- 1Key Laboratory of Chinese Internal Medicine of Ministry of Education, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 3Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
- 4Department of Hospital Medicine, ThedaCare Regional Medical Center-Appleton, Appleton, WI, United States
- 5Department of Cardiology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 6College of Integrated Traditional Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, China
Background: Dyspnea is the most common presenting symptom among patients hospitalized for acute heart failure (AHF). Dyspnea relief constitutes a clinically relevant therapeutic target and endpoint for clinical trials and regulatory approval. However, there have been no widely accepted dyspnea measurement standards in AHF. By systematic review and mapping the current evidence of the applied scales, timing, and results of measurement, we hope to provide some new insights and recommendations for dyspnea measurement.
Methods: PubMed, Embase, Cochrane Library, and Web of Science were searched from inception until August 27, 2020. Randomized controlled trials (RCTs) with dyspnea severity measured as the endpoint in patients with AHF were included.
Results: Out of a total of 63 studies, 28 had dyspnea as the primary endpoint. The Likert scale (34, 54%) and visual analog scale (VAS) (22, 35%) were most widely used for dyspnea assessment. Among the 43 studies with detailed results, dyspnea was assessed most frequently on days 1, 2, 3, and 6 h after randomization or drug administration. Compared with control groups, better dyspnea relief was observed in the experimental groups in 21 studies. Only four studies that assessed tolvaptan compared with control on the proportion of dyspnea improvement met the criteria for meta-analyses, which did not indicate beneficial effect of dyspnea improvement on day 1 (RR: 1.16; 95% CI: 0.99–1.37; p = 0.07; I2 = 61%).
Conclusion: The applied scales, analytical approaches, and timing of measurement are in diversity, which has impeded the comprehensive evaluation of clinical efficacy of potential therapies managing dyspnea in patients with AHF. Developing a more general measurement tool established on the unified unidimensional scales, standardized operation protocol to record the continuation, and clinically significant difference of dyspnea variation may be a promising approach. In addition, to evaluate the effect of experimental therapies on dyspnea more precisely, the screening time and blinded assessment are factors that need to be considered.
Introduction
Dyspnea is the most common presenting symptom among patients hospitalized for acute heart failure (AHF); more specifically, the prevalence of dyspnea at rest was 38.0% in patients in North America and ≥70.1% in patients in the rest of the world (1). There is room for new therapies to improve the symptoms of AHF, given that 36–54.6% of patients do not experience moderate or marked dyspnea relief within 48 h after standard administration (2–5). Moreover, early dyspnea relief is reportedly associated with a better prognosis in patients with AHF (6, 7). Therefore, dyspnea relief constitutes a clinically relevant therapeutic target and endpoint for clinical trials and regulatory approval (8, 9). It is estimated that 46.67% of the clinical trials have used dyspnea as the primary endpoint for the evaluation of treatment efficacy in AHF (10). However, there are still no widely accepted dyspnea measurement standards in AHF.
