal sensitivity was assessed by using the cotton swab test.
Corneal neovascularization was defined as the presence of vessels in the cornea. The extension of cNV was quantified clinically as the number of the corneal quadrants showing neovessels (one, two, three, or four quadrants). cNV depth was evaluated clinically at the slit-lamp biomicroscopy. A cotton tip was used to evaluate corneal sensitivity, which was defined as normal, reduced, or absent (14).
The etiology of cNV was defined based on medical history, clinical suspicion, and/or confirmed microbiology testing. We identified two major groups: cNV associated with infectious keratitis or non-infectious cNV. The infectious keratitis group was further subclassified as follows: (i) viral keratitis: herpesvirus [herpes simplex virus (HSV) or varicella zoster virus (VZV)], adenovirus, and morbillivirus; (ii) bacterial keratitis: Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pneumoniae, and Chlamydia trachomatis; (iii) Acanthamoeba keratitis; and (iv) fungal keratitis. In those cases where no definitive diagnosis was reached, either microbiological or clinical, the diagnosis was noted as “non-determined (ND).” The non-infectious keratitis group consisted of the following diagnosis: (i) trauma; (ii) allergic keratoconjunctivitis; (iii) chronic (i.e., more than 2 days per week) contact lens wear; (iv) dry eye and other immune-mediated disorders (rosacea, Sjögren's syndrome, and rheumatoid arthritis-associated ulcers); (v) ocular cicatricial pemphigoid and epidermolysis bullosa; (vi) Stevens–Johnson syndrome and Lyell syndrome; (vii) genetic/congenital/acquired dystrophies including keratoconus; (viii) chemical caustication; (ix) neurotrophic/neuroparalytic keratitis; (x) postocular surgery; and (xi) pterygium.
Statistical Analysis
The Kolmogorov–Smirnov, D'Agostino–Pearson, and Shapiro–Wilk tests for normality failed for all considered variables and non-parametric testing was performed on the data. Multiple comparison tests of the Kruskal–Wallis and Dunn were applied to test the association between the visual acuity and cNV. In this case, statistical analysis was performed by using GraphPad Prism version 5.0 (GraphPad 22 Software, La Jolla, California, United States of America). Data are expressed as mean ± SD.
In addition, we evaluated the potential association between the corneal neovascularization severity and visual acuity by using multiple regression with logMAR as the dependent variable and adjusting for age, gender, keratoconus, herpetic keratitis, penetrating keratoplasty, trauma, and phacoemulsification + intraocular lens. Sensitivity and inflammation type (blepharitis, hyperemia, or both) were excluded from the models because of the very large number of missing values. When included, however, they did not affect the significant variables.
The validity of final regression models (left and right eye) was assessed as follows. The assumption of constant error variance was checked graphically, plotting Pearson residuals vs. fitted values, and, formally, using the Cook–Weisberg test for heteroskedasticity. Since Cook and Weisberg's test for heteroskedasticity was borderline significant, we used Huber/White robust SEs in the final models. High leverage observations were identified by calculating the Pearson, standardized, and studentized residuals, Cook's D influence, and the hat diagonal matrix. We found only 15 high-leverage observations excluding which we noted no substantial changes.
A multiple regression was also fit to evaluate the potential independent predictors of sensitivity. The same above criteria to set the final models (left and right eye) were used.
Statistical significance was defined as a two-sided p < 0.05 for all the analyses, which were performed by using STATA 13.1 (StataCorp LLC, College Station, Texas, United States of America).
Results
Out of 13,493 patients who visited the Cornea and Ocular Surface Unit of the San Raffaele Scientific Institute, Milan, Italy between January 1, 2004, and December 31, 2018, a total of 1,406 (10.4; 95% CI 0.099–0.109) Caucasian patients (1,576 eyes) were diagnosed with cNV and included in the study. One thousand and sixty six patients showed monolateral cNV and 340 bilateral cNV. The mean age of the patient was 50.9 ± 20.2 years; males were 51.3% and females were 48.7%. LogMAR BSCVA was retrieved in 1,539 out of 1,576 eyes (37 eyes were not tested for BSCVA). Grading of cNV was performed in 1,456 eyes. The corneal sensitivity measurements were retrieved in 305 eyes (19.4%). The demographics of the patient are summarized in Table 1.
