
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 08 October 2021
Sec. Rheumatology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.730273
This article is part of the Research TopicJuvenile Spondyloarthritis: from Basic Science to Clinical TranslationView all 9 articles
Spondyloarthritis (SpA) is a group that includes a wide spectrum of clinically similar diseases manifested by oligoarticular arthritis and axial or peripheral ankylosis. Although axial SpA is predominant in Caucasians and adult-onset patients, juvenile-onset and Latin American patients are characterized by severe peripheral arthritis and particularly foot involvement. The peripheral involvement of SpA can vary from tarsal arthritis to the most severe form named ankylosing tarsitis (AT). Although the cause and etiopathogenesis of axSpA are often studied, the specific characteristics of pSpA are unknown. Several animal models of SpA develop initial tarsitis and foot ankylosis as the main signs, emphasizing the role of foot inflammation in the overall SpA spectrum. In this review, we attempt to highlight the clinical characteristics of foot involvement in SpA and update the knowledge regarding its pathogenesis, focusing on animal models and the role of mechanical forces in inflammation.
Spondyloarthritis (SpA) is a group of chronic inflammatory diseases of the entheses and the synovial membrane of the joints, tendons, and bursae that affects the spine, the sacroiliac joint, and peripheral sites (1, 2). Currently, SpA is known as axial SpA (axSpA) (3, 4) or peripheral (pSpA) (5). Ankylosing spondylitis (AS) represents the most severe form of SpA in which episodes of disease activity merge with chronic irreversible manifestations such as bone proliferation and ankylosis. According to ASAS classification, the “SpA” name is kept, and ankylosing spondylitis (AS) corresponds to radiographic axSpA (r-axSpA). While axSpA predominates at onset and through disease's course in European and European descendants (6), the combination of axSpA with pSpA is the clinical pattern most frequently found in Latin America (7–16), India (17, 18), the Middle East (19), and Asia (20).
In the past, studies on SpA referred to peripheral involvement in children, adolescents, and young adults (21, 22). Usually, peripheral involvement was described as an asymmetrical affection of the lower limbs. Regarding adult-onset disease, particularly AS, the peripheral disease became recognized in the mid-1970s (23, 24) as part of AS and disorders such as reactive arthritis (ReA) (formerly Reiter's syndrome), psoriatic arthritis (PsA), Crohn's disease, ulcerative colitis, and undifferentiated SpA.
Amor et al. (25) first included peripheral arthritis of the lower limbs among the SpA criteria. Then, the European Spondyloarthropathy Study Group (ESSG) proposed a classification system with two entry arms axial and peripheral (26). This idea gained recognition in the ASAS classification as pSpA. In the meantime, mid-foot arthritis, enthesitis, or tarsitis appeared as important manifestations in adolescents or young adults with AS (27).
It was challenging to assess enthesopathy even though the concept of “the entheseal organ” turned fundamental in understanding the disease's pathophysiology (28–30). Synovitis and particularly enthesitis, have been the target for studying cellular infiltrates, pro-inflammatory cytokines, and bone proliferation. As discussed below, the mechanisms leading to such phenomena include mechanic forces that act upon mechano-receptors, HLA-B27, ERAP1, and IL-23. The approach to studying the disease's pathogenesis has been driven from two paths: throughout animal models and human surgical samples.
Mid-foot arthritis, also known as, tarsitis, is a prominent feature in adolescents and young adult males with SpA (31, 32). Most adolescents and young adults have recurrent lower-limb arthritis and enthesitis combined with <20% axial symptoms. Five to 10 years later, 75% of such patients fulfill the AS criteria (33, 34). In contrast to juvenile-onset SpA (JoSpA), adults have back pain and 5 to 10 years later inflammatory back pain alongside sacroiliitis on magnetic resonance imaging (MRI) and radiographic studies (5, 35–39). Identifying the characteristic involvement of peripheral arthritis and enthesitis and its differentiation from other forms of juvenile idiopathic arthritis (JIA) as early as possible allows the use of biologic disease-modifying anti-rheumatic drugs (bDMARDs) years before the appearance of the spinal and sacroiliac joints symptoms (21). The same applies to eligibility criteria and outcome measures in clinical trials on the efficacy and safety of bDMARDs. Besides peripheral disease, some other variables are predictive of SpA in children and adolescents; specifically, a family history of SpA, HLA-B27 positivity, and clinically the history or presence of foot enthesopathy and arthropathy uveitis, inflammatory back pain, and sacroiliitis (40–45).
Tarsitis presents with mid-foot pain and swelling and often swollen ankles, inflammation of the plantar fascia, and Achilles tendon enthesitis (Figure 1). Radiographic studies show a spectrum of findings, such as diffuse osteopenia, joint space narrowing, and bone ankylosis. Erosions and enthesophytes are found in the extraarticular entheses, such as Achilles' tendon and plantar fascia bone attachments (Figure 2). MRI shows bone edema, synovial sheath and bursae swelling, and abundant synovial fluid in the joint space (Figure 3).
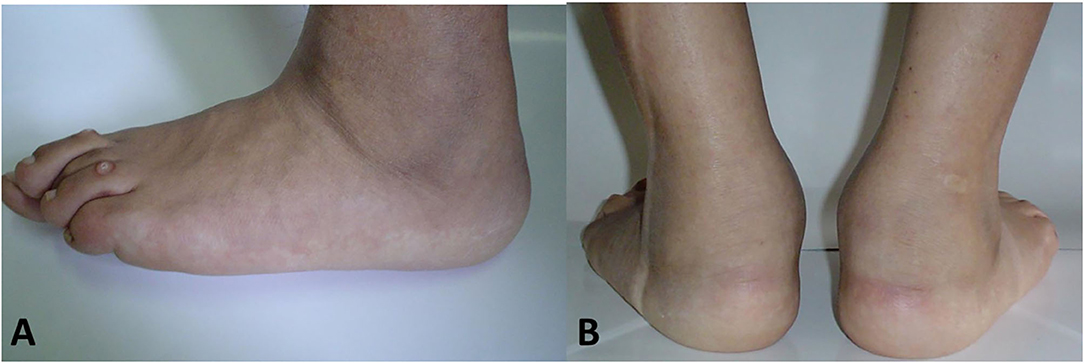
Figure 1. Lateral (A) and coronal (B) T-2 weighted-fat suppressed MR imaging showing edema in various tarsal bones, joint spaces and soft tissues in a 16 year old boy with chronic ankylosing tarsitis [Modified from Burgos-Vargas (46)].
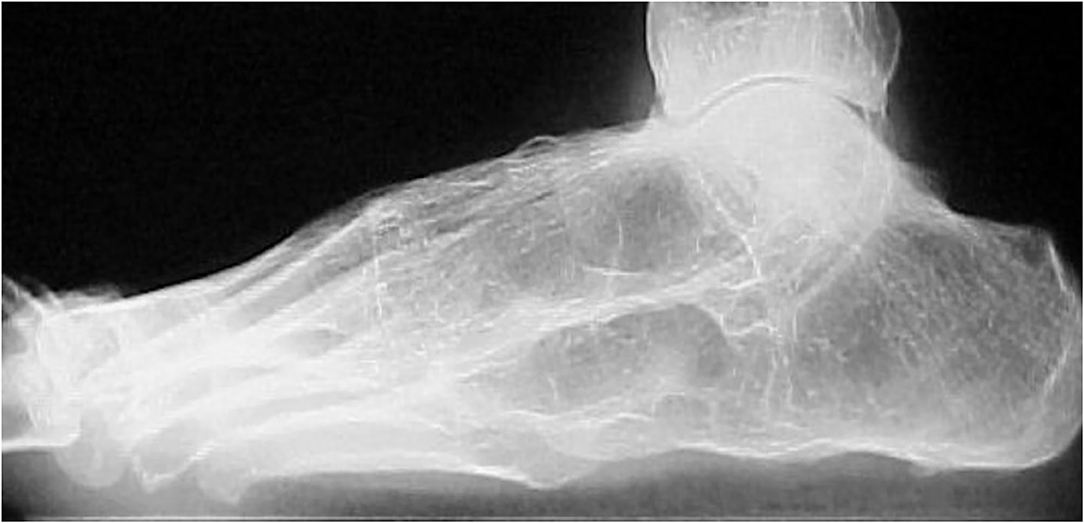
Figure 2. Chronic changes in a patient with JoSpA. Lateral view showing complete tarsal ankylosis and plantar enthesophytosis. Courtesy of Dr. Rubén Burgos-Vargas.

Figure 3. T-2 weighted-fat suppressed MR imaging showing edema in various tarsal bones, joint spaces and soft tissues in a 16 year old boy with chronic ankylosing tarsitis [Modified from Burgos-Vargas (46)].
The most severe cases are those evolving into ankylosing tarsitis (AT), a condition characterized by a partial or complete fusion of the tarsal bones and by the formation of bone bridges resembling in certain aspects the long-term changes of the sacroiliac and particularly the spine of AS patients (Figure 2).
Besides our descriptions of Mexicans with JoSpA, there are sporadic descriptions of tarsal involvement in other geographic localization and ethnic groups. For example, unilateral ankylosis of the tarsal bones was described in a 19 year-old male with AS diagnosed at the age of 15 who had several mid-foot episodes of arthritis; unilaterality was attributed to radiotherapy (47). In another report, 15 of 40 patients with JoSpA that underwent therapeutic immobilization of the feet developed tarsometatarsal fusion (48).