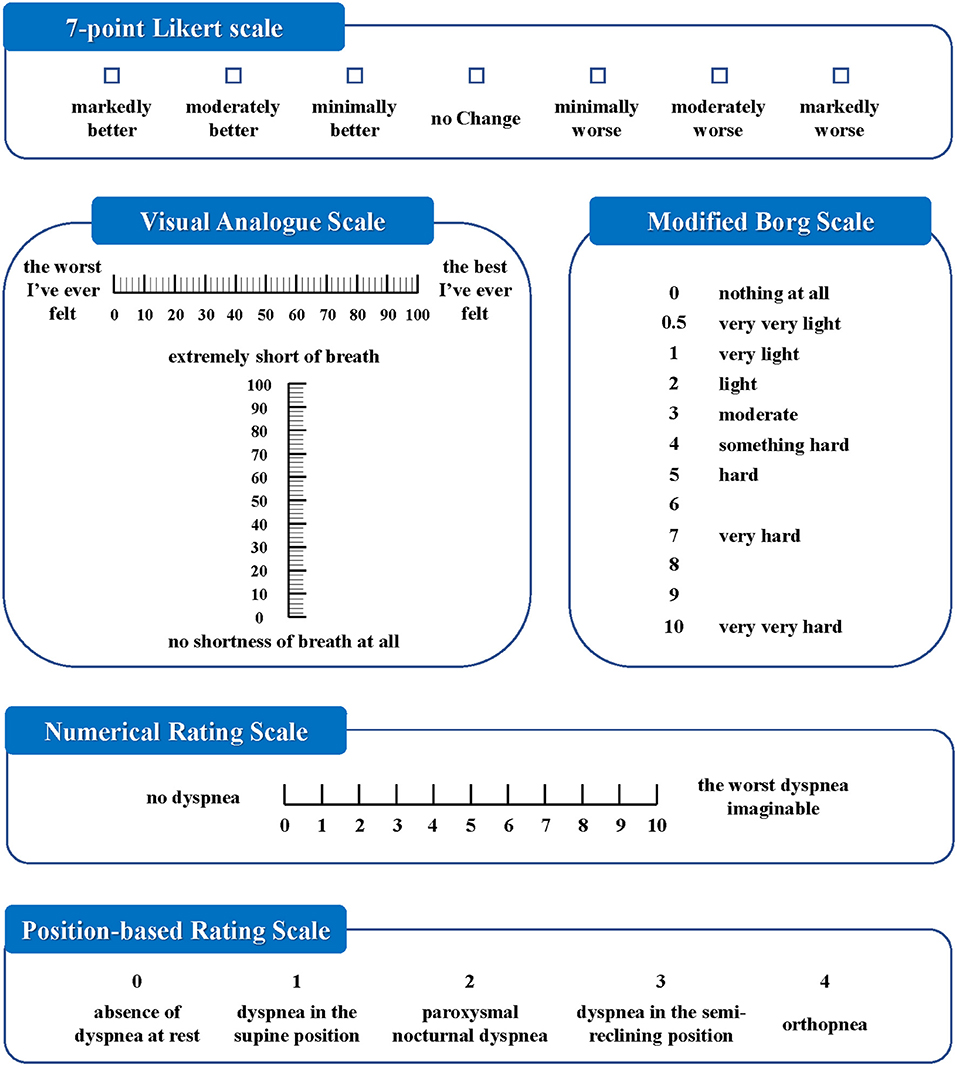
A narrative review published in 2010 described the strengths and weaknesses of different dyspnea measurement scales in AHF clinical trials, such as the Likert scale, visual analog scale (VAS), Borg scale, and dyspnea severity score (DSS) (8). Likert scales consist of 3-, 5-, or 7-point scales that ask patients to rate their feelings on a categorical spectrum. While the VAS asks patients to report or mark on a 0–100 mm line, and the distance from the 0-level of the scale was measured. The modified Borg scale is a 12-point scale in which words describing increasing degrees are assigned numbers of 0, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 (11, 12). The DSS was developed specifically to standardize dyspnea measurements in patients with AHF. It consists of asking patients to rate their level of dyspnea on a 5-point Likert scale in each category of provocative movement, which has patients sitting upright with oxygen, sitting upright without oxygen, lying supine without oxygen, walking 50 m as fast as possible, and a post-6-min walk test. The DSS ranges from 1 to 25 and essentially carries out the measurement when patients can no longer progress in performance (13).
Although a decade has passed since then, the best scales of dyspnea measurement in AHF are still not clear, neither are the timing and corresponding effects of measurement. These are of significant importance to the trial design and efficacy evaluation. Therefore, we aim to systematically review and map the current evidence of dyspnea measurement in patients with AHF in randomized controlled trials (RCTs), with the hope to provide some new insights and recommendations for dyspnea measurement.
