- 1Department of Critical Care Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Clinical Epidemiology and EBM Unit, National Clinical Research Center for Digestive Disease, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Background: The goal of our study was to evaluate the association of sex and in-hospital mortality in patients with septic shock in Beijing, China.
Materials and Methods: We analyzed 3,643 adult patients with septic shock from January 1, 2019, to Dec 31, 2019, in all secondary and tertiary hospitals in Beijing. Study data were retrospectively extracted from the Quality Control Center of Beijing Municipal Health Commission.
Results: There were 2,345 (64.37%) male and 1,298 (35.63%) female patients. Compared to male patients, female patients with septic shock had a higher in-hospital mortality rate (55.54 vs. 49.29%, p < 0.01). The median length of hospitalization stay for male patients was 22.71 days, while that for female patients was 19.72 days (p > 0.01). Male patients had a higher prevalence of pulmonary infection (68.8 vs. 31.2%, p < 0.01). The B values of sex in univariate and multivariate logistic regression were −0.251 and −0.312, respectively. Men had a lower likelihood of hospital mortality than women (OR = 0.732, 95% CI = 0.635–0.844, p = 0.000).
Conclusions: Female patients with septic shock had a higher risk of dying in the hospital than male patients.
Introduction
Sex is increasingly recognized as a key factor in trauma (1), coronary heart disease (2), autoimmune disease (3), cancer, mental disorder (4) and other medical conditions. A number of studies suggest that a patient's gender may influence both the provision of care as well as outcomes. Critical care is not immune to such bias (5).
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (6). Septic shock is a complex inflammatory crisis associated with a high rate of mortality (7). Sepsis and septic shock are major health care problems, affecting millions of people around the world each year, resulting in the death of as many as one in four patients (and often more) (6). Recently, several studies have evaluated the effect of gender for patients with sepsis or septic shock. However, reports on the sex and mortality of sepsis/septic shock have shown conflicting results (8–11). The goal of this study was to evaluate the association of sex and in-hospital mortality in patients with septic shock in Beijing, China.
Materials and Methods
Study Population
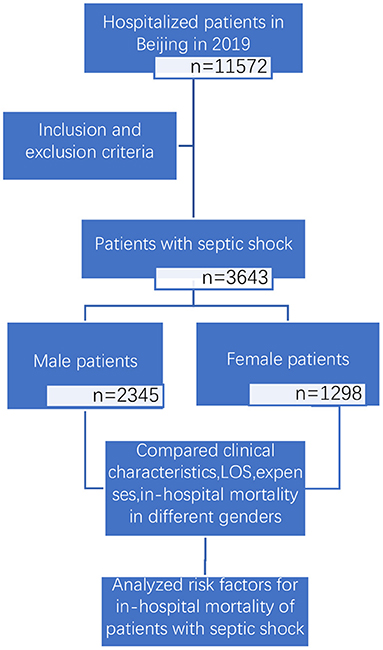
We conducted a retrospective and observational study (study flow chart shown in Figure 1). Based on the principal discharge diagnosis, patients with septic shock based on the sepsis-3.0 definition were enrolled by reviewing the inpatient lists from January 1, 2019, to Dec 31, 2019, in all secondary and tertiary hospitals in Beijing. The only exclusion criterion was an age <18 years old. This study was approved by the ethics committee of Beijing Friendship Hospital (No. 2021-P2-184-01) and granted a waiver of informed consent.
Data Collection
Study data were retrospectively extracted from the Quality Control Center of Beijing Municipal Health Commission. Data elements were collected from the cover sheet of medical records, including patient' demographics, medical history, expenses, length of hospital stay, hospital level and diagnosis discharge form.
Study Variables
Discharge forms included the following: recovered and discharged, discharged without recovery, referral, and death. We defined the first three conditions as “alive” and calculated the in-hospital mortality of patients with septic shock.
The race of patients was categorized as “Han” or “non-Han”. Hospital levels were categorized as “Tertiary hospitals” or “Secondary hospitals” which were determined officially.
The insurance of patients was categorized as “medical insurance” or “self-pay”. “Medical insurance” included Urban Employee Basic Medical Insurance and Urban Resident Basic Medical Insurance, New Rural Cooperative Medical Insurance or Business insurance.
