- 1Ocular Proteomics Platform, Singapore Eye Research Institute, Singapore, Singapore
- 2Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Branch of National Clinical Research Center for Ocular Disease, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin, China
- 3Department of Ophthalmology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 4Ophthalmology and Visual Sciences Academia Clinical Program, Duke-National University of Singapore Medical School, Singapore, Singapore
Purpose: Tear film lipid layer (TFLL) plays a vital role in maintaining the tear film stability and, thus, the lipid composition of the tears could greatly affect the physiological function and biophysical integrity of the tear film. The objective of this study is to assess the tear lipid composition of the patients receiving laser-assisted in situ keratomileusis (LASIK), femtosecond LASIK (FS-LASIK), or sub-Bowman's keratomileusis (SBK) surgery preoperatively and postoperatively.
Methods: Tear samples were collected from the left eye of the patient who receiving LASIK (n = 10), FS-LASIK (n = 10), or SBK (n = 10) surgery in week 0, week 1, week 4, and week 52. A rapid direct injection shotgun lipidomics workflow, MS/MSALL (<2 min/sample), was applied to examine the tear lipidome.
Results: In week 52, the SBK group demonstrated a similar lipidome profile compared to week 0, while the FS-LASIK and LASIK groups shifted away from week 0. Two lipids, ganglioside (GD3) 27:4 and triacylglycerol (TAG) 59:3, were found to be associated with the lipidome changes preoperatively and postoperatively. No statistical significance was found in the overall lipid classes from the FS-LASIK group. The LASIK group showed significant alteration in the phospholipid and sphingolipid over time, while the SBK group demonstrated a significant difference in the (O-acyl)-ω-hydroxy fatty acid (OAHFA) and phospholipid.
Conclusion: LASIK showed the greatest impact on the tear lipidome changes over time, while SBK demonstrated minimal impact among the three types of refractive surgeries after 1 year.
Introduction
The thin layer of the tear film covers the anterior surface of the cornea and serves the critical functions in maintaining the proper ocular function and health. Its main roles include moistening the mucous membrane, nourishing the avascular corneal, flushing out the contaminants and irritants, and providing a smooth surface for the visual acuity (1, 2). It has been proposed that the tear film is composed of three layers: an inner mucin layer, a middle aqueous layer, and an outer tear film lipid layer (TFLL). Lam et al. further divided the lipid layer into two sublayers: the superficial sublayer mainly consisting of the non-polar lipids and an inner amphiphilic sublayer facilitating the interaction between the polar and non-polar components of the tears (3). TFLL is vital for a stable tear film by preventing the tear film from evaporation (4). Therefore, the physiological function and biophysical integrity of the tear film would be greatly affected by the lipid composition. It was a challenging task to fully evaluate the lipid profile of the tear samples considering the small amount of the materials obtained from the humans, the diversity of the lipid species, and the complexities of the qualitative and quantitative lipidomics analysis (5). Nevertheless, the lipid composition of the tear film has been extensively studied (6–8). The high sensitivity of the mass spectrometry (MS) in analyzing the low sample volumes makes it a preferred approach in the biomedical research to decipher the fine changes of the lipid metabolism in the ocular and nonocular disorders, for example, Meibomian gland dysfunction, dry eye syndrome (9), and multiple sclerosis (10).
There are two techniques used for the excimer laser refractive correction procedures: surface or stromal ablation. The shallow cornea disruption in the surface ablation procedures such as photorefractive keratectomy (PRK), laser epithelial keratomileusis (LASEK), and epithelial laser-assisted in situ keratomileusis (Epi-LASIK) results in a lower incidence of the surgery-induced dry eye and provides more stability for the thinner cornea, implicating the better biomechanical outcomes. However, greater discomfort caused by the wound response and delayed vision recovery would still be the major problems for the surface ablation technique (11, 12). In contrast, the stromal ablation surgeries such as LASIK, femtosecond LASIK (FS-LASIK), and sub-Bowman's keratomileusis (SBK) have the advantages of essentially immediate vision correction, quick recovery, and very little to no discomfort (13). These three types of the refractive surgery use the corneal flap creation procedure to maintain the integrity of the corneal structures such as the Bowman's layer and the epithelium (14). However, the risk of post-LASIK keratectasia was elevated for the patients with moderate to high myopia due to the thicker flap (110–160 μm) (15, 16). SBK was developed from LASIK by using a mechanical microkeratome to create a thinner corneal flap (90–110 μm) and more planar in shape compared with the conventional LASIK approach (17). This approach is an evolutive procedure that increased the biomechanical stability of LASIK and reduced the pain experience of PRK (18, 19). The variation of the flap thicknesses and flap diameters by using a mechanical microkeratome in LASIK and SBK approaches was still a problem, despite their safeness and effectiveness. Corneal flap creation has become a more predictable and safe procedure with the introduction of FS-LASIK (20, 21).
Dry eye is a common symptom after LASIK surgery. It is believed that postsurgical development of the dry eye is closely related to the surgical cut of the corneal nerve fibers during the flap creation (22) and associated with the degree of preoperative myopia and the depth of laser treatment (23). The loss of the corneal innervation could affect the lacrimal function unit (LFU) (24), corneal blinking, and blinking of the Meibomian gland reflexes, resulting in the decreased aqueous and lipid tear secretion and mucin expression (25). Patel et al. showed that the tear lipid layer became thinner after LASIK (26). In general, patients receiving FS-LASIK surgery demonstrated the stable tear film compared with the mechanical microkeratome group (27, 28).
Although the tear lipids have been widely studied, a comprehensive lipidomics study examining the tear lipid profiles before and after the refractive surgery is still lacking. In this study, a technique specifically designed for the global lipidomics, MS/MSALL, was used to assess the tear lipidome prior to and after the refractive surgery (29–31). This technique collects all the precursor ions in the Q1 quadrupole and the collision-induced dissociation is carried out in Q2 quadrupole while collecting all the high-resolution MS/MS spectra at a high speed (29, 30). MS/MSALL is highly reproducible, bias free, and requires no method development (32). Given the important role of TFLL in maintaining the proper ocular function and easy accessibility of the tear samples, the tear lipid compositions of the patients taken FS-LASIK, LASIK, or SBK surgery preoperatively (week 0) and postoperatively (week 1, week 4, and week 52) were investigated by using MS/MSALL in this study.
Methods and Materials
Sample Collection
The study was approved by the Tianjin Medical University Institutional Review Board and was conducted according to the Declaration of Helsinki. The signed consent forms were obtained from the participating volunteers. The criteria for this study include: (1) the age of the participants should be over 18 years old; (2) a stable refractive error in the last 1 year; (3) soft contact lenses had not been worn for more than 1 week; (4) rigid contact lenses had not been worn for more than 2 weeks; (5) no history of eye disease or eye surgery; (6) no systemic connective tissue disease or autoimmune disease; (7) no other systemic diseases (such as diabetes, seborrheic dermatitis, or hyperlipidemia); (8) postoperative corneal stromal bed thickness was >250 μm; and (9) no breastfeeding or pregnancy. All the patients were explained about the advantages, disadvantages, and the risk of the three types of surgeries. The type of surgery to be carried out in each patient was based on the preference of the patient. Clinical examinations for the patients receiving FS-LASIK (n = 10), LASIK (n = 10), or SBK (n = 10) surgery included Schirmer test (without anesthesia), tear breakup time (TBUT), and corneal fluorescein staining. Corneal staining was graded from 0 to 5 according to the Oxford schema (33). Tear samples were collected by using the Schirmer strips from both the eyes of the patients and the strips were stored at −80°C until further analysis. Postregime for all the patients is the same. Topical medications after surgery consisted of fluorometholone eye drops four times daily for 1 week and tapered over 4 weeks, levofloxacin eye drops three times per day for 3 days, and artificial tears four times daily for 1–3 months depending on the severity of the postsurgical dry eye symptoms.