Methods
Information Sources and Search Strategy
This study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) reporting guidelines. Guided by the information specialists, two authors conducted a systematic search of the literature in PubMed, Embase, Cochrane Library, and Web of Science databases from inception until August 27, 2020. The search strategy in PubMed is available in eMethods in the Supplementary Material. No language and publication status restrictions were applied. Conference abstract, research protocol, and protocol registration information were screened for further potentially relevant studies. The reference lists of relevant reviews were searched to ensure literature saturation.
Eligibility Criteria
Two authors independently reviewed the abstracts and retrieved the papers that fulfilled the criteria for closer scrutiny. The inclusion criteria were as follows: (i) The study was an RCT involving human participants with AHF (ii) The dyspnea severity was measured as an endpoint, and (iii) The original research article, conference abstract, research protocol, and registration information were used to identify qualified studies. The exclusion criteria were as follows: (i) repetitive reports of the same study (were included as one study as only) (ii) the measurement of dyspnea was not specified and (iii) the full texts were unavailable. In the event of disagreement, the consensus was achieved through discussion.
Data Extraction
Data extraction was performed independently by two authors using a designed form which included: first author, year and journal of publication, study design, study sites, trial acronym, intervention, comparison, duration of screening, whether dyspnea was a primary or secondary endpoint, whether dyspnea was a composite endpoint, description of dyspnea measurement, and the timing and results of dyspnea measurement. Any disagreements in data extraction were resolved by discussion.
Data Analysis and Quality Assessment
For results from more than three RCTs with the same intervention, the dyspnea measurement scale and the timing of measurement were synthesized for meta-analyses using review manager (RevMan5.3, The Cochrane Collaboration, Oxford, UK). For dichotomous outcomes, results were expressed as the risk ratio (RR) with the corresponding 95% confidence interval (CI). For continuous outcomes, results were described with the weighted mean difference (MD) and 95% CI. Heterogeneity was assessed using both the chi-square test (with P < 0.10 to indicate significant heterogeneity) and the I2 value (with I2 > 50% to indicate significant heterogeneity). Estimates with low heterogeneity (P > 0.10 and I2 < 50%) were pooled using a fixed-effect model. Otherwise, a random effect model was used. All the tests were two-sided, and P < 0.05 was considered statistically significant.