Comorbidities included hypertension, diabetes mellitus, ischemic heart disease, chronic kidney disease, liver disease, chronic pulmonary disease and malignant tumors. Hypertension was defined as having a history of hypertension, receiving antihypertensive therapy, or having a systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on admission. Diabetes mellitus was defined as having a previous or new diagnosis of diabetes mellitus, receiving oral hypoglycemic drug therapy or insulin therapy, or having a fasting blood glucose level ≥7.0 mmol/L (126 mg/dL) or hemoglobin A1c level ≥6.5%. Ischemic heart disease included angina pectoris and myocardial infarction. Chronic kidney disease (CKD) was defined as abnormal kidney structure or function persisting for longer than 3 months. Liver disease included viral hepatitis and autoimmune, metabolic or alcohol-related liver disorders. Chronic pulmonary disease included chronic respiratory disease, cor pulmonale and pulmonary circulatory disease.
The site of infection included the pulmonary, skin, urinary tract, gastrointestinal tract, abdominal cavity and bloodstream.
The primary endpoint in the study was in-hospital mortality, which was defined as death during hospitalization. The hospital length of stay (LOS) and expenses were selected as the secondary outcomes.
Statistical Methods
Categorical variables are presented as frequencies (n) and percentages (%). Continuous variables that conformed to a normal distribution are expressed as the mean ± standard deviation, and those that did not conform to a normal distribution are expressed as the median (interquartile range). An unpaired t test or Mann–Whitney U test was used to assess the statistical significance of differences between means or medians, where appropriate. The significance of differences for categorical variables was analyzed using the Chi-squared test. To evaluate the relationship between sex and in-hospital mortality, univariate and multivariate logistic regression analyses were performed. All statistical analyses were performed using SPSS version 25.0. Two-sided P < 0.01 were considered statistically significant.
Results
Patient Characteristics
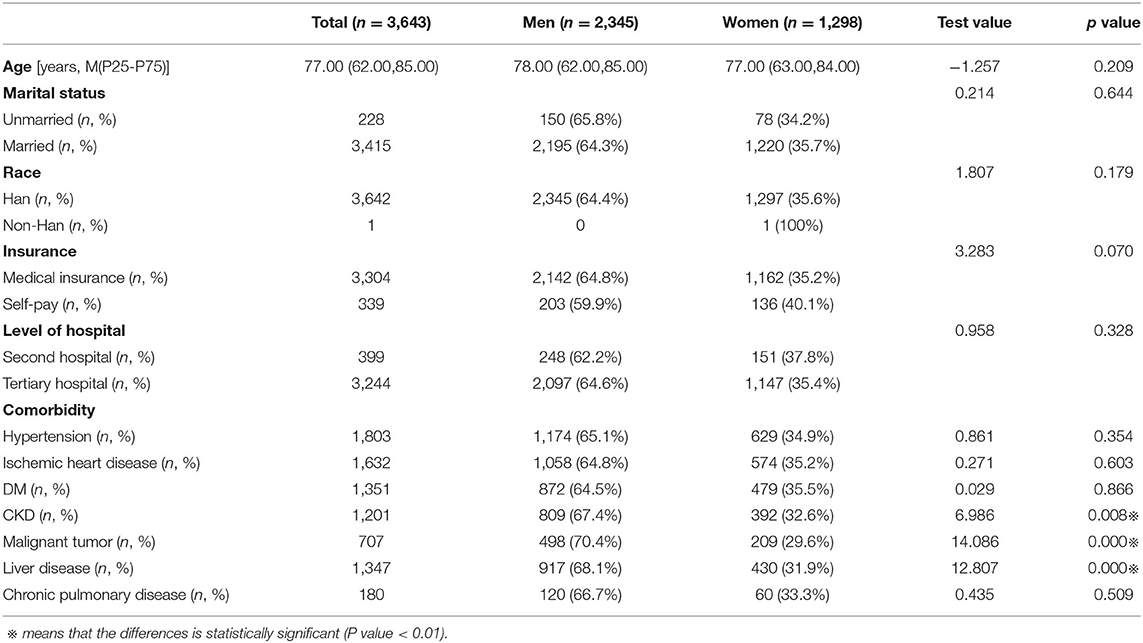
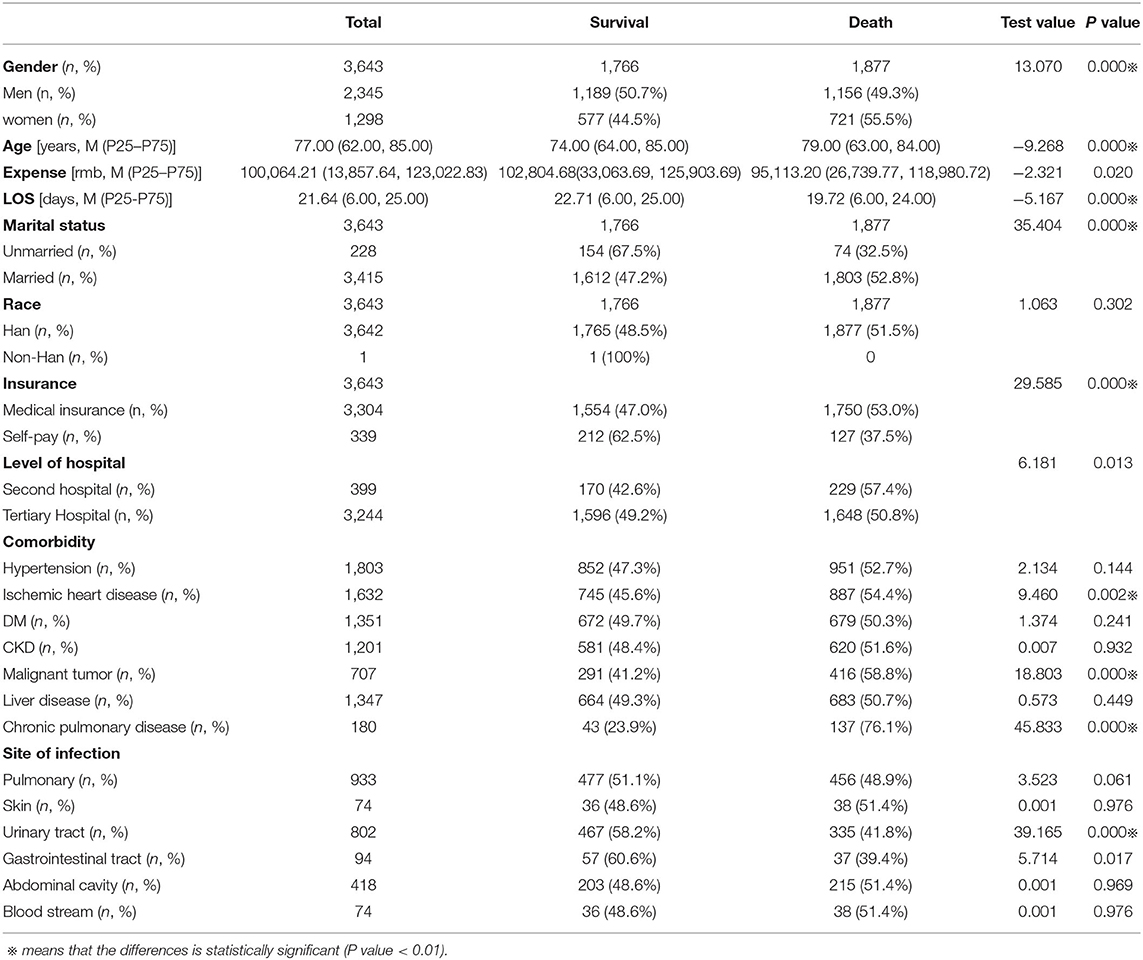
Among 3,643 patients with septic shock who were included in this study, 2,345 (64.37%) were male, and 1,298 (35.63%) were female. The clinical characteristics of the study population are summarized in Table 1.
The mean ages of male and female patients were 78.00 (62.00, 85.00) and 77.00 (63.00, 84.00) years, respectively. Men had a higher prevalence of malignant tumors (70.4 vs. 29.6%, p < 0.01), chronic kidney disease (67.4 vs. 32.6%, p < 0.01) and liver disease (68.1 vs. 31.9%, p < 0.01).