Table 1
| Variables | Total sample (n = 1,406) | Left eye | Right eye |
|---|---|---|---|
| Male gender, % | 51.3 | ||
| Mean age in years (SD) | 50.9 (20.2) | ||
| Age class, % | |||
| <18 y | 5.6 | ||
| 18–64.99 y | 65.7 | ||
| ≥65 y | 28.9 | ||
| cNV side, % | |||
| - Left | 35.6 | ||
| - Right | 40.2 | ||
| - Both | 24.2 | ||
| cNV sector, % | – | (n = 687) | (n = 769) |
| −1 | 32.6 | 33.9 | |
| −2 | 32.0 | 32.2 | |
| −3 or 4 | 35.4 | 33.8 | |
| cNV type, % | – | (n = 765) | (n = 819) |
| - Superficial | 64.7 | 64.8 | |
| - Deep | 22.0 | 21.9 | |
| - Mixed | 13.3 | 13.3 | |
| Inflammation, % | (n = 620) | ||
| - Blepharitis | 31.8 | ||
| - Hyperemia | 46.0 | ||
| - Both | 22.3 | ||
| Visual acuity | (n = 741) | (n = 798) | |
| MeanlogMAR (SD) | – | 0.88 (0.98) | 0.88 (0.96) |
| Sensitivity, % | – | (n = 149) | (n = 156) |
| - Absent | 25.5 | 30.8 | |
| - Reduced | 36.9 | 36.5 | |
| - Normal | 37.6 | 32.7 | |
| Diseases*, % | (n = 1,254) | ||
| - HSV keratitis | 22.6 | ||
| - Penetrating keratoplasty | 17.4 | ||
| - Keratoconus | 14.8 | ||
| - Trauma | 11.5 | ||
| - Keratitis, nc | 9.2 | ||
| - Phacoemulsification + intraocular lens** | 8.6 | ||
| - Failure** | 7.9 | ||
| - Contact lens | 5.3 | ||
| - Bacterial keratitis | 3.1 | ||
| - Amebic keratitis | 3.0 | ||
| - Leucoma, nc | 3.0 | ||
| - Keratopia | 2.8 | ||
| - Lamellar keratoplasty | 2.7 | ||
| - Dry eye | 2.7 | ||
| - Keratoconjuntivitis | 2.5 | ||
| - Ulcer | 2.2 | ||
| - Pemphigoid | 1.9 | ||
| - Pterygium | 1.6 | ||
| - Neurotrophic keratitis | 1.6 | ||
| - Fuchs syndrome | 1.3 | ||
| - Fungal keratitis | 1.0 | ||
| - Salzmann syndrome | 1.0 | ||
| - Autoimmune keratitis** | 0.9 | ||
| - Steven Johnson syndrome | 0.8 | ||
| - Sjorgen syndrome | 0.8 | ||
| - Trans-pars plana vitrectomy | 0.7 | ||
| - Glaucoma | 0.6 | ||
| - Aniridia | 0.6 | ||
| - Lyell syndrome | 0.6 | ||
| - Viral keratitis excluding HSV | 0.6 | ||
| - Terrien syndrome | 0.5 | ||
| - Graft vs. host disease | 0.3 | ||
| - Keratoglobus | 0.2 | ||
| - Lasik | 0.2 | ||
| - Coffin Siris syndrome | 0.1 | ||
| - Bowen syndrome | 0.1 | ||
| - Vogt syndrome | 0.1 | ||
| - Goldenhar syndrome | 0.1 | ||
| - Francois syndrome | 0.1 |
Descriptive analysis of the sample of the patients with corneal neovascularization (cNV).
Patients may have been diagnosed with more than one disease.
Total sample = 1,406.
nc, not classified.
Etiology of Corneal Neovascularization