A metal spatula was used to collect the expressed meibum, which was generated by gentle squeezing the eyelids of the volunteers. Meibum lipids were eluted by washing the spatula thoroughly with chloroform and the lipid extracts were subjected to dry by using a miVac sample concentrator (Genevac, Ipswich, UK). The dried samples were stored at −80°C until further analysis.
Lipid Extraction
The protocol for the lipid extraction was adopted from the previous work with some modifications (34). The first 15 mm of Schirmer strips were cut into the fine pieces (~2 mm) in the glass tubes. About 200 μL of methanol containing 50 μg/ml butylated hydroxytoluene (BHT) and 25 ng/ml myristic-d27 acid were added to the glass tube followed by 600 μL methyl tert-butyl ester (MTBE). The mixture was then incubated at 20°C for 30 min with a mixing speed of 900 rpm. About 180 μL of water was added for the phase separation. After thoroughly mixing the sample, the suspension is centrifuged for 10 min at 10°C with a speed of 2,000 g. The upper phase containing lipid was then transferred into a collection vial and dried down.
Direct Injection MS/MSALL Data Acquisition
Lipid extract was reconstituted in 100 μL methanol/chloroform (2:1, v/v) with 5 mM ammonium acetate and the sample was automatically loaded and directly delivered to the electrospray ionization (ESI) source by using the ACQUITY UPLC I-Class System (Waters Corporation, Milford, Massachusetts, USA). The running buffer was methanol/isopropanol (3:1, v/v) with 5 mM ammonium acetate and the flow rate was 30 μL/min. The MS/MSALL acquisition experiment was carried out on the SCIEX TripleTOF 5600 System (SCIEX, Framingham, Massachusetts, USA) in both the positive and negative polarities for the complete lipidome coverage. The parameter settings for ESI source included nebulizing gases (GS1) at 25, heating gases (GS2) at 10, curtain gas (CUR) at 20, temperature at 250°C, and ion spray voltage floating at 5,500 V for positive ionization and −4,500 V for negative ionization, respectively. The atmospheric pressure chemical ionization (APCI) probe and inlet were connected to an external calibrant delivery system (CDS) delivering the mass calibration solution for MS and MS/MS. The Analyst® TF 1.7 software (SCIEX, Framingham, Massachusetts, USA) was used to acquire the data from MS/MSALL. The mass range for time-of-flight MS (TOFMS) was from 200 to 1,200 m/z and the accumulation time was 300 ms, followed by 1,000 MS/MS spectra from 200.050 to 1,200.049 m/z in 1 Da steps. The accumulation time for the product ion scan was 100 ms and the collision energy was set to 50 ± 30 eV for positive polarity and −45 ± 30 eV for negative polarity, respectively. The total run time for one MS/MSALL acquisition was <2 min.
Raw Data Processing
The lipid identification and quantitation were performed by using the LipidView™ software 1.2 (SCIEX, Framingham, Massachusetts, USA) with a built-in library containing glycerolipids, phospholipids, sphingolipids, sterol lipids, and fatty acyls. A targeted search list for the wax esters was also included. A background subtraction by using the Schirmer strip and solvent was applied to the sample.
Statistical Analysis
The results are expressed as mean ± SD. Clinical characteristics were compared among LASIK, FS-LASIK, and SBK participants by the chi-squared test, a one-way ANOVA, or the Kruskal–Wallis one-way ANOVA as appropriate. The analysis of the lipids over the course of time was conducted by using the ANOVA by R programming (35). The principal component analysis (PCA) and partial least squares-discriminant analysis (PLS-DA) were carried out by the MetaboAnalyst 4.0 (Xia Lab@McGill, Quebec, Canada) (36) and the SIMCA 13.0.3 (Umetrics, Sweden, UK).
Results
Clinical Characteristics of the Patients
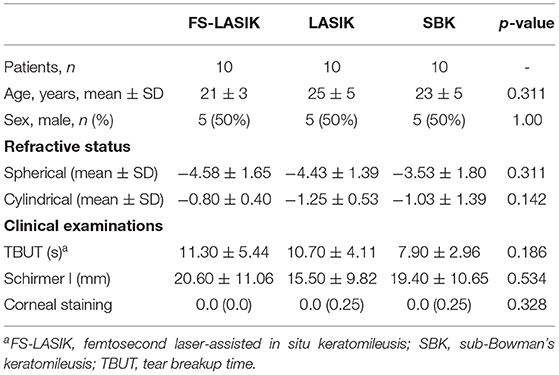
Table 1 shows the characteristics of the recruited patients in this study. There is no significant difference in age, gender, spherical, cylindrical, TBUT, Schirmer test, or corneal staining results among LASIK, FS-LASIK and SBK group prior to the refractive surgery. The assessment of TBUT, Schirmer test, and corneal staining for the patients receiving FS-LASIK, LASIK, or SBK surgery in week 0, week 1, week 4, and week 52 was shown in Figure 1. A significant difference in the corneal staining was noted in the FS-LASIK group over time. There was also a significant difference in the Schirmer test for the SBK group.
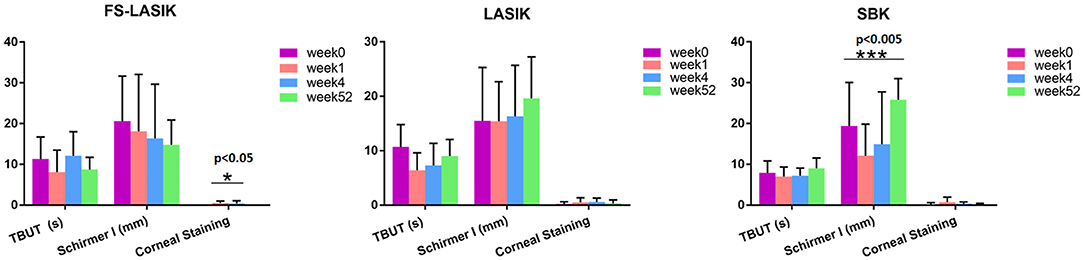
Figure 1. Clinical examination of the patients receiving femtosecond laser-assisted in situ keratomileusis (FS-LASIK), LASIK, and sub-Bowman's keratomileusis (SBK) surgery in week 0, week 1, week 4, and week 52, respectively. *p < 0.05, **p < 0.01, ***p < 0.005.
Lipid Detection by Using MS/MSALL
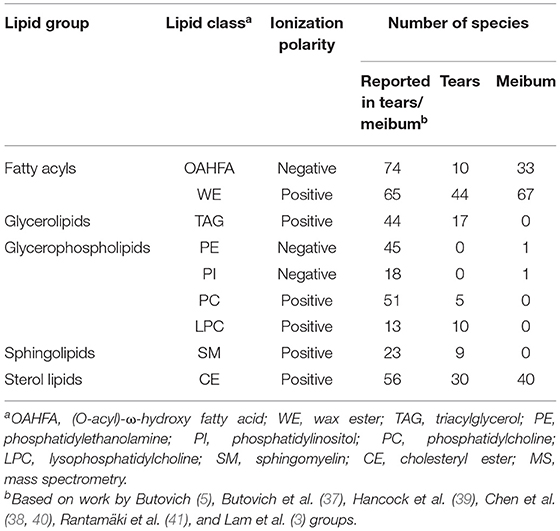
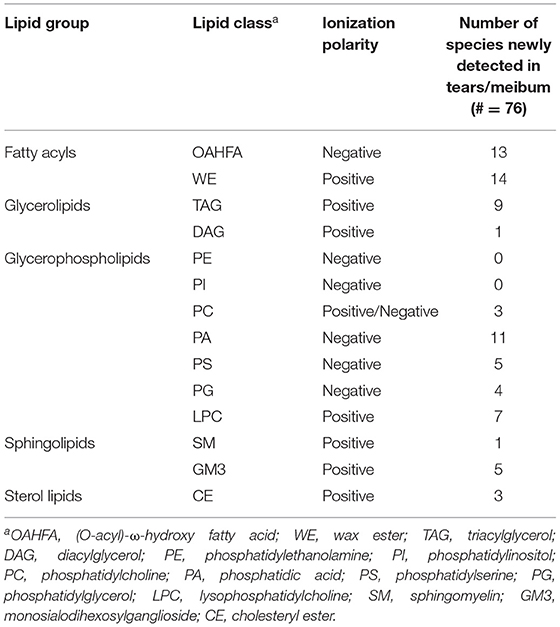
To demonstrate the reasonable coverage of the lipid species detected by this ultra-fast MS/MSALL method, several normal human tears and the meibum samples were evaluated. In this study, a list of the lipid species detected in the tears or meibum by using MS/MSALL technique was compared with the previous publications (3, 5, 37–41) and the result was shown in Table 2 indicating that this technique is capable of detecting the major lipid classes. We also found 76 new lipid species by using MS/MSALL method including (O-acyl)-ω-hydroxy fatty acids (OAHFAs), wax ester (WE), triacylglycerol (TAG), diacylglycerol (DAG), phosphatidylcholine (PC), phosphatidic acid (PA), phosphatidylserine (PS), phosphatidylglycerol (PG), lysophosphatidylcholine (LPC), sphingomyelin (SM), monosialodihexosylganglioside (GM3), and cholesteryl ester (CE) (Table 3). In total, around 300 lipid species that are present in ≥75% of the samples were detected in this study.