The methodological quality for the RCTs was assessed independently by the two authors based on Cochrane risk-of-bias criteria, and each quality item was graded as low, high, or unclear risk. The seven items used to evaluate bias in each trial included the randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
Results
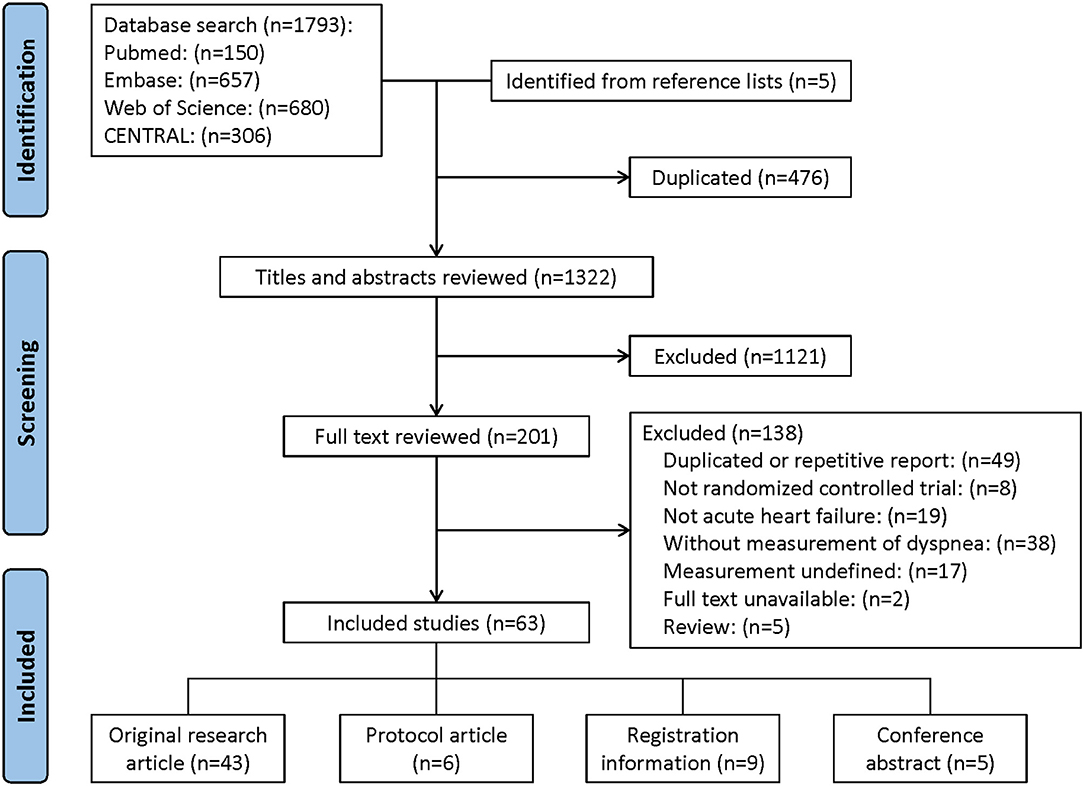
The results of the reference selection and data extraction process are summarized in Figure 1. In all, 1,793 references were identified through database searching and five articles were identified from the reference lists. After a review of titles and abstracts, 201 references were considered potentially eligible and full texts were reviewed. Ultimately, a total of 63 studies were included for data extraction.
Overview of Dyspnea Measurement
Of the 63 included RCTs, 28 studies used dyspnea as the primary endpoint, and of these, seven studies used dyspnea as the composite endpoint. Of the included studies, 26 studies used dyspnea as the secondary endpoint and nine studies did not specify the primary or secondary endpoint.
The severity of dyspnea was mostly assessed by patients themselves. Physician assessment of orthopnea or dyspnea on exertion was applied in 10 studies, and objective measurements such as pulmonary capillary wedge pressure and peak expiratory flow rate were used in six studies. With respect to the procedure of dyspnea assessment, only five studies described information about supplemental oxygen use, and nine studies described the posture of the patient during dyspnea assessment.
Dyspnea Measurement Scales
A total of eight dyspnea measurement scales were used in the included studies (Figures 2, 3). The Likert scale was the most widely used measurement scale of dyspnea in patients with AHF, and the 7-point Likert scale accounted for a large proportion. This scale asks patients to rate their level of dyspnea improvement directly on a 7-point categorical spectrum, ranging from “markedly better” to “markedly worse” (14). It can also act as an anchor to identify clinically important differences when used with continuous scales (15).
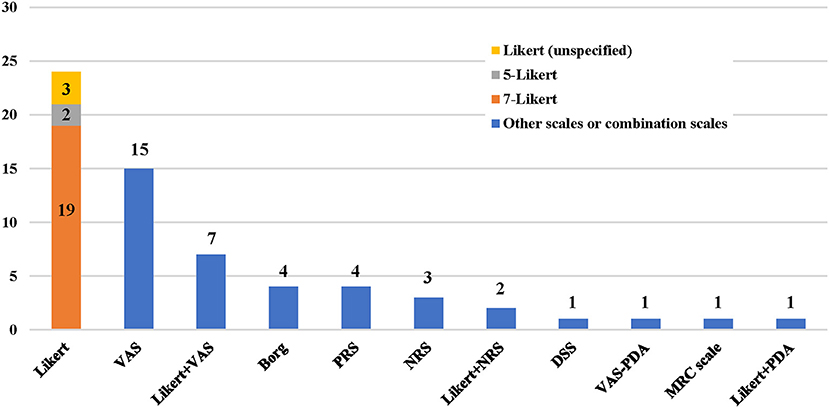
Figure 2. Application frequency of different dyspnea measurement scales. VAS, visual analog scale; PRS, position-based rating scale; NRS, numerical rating scale; DSS, dyspnea severity score; PDA, provocative dyspnea assessment; MRC, medical research council scale.