The etiology of cNV was most frequently non-infectious in both the monolateral (48.9%) and bilateral (76.8%) cases (Figure 1A).
Figure 1
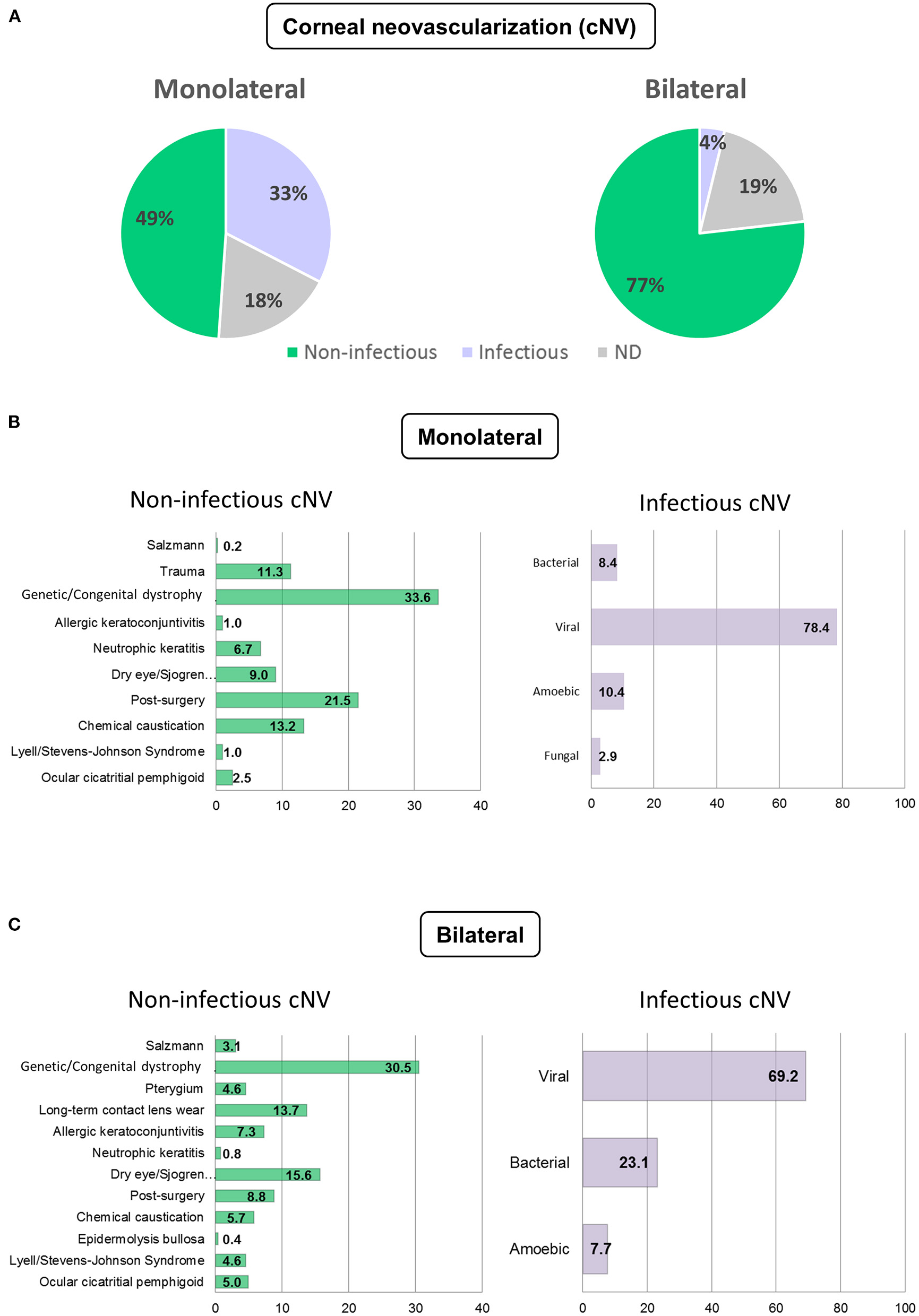
Etiology of corneal neovascularization (cNV). Etiology of monolateral and bilateral cNV that are classified as infectious, non-infectious, and non-determined (ND) (A) Distribution of the patients with infectious and non-infectious keratitis in monolateral (B) and bilateral (C) cNV.
In case of monolateral cNV, the etiology was infectious keratitis in 32.6% of the cases and undetermined in 18.5% of the cases. Viral keratitis (69.3%) was followed by bacterial (23.1%) and amebic (7.7%). Among non-infectious keratitis, the most prevalent diagnoses were genetic/congenital dystrophies, including aniridia, Fuchs and Terrien dystrophies, keratoconus, and others (33.6%). This was followed by cNV occurring after ocular surgery (21.5%), chemical caustication (13.2%), trauma (11.3%), cNV associated with dry eye, and other autoimmune diseases (9%). Ocular surgeries included: phacoemulsification and extracapsular cataract extraction (ECCE) with intraocular lens (IOL) implant, trans pars plana vitrectomy (TPPV), refractive surgery, pterygium removal, lamellar keratoplasty (LK), and penetrating keratoplasty (PK). Other causes of less prevalent non-infectious cNV were: neurotrophic keratitis (6.7%), ocular cicatricial pemphigoid (2.5%), Lyell and Stevens–Johnson syndromes (1%), and allergic keratoconjunctivitis (1%). These data are summarized in Figure 1B.
The etiology for bilateral cNV was non-infectious in 76.8% of the cases, infectious in 3.8% of the cases, and undetermined in 19.4% of the cases. Viral keratitis was the most prevalent infectious cause (69.3%) followed by bacterial (23.1%) and amebic (7.7%). Among non-infectious etiologies, genetic/congenital dystrophies were prevalent (30.5%) followed by dry eye, isolated or associated with Sjögren's syndrome, rosacea, or rheumatoid arthritis (15.6%). Long-term contact lens wear accounted for 13.7% of the cases, ocular surgeries for 8.8% of the cases, allergic conjunctivitis for 7.3% of the cases, bilateral chemical burns for 5.7% of the cases, while ocular cicatricial pemphigoid and Stevens–Johnson syndrome accounted for 5 and 4.6% of the cases, respectively. Other less frequent causes of non-infectious cNV are listed in Figure 1C. Categorization of cNV of the most frequent diseases is provided in Supplementary Figure 1.