Multivariate Analysis of the Lipidomic Profile
Multivariate analysis including PCA and PLS-DA was applied in this study to examine the pattern of the lipidomic profiles over time (week 0, week 1, week 4, and week 52). The tight cluster of the quality control (QC) samples in PCA score plot indicated the robustness of our direct injection MS/MSALL data acquisition platform (Supplementary Figure 1). An overview of the lipidomic profiles preoperatively and postoperatively from the FS-LASIK, LASIK, and SBK groups was shown in Figure 2 by using the PLS-DA score plots. There was ample overlap among week 0, week 1, and week 4 in the FS-LASIK group, implicating there was no clear difference among these three time points. In contrast, the lipidomic profiles of the LASIK and SBK groups in week 1 and 4 were distinctly separated compared to week 0. Interestingly, an overlap between week 0 and 52 was observed in the SBK group. On the other hand, the LASIK group showed a larger difference compared to the FS-LASIK group between week 0 and 52. In addition, the first two components of the PLS-DA model for the three types of surgeries can only explain ~20% of covariance among the different time points. Individual variations and small sample size might be one reason for this covariance. The above findings revealed that in week 52, the SBK group demonstrated a similar lipidome profile compared to week 0, while the FS-LASIK and LASIK groups shifted away from week 0.
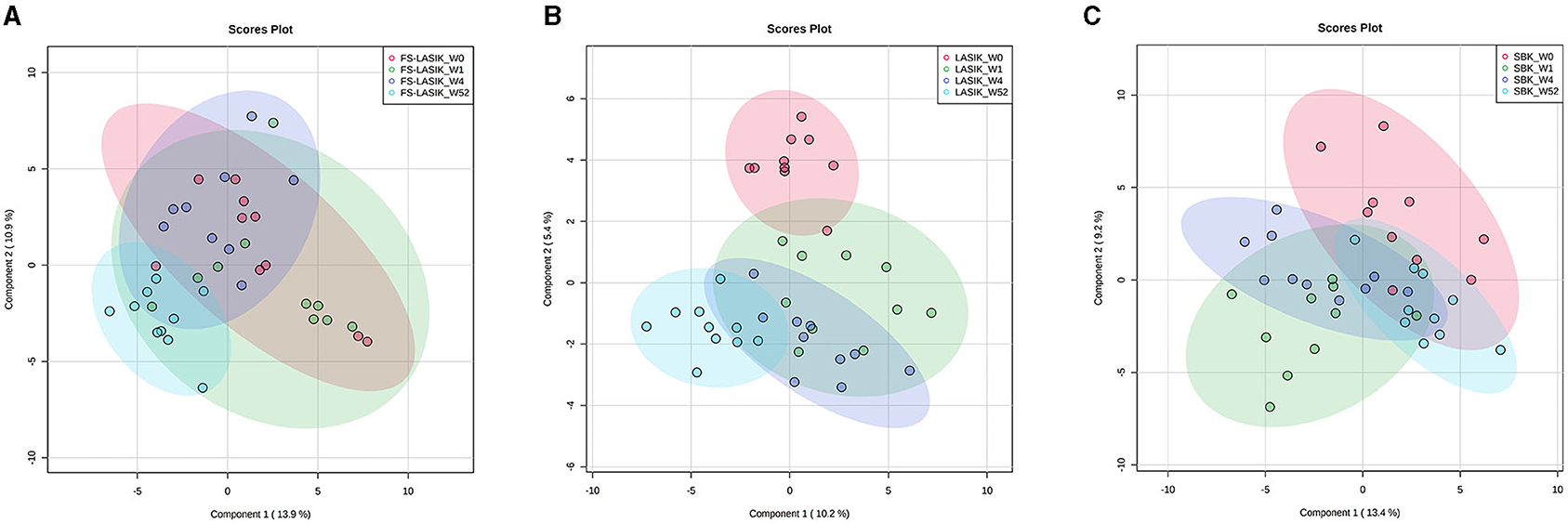
Figure 2. The partial least squares-discriminant analysis (PLS-DA) score plots showing the lipid profiles in week 0, week 1, week 4, and week 52 from the FS-LASIK, LASIK, and SBK groups, respectively. (A) FS-LASIK; (B) LASIK; (C) SBK. Red circle: week 0; Green circle: week 1; Purple circle: week 4; and Blue circle: week 52.
The loading plot of the PLS-DA model is complementary to the score plot and summarizes how the lipids relate to each subgroup. By examining the corresponding loading plot of the PLS-DA model (Supplementary Figure 2), TAG 59:3 is closely associated with the FS-LASIK and LASIK groups in week 52. In addition, GD3 27:4, a disialoganglioside with the three glycosyl groups, is closely associated with the FS-LASIK and SBK groups in week 1. Interestingly, this lipid is associated with the lipidomics profile of the LASIK group in week 4. The levels of these two lipids in the FS-LASIK, LASIK, and SBK groups preoperatively and postoperatively were shown in Figure 3.