Chinese and French large cohorts of patients with JoSpA have shown tarsitis in around 6 to 9% (49, 50). Data from India indicate that involvement of the mid-foot is common and severe (17). Likewise, tarsal bone ankylosis was seen in patients with oligoarticular juvenile rheumatoid arthritis and back pain from India (51) and Turkey (52, 53).
Based on the New York classification of sacroiliitis (5, 54), our group has developed an equivalent grading of radiographic parameters of classification and interpretation of tarsitis (39). As a result, the Spondyloarthritis Tarsal Radiographic Index (SpA-TRI) has good sensitivity and specificity to evaluate structural but no inflammatory changes (39, 48).
Tarsal ankylosis (defined as tibiotarsal, intertarsal, or tarsometatarsal ankylosis) occurred in 18% of patients with juvenile rheumatoid arthritis (JRA) and 23% of adult-onset patients with rheumatoid arthritis. In another study, tarsal ankylosis accounted for 25% of the seropositive and seronegative polyarticular JRA, 9 and 19% of the pauciarticular and systemic (51). Interestingly, most of these cases also had carpal ankylosis, and none had SpA. In addition, Tarsal and carpal ankylosis occurred in adult patients with JRA from India (51). Compared with patients without radiographic sacroiliitis, around 40% of such patients with radiographic tarsitis graded 3 or 4 with the SpA tarsal radiographic index (55).
The characteristics of SpA in adults are inflammation and enthesitis, followed by bone proliferation and ankylosis involving the sacroiliac and spinal joints (56). While the cause and mechanisms involved in inflammation are partially known, those participating in bone proliferation remained unidentified. Research advances are associated with new treatment forms, which may induce remission of inflammation but not halter bone formation (57). Additionally, many studies focus on the axial skeleton and very little on peripheral sites. In contrast, striking findings occur in the mid and hindfoot in many animal models, highlighting a window of opportunity to study human samples from peripheral joints.
Unfortunately, pediatric and adult rheumatologists in our clinic and probably from other centers have neglected midfoot involvement despite its severity and consequences. Most people think of the ankle and metatarsophalangeal joints when children and even adults complain of midfoot/tarsus pain and swelling. The connection between the exacerbated inflammatory responses and the abnormal residual ossification remains a potential field to improve our therapeutic approach. Blocking the mechanisms subjacent to bone proliferation could improve the overall prognosis significantly in our patients.
Interest in peripheral arthritis as a critical manifestation of SpA developed in parallel with studies on psoriasis and psoriatic arthritis (PsA) and descriptions of enthesitis and dactylitis (24, 58, 59). Dactylitis often occurred in single toes as a companion to nail psoriasis. Recently, peripheral arthritis appeared again in clinical descriptions (48). An international study of 4,465 patients with SpA found that nearly 70% of the participants had at least one episode of peripheral arthritis (48, 60). Data splitting yielded 57% with arthritis, enthesitis in 44%, and dactylitis in 15%. The study confirmed the highest percentage of peripheral manifestations in around 80% of patients in Latin America, dactylitis in 37% of PsA, and enthesitis in 65% of JoSpA. Mid-foot involvement or tarsitis occurred in rank order in 13% of pSpA, 10% of PsA, 9% of reactive arthritis (ReA) and inflammatory bowel disease (IBD), and 5% of axSpA. The proportion of tarsitis in JoSpA was 19%. Per geographic region, tarsitis occurred in 24% of patients from Latin America. In children, inflammatory clinical events may progress throughout the years and end in bone ankylosis. We proposed three stages to approach the very early symptoms of the “Prodrome” stage, which evolve and progress in a rather slow and recurrent “continuum” of disease, ending up with bone ankylosis (Figure 4).
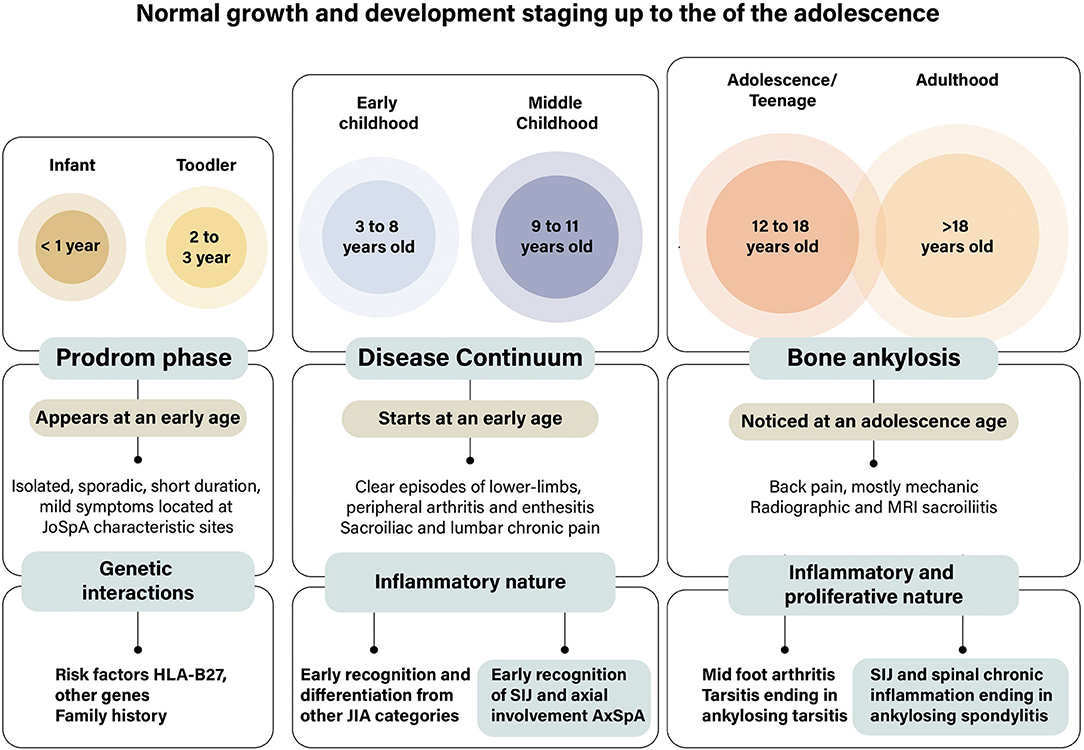
Figure 4. Stages in the development of foot affection in juvenile-onset spondyloarthritis. We proposed three stages to approach the early symptoms of foot affections in patients with JoSpA. The duration of each of the stages is certainly unknown since information is scarce. Few symptoms, such as isolated, sporadic, short duration, episodes of joint pain often considered growing pains, characterize the prodrome stage. Around the age of 8 years starts the inflammatory stage of the disease, which is mostly characterized by arthritis, enthesitis, and axial involvement; this is the disease “continuum” stage, which merged with tarsal ankylosis in the adolescent years or sacroiliac and spinal ankylosis in early adulthood. This is a clinical picture needing differentiation from other diseases, particularly from subgroups of juvenile idiopathic arthritis. At the same time, the relation between children and adult diseases should be established via de sacroiliac and spinal demonstration in children. These events take place when there are several changes in growth and development in childhood and adolescence.
There is very scarce information addressing the pathogenesis of peripheral and foot affection on SpA; nonetheless, genetic association studies have revealed that the HLA-B27 predisposition is shared with axSpA and that other genes like LMP2 (61), ERAP-1, ERAP-2 (62) and class II MHC are involved (63).
Although peripheral osteoproliferation seems to be the main problem in these patients, very few studies focus on the pathogenesis of tissue inflammation and proliferation (2). We have previously analyzed tendon, enthesis, and joint samples from the midfoot of Mexican patients with ankylosing tarsitis (AT) (64). Our results revealed a scarce leukocyte infiltration accompanied by an osteoid intrusion in the extracellular matrix (ECM), suggesting that, probably, intramembranous ossification of the enthesis and subchondral osteoproliferation could take place. We also found an important expression of bone lineage proteins like osteopontin (OPN) and osteocalcin (OCN) in mesenchymal tissues as well as parathyroid hormone-related protein (PTHrP) and basic sialoprotein on bone tissues. The role of osteocalcin in physiological and pathological bone formation remains an important question in SpA (65, 66); however, its expression on entheseal cells might involve its participation in inducing an osteoblastic phenotype (67).
Remarkably, in different animal models of SpA, midfoot arthritis and ossification are the main clinical features that can happen either before or simultaneously that axial arthritis. Animal models of transgenic animals like the HLA-B27-transgenic rats develop spontaneous arthritis in the hind paws accompanied by spondylitis, uveitis, and gut inflammation, resembling human disease (68). Interestingly, a transgenic model of TNF overexpression in mice (TNFΔARE) is characterized by Crohn's-like ileitis, midfoot ossification and inflammation, sacroilitis, and spinal ossification (69, 70) that worsens with increased mechanical stress and can develop in the absence of mature T or B cells (71). A more recently described model involves the transmembrane expression of TNF; in this model, animals develop a disease characterized by axial and peripheral enthesitis with abundant leukocyte infiltration (72). These experimental approaches resemble human disease and point to the importance of peripheral arthritis and enthesitis in the onset of the disease with an interesting involvement of immune pathways and mechanical forces.