Visual Acuity and Corneal Neovascularization
Out of the 1,539 eyes in which visual acuity was measured, cNV extension quantification was available for 1,229 eyes, which were used for this analysis. Eyes affected with one quadrant of cNV were significantly more likely to have better BSCVA than eyes presenting with three-fourths of the quadrants (p < 0.001). Similarly, eyes with two quadrants had significantly better BSCVA than eyes presenting with three-fourths of the quadrants of cNV (p < 0.001) (Figure 2A). Specifically, the media LogMAR was 0.65 ± 0.83, 0.74 ± 0.87, and 1.24 ± 1.08 in cNV one, two, and three-fourths of the quadrants, respectively. Moreover, severe cNV (three-fourths of the quadrants) was a significant predictor of low visual acuity (p < 0.001) (Table 2). In addition, the age of the patients with cNV was also negatively correlated with visual acuity (p < 0.001), while gender was not determinant (Table 2). Finally, superficial, deep, or mixed cNV occurred in 856, 281, and 190 eyes, respectively. We observed that, despite the amount of quadrants affected, most cNV was superficial (p < 0.05) (Table 3). In addition, eyes affected with superficial cNV presented significantly better BSCVA compared to eyes with deep and mixed cNV suggesting that lower visual acuity was associated with the extension and depth of cNV (p < 0.005) (Figure 2B and Table 4).
Figure 2
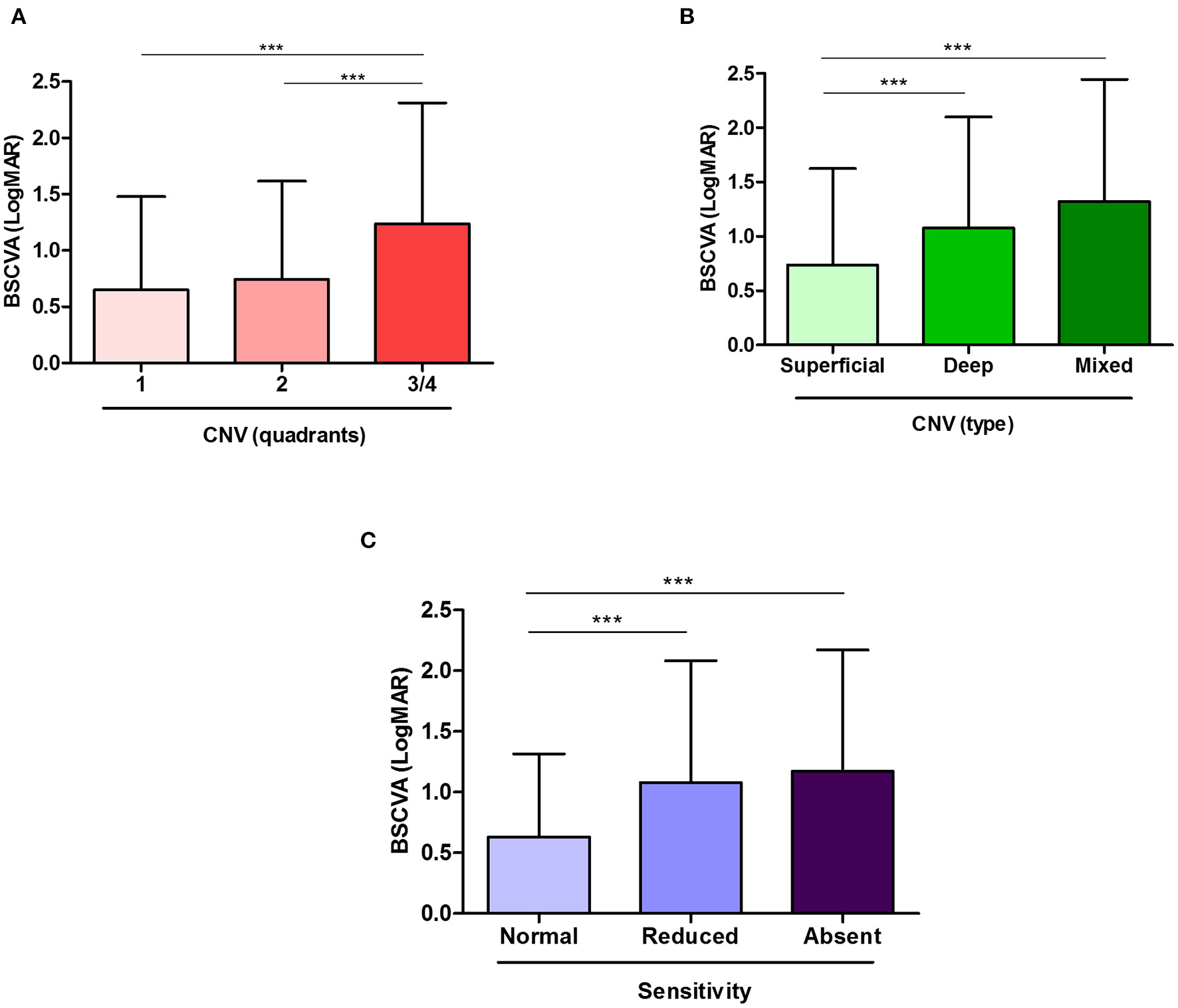
Visual acuity is affected by the extent of corneal neovascularization (cNV) and the decrease in corneal sensitivity. Worse best spectacle corrected visual acuity (BSCVA), observed as an increase in logMAR, is associated with increasing cNV extent (A) and location (B). In addition, the patients with reduced or absent corneal sensitivity showed worse BSCVA (C). Results are expressed as mean ± SD. Statistical analysis was performed by using multiple comparison tests of the Kruskal–Wallis and Dunn's. ***p < 0.001.