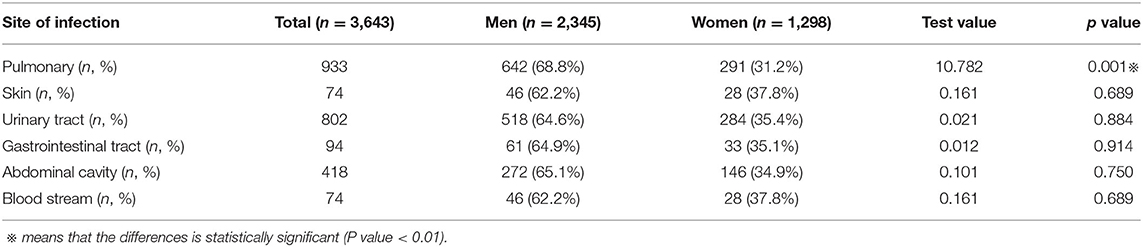
Site of Infection in Different Genders
The main site of infection leading to septic shock was the pulmonary system. Men had a higher prevalence of pulmonary infection (68.8 vs. 31.2%, p < 0.01) (see Table 2).
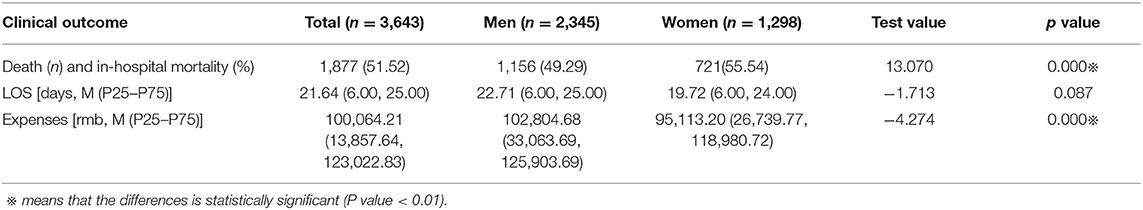
The Differences in Clinical Outcomes by Sex
We analyzed the clinical outcomes in male and female patients with septic shock (see Table 3). The in-hospital mortality rate was higher in women than in men (55.54 vs. 49.29%, p < 0.01). Meanwhile, male patients had higher hospital expenses (p < 0.01) and longer stays (p > 0.01) at the hospital.
Sex and In-hospital Mortality
We divided patients with septic shock into two groups according to different clinical outcomes (death or survival in the hospital) and compared the data of the two groups (see Table 4). We found that sex, age, length of stay, marital status, medical insurance status, chronic pulmonary disease, malignant tumor, ischemic heart disease, and urinary tract infection were significantly different between the two groups (p < 0.01).
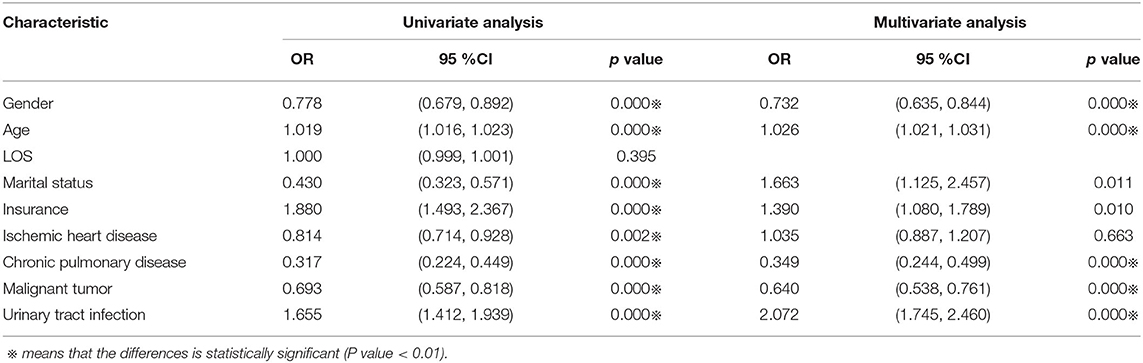
We then performed univariate and multivariate logistic regression on the above different indicators (see Table 5). To examine the association between sex and in-hospital mortality, logistic regression models were used to adjust for patients' clinical characteristics, including sex, age, length of stay, marital status, medical insurance status, chronic pulmonary disease, malignant tumor, ischemic heart disease, and urinary tract infection.
In univariate logistic regression, the B value of gender was −0.251, whereas in multivariate regression the B value of sex was −0.312. The results suggested that after adjusting the covariates, the correlation between gender and in-hospital death was greater. Male patients had a lower likelihood of hospital mortality than female patients (OR = 0.732, 95% CI = 0.635–0.844, p = 0.000).
Discussion
In this large, hospital-based registry for male and female patients discharged with septic shock in Beijing China, we observed that men with septic shock were more likely to suffer from chronic diseases (such as hypertension, DM, ischemic heart disease, chronic kidney disease, chronic pulmonary disease, liver disease, malignant tumor) and had higher hospital expenses and longer stays at the hospital. However, the in-hospital mortality rate of male patients with septic shock was lower than that of female patients.