Another noteworthy model of SpA is induced after the transgenic edition of ZAP70 in SKG (Sakaguchi) mice, which develop SpA and Crohn's-like ileitis after the injection of microbial compounds like curdlan or zymosan (73, 74). In addition, the animals of other induced models like proteoglycan-induced arthritis (PGIA) (75–77), collagen-antibodies induced arthritis (CAIA) (70, 71, 78, 79), and DBA mice (80–85) also can show a certain degree of midfoot inflammation and, in chronic models, a severe ossification, accompanied by overexpression of inflammatory cytokines like IL-1B, IL-12B, IL-17A, and IL-6.
Experimental evidence from the IL-23 minicircle overexpression model points to an essential involvement of tendon and ligaments through altered stromal cell function, myeloid cell responsiveness, or gamma delta (γδ) T cell-dependent mechanisms (78). Furthermore, firm evidence for a link with mechanical stress has arisen from hind limb unloading vs. voluntary running experiments that decreased or increased mechanical stress. The studies firmly showed that both in the TNFΔARE model (70) and CAIA (71), unloading prohibits arthritis onset, whereas the reverse was observed under voluntary running conditions. Intriguingly, while unloading prohibited the onset of arthritis in collagen-induced arthritis (CIA), it did not interfere with the development of anti-collagen antibodies, indicating that mechanostress regulates joint inflammation but uncouples it from induction of autoantibodies (71). The mechanostress effect is also apparent in the absence of adaptive immunity, suggesting that tissue-resident stroma may account for it. This is intriguing as several studies have pointed to a crucial role for entheseal and skin γδ T cells in the onset of IL-23-driven PsA both on skin and joints (86–88).
Several animal models point that canonical T cells appear not to be indispensable for mechanostress induced inflammation. In line with this, in vitro stretch of tendon and ligament-stromal cells induce an array of pro-inflammatory mediators, several of which are shared with skin keratinocytes. They include chemokines, cytokines, and several danger-associated molecular patterns (DAMPs). The induction of CCL2, for example, was shown to recruit classical monocytes. Mechanostress also led to a marked activation of complement, which attenuated mechanostress induced inflammation (89).
A summary of the characteristics of animal models that can present midfoot inflammation and ossification is showed in Table 1. Interestingly, many mechanisms can be involved in the development of SpA, and although animal models have provided much of the current information, several studies on human samples reveal a complex immune network that modulates the response to risk factors and enhancing elements toward the onset of the disease (Figure 5).
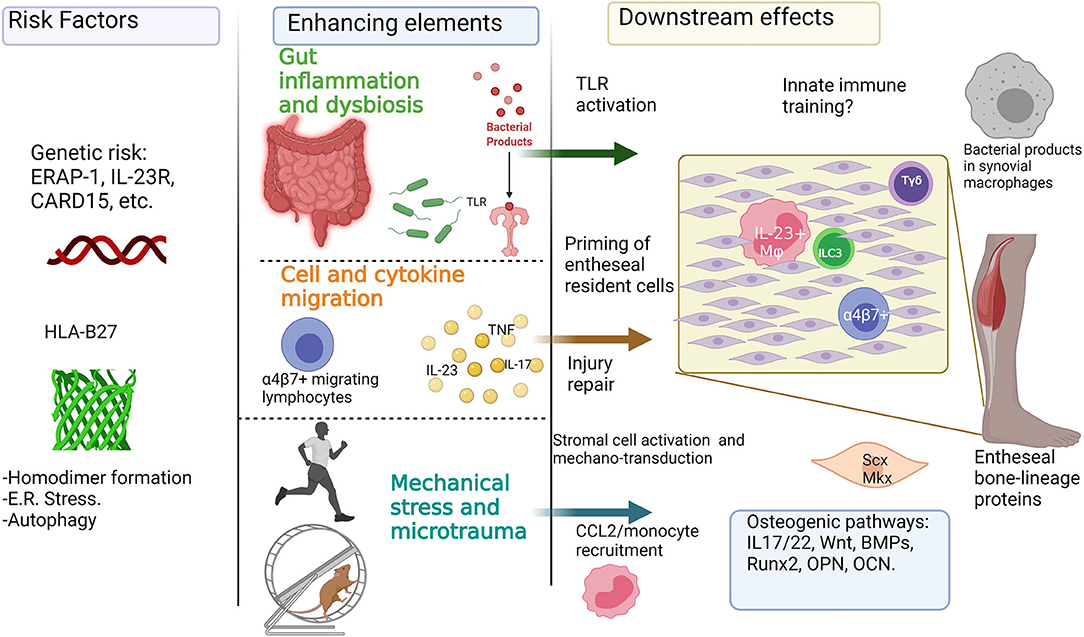
Figure 5. Inflammatory and mechanical factors in the onset of enthesitis: Genetic predisposition for enthesitis can be represented by HLA-B27 and its role in the endoplasmic reticulum (E.R.) stress, autophagy, and homodimer formation, and also by other polymorphisms in genes like ERAP-1 and IL-23R. Further enhancing elements involved in the onset of enthesitis include the role of the “leaky gut” syndrome, intestinal dysbiosis, and the activation of gut-homing lymphocytes. Additionally, mechanical stress can play a key role in enhancing inflammation and osteoproliferation through injury repair mechanisms, production of chemokines like CCL2, and the activation of stromal cells like tenocytes. The previous can be added to the potential role of osteogenic pathways like Wnt, BMP, and Runx2. This figure was done with biorender (Biorender.com).
Genetic studies have provided a significant step in the discovery of potential triggers of SpA pathogenesis. The most studied gene for SpA susceptibility is the class I histocompatibility molecule HLA-B27 (90, 91), which, previously was considered as a potential link with “arthritogenic peptides” presented to self-reactive lymphocytes; nevertheless, this has not been proven, and current theories postulate other roles like the induction of endoplasmic- reticulum (ER) stress (92) and homodimer formation (93).
Studies in animal models of transgenic HLA-B27 rats and mice (68) revealed that this molecule tends to misfold during its synthesis in the endoplasmic reticulum (ER) (94), causing ER stress, activation of the unfolded protein response (UPR) (95) and the induction of the inflammatory cytokine IL-23 (92). However, UPR activation has not been proven in humans, as UPR markers are not increased in samples of patients of SpA (96), and instead, some reports suggest that IL-23 production could be related to an increase of autophagy markers in the gut (97). Furthermore, killer immunoglobulin receptors (KIR) expressed on NK and Th17 cells can recognize aberrant B27 homodimers (98, 99), and therefore, induce IL-17 production. Remarkably, heavy chain homodimers have been found in patients' gut and vertebral joints (100).
Although most of the cellular and molecular mechanisms related to the initial triggers and the amplification of inflammation in SpA are known for animal models and in vitro research, the use of bDMARDs has allowed basic and clinical researchers to study the role of several cytokines in the human SpA. The first and most known biologic target is the pro-inflammatory cytokine TNF, and TNF inhibitors (TNFi) are currently the most used treatment for the disease. The use of TNFi confirmed its therapeutic effect in adult patients with AS (38) and prevented proliferation and structural progression after 8 years (101). Although JoSpA patients can also benefit from TNFi (102), there are no reports of osteoproliferation in these patients' peripheral joints.
Type 3 immunity (mediated by IL-23, IL-17, and IL-22) also plays an essential role in the pathogenesis of SpA. IL-17 is a pro-inflammatory cytokine involved in animal models of SpA (74, 78, 99) and increased in patients' blood and synovial fluid (103–106). The pro-inflammatory and destructive effects of IL-17 have been associated with synovitis, enthesitis, and bowel inflammation (107–109). Also, animal models of IL-23 minicircle injection (78) and SKG mice (74) mainly depend on IL-17. The therapeutic inhibition of this cytokine has significant effects on disease activity and vertebral inflammation (110), although, again, few reports focus on peripheral symptoms, and the existing ones only evaluate PsA patients (111, 112).
IL-23 is a cytokine involved in the differentiation and maintenance of the Th17 phenotype (113). Its role in SpA has become highly relevant for the scientific community since, in 2012, it was published that the over-expression of this cytokine with DNA minicircles in mice could induce a spontaneous model of SpA-like disease (78). In the report by Sherlock et al., this cytokine could act on a previously undescribed population of entheseal resident CD3+, RORγt+, Sca1+, CD4–, CD8–, IL−23R+ T cells. Several groups around the world have tried to identify such entheseal, IL-23 responsive cells, and it has been suggested that invariant-receptor natural-killer T cells (iNKT) (114), Th17 (99), mucosal-associated invariant T (MAIT) cells (107), Tγδ (103), and type 3 innate lymphoid (ILC3) cells (104) could be responsible for the IL-23 role on inflammation and osteoproliferation. Strikingly, when the inhibition of IL-23 was taken to the clinics, it did not result in any therapeutic benefit (115), suggesting that probably, there are IL-17 producing cells independently of the IL-23 status (116, 117) or that IL-23 is involved in very early steps of SpA induction (118).