Table 2
| Variables | Left eye | Right eye | ||
|---|---|---|---|---|
| Regression coefficient (95% CI) | p * | Regression coefficient (95% CI) | p * | |
| Age, 1-year increase | 0.011 (0.006; 0.015) | <0.001 | 0.011 (0.007; 0.014) | <0.001 |
| Male gender | 0.053 (−0.113; 0.218) | 0.5 | −0.009 (−0.155; 0.137) | 0.9 |
| cNV, sector 1 | 0 (Ref. cat.) | – | 0 (Ref. cat.) | – |
| cNV, sector 2 | 0.114 (−0.068; 0.296) | 0.2 | 0.054 (−0.106; 0.214) | 0.5 |
| cNV, sector 3-4 | 0.542 (0.348; 0.735) | <0.001 | 0.512 (0.326; 0.698) | <0.001 |
Multivariate analysis predicting visual acuity (logMAR).
Multiple regression with robust SEs. Number of observations: 493 (left eye) or 576 (right eye); R-squared: 0.206 (left eye) or 0.204 (right eye). Inflammation and sensitivity were excluded from the models because of the very large number of missing values. When included, however, they did not affect significant variables. An increase in logMAR regression coefficient corresponds to a decrease in visual acuity.
Table 3
| Variables | Left eye | Right eye | ||||||
|---|---|---|---|---|---|---|---|---|
| cNV 1 | cNV 2 | cNV 3-4 | p * | cNV 1 | cNV 2 | cNV 3-4 | p * | |
| (n = 224) | (n = 220) | (n = 243) | (n = 261) | (n = 248) | (n = 260) | |||
| Male gender, % | 45.1 | 49.6 | 50.6 | 0.5 | 51.3 | 52.0 | 50.4 | 0.9 |
| Mean age in years (SD) | 47.6 (19.8) | 50.9 (18.7) | 52.5 (20.2) | 0.03 | 47.4 (20.8) | 49.8 (21.0) | 52.4 (20.0) | 0.03 |
| Bilateral CNV, % | 26.3 | 38.6 | 51.0 | <0.001 | 23.0 | 35.1 | 46.9 | <0.001 |
| cNV sector on the other eye, % | ||||||||
| −1 | 69.0 | 14.6 | 5.8 | <0.001 | 67.8 | 14.8 | 5.0 | <0.001 |
| −2 | 20.7 | 69.5 | 9.9 | 20.3 | 70.4 | 10.7 | ||
| −3/4 | 10.3 | 15.6 | 84.3 | 11.9 | 14.8 | 84.3 | ||
| cNV type, % | (n = 201) | (n = 200) | (n = 225) | 0.05 | (n = 230) | (n = 219) | (n = 242) | 0.001 |
| - Superficial | 59.2 | 68.0 | 64.4 | 64.4 | 67.6 | 62.0 | ||
| - Deep | 27.9 | 18.5 | 17.8 | 27.8 | 16.0 | 20.2 | ||
| - Mixed | 12.9 | 13.5 | 17.8 | 7.8 | 16.4 | 17.8 | ||
| Visual acuity | (n = 201) | (n = 189) | (n = 190) | <0.001 | (n = 232) | (n = 204) | (n = 213) | <0.001 |
| MeanlogMAR (SD) | 0.59 (0.76) | 0.74 (0.94) | 1.24 (1.08) | 0.71 (0.88) | 0.75 (0.80) | 1.23 (1.07) | ||
| Sensitivity, % | (n = 39) | (n = 33) | (n = 40) | 0.04 | (n = 60) | (n = 43) | (n = 35) | 0.6 |
| - Absent | 20.5 | 21.2 | 42.5 | 25.0 | 34.9 | 31.4 | ||
| - Reduced | 38.5 | 57.6 | 30.0 | 35.0 | 39.5 | 40.0 | ||
| - Normal | 41.0 | 21.2 | 27.5 | 40.0 | 25.6 | 28.6 | ||
| Most frequent diseases, % | (n = 196) | (n = 180) | (n = 214) | (n = 232) | (n = 220) | (n = 230) | ||
| - HSV keratitis | 27.0 | 21.7 | 12.1 | 0.001 | 26.7 | 22.3 | 13.9 | 0.003 |
| - Keratoconus | 16.8 | 15.6 | 12.1 | 0.4 | 16.8 | 15.9 | 12.2 | 0.4 |
| - Penetrating keratoplasty | 13.8 | 16.7 | 19.2 | 0.3 | 15.5 | 16.4 | 14.4 | 0.8 |
| - Trauma | 6.1 | 7.2 | 12.6 | 0.04 | 8.2 | 12.7 | 17.8 | 0.008 |
| - Phacoemulsification + intraocular lens | 5.8 | 5.9 | 9.5 | 0.2 | 6.1 | 8.1 | 8.1 | 0.6 |
Association between corneal neovascularization (cNV) severity and the selected variables: univariate analyses.