The VAS, which is understood to sensitively quantify changes in dyspnea severity, is the second-most widely used measurement scale of dyspnea in patients with AHF. The ends of the straight horizontal line are defined as the extreme limits of the parameter to be measured, oriented from the left 0 to the right 100, where 0 was the worst and 100 was the best that the breathing of the patient had ever felt (16). In some studies, the vertical numerical continuum was used, wherein “no shortness of breath at all” was placed at the bottom of the scale and “extremely short of breath” was placed at the top of the scale (17).
The numerical rating scale (NRS) is a segmented numeric version of the VAS in which a respondent selects a whole number from 0 to 10, with 0 being no dyspnea and 10 being the worst dyspnea imaginable (3). A pilot study reported that NRS and VAS showed good agreement when assessing dyspnea severity in the emergency department (18).
Some scales involved statuses such as when a patient experienced dyspnea, respective to the position, provocative movement, and oxygen supply. The position-based rating scale (PRS) assessed dyspnea with the combination of the position and symptom of patients, i.e., absence of dyspnea at rest, dyspnea in the supine position, paroxysmal nocturnal dyspnea, dyspnea in the semireclining position, and orthopnea (19).
The provocative dyspnea assessment (PDA) scale refers to an ordered approach to assess dyspnea across a series of conditions that are increasingly difficult for a patient to tolerate. It may provide a robust profile of dyspnea that is sensitive to change. However, in the RED-ROSE trial, exercise provocation proved to have unacceptable feasibility in the AHF cohort (20). Therefore, some researchers modified it and proposed the VAS-PDA. The subjects assessed their dyspnea severity using VAS in up to three positions as tolerated at each time point, with a score of 0 indicating no dyspnea and a score of 100 indicating very severe dyspnea. Position-1: sitting upright on supplemental oxygen. Position-2: sitting upright off oxygen. Position-3: lying supine off oxygen. Subjects acclimated at each position for 5 min. This created a summed scaled score that ranged from the best dyspnea (0 at all 3 positions = 0) to the worst (100 at all 3 positions = 300) (21).
The medical research council (MRC) scale was developed for grading the effect of dyspnea on daily activities. It comprises five items: 1 (experiencing shortness of breath only during vigorous exercise); 2 (experiencing shortness of breath when walking briskly or ascending a gentle slope); 3 (walking slower than other people their age due to shortness of breath or having to stop to catch their breath even when walking slowly); 4 (stopping to catch their breath after walking <100 m or after a few minutes); and 5 (experiencing so much shortness of breath that they no longer leave the home, or experiencing shortness of breath when getting dressed) (22). In one study, this scale was not sensitive enough for patients with AHF to track responses to therapy during a single hospital stay (8).
Timing and Results of Dyspnea Measurement
Among the 43 RCTs (3, 4, 14, 16, 17, 19, 21–57) with results reported in original research articles, 23 studies mentioned the duration of screening and in 91.3% of studies, the screening was within 24 h from symptom presentation. The dyspnea was assessed most frequently on days 1, 2, 3, and 6 h after randomization or when the study therapy was given (Figure 4).