Almost half of the patients with SpA have microscopical subclinical gut inflammation (119), and there is a strong association of IBD with SpA. Whether or not this relationship is involved in the foot involvement of JoSpA remains unknown. Nonetheless, the increased severity and frequency of tarsitis in developed countries is probably associated with a higher incidence of intestinal infections. The relationship between gut inflammation and arthritis or enthesitis is a challenging research topic that includes intestinal dysbiosis and cell migration (120–122). It has been suggested that some inflammatory mediators like cytokines and leukocytes can be originated in the gut, and such could be the case of ILC3 cells (104) or Tγδ cells (123).
Recently, our group described that a population of Tγδ cells expressing the gut-homing integrin a4β7 is enriched in the peripheral blood of patients with axSpA and that this population has an increased expression of TLR2 and TLR4, which might induce them to a pre-activation state and an enhanced response to pathogenic molecular patterns (123). The characteristics of this population are still unknown; nevertheless, further studies are needed to address a possible migration phenomenon.
SpA has been linked to a strong genetic predisposition (124, 125) and certain micro-organisms interplay with the immune system (126, 127), with reactive arthritis as a prototypic example, although this association is not always that clear in a significant fraction of patients. Similarly, what drives the joint-centered inflammation in this spectrum of diseases has been a longstanding enigma in the field. Previous work from our group has revealed an interplay between infections and SpA (128). Specifically, we described the presence of bacterial DNA in synovial macrophages (129) and antibody and cellular immune response against the enterobacterial heat shock protein-60 (HSP60) in blood and synovial fluid samples (127).
While many residual questions remain to date, some recent concepts have arisen that at least partially address why some joints or joint structures is typically associated with spondyloarthritis. Spondyloarthritides is notoriously known to affect enthesis, particularly those of lower limbs such as Achilles Tendons or fascia plantaris (130, 131). This is a feature that at least clinically is considered a hallmark of the spondyloarthritides spectrum. These clinical concepts are supported by a large body of imaging data demonstrating not only soft tissue swelling but also associated osteitis. These observations highlight the importance of the functional unit formed between muscle, tendons, the entheseal part, and the underlying bone. The tissues connecting muscle to bone (tendons) or bone to bone (ligaments) are specialized to permit the transmission of mechanical forces. Despite this, few mechanistic studies have addressed how mechanical forces may drive the onset of joint inflammation.
Healthy tendons and ligaments contain several unique cell types to ensure their homeostasis. They contain stromal cells, referred to as tenocytes, that constitute the majority of cell types within healthy tendons and ligaments. Their primary role is to control the extracellular matrix synthesis by producing collagen or degrade them by releasing proteases (132, 133). Tenocytes are notoriously mechanosensitive cells mediated at least in part by the transcription factors scleraxis (Scx) and mohawk (Mkx), which drive the expression of mechanical stress-activated genes, extracellular matrix genes (e.g., collagen) or adhesion molecules. These elements can interact with the circulating or resident immune system, and although enthesis resident immune cells are scarce, there are rare T cell subsets such as γδT cells and ILC3s, also, IL-23 producing CD14+ myeloid cells have been described (133).
The role of these immune cells in a steady state is relatively poorly understood but is thought to play a role in tendon and ligament repair. Despite the undeniable role of mechanical loading on tendon and ligament homeostasis in health, several observations have indicated that mechanical stress also leads to inflammation. Thus, healthy individuals exposed to intense physical activities (athletes, military recruits) may often develop bone marrow edema on sacroiliac joint imaging with many resemblances to acute inflammatory lesions noted in SpA patients (134, 135). Not surprisingly, mechanical stress has also been linked to inflammatory rheumatic diseases such as RA, PsA, and AS (136). Here, physical trauma has been associated with disease initiation and structural progression (137, 138).
In sum, mechanical forces display a myriad of effects on skin, tendon, and ligaments, reflecting a crosstalk between stromal and immune cells. However, there are still several outstanding questions. The threshold between normal mechanical loading and pathological stress is poorly defined, and whether mechanostress-induced inflammation in PsA reflects altered response to normal or rather exposure to supraphysiological levels of mechanical stress is currently unclear (139). Alternatively, the resolution of inflammation induced by mechanostress may also be impaired, although the underlying mechanisms are still relatively unclear.
Anatomically, the foot has 28 bones and 31 joints into three large areas: the forefoot (metatarsals and phalanges), the mid-foot (cuboid, navicular, and cuneiforms), and the hindfoot (calcaneus and talus). The foot and the pelvis are the most important weight-bearing structures in spreading loads through the spine, lower limbs, the tarsal areas, and the foot arches. Therefore, mechanical forces could drive structural damage in a similar way to the experimental models described before.
Certainly, future studies are pointing to a potential role of mechanical stress and microtrauma on inflammation and bone formation; However, some questions remain open about the interaction of these elements in the initiation of tissue repair mechanisms and ossification, there is a need to explore if inflammation and mechanostress act as sequential factors, enhancing elements or independent pathways.
Although little is known regarding the specific mechanisms of osteoproliferation in SpA, this phenomenon is probably derived from inflammation, according to radiographic studies. It has been postulated that HLA-B27 homodimers can be recognized by killer immunoglobulin-like receptors (KIR) expressed on Th17 and NK cells and that these cells can produce IL-17 mediated responses that link HLA-B27 with inflammation (91, 140). These homodimers have been found in spinal joints; nevertheless, it has not been explored if they can be found in peripheral joints.
The cytokine IL-22 is a master regulator of epidermal proliferation and barrier integrity, both in the gut and the skin; this capacity to induce proliferation is not restricted to epidermal tissues, as it has shown to interact with joint stromal cells. In the mice model of IL-23 overexpression (78), it has been reported that IL-22 (which can be either produced downstream of the IL-23 effect or independently) can induce the expression of bone growth molecules such as Akp3, Cebpb, Wnt10b, Wnt3a, and Gli1. This cytokine can also induce the mineralization and ossification of mesenchymal stem cells (141) and induce keratinocyte and fibroblast proliferation in psoriasis (142). IL-22 can become a potential therapeutic target to prevent the bone formation in the spondyloarthritides, although its effect on barrier integrity and host defense make this a difficult step.
Remarkably, there are two pathways of bone formation, endochondral and pseudomembranous ossification, and both can participate in the pathogenic bone formation of SpA patients (67, 143–145). Intense research has elucidated some pathways related to bone formation in SpA, such as the Wnt and bone morphogenetic protein (BMP) pathways (84, 146, 147). Results from the animal model of aging DBA/1 mice showed that the injection of the BMP-inhibitor nogging significantly decreases ossification and entheseal cell formation at peripheral joints. The role of BMP in peripheral SpA is also reinforced by the evidence of smad 1/5 activity in Achilles tendon's enthesis samples of SpA patients (84).
The Wnt signaling pathway is a key regulator of bone formation that can be altered in diseases like osteoarthritis (OA), rheumatoid arthritis (RA), and SpA (146). Remarkably, the Wnt inhibitor Dickkopf-1 (Dkk-1) is downregulated in patients with AS (148); therefore, a higher Wnt activity has been related to a pro-osteogenic phenotype as reviewed elsewhere (146, 149).
Sclerostin (encoded by the sost gene) is another Wnt inhibitor that can also inhibit BMP function (150) with an important role in SpA pathogenesis. Immunohistochemical analysis of zygapophyseal joints of SpA patients has revealed a very low sclerostin expression compared to samples from OA and RA patients or healthy subjects (151). The levels of sclerostin and anti-sclerostin antibodies can be useful as biomarkers for axial disease (152). However, there are no reports of its involvement in peripheral affection and tarsal ankylosis.
Although the question about the effect of inflammation and biologic therapy in bone formation is a controversial topic (153), preclinical studies revealed that TNF inhibitors could modulate Dkk-1 activity (148) and strikingly, recent studies suggest that the inhibition of the IL-12 and IL-23 pathway with Ustekinumab can increase Wnt activity (154).
The potential role of inflammation and mechanical stress in the onset of peripheral enthesitis is shown in Figure 5.
Even though peripheral symptoms of SpA and, particularly those of juvenile-onset patients, are widely recognized, there are still many questions regarding the behavior of structural evolution, bDMARD response, association with gut dysbiosis/microbiota, and immune-mediated pathogenesis. There is a current need for more profound studies in all these fields, considering that demographic and clinical characteristics might be recognized and considered as core manifestations of the disease. In this review, we emphasized the critical role of foot affection in JoSpA patients. We attempted to focus on these manifestations as potential early diagnostic elements and on these manifestations as potential early diagnostic elements and prospective sites for translational studies.
The peripheral approach of areas in which physiopathological events occur warrants a potential site for in-situ study of the SpA, providing a remarkable opportunity to deepen on the mechanical triggers that influence the proliferation of what can be considered as a dynamic anatomic-functional “foot unit.” The relationship between gut dysbiosis and peripheral SpA is still poorly understood. Although many reports analyze these factors separately, there is a lack of integrating elements that explain this interaction. Moreover, the effect of mechanical stress probably acts as an enhancing factor for previously primed immune and environmental elements.
RB-V conceived the idea and directed the project. JR-L and RB-V revised the final version and prepared the figures. All the authors contributed equally to the writing of the manuscript.