Kruskal–Wallis test for continuous variables, chi-squared test for categorical ones.
Table 4
| cNV type | Left eye | Right eye | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Superficial | Deep | Mixed | p * | Superficial | Deep | Mixed | p * |
| (n = 495) | (n = 168) | (n = 102) | (n = 531) | (n = 179) | (n = 109) | |||
| Male gender, % | 48.9 | 49.4 | 44.1 | 0.6 | 50.1 | 52.5 | 52.3 | 0.8 |
| Mean age in years (SD) | 49.5 (19.9) | 50.7 (18.9) | 52.6 (19.1) | 0.3 | 48.5 (21.3) | 49.6 (19.9) | 57.6 (18.2) | <0.001 |
| Visual acuity | (n = 430) | (n = 142) | (n = 81) | (n = 455) | (n = 141) | (n = 95) | ||
| Mean logMAR (SD) | 0.75 (0.91) | 1.03 (1.00) | 1.23 (1.10) | <0.005 | 0.73 (0.86) | 1.12 (1.05) | 1.40 (1.14) | <0.005 |
| Most frequent diseases, % | (n = 420) | (n = 157) | (n = 91) | (n = 462) | (n = 163) | (n = 101) | ||
| - HSV keratitis | 13.1 | 25.5 | 30.8 | <0.005 | 12.6 | 31.3 | 31.7 | <0.005 |
| - Penetrating keratoplasty | 16.2 | 14.0 | 14.3 | 0.8 | 12.3 | 17.2 | 15.8 | 0.3 |
| - Keratoconus | 17.9 | 12.1 | 5.5 | 0.006 | 17.8 | 12.9 | 5.9 | 0.007 |
| - Trauma | 7.9 | 7.6 | 15.4 | 0.06 | 12.8 | 8.6 | 16.8 | 0.13 |
| - Phacoemulsification + intraocular lens | 7.1 | 10.2 | 8.8 | 0.4 | 7.9 | 8.4 | 9.2 | 0.9 |
Association between corneal neovascularization (cNV) type and the most frequent diagnoses: univariate analyses.
Kruskal–Wallis test for continuous variables, chi-squared test for categorical ones.
Corneal Anesthesia and BSCVA
Out of 1,229 eyes where cNV was quantified, 253 eyes were also tested for corneal sensitivity, which were used for this analysis. BSCVA (LogMAR) was significantly lower (i.e., better vision) in eyes that presented normal corneal sensitivity (0.63 ± 0.68, N = 82) compared to the ones that showed reduced (1.08 ± 1.00, N = 100) or completely absent (1.17 ± 1.00, N = 71) (p < 0.001) sensitivity (Figure 2C). Moreover, we found that in the patients with cNV, lower visual acuity is a predictor of reduced corneal sensitivity (p < 0.05) (Supplementary Table 1).
Corneal Neovascularization and Sensitivity
Out of 305 eyes where corneal sensitivity was measured, cNV was quantified in 203 eyes, which was included for the analysis. Table 3 shows the loss of corneal sensitivity that was associated with the extent of cNV (p < 0.05). In other words, the majority of the patients affected with cNV one quadrant presented normal sensitivity, while most of the patients with severe cNV (third-fourths of the quadrants) displayed anesthesia (p < 0.05). Therefore, cNV extent resulted in a significant predictor of lower corneal sensitivity (p < 0.05) (Supplementary Table 1).
Discussion
Corneal neovascularization is the second cause of blindness worldwide (5) and an area of significant medical need. Current treatments include topical application of corticosteroids or non-steroidal anti-inflammatory agents, which can be associated with serious side effects such as ocular hypertension, posterior cataract induction, delayed healing, or corneal melting, respectively (4). Recently, vascular endothelial growth factor (VEGF) inhibitors such as ranibizumab (Lucentis; Genentech), and bevacizumab (Avastin; Genentech) have been proposed as a treatment for cNV with encouraging results (15, 16).
Inhibition of cNV has been considered clinically beneficial, although weak evidence supports the efficacy of the anti-cNV treatments in ameliorating vision (17). In any case, the real impact of cNV, and its extension, on visual acuity remains largely unknown. For this reason, we aimed to quantify the impact of cNV on visual acuity in a large population. We show that BSCVA is significantly and negatively affected by cNV in our cohort of 1,406 patients. To the best of our knowledge, only one prior report has assessed the impact of cNV on visual acuity and has found it reduced in 12% of patients affected with cNV (6), which is very similar to the prevalence reported in 10.4% of the patients. However, the limited number of the patients considered in that study (35 subjects out of 845 subjects) and the different population (general ophthalmology as opposed to cornea clinic) and the absence of cNV grading make it difficult to compare the two studies.