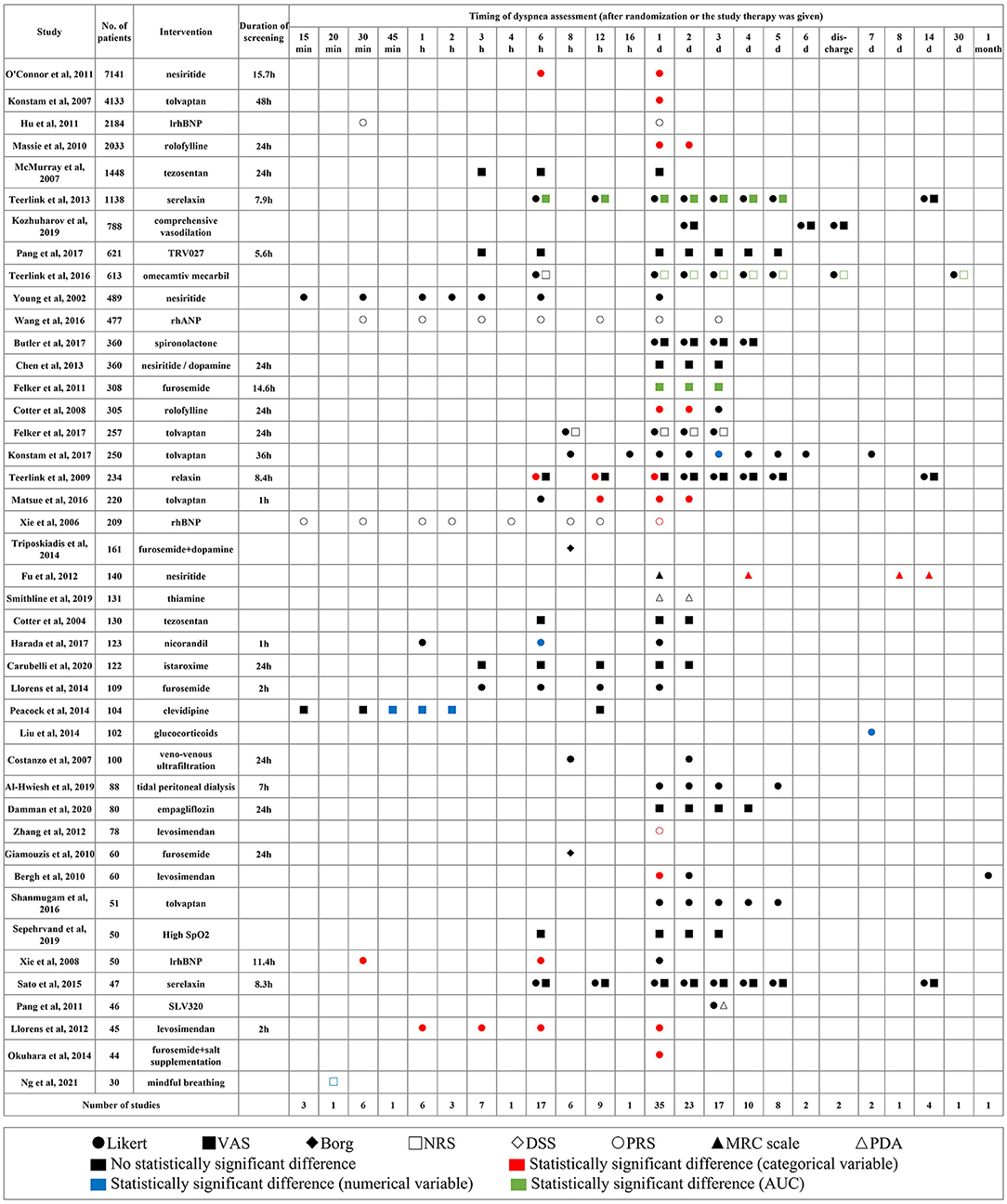
Figure 4. Applied scales, timing, and results of dyspnea measurement in different studies. Shapes represent different scales, colors represent different types of variables, and the statistical significance of results. VAS, visual analog scale; PRS, position-based rating scale; NRS, numerical rating scale; DSS, dyspnea severity score; PDA, provocative dyspnea assessment; MRC, medical research council scale; AUC, area under the curve.
Compared with control groups, better dyspnea relief was observed in experimental groups in 21 studies (p < 0.05), half of which came from proportions of dyspnea improvement measured by the Likert scale. However, improvements on dyspnea were not consistent when measured by different scales in the same study.