JR-L, CP-T, and RB-V receive a scholarship from Sistema Nacional de Investigadores (SNI- CONACYT). JR-L receives support from PEE, PEI, and SIJA-UNAM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dougados M, Baeten D. Spondyloarthritis. Lancet. (2011) 377:2127–37. doi: 10.1016/S0140-6736(11)60071-8
2. Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Prim. (2015) 1:1–17. doi: 10.1038/nrdp.2015.13
3. Rudwaleit M, Landewé R, Van Der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis. (2009) 68:770–6. doi: 10.1136/ard.2009.108217
4. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. (2009) 68:777–83. doi: 10.1136/ard.2009.108233
5. Rudwaleit M, Van Der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. (2011) 70:25–31. doi: 10.1136/ard.2010.133645
6. Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global prevalence of spondyloarthritis: a systematic review and meta-regression analysis. Arthritis Care Res. (2016) 68:1320–31. doi: 10.1002/acr.22831
7. Benegas M, Muñoz-Gomariz E, Font P, Burgos-Vargas R, Chaves J, Palleiro D, et al. Comparison of the clinical expression of patients with ankylosing spondylitis from Europe and Latin America. J Rheumatol. (2012) 39:2315–20. doi: 10.3899/jrheum.110687
8. Lau CS, Burgos-Vargas R, Louthrenoo W, Mok MY, Wordsworth P, Zeng QY. Features of spondyloarthritis around the world. Rheum Dis Clin North Am. (1998) 24:753–70. doi: 10.1016/S0889-857X(05)70040-5
9. Citera G, Bautista-Molano W, Peláez-Ballestas I, Azevedo VF, Perich RA, Méndez-Rodríguez JA, et al. Prevalence, demographics, and clinical characteristics of Latin American patients with spondyloarthritis. Adv Rheumatol. (2021) 61:2. doi: 10.1186/s42358-020-00161-5
10. Burgos-Vargas R, Peláez-Ballestas I. Epidemiology of spondyloarthritis in México. American J Med Sci. (2011) 341:298–300. doi: 10.1097/MAJ.0b013e31820f8d0a
11. Peláez-Ballestas I, Navarro-Zarza JE, Julian B, Lopez A, Flores-Camacho R, Casasola-Vargas JC, et al. A community-based study on the prevalence of spondyloarthritis and inflammatory back pain in Mexicans. J Clin Rheumatol. (2013) 19:57–61. doi: 10.1097/RHU.0b013e3182862e65
12. Peláez-Ballestas I, Pons-Estel BA, Burgos-Vargas R. Epidemiology of rheumatic diseases in indigenous populations in Latin-Americans. Clin Rheumatol. (2016) 35:1. doi: 10.1007/s10067-016-3298-6
13. Buschiazzo E, Maldonado-Cocco JA, Arturi P, Citera G, Berman A, Nitsche A, et al. Epidemiology of spondyloarthritis in Argentina. Am J Med Sci. (2011) 341:289–92. doi: 10.1097/MAJ.0b013e31820f8cc3
14. Gallinaro AL, Ventura C, Barros PDS, Gonçalves CR. Espondiloartrites: análise de uma série Brasileira comparada a uma grande casuística Ibero-Americana (estudo RESPONDIA). Rev Bras Reumatol. (2010) 50:581–9. doi: 10.1590/S0482-50042010000500009
15. Sampaio-Barros PD. Epidemiology of spondyloarthritis in Brazil. Am J Med Sci. (2011) 341:287–8. doi: 10.1097/MAJ.0b013e31820f8caf
16. Valle-Oñate R, Candia L, Romero-Sánchez C, Iglesias-Gamarra A, Caballero-Uribe CV, Santos-Moreno P, et al. Epidemiology of spondyloarthritis in Colombia. Am J Med Sci. 341:293–4. doi: 10.1097/MAJ.0b013e31820f8cdb
17. Malaviya AN, Agrawal N, Patil NS. Clinical characteristics of peripheral spondyloarthritis without psoriasis, inflammatory enteropathy or preceding infection, from a single rheumatology clinic in northern India. Clin Rheumatol. (2017) 36:2613–8. doi: 10.1007/s10067-017-3720-8
18. Malaviya AN, Rawat R, Agrawal N, Patil NS. The nonradiographic axial spondyloarthritis, the radiographic axial spondyloarthritis, and ankylosing spondylitis: the tangled skein of rheumatology. Int J Rheumatol. (2017) 2017:1824794. doi: 10.1155/2017/1824794
19. Saeed MA, Ahmed H, Faiq M, Aslam Z, Elaine Anwer Khan S, Batool S, et al. Prevalence of inflammatory back pain and radiographic axial spondyloarthritis in a semi-urban community of Lahore, Pakistan. Int J Rheum Dis. (2021) 24:207–15. doi: 10.1111/1756-185X.14030
20. Hur J-W, Ko KM, Park K-S, Hong S-J, Kim H-S, Lee M-S. Real-world experiences of the diagnosis process in Korean patients with ankylosing spondylitis based on a self-report questionnaire. J Int Med Res. (2021) 49:030006052110042. doi: 10.1177/03000605211004201
21. Ansell BM. Chronic arthritis in childhood. Ann Rheum Dis. (1978) 37:107–120. doi: 10.1136/ard.37.2.107
22. Schaller J, Bitnum S, Wedgwood RJ. Ankylosing spondylitis with childhood onset. J Pediatr. (1969) 74:505–16. doi: 10.1016/S0022-3476(69)80032-6
23. Moll J, Haslock I, Macrae I, Wright V. Associations between ankylosing spondylitis, psoriatic arthritis, ‘ ‘Reiter's disease, the intestinal arthropathies, and “Behcet's syndrome. Med. (1974) 53:343–64. doi: 10.1097/00005792-197409000-00002
24. Moll JMH. “The leeds idea”: an historical account of the spondarthritis concept. Reumatismo. (2007) 59:13–18. doi: 10.4081/reumatismo.2007.1s.13
25. Amor B, Dougados M, Mijiyawa M. Criteres de classification des spondyloarthropathies. Rev Rhum Mal Osteoartic. (1990) 57:85–89.
26. Dougados M, Linden S Van Der, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. (1991) 34:1218–27. doi: 10.1002/art.1780341003
27. Burgos-Vargas R, Naranjo A, Castillo J, Katona G. Ankylosing spondylitis in the Mexican mestizo: patterns of disease according to age at onset. J Rheumatol. (1989) 16:186–91.
28. Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. (2001) 199:503–26. doi: 10.1046/j.1469-7580.2001.19950503.x
29. Shaw HM, Vázquez OT, McGonagle D, Bydder G, Santer RM, Benjamin M. Development of the human Achilles tendon enthesis organ. J Anat. (2008) 213:718–24. doi: 10.1111/j.1469-7580.2008.00997.x
30. Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The “enthesis organ” concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. (2004) 50:3306–13. doi: 10.1002/art.20566
31. Burgos-Vargas R, Gutiérrez-Suárez R. Juvenile ankylosing spondylitis. In: Elzouki AY, Harfi HA, Nazer HM, Stapleton FB, Oh W, Whitley RJ, editors. Textbook of Clinical Pediatrics. Berlin; Heidelberg: Springer Berlin Heidelberg (2007). p. 1601–9.
32. Burgos-Vargas R, Zquez-Mellado JV. The early clinical recognition of juvenile-onset ankylosing spondylitis and its differentiation from juvenile rheumatoid arthritis. Arthritis Rheum. (1995) 38:835–44. doi: 10.1002/art.1780380618
33. Burgos-Vargas R, Clark P. Axial involvement in the seronegative enthesopathy and arthropathy syndrome and its progression to ankylosing spondylitis. J Rheumatol. (1989) 16:192–7.
34. Cabral DA, Oen KG, Petty RE. SEA syndrome revisited: a longterm followup of children with a syndrome of seronegative enthesopathy and arthropathy. J Rheumatol. (1992) 19:1282–5.
35. Rudwaleit M, Landewé R, Sieper J. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. (2016) 375:1302–3. doi: 10.1056/NEJMc1609622
36. Maksymowych WP, Lambert RGW, Østergaard M, Pedersen SJ, Machado PM, Weber U, Bennett AN, et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis. (2019) 78:1550–8. doi: 10.1136/annrheumdis-2019-215589
37. Rudwaleit M. Spondyloarthropathies: identifying axial SpA in young adults with chronic back pain. Nat Rev Rheumatol. (2016) 12:378–80. doi: 10.1038/nrrheum.2016.98
38. Poddubnyy D, Rudwaleit M. Early spondyloarthritis. Rheum Dis Clin North Am. (2012) 38:387–403. doi: 10.1016/j.rdc.2012.04.007
39. Dougados M, ‘d'Agostino MA, Benessiano J, Berenbaum F, Breban M, Claudepierre P, Combe B, et al. The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Jt Bone Spine. (2011) 78:598–603. doi: 10.1016/j.jbspin.2011.01.013
40. Flatø B, Hoffmann-Vold AM, Reiff A, Førre Ø, Lien G, Vinje O. Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum. (2006) 54:3573–82. doi: 10.1002/art.22181
41. Selvaag AM, Flatø B, Dale K, Lien G, Vinje O, Smerdel-Ramoya A, et al. Radiographic and clinical outcome in early juvenile rheumatoid arthritis and juvenile spondyloarthropathy: a 3-year prospective study. J Rheumatol. (2006) 33:1382–91. doi: 10.1002/art.10783
42. Flatø B, Smerdel A, Johnston V, Lien G, Dale K, Vinje O, et al. The influence of patient characteristics, disease variables, and HLA alleles on the development of radiographically evident sacroiliitis in juvenile idiopathic arthritis. Arthritis Rheum. (2002) 46:986–94. doi: 10.1002/art.10146
43. Stoll ML, Bhore R, Dempsey-Robertson M, Punaro M. Spondyloarthritis in a pediatric population: risk factors for sacroiliitis. J Rheumatol. (2010) 37:2402–8. doi: 10.3899/jrheum.100014
44. Huerta-Sil G, Casasola-Vargas JC, Londoño JD, Rivas-Ruíz R, Chávez J, Pacheco-Tena C, et al. Low grade radiographic sacroiliitis as prognostic factor in patients with undifferentiated spondyloarthritis fulfilling diagnostic criteria for ankylosing spondylitis throughout follow up. Ann Rheum Dis. (2006) 65:642–6. doi: 10.1136/ard.2005.043471
45. Burgos-Vargas R, Vázquez-Mellado J, Cassis N, Duarte C, Casarín J, Cifuentes M, et al. Genuine ankylosing spondylitis in children: a case-control study of patients with early definite disease according to adult onset criteria. J Rheumatol. (1996) 23:2140–7.