Previous animal and human studies indicated that females have advantageous immunologic and cardiovascular responses during infectious challenge, which means a higher sepsis incidence in males than in females. However, clinical studies on sex and mortality among critically ill sepsis patients have shown conflicting results (12). This may be related to the differences in study design, sample sizes, population included in the studies (ICU patients or non-ICU patients) and the research methods.
To explain the results in our study, we tried to analyze the following possible mechanisms. Estrogens have been proven to have a direct protective effect on vascular endothelial cells (13), inhibit endothelial cell apoptosis, induce endothelial cell proliferation and migration, and promote microvessel regeneration (14). However, estrogens also have physiologic actions that could be detrimental in sepsis (15). In studies of gender-specific responses to endotoxin, there were higher estrogen concentrations in elderly critically ill women than in younger critically ill women, as well as elevated estrogen concentrations in critically ill men, plus the association of higher estrogen levels with higher mortality in both women and men (16, 17). Non-biological explanations for our findings must also be considered. Previous studies (5, 18, 19) suggest that female patients with sepsis/septic shock received less medical care than male patients, and the proportion of withheld or withdrawn treatment was greater for female than for male patients. Although our study did not include data about treatment, it may also be one of the reasons why the in-hospital mortality of female patients with septic shock is higher than that of male patients (20). Sex differences in sites of infection were observed in the study, but similar to the hospital mortality difference, it is unclear whether they originate from gender differences in biology, comorbidity, or medical assessment and care.
The study was a large retrospective study of sample size, making the results credible. Many previous studies (18–21) indicated that the authors only had access to data for patients who presented with sepsis in the ED or ICU. However, in clinical practice, not all patients with septic shock receive treatment in the ED or ICU, so some cases may be missed. Our study included all patients with septic shock in all departments compared with other studies.
Limited by our current capabilities and the extent to which the database can be used, our research discovered a phenomenon but cannot fully explain its pathophysiological mechanism. Information on medical care during hospitalization cannot be fully indicated. Whether to adopt standardized treatment is very important for clinical prognosis. The results of this study can provide ideas and evidence for follow-up research. The patients in our study were middle-aged or elderly, which may not fully represent the characteristics of the entire adult population. This is a limitation of retrospective research. In our study, the death group was older than the alive group. As age increased, mortality also increased. We should consider the impact of age on mortality. However, there was no statistically significant difference in age between male and female patients. The impact of sex on in-hospital mortality was adjusted by logistic regression. In multivariate logistic regression, the B value of sex was greater than that in univariate regression. Based on this, we believe that the conclusions are valid.
Conclusion
In this study, female patients with septic shock had a higher in-hospital mortality than male patients. This difference remained after multivariable adjustment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Beijing Friendship Hospital Ethics Committee (No. 2021-P2-184-01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MLD and XZ contributed to conception and design of the study. ZL, PL, and NZ organized the database. NZ and XZ performed the statistical analysis. XZ wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JL declared a shared affiliation with the authors to the handling editor at the time of the review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors express special thanks to Dr. Dongchen Guo and Dr. Haiman Wang, who helped this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.733410/full#supplementary-material
Abbreviations
LOS, length of hospital stay; ICU, Intensive care unit.