We also found that BSCVA is progressively reduced with an increasing extension of cNV. We would like to clarify that this does not mean that reducing the extent of cNV (therapeutically) may necessarily result in the improvement of BSCVA. In fact, vessel leaking of calcium or blood could irreversibly impair corneal clarity, and, hence, vision. Our study, however, suggests the importance of modulating cNV in its earlier stages as its progression to involve the entire cornea is indeed associated with worse vision. Additionally, we observed a significant correlation between cNV and loss of corneal sensitivity in line with prior reports in the animal models (18). The link underlying corneal nerve loss/dysfunction and cNV is not clear. It is known that corneal infections (19) or dry eye (20) is associated with altered nerve morphology and corneal sensitivity. One could hypothesize that neovessels induce corneal edema and, hence, cause peripheral nerve degeneration. Additionally, it is possible that normal nerves secrete anti-angiogenic factors such as pigment epithelium-derived factor (18), which are lost after their degeneration. Acute damage of corneal nerves and subsequent release of substance P could also promote cNV (21). Finally, it is possible that massive leukocyte infiltration, as it occurs during corneal inflammation, induces cNV and nerve disruption. Indeed, it has been reported that reduction of corneal nerve density is associated with an increased leukocyte infiltration in the conditions commonly associated with cNV (22).
Regardless of the mechanism(s) involved, this study suggests that corneas affected with cNV should be routinely checked for abnormal sensitivity to rule out concomitant neurotrophic disease.
Finally, we found that eyes with corneal anesthesia had worse BSCVA as opposed to eyes with normal corneal sensitivity. This suggests that vision reduction in patients with cNV could have multiple causes beyond the obvious effect of opacity induced by vessel growth into the cornea. In fact, impairment and/or loss of corneal sensitivity is associated with tear film alteration, punctate keratopathy, or ulcers, which can all reduce visual acuity. The finding that cNV patients exhibited altered nerve function should be taken into account, since anti-VEGF treatments, which have been proposed for cNV, are neurotoxic (23). Interestingly, VEGF neutralization with bevacizumab resulted in downregulation of nerve growth factor and delayed wound healing (24).
In summary, the most prevalent etiology of cNV in our population was non-infectious corneal dystrophies/degenerations. This group included, not surprisingly, the patients affected with chronic corneal edema or aniridia. The finding that the patients with keratoconus were also affected could be associated with extensive contact lens use in this group. On the other hand, infectious keratitis was the second cause of monolateral cNV and the third cause of bilateral cNV. This is in line with prior literature, which confirms a lower prevalence of infectious keratitis in developed countries (25). Among infections, herpes keratitis was the most prevalent, which consolidates prior reports (7). Of note, the most common diagnosis among the patients affected with most severe (third-fourths of the quadrants) cNV were penetrating keratoplasty and trauma (16.7 and 15.3%, respectively) followed by keratoconus (12.2%) (Supplementary Material).
We acknowledge that this study did not consider the central extension of cNV, as it was not possible to retrieve such information in this large cohort of patients. However, this study shows that higher cNV density (and, specifically, deep and extensive cNV) is associated with a lower vision that underlines the relevance of cNV-associated disorders as major drivers of visual impairment.
Funding
This work was supported by the institutional funding.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by mds. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
GF and PR contributed to conception and design of the study. RL, GT, and PF organized the database. LM and CA performed the statistical analysis. RL, PF, and GF analyzed the data. RL and GF wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.733538/full#supplementary-material
References
1.
Cursiefen C Colin J Dana R Diaz-Llopis M Faraj LA Garcia-Delpech S et al . Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: outcome of an expert roundtable. Br J Ophthalmol. (2012) 96:3–9. 10.1136/bjo.2011.204701
2.
Cursiefen C Hofmann-Rummelt C Küchle M Schlötzer-Schrehardt U . Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. (2003) 87:101–6. 10.1136/bjo.87.1.101
3.
Cursiefen C Chen L Dana MR Streilein JW . Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. (2003) 22:273–81. 10.1097/00003226-200304000-00021
4.
Azar DT . Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. (2006) 104:264–302.
5.
Skobe M Dana R . Blocking the path of lymphatic vessels. Nat Med. (2009) 15:993–4. 10.1038/nm0909-993
6.
Colby K Adamis A . Prevalence of corneal vascularization in a general eye service population. Invest Ophthalmol Vis Sci. (1996) 37:2734.
7.
Lee P Wang CC Adamis AP . Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. (1998) 43:245–69. 10.1016/S0039-6257(98)00035-6
8.
Nomura K Nakao M Matsubara K . Subjective symptom of eye dryness and lifestyle factors with corneal neovascularization in contact lens wearers. Eye Contact Lens. (2004) 30:95–8. 10.1097/01.ICL.00000117256.77329.06
9.
Murube J . Aniridia and the ocular surface. Ocul Surf. (2004) 2:55–7. 10.1016/S1542-0124(12)70144-1
10.