46. Burgos-Vargas R. The assessment of the Spondyloarthritis International Society concept and criteria for the classification of axial spondyloarthritis and peripheral spondyloarthritis: a critical appraisal for the pediatric rheumatologist. Pediatr Rheumatol Online J. (2012) 10:14. doi: 10.1186/1546-0096-10-14
47. Roth RD. Tarsal ankylosis in juvenile ankylosing spondylitis. J Am Podiatr Med Assoc. (1986) 76:514–8. doi: 10.7547/87507315-76-9-514
48. López-Medina C, Moltó A, Dougados M. Peripheral manifestations in spondyloarthritis and their effect: an ancillary analysis of the ASAS-COMOSPA study. J Rheumatol. (2020) 47:211–7. doi: 10.3899/jrheum.181331
49. Goirand M, Breton S, Chevallier F, Duong NP, Uettwiller F, Melki I, et al. Clinical features of children with enthesitis-related juvenile idiopathic arthritis / juvenile spondyloarthritis followed in a French tertiary care pediatric rheumatology centre. Pediatr Rheumatol. (2018) 16:21. doi: 10.1186/s12969-018-0238-9
50. Huang F, Zhang J, Zhu J, Guo J, Yang C. Juvenile spondyloarthropathies: the Chinese experience. Rheum Dis Clin North Am. (2003) 29:531–47. doi: 10.1016/S0889-857X(03)00048-6
51. Narayanan K, Rajendran CP, Porkodi R, Shanmuganandan K. A follow-up study of juvenile rheumatoid arthritis into adulthood. J Assoc Phys India. (2002) 50:1039–41.
52. Erdem CZ, Sarikaya S, Erdem LO, Ozdolap S, Gundogdu S. MR imaging features of foot involvement in ankylosing spondylitis. Eur J Radiol. (2005) 53:110–9. doi: 10.1016/j.ejrad.2004.03.013
53. Ozaras N, Havan N, Poyraz E, Rezvani A, Aydin T. Functional limitations due to foot involvement in spondyloarthritis. J Phys Ther Sci. (2016) 28:2005–8. doi: 10.1589/jpts.28.2005
54. Feldtkeller E, Khan MA, Van Der Heijde D, Van Der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. (2003) 23:61–6. doi: 10.1007/s00296-002-0237-4
55. Pacheco-Tena C, Londoño JD, Cazarín-Barrientos J, Martínez A, Vázquez-Mellado J, Moctezuma JF, et al. Development of a radiographic index to assess the tarsal involvement in patients with spondyloarthropathies. Ann Rheum Dis. (2002) 61:330–4. doi: 10.1136/ard.61.4.330
56. Ehrenfeld M. Spondyloarthropathies. Best Pract Res Clin Rheumatol. (2012) 26:135–45. doi: 10.1016/j.berh.2012.01.002
57. Sari I, Haroon N. Disease modification in axial spondyloarthritis. Best Pract Res Clin Rheumatol. (2018) 32:427–39. doi: 10.1016/j.berh.2019.02.007
58. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. (2006) 54:2665–73. doi: 10.1002/art.21972
59. Moll JMH, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. (1973) 3:55–78. doi: 10.1016/0049-0172(73)90035-8
60. López-Medina C, Molto A, Sieper J, Duruöz T, Kiltz U, Elzorkany B, et al. Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: results of the worldwide, cross-sectional ASAS-PerSpA study. Open. (2021) 7:1450. doi: 10.1136/rmdopen-2020-001450
61. Maksymowych WP, Jhangri GS, Gorodezky C, Luong M, Wong C, Burgos-Vargas R, et al. The LMP2 polymorphism is associated with susceptibility to acute anterior uveitis in HLA-B27 positive juvenile and adult Mexican subjects with ankylosing spondylitis. Ann Rheum Dis. (1997) 56:488–492. doi: 10.1136/ard.56.8.488
62. Londono J, Santos AM, Rueda JC, Calvo-Paramo E, Burgos-Vargas R, Vargas-Alarcon G, et al. Association of ERAP2 polymorphisms in Colombian HLA-B27+ or HLA-B15+ patients with SpA and its relationship with clinical presentation: axial or peripheral predominance. RMD Open. (2020) 6:1–8. doi: 10.1136/rmdopen-2020-001250
63. Ploski R, Flat B, Vinje O, Maksymowych W, Thorsby E. Association to HLA-DRB l “O8”, for an HLA-linked proteasome gene in juvenile ankylosing spondylitis. Hum Immunol. (1995) 96:88–96. doi: 10.1016/0198-8859(95)00063-A
64. Ramírez González R, Soto Abraham MV, Ugalde Viteli A, Pacheco Tena C, Burgos-Vargas R. Identificación de sitios ideales para el estudio histopatológico de la tarsitis en pacientes con espondiloartropatías. Reumatol Clin. (2006) 2:164–7. doi: 10.1016/S1699-258X(06)73041-8
65. Kwon SR, Jung KH, Lim MJ, Son MJ, Choi BH, Park SG, et al. The effect of anti-TNF treatment on osteoblastogenesis in ankylosing spondylitis: the number of circulating osteoblast-lineage cells in peripheral blood decreased after infliximab therapy in patients with ankylosing spondylitis. Clin Exp Rheumatol. (2017) 35:837–43. doi: 10.1007/s00296-011-1981-0
66. Jo S, Lee EJ, Nam B, Kang J, Lee S, Youn J, et al. Effects of dihydrotestosterone on osteoblast activity in curdlan-administered SKG mice and osteoprogenitor cells in patients with ankylosing spondylitis. Arthritis Res Ther. (2020) 22:121–22. doi: 10.1186/s13075-020-02217-9
67. Pacheco-tena C, Pérez-tamayo R, Pineda C, González-chávez SA, Quiñonez-flores C, Vitelly AU, et al. Bone lineage proteins in the entheses of the midfoot in patients with spondyloarthritis. J Rheumatol. (2014) 42:630–7. doi: 10.3899/jrheum.140218
68. Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell. (1990) 63:1099–12. doi: 10.1016/0092-8674(90)90512-D
69. Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU- rich elements: implications for joint and gut-associated immunopathologies. Immunity. (1999) 10:387–98. doi: 10.1016/S1074-7613(00)80038-2
70. Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. (2014) 73:437–45. doi: 10.1136/annrheumdis-2013-203643
71. Cambré I, Gaublomme D, Burssens A, Jacques P, Schryvers N, De Muynck A, et al. Mechanical strain determines the site-specific localization of inflammation and tissue damage in arthritis. Nat Commun. (2018) 9:4613. doi: 10.1038/s41467-018-06933-4
72. Kaaij MH, van Tok MN, Blijdorp IC, Ambarus CA, Stock M, Pots D, et al. Transmembrane TNF drives osteoproliferative joint inflammation reminiscent of human spondyloarthritis. J Exp Med. (2020) 217:e20200288. doi: 10.1084/jem.20200288
73. Ruutu M, Thomas G, Steck R, Degli-Esposti MA, Zinkernagel MS, Alexander K, et al. β-Glucan triggers spondylarthritis and Crohn's disease-like ileitis in SKG mice. Arthritis Rheum. (2012) 64:2211–22. doi: 10.1002/art.34423
74. Benham H, Rehaume LM, Hasnain SZ, Velasco J, Baillet AC, Ruutu M, et al. Interleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol. (2014) 66:1755–67. doi: 10.1002/art.38638
75. Haynes KR, Pettit AR, Duan R, Tseng HW, Glant TT, Brown MA, et al. Excessive bone formation in a mouse model of ankylosing spondylitis is associated with decreases in Wnt pathway inhibitors. Arthritis Res Ther. (2012) 14:R253. doi: 10.1186/ar4096
76. Bárdos T, Szabó Z, Czipri M, Vermes C, Tunyogi-Csapó M, Urban RM, et al. A longitudinal study on an autoimmune murine model of ankylosing spondylitis. Ann Rheum Dis. (2005) 64:981–7. doi: 10.1136/ard.2004.029710
77. Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms, and genetics. Crit Rev Immunol. (2003) 23:199–250. doi: 10.1615/CritRevImmunol.v23.i3.20
78. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+ CD4- CD8- entheseal resident T cells. Nat Med. (2012) 18:1069–76. doi: 10.1038/nm.2817
79. Banda NK. An accidental discovery of a new potential mouse model of the axial spondyloarthritis. J Immunol. (2019) 202:133.