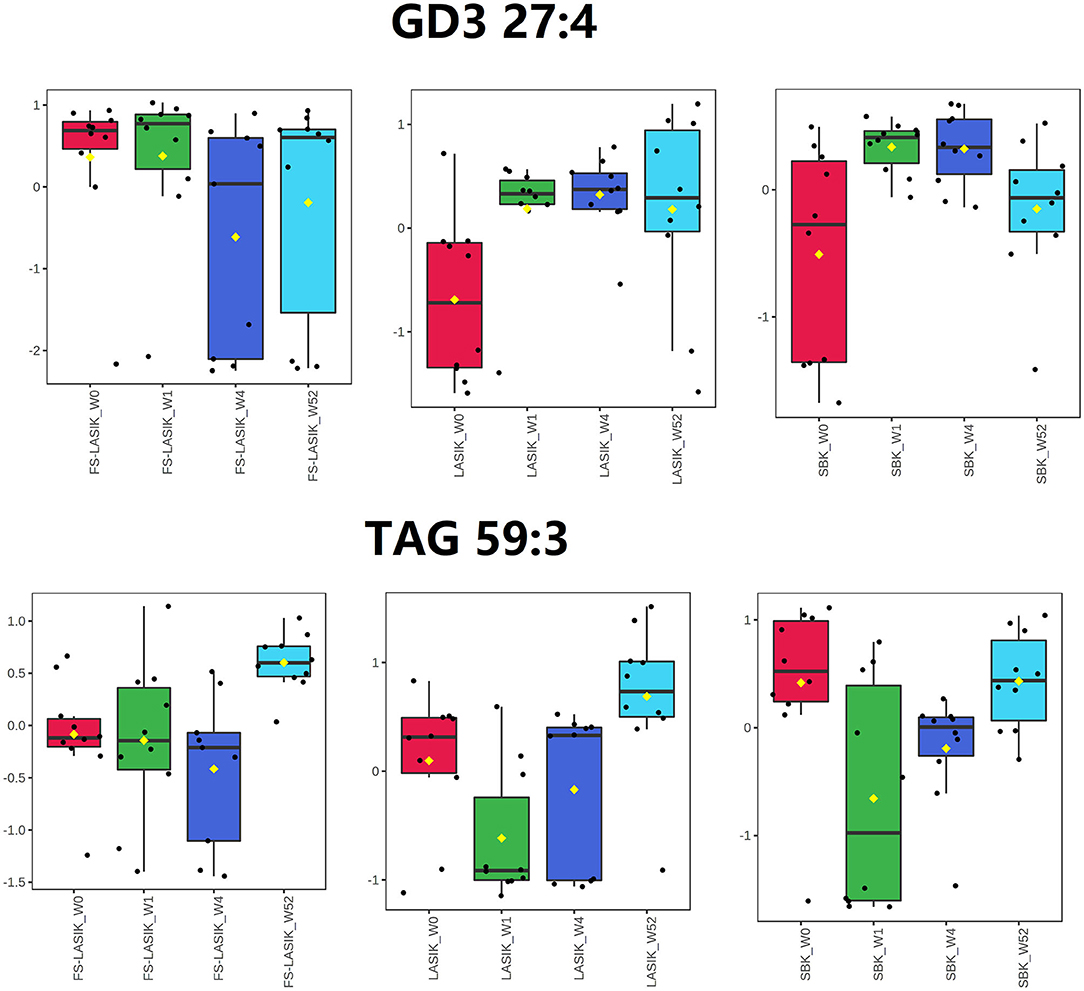
Figure 3. Box and whisker plots showing the intensity changes over time for GD3 27:4 and triacylglycerol (TAG) 59:3.
Comparison of the Lipid Classes and Lipid Species Among the Refractive Surgeries
The levels of the major lipid classes found in the FS-LASIK, LASIK, and SBK groups in week 0, week 1, week 4, and week 52 were shown in Figure 4. In this study, the major lipid classes include OAHFAs, non-polar lipid, phospholipid, sphingolipid, and lysophospholipid. No statistical significance was found in the overall lipid classes from the FS-LASIK group. The LASIK group showed significant alteration in the phospholipid and sphingolipid over time, while the SBK group demonstrated a significant difference in the OAHFA and phospholipid.
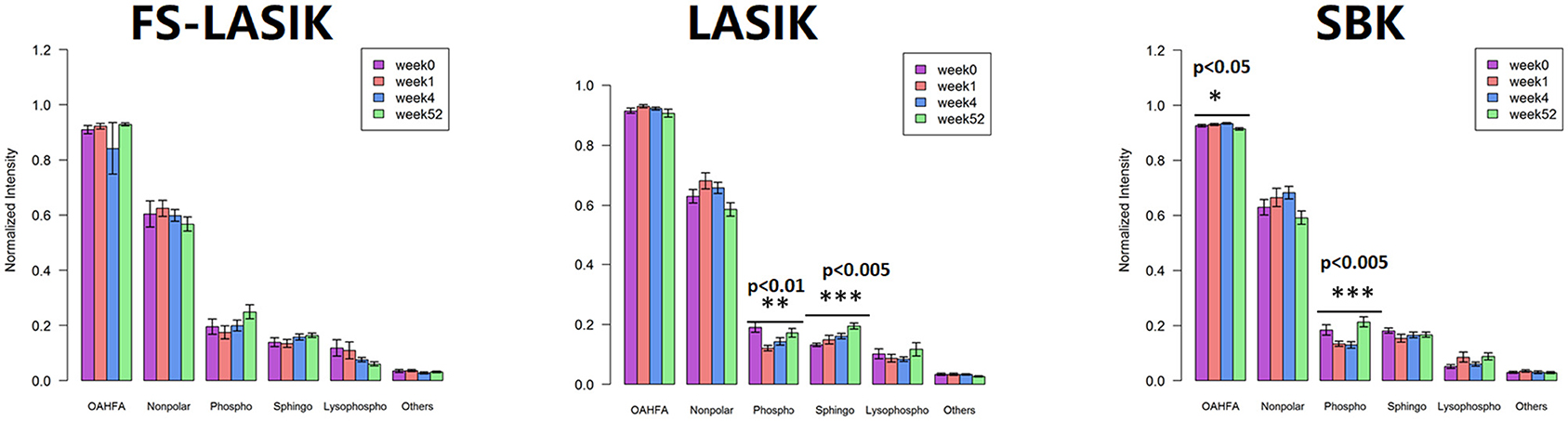
Figure 4. Overall tear lipid classes at week 0, week 1, week 4, and week 52 from the FS-LASIK, LASIK, and SBK groups. *p < 0.05, **p < 0.01, ***p < 0.005.
Individual lipid species belonging to non-polar lipid, phospholipid, and lysophospholipid in week 0, week 1, week 4, and week 52 were also examined in this study and the quantitative comparison from the FS-LASIK, LASIK, and SBK groups was shown in Figure 5. The levels of DAG in the FS-LASIK and WE in the SBK groups were significantly changed over time (Figures 5A,C). Most of the difference in the phospholipids was observed in PC from the LASIK and SBK groups with statistical significance. No significant change was detected in lysophospholipid.
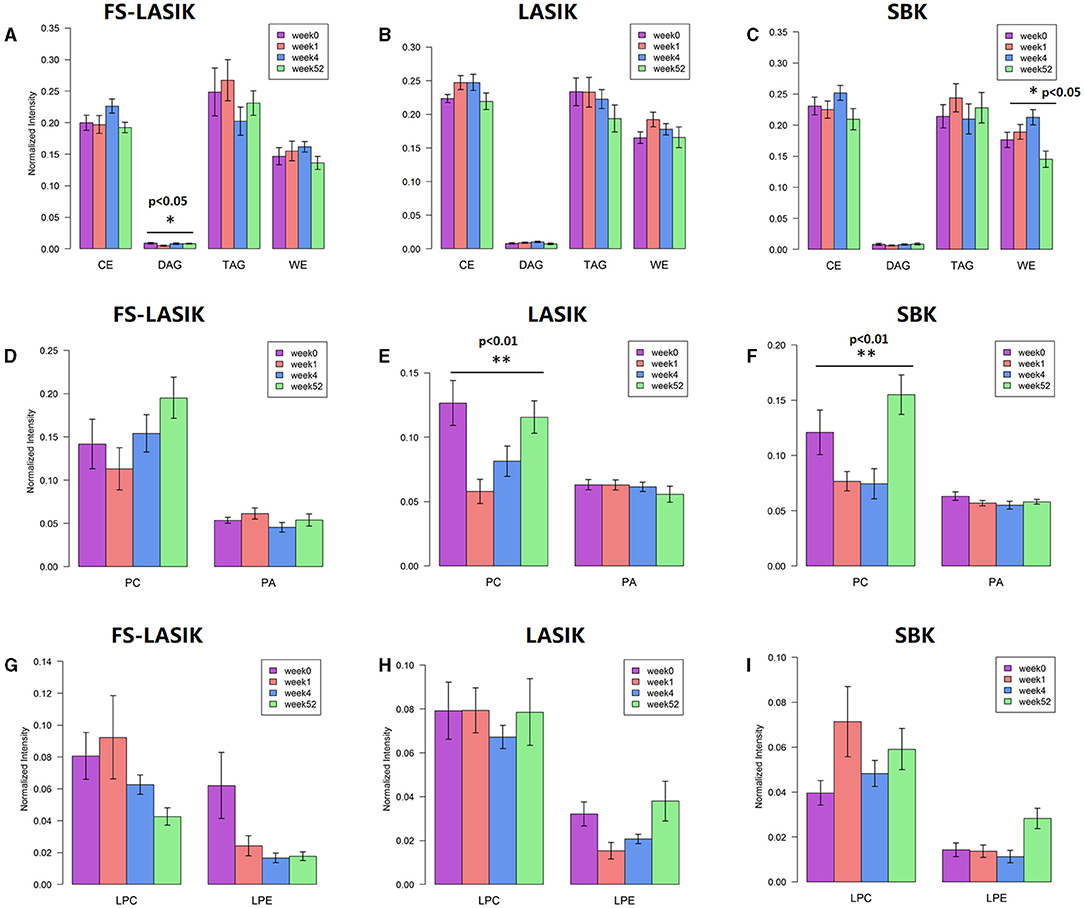
Figure 5. Individual lipid classes found in the FS-LASIK, LASIK, and SBK groups at week 0, week 1, week 4, and week 52, respectively. (A–C) Non-polar lipid; (D–F) phospholipid; (G–I) lysophospholipid. *p < 0.05, **p < 0.01, ***p < 0.005.