Vera LS Gueudry J Delcampe A Roujeau JC Brasseur G Muraine M . In vivo confocal microscopic evaluation of corneal changes in chronic stevens-johnson syndrome and toxic epidermal necrolysis. Cornea. (2009) 28:401–7. 10.1097/ICO.0b013e31818cd299
11.
Bachmann B Taylor RS Cursiefen C . Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. (2010) 117:39. 10.1016/j.ophtha.2010.01.039
12.
Dana MR Schaumberg DA Kowal VO Goren MB Rapuano CJ Laibson PR et al . Corneal neovascularization after penetrating keratoplasty. Cornea. (1995) 14:604–9. 10.1097/00003226-199511000-00014
13.
Holladay JT . Proper method for calculating average visual acuity. J Refract Surg. (1997) 13:388–91. 10.3928/1081-597X-19970701-16
14.
Kinard KI Smith AG Singleton JR Lessard MK Katz BJ Warner JEA et al . Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache. (2015) 55:543–9. 10.1111/head.12547
15.
Dastjerdi MH Al-Arfaj KM Nallasamy N Hamrah P Jurkunas U V Pineda R et al . Topical bevacizumab in the treatment of corneal neovascularization; results of a prospective, open-label, noncomparative study. Arch Ophthalmol. (2009) 127:381–9. 10.1001/archophthalmol.2009.18
16.
Ferrari G Dastjerdi MH Okanobo A Cheng SF Amparo F Nallasamy N et al . Topical ranibizumab as a treatment of corneal neovascularization. Cornea. (2013) 32:992–7. 10.1097/ICO.0b013e3182775f8d
17.
Bachmann B Taylor RS Cursiefen C . The association between corneal neovascularization and visual acuity: a systematic review. Acta Ophthalmol. (2013) 91:12–9. 10.1111/j.1755-3768.2011.02312.x
18.
Ferrari G Hajrasouliha AR Sadrai Z Ueno H Chauhan SK Dana R . Nerves and neovessels inhibit each other in the cornea. Investig Ophthalmol Vis Sci. (2013) 54:813–20. 10.1167/iovs.11-8379
19.
Müller RT Abedi F Cruzat A Witkin D Baniasadi N Cavalcanti BM et al . Degeneration and regeneration of subbasal corneal nerves after infectious keratitis: a longitudinal in vivo confocal microscopy study. Ophthalmology. (2015) 122:2200–9. 10.1016/j.ophtha.2015.06.047
20.
Xu KP Yagi Y Tsubota K . Decrease in corneal sensitivity and change in tear function in dry eye. Cornea. (1996) 15:235–9. 10.1097/00003226-199605000-00002
21.
Bignami F Giacomini C Lorusso A Aramini A Rama P Ferrari G . NK1 receptor antagonists as a new treatment for corneal neovascularization. Invest Ophthalmol Vis Sci. (2014) 55:6783–94. 10.1167/iovs.14-14553
22.
Cruzat A Witkin D Baniasadi N Zheng L Ciolino JB Jurkunas U V et al . Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Investig Ophthalmol Vis Sci. (2011) 52:5136–43. 10.1167/iovs.10-7048
23.
Yu CQ Zhang M Matis KI Kim C Rosenblatt MI . Vascular endothelial growth factor mediates corneal nerve repair. Investig Ophthalmol Vis Sci. (2008) 49:3870–8. 10.1167/iovs.07-1418
24.
Kim EC Lee WS Kim MS . The inhibitory effects of bevacizumab eye drops on NGF expression and corneal wound healing in rats. Investig Ophthalmol Vis Sci. (2010) 51:4569–73. 10.1167/iovs.09-4937
25.
Lam DSC Houang E Fan DSP Lyon D Seal D Wong E et al . Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye. (2002) 16:608–18. 10.1038/sj.eye.6700151
Summary
Keywords
corneal neovascularization, visual acuity, corneal sensitivity, retrospective study, epidemiology
Citation
Lasagni Vitar RM, Triolo G, Fonteyne P, Acuti Martellucci C, Manzoli L, Rama P and Ferrari G (2021) Epidemiology of Corneal Neovascularization and Its Impact on Visual Acuity and Sensitivity: A 14-Year Retrospective Study. Front. Med. 8:733538. doi: 10.3389/fmed.2021.733538
Received
30 June 2021
Accepted
13 September 2021
Published
14 October 2021
Volume
8 - 2021
Edited by
Lasse Dahl Ejby Jensen, Linköping University, Sweden
Reviewed by
Juan Alvarez De Toledo Elizalde, Clínica Oftalmológica Barraquer, Spain; Edward Wylegała, Medical University of Silesia, Poland
Updates
Copyright
© 2021 Lasagni Vitar, Triolo, Fonteyne, Acuti Martellucci, Manzoli, Rama and Ferrari.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giulio Ferrari ferrari.giulio@hsr.itPaolo Rama rama.paolo@hsr.it
This article was submitted to Ophthalmology, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.