80. Lories RJU, Matthys P, De Vlam K, Derese I, Luyten FP. Ankylosing enthesitis, dactylitis, and onychoperiostitis in male DBA/1 mice: a model of psoriatic arthritis. Ann Rheum Dis. (2004) 63:595–8. doi: 10.1136/ard.2003.013599
81. Nordling C, Karlsson-Parra A, Jansson L, Holmdahl R, Klareskog L. Characterization of a spontaneously occurring arthritis in male DBA/1 mice. Arthritis Rheum. (1992) 35:717–22. doi: 10.1002/art.1780350619
82. Corthay A, Hansson A-S, Holmdahl R. T lymphocytes are not required for the spontaneous development of entheseal ossification leading to marginal ankylosis in the DBA/1 mouse. Arthritis Rheum. (2000) 43:844–51. doi: 10.1002/1529-0131(200004)43:4<844::AID-ANR15>.0.CO;2-B
83. Quiñonez-Flores CM, López-Loeza SM, Pacheco-Tena C, Muñoz-Morales PM, Acosta-Jiménez S, González-Chávez SA. Stability of housekeeping genes in inflamed joints of spontaneous and collagen-induced arthritis in DBA/1 mice. Inflamm Res. (2021) 70:619–32. doi: 10.1007/s00011-021-01453-2
84. Lories RJU, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. (2005) 115:1571–9. doi: 10.1172/JCI23738
85. Holmdahl R, Jansson L, Andersson M, Jonsson R. Genetic, hormonal and behavioural influence on spontaneously developing arthritis in normal mice. Clin Exp Immunol. (1992) 88:467–72. doi: 10.1111/j.1365-2249.1992.tb06473.x
86. Reinhardt A, Yevsa T, Worbs T, Lienenklaus S, Sandrock I, Oberdörfer L, et al. Interleukin-23–dependent γ/δ T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. (2016) 68:2476–86. doi: 10.1002/art.39732
87. Watad A, Bridgewood C, McGonagle DG. Response to: â € correspondence to â € normal human enthesis harbours conventional CD4+ and CD8+ T cells with regulatory features and inducible IL-17A and TNF expression” by Wang and Ma. Ann Rheum Dis. (2020) doi: 10.1136/annrheumdis-2020-219047. [Epub ahead of print].
88. Bridgewood C, Sharif K, Sherlock J, Watad A, McGonagle D. Interleukin-23 pathway at the enthesis: the emerging story of enthesitis in spondyloarthropathy. Immunol Rev. (2020) 294:27–47. doi: 10.1111/imr.12840
89. Cambré I, Gaublomme D, Schryvers N, Lambrecht S, Lories R, Venken K, et al. Running promotes chronicity of arthritis by local modulation of complement activators and impairing T regulatory feedback loops. Ann Rheum Dis. (2019) 78:787–95. doi: 10.1136/annrheumdis-2018-214627
90. Vermeire S, Sandborn WJ, Danese S, Hébuterne X, Salzberg BA, Klopocka M, et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. (2017) 390:135–44. doi: 10.1016/S0140-6736(17)30930-3
91. Bowness P. Hla-B27. Annu Rev Immunol. (2015) 33:29–48. doi: 10.1146/annurev-immunol-032414-112110
92. DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. (2009) 60:2633–43. doi: 10.1002/art.24763
93. Wong-Baeza I, Ridley A, Shaw J, Hatano H, Rysnik O, McHugh K, et al. KIR3DL2 Binds to HLA-B27 dimers and free H chains more strongly than other HLA class I and promotes the expansion of T cells in ankylosing spondylitis. J Immunol. (2013) 190:3216–24. doi: 10.4049/jimmunol.1202926
94. Dangoria NS, Delay ML, Kingsbury DJ, Mear JP, Ziegler A, Colbert RA. HLA-B27 Misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum*. J Biol Chem. (2002) 277:23459–68. doi: 10.1074/jbc.M110336200
95. Turner MJ, DeLay ML, Bai S, Klenk E, Colbert RA. HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: implications for the pathogenesis of spondylarthritis-like disease. Arthritis Rheum. (2007) 56:215–23. doi: 10.1002/art.22295
96. Neerinckx B, Carter S, Lories R. IL-23 expression and activation of autophagy in synovium and PBMCs of HLA-B27 positive patients with ankylosing spondylitis. Response to:' Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of pati. Ann Rheum Dis. (2014) 73:e68. doi: 10.1136/annrheumdis-2014-206277
97. Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, et al. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. (2014) 73:1566–74. doi: 10.1136/annrheumdis-2012-202925
98. Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, et al. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. (2002) 46:2972–82. doi: 10.1002/art.10605
99. Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. (2011) 186:2672–80. doi: 10.4049/jimmunol.1002653
100. Rysnik O, McHugh K, van Duivenvoorde L, van Tok M, Guggino G, Taurog J, et al. Non-conventional forms of HLA-B27 are expressed in spondyloarthritis joints and gut tissue. J Autoimmun. (2016) 70:12–21. doi: 10.1016/j.jaut.2016.03.009
101. Baraliakos X, Haibel H, Listing J. Radiographic progression in ankylosing spondylitis - results after up to 8 years of anti-TNF treatment. Ann Rheum Dis. (2011) 46:1450–3. doi: 10.1093/rheumatology/kem166
102. Hugle B, Burgos-Vargas R, Inman RD, ‘O'Shea F, Laxer RM, Stimec J, Whitney-Mahoney K, et al. Long-term outcome of anti-tumour necrosis factor alpha blockade in the treatment of juvenile spondyloarthritis. Clin Exp Rheumatol. (2014) 32:424–31.
103. Kenna TJ, Davidson SI, Duan R, Bradbury LA, McFarlane J, Smith M, et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. (2012) 64:1420–29. doi: 10.1002/art.33507
104. Ciccia F, Guggino G, Rizzo A, Saieva L, Peralta S, Giardina A, et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis. (2015) 74:1739–47. doi: 10.1136/annrheumdis-2014-206323
105. Mei Y, Pan F, Gao J, Ge R, Duan Z, Zeng Z, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. (2011) 30:269–73. doi: 10.1007/s10067-010-1647-4
106. Limón-Camacho L, Vargas-Rojas MI, Vázquez-Mellado J, Casasola-Vargas J, Moctezuma JF, Burgos-Vargas R, et al. In vivo peripheral blood pro-inflammatory T cells in patients with ankylosing spondylitis. J Rheumatol. (2012) 39:830–5. doi: 10.3899/jrheum.110862
107. Gracey E, Qaiyum Z, Almaghlouth I, Lawson D, Karki S, Avvaru N, et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann Rheum Dis. (2016) 75:2124–32. doi: 10.1136/annrheumdis-2015-208902
108. Zhang L, Li Y-gang, Li Y-hua, Qi L, Liu X-guang, Yuan C-zhong, et al. Increased frequencies of th22 cells as well as th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS ONE. (2012) 7:e31000. doi: 10.1371/journal.pone.0031000
109. Smith JA, Colbert RA. The interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. (2014) 66:231–41. doi: 10.1002/art.38291
110. Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, Van Der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. (2013) 382:1705–13. doi: 10.1016/S0140-6736(13)61134-4
111. McInnes IB, Behrens F, Mease PJ, Kavanaugh A, Ritchlin C, Nash P, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. (2020) 395:1496–505. doi: 10.1016/S0140-6736(20)30564-X
112. McGonagle DG, McInnes IB, Kirkham BW, Sherlock J, Moots R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: recent advances and controversies. Ann Rheum Dis. (2019) 78:1167–78. doi: 10.1136/annrheumdis-2019-215356
113. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. (2014) 14:585–600. doi: 10.1038/nri3707
114. Jacques P, Venken K, Van Beneden K, Hammad H, Seeuws S, Drennan MB, et al. Invariant natural killer T cells are natural regulators of murine spondylarthritis. Arthritis Rheum. (2010) 62:988–99. doi: 10.1002/art.27324
115. Baeten D, Østergaard M, Wei JCC, Sieper J, Järvinen P, Tam LS, Salvarani C, et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann Rheum Dis. (2018) 77:1295–302. doi: 10.1136/annrheumdis-2018-213328
116. Cuthbert RJ, Watad A, Fragkakis EM, Dunsmuir R, Loughenbury P, Khan A, et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis. (2019) 78:1559–65. doi: 10.1136/annrheumdis-2019-215210
117. Siebert S, Millar NL, McInnes IB. Why did IL-23p19 inhibition fail in AS: a tale of tissues, trials or translation? Ann Rheum Dis. (2019) 78:1015–8. doi: 10.1136/annrheumdis-2018-213654
118. van Tok MN, Na S, Lao CR, Alvi M, Pots D, van de Sande MGH, et al. The initiation, but not the persistence, of experimental spondyloarthritis is dependent on interleukin-23 signaling. Front Immunol. (2018) 9:1550. doi: 10.3389/fimmu.2018.01550
119. Van Praet L, Van Den Bosch FE, Jacques P, Carron P, Jans L, Colman R, et al. Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis. (2013) 72:414–7. doi: 10.1136/annrheumdis-2012-202135