Discussion
Tear samples have gained popularity in the investigation of disease pathogenesis (1), progression (7), and treatment response (42) due to its quick and non-invasive collection. The content of tear is a dynamic reflection of the ocular surface and, therefore, the lipidomic profiling analysis of the tear could provide information of the physiological, nutritional, and health status of an individual. A high-resolution MS/MSALL shotgun lipidomics analysis was applied here to investigate the tear samples from the patients receiving LASIK, FS-LASIK, or SBK surgery preoperatively and postoperatively.
In this study, direct injection-based MS shotgun lipidomics combined with liquid pump and autosampler from liquid chromatography (LC) with MS to perform the rapid lipidomic profiling. Comprehensive profiling and quantitation of lipid species could be achieved by this approach without the front-end chromatography separation (43). It captured every precursor ion by high-resolution MS/MS without missing any information. Therefore, MS/MSALL could obtain quantification information with no method development required for all the species in a single analysis. The use of a fully automated sampler and short run time (around 2 min for each sample) makes high-throughput sample analysis applicable for future clinical applications. Lastly, this approach allows for the detection of most lipid species reported in the literature and some new lipid species, implicating the feasibility of the direct injection based-MS shotgun lipidomics.
Dry eye after the stromal ablation surgeries is closely related to the corneal denervation. The stromal and sub-basal nerves are both severed during the flap creation, except those located at the flap hinge. As a consequence of the severed nerves, a reduction in the tear film stability and dry eye symptoms may occur (44). In this study, we only detected the significant changes in the corneal staining that results in the FS-LASIK group and the Schirmer test in the SBK group. Dry eye after the laser corneal refractive surgery is considered the most common complication with clinical signs such as positive vital staining of the ocular surface, decreased TBUT and Schirmer test value, reduced corneal sensitivity, and decreased functional visual acuity (45). However, previous literature is inconsistent with respect to the tear film stability after the refractive surgery. Some have reported that TBUT and Schirmer test value were diminished in both the microkeratome and femtosecond laser-created flaps (46–49), while others found no significant changes in TBUT and Schirmer test or noted a slight but insignificant increase in TBUT (50–53). There is also a discrepancy in the corneal staining that results after the refractive surgery. Some research groups observed the elevated corneal staining after 1 week of the refractive surgery and it recovered to the baseline levels after 1 month (54, 55). In contrast, Bower et al. reported significantly higher cornea staining for up to 12 months (56). In this study, corneal staining was elevated in week 1 and almost returned to the preoperative levels in week 52 among all the three types of refractive surgery. The sub-basal nerves left in the flap will undergo a degenerative process other than a sudden vanishing after surgery (57, 58). Furthermore, Wilson suggested that the punctate epithelial erosions after surgery may be attributed to the neurotrophic epitheliopathy (50). The creation of the flap by using the microkeratome was irregular and thick compared with the femtosecond laser (59) and, thus, more sub-basal nerves were disrupted and undergoing regeneration after the microkeratome application. The total number of the sub-basal nerve was reported to be negatively correlated with corneal staining (60). It could be a possible reason for the significant changes of the corneal staining overtime in the FS-LASIK group. Another factor to be considered for this observation is the difference in suction time. A femtosecond laser had a longer suction time (~56 s) compared to laser (~40 s) and microkeratome (~20 s) (61). Therefore, the cells of the ocular surface including conjunctival goblet cells may have an increased risk of damage in the FS-LASIK group. In addition, the energy attenuation property of the femtosecond laser during the flap creation process may result in the incomplete dissociation of the corneal flap margin. Lastly, small sample size and individual variation may also affect the clinical observations in this study.
The SBK group showed higher TBUT and Schirmer test value in week 52 compared with preoperation, while the FS-LASIK and LASIK groups had lower TBUT in week 52, implicating the recovery of the SBK group in week 52. This finding is consistent with the PLS-DA score plot results, which demonstrated a similar lipidome profile in week 52 and 0 for the SBK group. The accordance between the clinical examination and lipidomics results indicated that the occurrence and development of dry eye after the refractive surgery are closely related to the lipidome alteration. The creation of the corneal flap is the most critical element in LASIK surgery. FS-LASIK performed the corneal flap creation by using the femtosecond laser, while LASIK and SBK used a mechanical microkeratome. SBK can create a thinner corneal flap compared to LASIK and, hence, increase the available residual stromal bed, preserve corneal tissue, and reduce stromal nerve damage (62, 63). It has been reported that the femtosecond laser could directly trigger the apoptosis of the keratocytes and the corneal flap must be separated bluntly after the application of the femtosecond laser (64). This additional mechanical flap dissection might induce an extra injury for the corneal nerve or tissue cell and, thus, affect the recovery rate. The different patterns of lipidome among the three types of surgeries might also be due to the various tissue reactions caused by the laser or microkeratome application. Zhang et al. found that the corneal sub-basal nerve fibers repairing in the SBK group were faster compared to the FS-LASIK group (17). In addition, the anatomy results of the corneal nerve showed that SBK surgery could conserve more nerve branches compared to FS-LASIK surgery. This study indicates that the thinner corneal flap creation might accelerate the recovery of the lipidomic profile to preoperation after 1 year compared to the lipidome of the SBK group to the FS-LASIK and LASIK groups in week 0 and 52, respectively (Figure 2).
In this study, GD3 27:4 was highly expressed in week 1 compared to week 0 in the LASIK and SBK groups, while the difference between week 0 and 1 in the FS-LASIK group was not obvious. This finding was in accordance with the PLS-DA score plot results that showing the different patterns between week 0 and 1 in the FS-LASIK, LASIK, and SBK groups, respectively. Gangliosides are a family of acidic glycosphingolipids and GD3 is a minor ganglioside in most normal tissue. Gangliosides might play a role in the biological processes related to the retinal physiology and vision disorders involving the loss of photoreceptors or pathological retinal neovascularization (65). The expression of GD3 ganglioside increases during the development and in pathological conditions (66). The 3G5 antigen, a ganglioside, is reported to be a useful marker for the identification of the corneal keratocytes and for documenting their response to stress associated with the wound healing (67). A previous study reported that FS-LASIK and SBK showed a little more severe keratocyte reaction compared to LASIK after 1–3 months of surgery due to the thinner corneal flap creation (17). The above observations indicate that the changes of GD3 27:4 levels might be associated with the wound healing after the refractive surgery.