References
1. Marcolini EG, Albrecht JS, Sethuraman KN, Napolitano LM. Gender disparities in trauma care: how sex determines treatment, behavior, and outcome. Anesthesiol Clin. (2019) 37:107–17. doi: 10.1016/j.anclin.2018.09.007
2. Hao Y, Liu J, Liu J, Yang N, Smith SC Jr, Huo Y, et al. Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome. Circulation. (2019) 139:1776–85. doi: 10.1161/CIRCULATIONAHA.118.037655
3. Golden LC, Voskuhl R. The importance of studying sex differences in disease: The example of multiple sclerosis. J Neurosci Res. (2017) 95:633–43. doi: 10.1002/jnr.23955
4. Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, et al. Understa. Alzheimer's Dement. (2018) 14:1171-1183. doi: 10.1016/j.jalz.2018.04.008
5. Elizabeth Wilcox M, Donnelly JP, Lone NI. Understanding gender disparities in outcomes after sepsis. Intensive Care Med. (2020) 46:796–8. doi: 10.1007/s00134-020-05961-3
6. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. (2017) 45:486–552. doi: 10.1007/s00134-017-4683-6
7. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. (2018) 392:75–87. doi: 10.1016/S0140-6736(18)30696-2
8. Madsen TE, Simmons J, Choo EK, Portelli D, McGregor AJ, Napoli AM. The DISPARITY Study: do gender differences exist in Surviving Sepsis Campaign resuscitation bundle completion, completion of individual bundle elements, or sepsis mortality? J Crit Care. (2014) 29:473.e7–11. doi: 10.1016/j.jcrc.2014.01.002
9. Xu J, Tong L, Yao J, Guo Z, Lui KY, Hu X, et al. Association of sex with clinical outcome in critically ill sepsis patients: a retrospective analysis of the large clinical database MIMIC-III. Shock. (2019) 52:146–51. doi: 10.1097/SHK.0000000000001253
10. Combes A, Luyt CE, Trouillet JL, Nieszkowska A, Chastre J. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med. (2009) 37:2506–11. doi: 10.1097/CCM.0b013e3181a569df
11. van Vught LA, Scicluna BP, Wiewel MA, Hoogendijk AJ, Klein Klouwenberg PMC, Ong DSY, et al. MARS Consortium. Association of gender with outcome and host response in critically ill sepsis patients. Crit Care Med. (2017) 45:1854–62. doi: 10.1097/CCM.0000000000002649
12. Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, et al. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit Care. (2013) 17:R50. doi: 10.1186/cc12570
13. Shufelt CL, Pacheco C, Tweet MS, Miller VM. Sex-specific physiology and cardiovascular disease. Adv Exp Med Biol. (2018) 1065:433–54. doi: 10.1007/978-3-319-77932-4_27
14. Montt-Guevara MM, Palla G, Spina S, Bernacchi G, Cecchi E, Campelo AE, et al. Regulatory effects of estetrol on the endothelial plasminogen pathway and endothelial cell migration. Maturitas. (2017) 99:1–9. doi: 10.1016/j.maturitas.2017.02.005
15. Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. (1991) 88:5134–8. doi: 10.1073/pnas.88.12.5134.PMID:2052592
16. May AK, Dossett LA, Norris PR, Hansen EN, Dorsett RC, Popovsky KA, et al. Estradiol is associated with mortality in critically ill trauma and surgical patients. Crit Care Med. (2008) 36:62–8. doi: 10.1097/01.CCM.0000292015.16171.6D
17. Angstwurm MW, Gaertner R, Schopohl J. Outcome in elderly patients with severe infection is influenced by sex hormones but not gender. Crit Care Med. (2005) 33:2786–93. doi: 10.1097/01.ccm.0000190242.24410.17
18. Valentin A, Jordan B, Lang T, Hiesmayr M, Metnitz PG. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med. (2003) 31:1901–7. doi: 10.1097/01.CCM.0000069347.78151.50
19. Fowler RA, Sabur N, Li P, Juurlink DN, Pinto R, Hladunewich MA, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. (2007) 177:1513–9. doi: 10.1503/cmaj.071112
20. Pietropaoli AP, Glance LG, Oakes D, Fisher SG. Gender differences in mortality in patients with severe sepsis or septic shock. Gend Med. (2010) 7:422–37. doi: 10.1016/j.genm.2010.09.005
Keywords: septic shock, sex, in-hospital mortality, risk factor, cover sheet of medical records
Citation: Zhou X, Zeng N, Liu P, Liu Z and Duan M (2021) Sex Differences in In-hospital Mortality of Patients With Septic Shock: An Observational Study Based on Data Analysis From a Cover Sheet of Medical Records in Beijing. Front. Med. 8:733410. doi: 10.3389/fmed.2021.733410
Received: 30 June 2021; Accepted: 14 September 2021;
Published: 11 October 2021.
Edited by:
Luo Zhe, Fudan University, ChinaReviewed by:
Fengxue Zhu, Peking University People's Hospital, ChinaJingyuan Liu, Capital Medical University, China
Copyright © 2021 Zhou, Zeng, Liu, Liu and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meili Duan, ZG1laWxpJiN4MDAwNDA7Y2NtdS5lZHUuY24=
 Xiao Zhou
Xiao Zhou Na Zeng2
Na Zeng2 Pei Liu
Pei Liu Zhuang Liu
Zhuang Liu Meili Duan
Meili Duan