120. Gracey E, Dumas E, Yerushalmi M, Qaiyum Z, Inman RD, Elewaut D. The ties that bind: skin, gut and spondyloarthritis. Curr Opin Rheumatol. (2018) 1:62–9. doi: 10.1097/BOR.0000000000000569
121. Gilis E, Mortier C, Venken K, Debusschere K, Vereecke L, Elewaut D. The role of the microbiome in gut and joint inflammation in psoriatic arthritis and spondyloarthritis. J Rheumatol Suppl. (2018) 94:36–9. doi: 10.3899/jrheum.180135
122. Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. (2017) 76:1123–32. doi: 10.1136/annrheumdis-2016-210000
123. Romero-López JP, Gómez-Martínez D, Domínguez-López ML, Jiménez-Zamudio L, Casasola-Vargas JC, Burgos-Vargas R, et al. Differential expression of TLR2 and TLR4 in α4β7-positive leukocytes of patients with axial spondyloarthritis. Rheumatology. (2018) 60:837–48. doi: 10.1093/rheumatology/kez364
124. Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, Pointon JJ, et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet. (2010) 42:123–7. doi: 10.1038/ng.513
125. Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet. (2013) 45:730–8. doi: 10.1038/ng.2667
126. Romero-López JP, Domínguez-López ML, Burgos-Vargas R, García-Latorre E. Stress proteins in the pathogenesis of spondyloarthritis. Rheumatol Int. (2019) 39:595–604. doi: 10.1007/s00296-018-4070-9
127. Domínguez-López ML, Burgos-Vargas R, Galicia-Serrano H, Bonilla-Sánchez MT, Rangel-Acosta HH, Cancino-Díaz ME, et al. IgG antibodies to enterobacteria 60 kDa heat shock proteins in the sera of HLA-B27 positive ankylosing spondylitis patients. Scand J Rheumatol. (2002) 31:260–5. doi: 10.1080/030097402760375133
128. Martínez A, Pacheco-Tena C, Vázquez-Mellado J, Burgos-Vargas R. Relationship between activity and infection in patients with spondyloarthropathies. Ann Rheum Dis. (2004) 63:1338–40. doi: 10.1136/ard.2003.011882
129. Pacheco-Tena C, Alvarado De La Barrera C, López-Vidal Y, Vázquez-Mellado J, Richaud-Patin Y, Amieva R, et al. Bacterial DNA in synovial fluid cells of patients with juvenile onset spondyloarthropathies. Rheumatology. (2001) 40:920–7. doi: 10.1093/rheumatology/40.8.920
130. McGonagle D, Wakefield RJ, Ai LT, ‘D'Agostino MA, Toumi H, Hayashi K, Emery P, et al. Distinct topography of erosion and new bone formation in achilles tendon enthesitis: implications for understanding the link between inflammation and bone formation in spondylarthritis. Arthritis Rheum. (2008) 58:2694–699. doi: 10.1002/art.23755
131. McGonagle D, Lories RJU, Tan AL, Benjamin M. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. (2007) 56:2482–91. doi: 10.1002/art.22758
132. Millar NL, Murrell GAC, Mcinnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. (2017) 13:110–22. doi: 10.1038/nrrheum.2016.213
133. Gracey E, Burssens A, Cambré I, Schett G, Lories R, McInnes IB, et al. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat Rev Rheumatol. (2020) 16:193–207. doi: 10.1038/s41584-019-0364-x
134. Varkas G, De Hooge M, Renson T, De Mits S, Carron P, Jacques P, et al. Effect of mechanical stress on magnetic resonance imaging of the sacroiliac joints: assessment of military recruits by magnetic resonance imaging study. Rheumatology. (2018) 57:508–13. doi: 10.1093/rheumatology/kex491
135. Renson T, Renson T, Depicker A, De Craemer AS, De Craemer AS, Deroo L, et al. High prevalence of spondyloarthritis-like MRI lesions in postpartum women: a prospective analysis in relation to maternal, child and birth characteristics. Ann Rheum Dis. (2020) 79:929–34. doi: 10.1136/annrheumdis-2020-217095
136. Ospelt C, Frank-Bertoncelj M. Why location matters-site-specific factors in rheumatic diseases. Nat Rev Rheumatol. (2017) 13:433–42. doi: 10.1038/nrrheum.2017.96
137. Van Mechelen M, Lories R. Spondyloarthritis on the move: biomechanical benefits or harm. Curr Rheumatol Rep. (2020) 22:35. doi: 10.1007/s11926-020-00913-8
138. Van Mechelen M, Lories RJ. Microtrauma: no longer to be ignored in spondyloarthritis? Curr Opin Rheumatol. (2016) 28:176–80. doi: 10.1097/BOR.0000000000000254
139. Debusschere K, Cambré I, Gracey E, Elewaut D. Born to run: the paradox of biomechanical force in spondyloarthritis from an evolutionary perspective. Best Pract Res Clin Rheumatol. (2017) 31:887–94. doi: 10.1016/j.berh.2018.07.011
140. Ridley A, Hatano H, Wong-Baeza I, Shaw J, Matthews KK, Al-Mossawi H, et al. Activation-Induced Killer Cell Immunoglobulin-like Receptor 3DL2 Binding to HLA-B27 Licenses Pathogenic T Cell Differentiation in Spondyloarthritis. Arthritis Rheumatol. (2016) 68:901–14. doi: 10.1002/art.39515
141. El-Zayadi AA, Jones EA, Churchman SM, Baboolal TG, Cuthbert RJ, El-Jawhari JJ, et al. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology. (2017) 56:488–93. doi: 10.1093/rheumatology/kew384
142. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. (2009) 119:3573–85. doi: 10.1172/JCI40202
143. Pacheco-Tena C, González-Chávez SA, Quiñonez-Flores C, Burgos-Vargas R. Bone proliferation in ankylosing tarsitis might involve mechanical stress, and hormonal and growth factors. J Rheumatol. (2015) 42:2210. doi: 10.3899/jrheum.150475
144. Appel H, Sieper J. Analysis of bone samples from patients with spondyloarthritides - identifying causes of new bone formation in axial spondyloarthritis. J Rheumatol. (2015) 42:561–3. doi: 10.3899/jrheum.150046
145. McGonagle D, Marzo-Ortega H, ‘O'Connor P, Gibbon W, Hawkey P, Henshaw K, Emery P. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann Rheum Dis. (2002) 61:534–7. doi: 10.1136/ard.61.6.534
146. Lories RJ, Corr M, Lane NE. To Wnt or not to Wnt: the bone and joint health dilemma. Nat Rev Rheumatol. (2013) 9:328–39. doi: 10.1038/nrrheum.2013.25
147. Van Mechelen M, Gulino GR, de Vlam K, Lories R. Bone disease in axial spondyloarthritis. Calcif Tissue Int. (2018) 102:547–58. doi: 10.1007/s00223-017-0356-2
148. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. (2007) 13:156–63. doi: 10.1038/nm1538
149. Corr M. Wnt signaling in ankylosing spondylitis. Clin Rheumatol. (2014) 33:759–62. doi: 10.1007/s10067-014-2663-6
150. Semenov MV, He X. LRP5 mutations linked to high bone mass diseases cause reduced LRP5 binding and inhibition by SOST. J Biol Chem. (2006) 281:38276–84. doi: 10.1074/jbc.M609509200
151. Appel H, Ruiz-Heiland G, Listing J, Zwerina J, Herrmann M, Mueller R, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. (2009) 60:3257–62. doi: 10.1002/art.24888
152. Luchetti MM, Ciccia F, Avellini C, Benfaremo D, Guggino G, Farinelli A, et al. Sclerostin and antisclerostin antibody serum levels predict the presence of axial spondyloarthritis in patients with inflammatory bowel disease. J Rheumatol. (2018) 45:630–7. doi: 10.3899/jrheum.170833
153. Maksymowych WP, Elewaut D, Schett G. Motion for debate: the development of ankylosis in ankylosing spondylitis is largely dependent on inflammation. Arthritis Rheum. (2012) 64:1713–19. doi: 10.1002/art.34442
154. Fiechter RH, de Jong HM, van Mens LJJ, Fluri IA, Tas SW, Baeten DLP, et al. IL-12p40/IL-23p40 blockade with ustekinumab decreases the synovial inflammatory infiltrate through modulation of multiple signaling pathways including MAPK-ERK and Wnt. Front Immunol. (2021) 12:611656. doi: 10.3389/fimmu.2021.611656
Keywords: spondyloarthritis, ankylosing tarsitis, juvenile onset spondyloarthritis, foot arthritis, mechanical stress
Citation: Romero-López JP, Elewaut D, Pacheco-Tena C and Burgos-Vargas R (2021) Inflammatory Foot Involvement in Spondyloarthritis: From Tarsitis to Ankylosing Tarsitis. Front. Med. 8:730273. doi: 10.3389/fmed.2021.730273
Received: 24 June 2021; Accepted: 06 September 2021;
Published: 08 October 2021.
Edited by:
Garifallia Sakellariou, University of Pavia, ItalyReviewed by:
Jeannette Kunz, Nazarbayev University School of Medicine, KazakhstanCopyright © 2021 Romero-López, Elewaut, Pacheco-Tena and Burgos-Vargas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rubén Burgos-Vargas, ci5idXJnb3MudmFyZ2FzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.