An increased level of TAG 59:3 in week 52 was observed in the FS-LASIK and LASIK group compared to week 0, while there was no obvious change in the SBK group. Similarly, a clear separation between week 0 and 52 in the FS-LASIK and LASIK groups was observed, while the lipidomic profile of the SBK group in week 0 and 52 overlapped as shown in the PLS-DA score plot. No association was found between TAG 59:3 and the lipidomic profile of the SBK group in week 52. Chen et al. reported the upregulated TAGs in the meibum of the patients with dry eyes (68). Higher TAG has been demonstrated to be related to the corneal nerve damage in the patients with idiopathic small fiber neuropathy (69). In addition, it has been reported that the recovery rate of the corneal sub-basal nerve fibers in the SBK group was faster compared to the FS-LASIK and LASIK groups (17). The higher levels of TAG 59:3 observed at week 52 in the FS-LASIK and LASIK groups suggest that TAG 59:3 might play a negative role in the corneal sub-basal nerve fiber repairing.
(O-acyl)-ω-hydroxy fatty acid, an amphiphilic component in tears, plays a role in orienting the molecules at the lipid/water interface and facilitating the interaction between the polar and non-polar components of the tears to maintain the tear film stability (70). Lam et al. reported that the level of OAHFA was positively correlated with TBUT, reductions in ocular evaporation rate, and degree of ocular discomfort in the patients with dry eyes (3, 42). In this study, an obvious decrease of OAHFA intensity in week 52 was observed, while the intensity at week 0, week 1, and week 4 did not change much (Figure 4C). However, an increase of TBUT in week 52 in the SBK groups was detected (Table 2) showing a discrepancy with the previous studies (3, 42). The inconsistency between this study and Lam study might be due to the different cohorts of the patients. Their findings were based on a cohort of the patients with Meibomian gland dysfunction, while this study recruited only the patients with myopia.
The non-polar lipids, including CE, DAG, TAG, and WE, reside on the surface of an aqueous film and, thus, prevent the excessive evaporation of the aqueous component in the tear film. The deficiencies of the non-polar lipids may play a critical role in the evaporative dry eye (71). We did not detect any significant changes of the overall non-polar lipids preoperatively and postoperatively in the three types of refractive surgery (Figure 4). Only a significant alteration of DAG in the FS-LASIK group (Figure 5A) and WE in the SBK group (Figure 5C) was observed when examining the lipid species of an individual within the non-polar lipids. WEs are a major component of TFLL and recent studies have shown that WE film can effectively retard the evaporation of water (72, 73). Lam et al. reported a positive correlation between high molecular mass of the WEs with unsaturated FA chains and corneal staining, implying that the alteration of WEs level in the patients with dry eye syndrome was dependent on their molecular masses and fatty acyl chain saturation (9). However, different patterns were observed between the expression levels of WE and TBUT and Schirmer test in the SBK group. The assessment of overall levels of WE despite the fatty acyl saturations and molecular mass in this study might be a possible reason for this observation. Therefore, further evidence is required to determine the role of WE prior to and after the refractive surgery.
Phospholipids, accounting for 5–20 mol% of all the lipids in tears (74), play an essential role in the surface-active behavior of the meibum-like lipid compositions (75) and, thus, maintain the function of TFLL. This class of lipid could act as an interface between the Meibomian oil and the aqueous layer, since it lies anterior to the aqueous components. The presence of this polar phospholipid interface is critical to the spreading of the non-polar lipid film over the aqueous layer (76). Lysophospholipids were released by the hydrolysis of the phospholipid and this process was catalyzed by phospholipase A2. We have detected the significant changes in the phospholipids in the LASIK and SBK groups. Peters et al. found that TBUT was improved by the presence of the phospholipids by using a model eye (77). The trends of TBUT in the LASIK and SBK groups over time are similar compared to the PCs, which is a major component of the phospholipid. Those facts implicated that PC is responsible for the alterations of the overall phospholipids in the LASIK and SBK groups.
Our study findings must be considered in light of their limitations. First, a relatively small sample size was assessed in this study. Most clinical examination characteristics preoperatively and postoperatively did not achieve the statistical significance, most likely due to the small sample size. Furthermore, the inclusion of several time points between week 4 and 52 would provide more information for the lipidomic profile changes over time.
In this study, a rapid direct injection shotgun lipidomics workflow (<2 min/sample) was developed to examine the human tear lipidome from the patients receiving LASIK, FS-LASIK, or SBK surgery preoperatively and postoperatively (week 0, week 1, week 4, and week 52). The PLS-DA score plots revealed that the lipidome of the SBK group in week 52 was similar compared to week 0, while the FS-LASIK and LASIK groups showed distinct separation between week 0 and 52. Two lipids, TAG 59:3 and GD3 27:4, were found to be associated with the pattern changes among the FS-LASIK, LASIK, and SBK groups. LASIK showed the greatest impact on the lipidome changes over time. SBK demonstrated minimal impact among the three types of the refractive surgery after 1 year of surgery. Those findings in this longitudinal study could potentially aid in the understanding of the impact of the refractive surgery on the stability of the tear film by examining the human tear lipidome.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Tianjin Medical University Eye Hospital; Tianjin Medical University Eye Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SZ and LZ designed the study and verify the underlying data. Preoperative patients were screened and surgeries were performed by SZ. YQ followed up the patients, collected tears from patients, and led the data collection. YG led the tear lipid layer analysis. YH and XL oversaw the research. YQ and YG wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation (NSFC #81970769, Beijing, China), the National Medical Research Council Centre (CG 2013 and CG 2017, Singapore), and the SingHealth Foundation (Singapore).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study is grateful for the experimental technical support provided by the Tianjin Medical University Eye Hospital and the Singapore Eye Research Institute.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.731462/full#supplementary-material
References
1. Zhou L, Beuerman RW. Tear analysis in ocular surface diseases. Prog Retin Eye Res. (2012) 31:527–50. doi: 10.1016/j.preteyeres.2012.06.002
2. Yazdani M, Elgstøen KBP, Rootwelt H, Shahdadfar A, Utheim ØA, Utheim TP. Tear metabolomics in dry eye disease: a review. Int J Mol Sci. (2019) 20:3755. doi: 10.3390/ijms20153755
3. Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. (2014) 55:289–98. doi: 10.1194/jlr.M044826
4. Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. (2020) 197:108115. doi: 10.1016/j.exer.2020.108115
5. Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. (2008) 49:3779–89. doi: 10.1167/iovs.08-1889
6. Cwiklik L. Tear film lipid layer: a molecular level view. Biochim Biophys Acta. (2016) 1858:2421–30. doi: 10.1016/j.bbamem.2016.02.020
7. Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A. ω-3 tear film lipids correlate with clinical measures of dry eye. Invest Ophthalmol Vis Sci. (2016) 57:2472–8. doi: 10.1167/iovs.16-19131
8. Sassa T, Tadaki M, Kiyonari H, Kihara A. Very long-chain tear film lipids produced by fatty acid elongase ELOVL1 prevent dry eye disease in mice. FASEB J. (2018) 32:2966–78. doi: 10.1096/fj.201700947R
9. Lam SM, Tong L, Reux B, Duan X, Petznick A, Yong SS, et al. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J Lipid Res. (2014) 55:299–306. doi: 10.1194/jlr.P041780
10. Cicalini I, Rossi C, Pieragostino D, Agnifili L, Mastropasqua L, Di Ioia M, et al. Integrated lipidomics and metabolomics analysis of tears in multiple sclerosis: an insight into diagnostic potential of lacrimal fluid. Int J Mol Sci. (2019) 20:1265. doi: 10.3390/ijms20061265
11. Wen D, McAlinden C, Flitcroft I, Tu R, Wang Q, Alió J, et al. Postoperative efficacy, predictability, safety, and visual quality of laser corneal refractive surgery: a network meta-analysis. Am J Ophthalmol. (2017) 178:65–78. doi: 10.1016/j.ajo.2017.03.013
12. Althomali TA. Comparison of microkeratome assisted sub-Bowman keratomileusis with photorefractive keratectomy. Saudi J Ophthalmol. (2017) 31:19–24. doi: 10.1016/j.sjopt.2017.01.004
13. Shortt AJ, Bunce C, Allan BDS. Evidence for superior efficacy and safety of LASIK over photorefractive keratectomy for correction of myopia. Ophthalmology. (2006) 113:1897–908. doi: 10.1016/j.ophtha.2006.08.013
14. Xu Z, Shen M, Hu L, Zhuang X, Peng M, Hu D, et al. The impact of flap creation methods for Sub-Bowman's Keratomileusis (SBK) on the central thickness of bowman's layer. PLoS ONE. (2015) 10:e0124996. doi: 10.1371/journal.pone.0124996
15. Tatar MG, Kantarci FA, Yildirim A, Uslu H, Colak HN, Goker H, et al. Risk factors in post-LASIK corneal ectasia. J Ophthalmol. (2014) 2014:204191. doi: 10.1155/2014/204191
16. Harissi-Dagher M, Frimmel SAF, Melki S. High myopia as a risk factor for post-LASIK ectasia: a case report. Digit J Ophthalmol. (2009) 15:9–13. doi: 10.5693/djo.01.2009.003
17. Zhang F, Deng S, Guo N, Wang M, Sun X. Confocal comparison of corneal nerve regeneration and keratocyte reaction between FS-LASIK, OUP-SBK, and conventional LASIK. Invest Ophthalmol Vis Sci. (2012) 53:5536–44. doi: 10.1167/iovs.11-8786
18. Durrie DS, Slade SG, Marshall J. Wavefront-guided excimer laser ablation using photorefractive keratectomy and sub-Bowman's keratomileusis: a contralateral eye study. J Refract Surg. (2008) 24:S77–84. doi: 10.3928/1081597X-20080101-14
19. Slade SG. Thin-flap laser-assisted in situ keratomileusis. Curr Opin Ophthalmol. (2008) 19:325–9. doi: 10.1097/ICU.0b013e328302cc77
20. Vaddavalli PK, Yoo SH. Femtosecond laser in-situ keratomileusis flap configurations. Curr Opin Ophthalmol. (2011) 22:245–50. doi: 10.1097/ICU.0b013e3283479ebd
21. Yuen LH, Chan WK, Koh J, Mehta JS, Tan DT. A 10-year prospective audit of LASIK outcomes for myopia in 37,932 eyes at a single institution in Asia. Ophthalmology. (2010) 117:1236–44. doi: 10.1016/j.ophtha.2009.10.042
23. De Paiva CS, Chen Z, Koch DD, Hamill MB, Manuel FK, Hassan SS, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. (2006) 141:438–45. doi: 10.1016/j.ajo.2005.10.006
24. Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. (2017) 15:438–510. doi: 10.1016/j.jtos.2017.05.011
25. Yu C, Li Y, Wang Z, Jiang Y, Jin Y. Comparison of corneal nerve regeneration and dry eye condition after conventional LASIK and femtosecond-assisted LASIK. Zhonghua Yan Ke Za Zhi. (2015) 51:188–92. doi: 10.3760/cma.j.issn.0412-4081.2015.03.008
26. Patel S, Perez-Santonja JJ, Alio JL, Murphy PJ. Corneal sensitivity and some properties of the tear film after laser in situ keratomileusis. J Refract Surg. (2001) 17:17–24. doi: 10.3928/1081-597X-20010101-02
27. Xia L-K, Yu J, Chai G-R, Wang D, Li Y. Comparison of the femtosecond laser and mechanical microkeratome for flap cutting in LASIK. Int J Ophthalmol. (2015) 8:784–90. doi: 10.3980/j.issn.2222-3959.2015.04.25
28. Sun C-C, Chang C-K, Ma DH-K, Lin Y-F, Chen K-J, Sun M-H, et al. Dry eye after LASIK with a femtosecond laser or a mechanical microkeratome. Optom Vis Sci. (2013) 90:1048–56. doi: 10.1097/OPX.0b013e31829d9905
29. Simons B, Kauhanen D, Sylvänne T, Tarasov K, Duchoslav E, Ekroos K. Shotgun lipidomics by sequential precursor ion fragmentation on a hybrid quadrupole time-of-flight mass spectrometer. Metabolites. (2012) 2:195–213. doi: 10.3390/metabo2010195
30. Rockwell HE, Gao F, Chen EY, McDaniel J, Sarangarajan R, Narain NR, et al. Dynamic assessment of functional lipidomic analysis in human urine. Lipids. (2016) 51:875–86. doi: 10.1007/s11745-016-4142-0
31. Gao F, McDaniel J, Chen EY, Rockwell H, Lynes MD, Tseng YH, et al. Monoacylglycerol analysis using MS/MSALL quadruple time of flight mass spectrometry. Metabolites. (2016) 6:25. doi: 10.3390/metabo6030025
32. Gao F, McDaniel J, Chen EY, Rockwell HE, Nguyen C, Lynes MD, et al. Adapted MS/MSALL shotgun lipidomics approach for analysis of cardiolipin molecular species. Lipids. (2018) 53:133–42. doi: 10.1002/lipd.12004
33. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. (2003) 22:640–50. doi: 10.1097/00003226-200310000-00008
34. Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl- tert -butyl ether for high-throughput lipidomics. J Lipid Res. (2008) 49:1137–46. doi: 10.1194/jlr.D700041-JLR200
35. R Core Team. A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2018). Available online at: https://www.r-project.org (accessed September 26, 2021).
36. Chong J, Soufan O, Caraus I, Xia J, Li C, Wishart DS, et al. MetaboAnalyst 40: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. (2018) 46:W486–94. doi: 10.1093/nar/gky310
37. Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. (2007) 42:765–76. doi: 10.1007/s11745-007-3080-2
38. Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci. (2010) 51:6220–31. doi: 10.1167/iovs.10-5687
39. Hancock SE, Ailuri R, Marshall DL, Brown SHJ, Saville JT, Narreddula VR, et al. Mass spectrometry-directed structure elucidation and total synthesis of ultra-long chain (O-acyl)-ω-hydroxy fatty acids. J Lipid Res. (2018) 59:1510–8. doi: 10.1194/jlr.M086702
40. Chen J, Green KB, Nichols KK. Quantitative profiling of major neutral lipid classes in human meibum by direct infusion electrospray ionization mass spectrometry. Investi Ophthalmol Vis Sci. (2013) 54:5730–53. doi: 10.1167/iovs.12-10317
41. Rantamäki AH, Seppänen-Laakso T, Oresic M, Jauhiainen M, Holopainen JM. Human tear fluid lipidome: from composition to function. PLoS ONE. (2011) 6:1–7. doi: 10.1371/journal.pone.0019553
42. Lam SM, Tong L, Duan X, Acharya UR, Tan JH, Petznick A, et al. Longitudinal changes in tear fluid lipidome brought about by eyelid-warming treatment in a cohort of meibomian gland dysfunction. J Lipid Res. (2014) 55:1959–69. doi: 10.1194/jlr.P051185
43. Gao F, McDaniel J, Chen EY, Rockwell HE, Drolet J, Vishnudas VK, et al. Dynamic and temporal assessment of human dried blood spot MS/MSALL shotgun lipidomics analysis. Nutr Metab. (2017) 14:1–12. doi: 10.1186/s12986-017-0182-6
44. Ang RT, Dartt DA, Tsubota K. Dry eye after refractive surgery. Curr Opin Ophthalmol. (2001) 12:318–22. doi: 10.1097/00055735-200108000-00013
45. Toda I. Dry eye after LASIK. Invest Ophthalmol Vis Sci. (2018) 59:DES109–115. doi: 10.1167/iovs.17-23538
46. Kalyvianaki MI, Katsanevaki VJ, Kavroulaki DS, Kounis GA, Detorakis ET, Pallikaris IG. Comparison of corneal sensitivity and tear function following Epi-LASIK or laser in situ keratomileusis for myopia. Am J Ophthalmol. (2006) 142:669–71. doi: 10.1016/j.ajo.2006.04.054
47. Barequet IS, Hirsh A, Levinger S. Effect of thin femtosecond LASIK flaps on corneal sensitivity and tear function. J Refract Surg. (2008) 24:897–902. doi: 10.3928/1081597X-20081101-08
48. Tanaka M, Takano Y, Dogru M, Toda I, Asano-Kato N, Komai-Hori Y, et al. Effect of preoperative tear function on early functional visual acuity after laser in situ keratomileusis. J Cataract Refract Surg. (2004) 30:2311–5. doi: 10.1016/j.jcrs.2004.02.086
49. Goto T, Zheng X, Klyce SD, Kataoka H, Uno T, Yamaguchi M, et al. Evaluation of the tear film stability after laser in situ keratomileusis using the tear film stability analysis system. Am J Ophthalmol. (2004) 137:116–20. doi: 10.1016/S0002-9394(03)00901-2
50. Wilson SE. Laser in situ keratomileusis–induced (presumed) neurotrophic epitheliopathy. Ophthalmology. (2001) 108:1082–7. doi: 10.1016/S0161-6420(01)00587-5
51. Donnenfeld ED, Solomon K, Perry HD, Doshi SJ, Ehrenhaus M, Solomon R, et al. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology. (2003) 110:1023–30. doi: 10.1016/S0161-6420(03)00100-3
52. Foo SK, Kaur S, Abd Manan F, Low AJ. The changes of tear status after conventional and wavefront-guided intraLASIK. Malaysian J Med Sci. (2011) 18:32–9. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3216215/
53. Petznick A, Chew A, Hall RC, Chan CML, Rosman M, Tan D, et al. Comparison of corneal sensitivity, tear function and corneal staining following laser in situ keratomileusis with two femtosecond laser platforms. Clin Ophthalmol. (2013) 7:591–8. doi: 10.2147/OPTH.S42266
54. Zhang C, Ding H, He M, Liu L, Liu L, Li G, et al. Comparison of early changes in ocular surface and inflammatory mediators between femtosecond lenticule extraction and small-incision lenticule extraction. PLoS ONE. (2016) 11:e0149503. doi: 10.1371/journal.pone.0149503
55. Mian SI, Shtein RM, Nelson A, Musch DC. Effect of hinge position on corneal sensation and dry eye after laser in situ keratomileusis using a femtosecond laser. J Cataract Refract Surg. (2007) 33:1190–4. doi: 10.1016/j.jcrs.2007.03.031
56. Bower KS, Sia RK, Ryan DS, Mines MJ, Dartt DA. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: manifestations, incidence, and predictive factors. J Cataract Refract Surg. (2015) 41:2624–34. doi: 10.1016/j.jcrs.2015.06.037
57. Linna TU, Vesaluoma MH, Pérez–Santonja JJ, Petroll WM, Alió JL, Tervo TMT. Effect of myopic LASIK on corneal sensitivity and morphology of subbasal nerves. Invest Ophthalmol Vis Sci. (2000) 41:393–7. Available online at: https://iovs.arvojournals.org/article.aspx?articleid=2199874
58. Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. (2010) 8:135–45. doi: 10.1016/S1542-0124(12)70224-0
59. Zhou Y, Zhang J, Tian L, Zhai C. Comparison of the Ziemer FEMTO LDV femtosecond laser and Moria M2 mechanical microkeratome. J Refract Surg. (2012) 28:189–94. doi: 10.3928/1081597X-20120208-01
60. Latifi G, Afshan AB, Beheshtnejad AH, Zarei-Ghanavati M, Mohammadi N, Ghaffari R, et al. Changes in corneal subbasal nerves after punctal occlusion in dry eye disease. Curr Eye Res. (2020) 46:777–83. doi: 10.1080/02713683.2020.1833349
61. Salomão MQ, Ambrósio Jr R, Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome vs. femtosecond laser. J Cataract Refract Surg. (2009) 35:1756–60. doi: 10.1016/j.jcrs.2009.05.032
62. Sun Y, Deng Y-P, Wang L, Huang Y-Z, Qiu L-M. Comparisons of morphologic characteristics between thin-flap LASIK and SBK. Int J Ophthalmol. (2012) 5:338–42. doi: 10.3980/j.issn.2222-3959.2012.03.17
63. Dawson DG, Grossniklaus HE, Edelhauser HF, McCarey BE. Biomechanical and wound healing characteristics of corneas after excimer laser keratorefractive surgery. J Refract Surg. (2008) 24:S90–6. doi: 10.3928/1081597X-20080101-16
64. Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. (2002) 43:3660–4. Available online at: https://iovs.arvojournals.org/article.aspx?articleid=2162391
65. Lydic TA, Busik J V, Reid GE. A monophasic extraction strategy for the simultaneous lipidome analysis of polar and non-polar retina lipids. J Lipid Res. (2014) 55:1797–809. doi: 10.1194/jlr.D050302
66. Malisan F, Testi R. GD3 ganglioside and apoptosis. Biochim Biophys Acta. (2002) 1585:179–87. doi: 10.1016/S1388-1981(02)00339-6
67. Stramer BM, Kwok MGK, Farthing-Nayak PJ, Jung J-C, Fini ME, Nayak RC. Monoclonal antibody (3G5)–defined ganglioside: cell surface marker of corneal keratocytes. Invest Ophthalmol Vis Sci. (2004) 45:807–12. doi: 10.1167/iovs.03-0256
68. Chen J, Keirsey J, Basso K, Nichols KK. Differentially expressed non-polar lipids in human meibum of dry eye disease. Invest Ophthalmol Vis Sci. (2015) 56:342. Available online at: https://iovs.arvojournals.org/article.aspx?articleid=2333286
69. Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. (2010) 223:245–50. doi: 10.1016/j.expneurol.2009.08.033
71. Bruna M, Breward CJW. The influence of non-polar lipids on tear film dynamics. J Fluid Mech. (2014) 746:565–605. doi: 10.1017/jfm.2014.106
72. Rantamäki AH, Javanainen M, Vattulainen I, Holopainen JM. Do lipids retard the evaporation of the tear fluid? Invest Ophthalmol Vis Sci. (2012) 53:6442–7. doi: 10.1167/iovs.12-10487
73. Rantamäki AH, Wiedmer SK, Holopainen JM. Melting points—the key to the anti-evaporative effect of the tear film wax esters. Invest Ophthalmol Vis Sci. (2013) 54:5211–7. doi: 10.1167/iovs.13-12408
74. Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film - a review. Exp Eye Res. (2015) 137:125–38. doi: 10.1016/j.exer.2015.05.002
75. Rantamäki AH, Holopainen JM. The effect of phospholipids on tear film lipid layer surface activity. Invest Ophthalmol Vis Sci. (2017) 58:149–54. doi: 10.1167/iovs.16-20468
76. Ham BM, Cole RB, Jacob JT. Identification and comparison of the polar phospholipids in normal and dry eye rabbit tears by MALDI-TOF mass spectrometry. Invest Ophthalmol Vis Sci. (2006) 47:3330–8. doi: 10.1167/iovs.05-0756
Keywords: lipidomics, refractive surgery, LASIK, FS-LASIK, SBK, PLS-DA
Citation: Gao Y, Qi Y, Huang Y, Li X, Zhou L and Zhao S (2021) Lipidomics Analysis of the Tears in the Patients Receiving LASIK, FS-LASIK, or SBK Surgery. Front. Med. 8:731462. doi: 10.3389/fmed.2021.731462
Received: 27 June 2021; Accepted: 24 September 2021;
Published: 27 October 2021.
Edited by:
Michael Mimouni, Rambam Health Care Campus, IsraelReviewed by:
Efrat Naaman, Rambam Health Care Campus, IsraelRashad Sukhtian, Rambam Health Care Campus, Israel
Copyright © 2021 Gao, Qi, Huang, Li, Zhou and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhou, zhou.lei@seri.com.sg; Shaozhen Zhao, zhaosz1997@sina.com
†These authors have contributed equally to this work and share first authorship
 Yan Gao
Yan Gao