The data from the Likert scale was usually analyzed as a categorical variable considering markedly improved and moderately improved as improvement responders. A few other studies inappropriately analyzed it as a numerical variable and calculated the mean and SD (58). The VAS and NRS were used to quantify persistent relief in dyspnea by the change in area under the curve (AUC) through day 3 or 5.
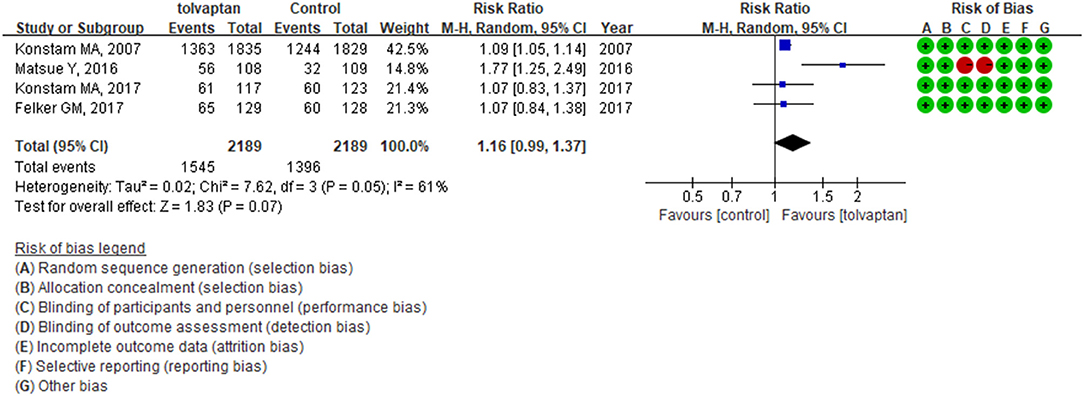
Four studies assessed tolvaptan compared with control on the proportion of dyspnea improvement and had divergent results. The synthesized results did not indicate the beneficial effect of tolvaptan on day 1 (RR: 1.16; 95% CI: 0.99–1.37; p = 0.07; I2 = 61%). The EVEREST trial had a much larger sample size, and the AQUAMARINE trial did not apply placebo control and blinding (3, 23). The TACTICS-HF trial and SECRET of CHF trial each produced similar results (Figure 5) (4, 35).
Discussion
Of the 63 included RCTs, the severity of dyspnea was mostly assessed by patients themselves. Dyspnea is the comprehensive real-world feeling of a patient and deserves full respect. Physician assessment or a single objective measurement is unable to replace the feelings of patients (59, 60); therefore, patient-reported dyspnea measurement scales have been widely used. The Likert scale and VAS were the most accepted tools as demonstrated in this article, which was consistent with the findings of previous reviews (8, 61). Compared with chronic heart failure, the choice of dyspnea measurement scales for patients with AHF emphasizes its usability and sensitivity. The Likert scale is more comprehensible and could directly discriminate the change of dyspnea severity. As a complement, VAS could sensitively quantify the degree of subjective feelings and allow continuous assessment. In the MEASURE-HF trial, the Likert measures of dyspnea initially improved rapidly (day 1, 2) with no significant improvement thereafter (day 7); whereas, the VAS measures of dyspnea improved continually throughout the length of hospital stay (62). Therefore, multiple dyspnea measurement scales should be used simultaneously to capture the entirety of the dyspnea symptom throughout the study.
It is generally believed that a reliable dyspnea measurement with standardized assessment procedures remains a critical unmet need in AHF research (10, 63). Provocative assessment is a reasonable approach, except that exercise provocation has unacceptable feasibility in patients with AHF. However, it is necessary to define the body position and oxygen use of the patient during dyspnea assessment, which was seldom reported in available reports. Furthermore, instead of simply adding up the scores achieved under different conditions, analyzing the scores under separate conditions can make the results more easily understood or interpretable. Regarding the timing of dyspnea measurement, our review showed that it was most frequently on days 1, 2, 3, and 6 h after randomization or when the study therapy was given. It is obvious that the diverse measurement scales, analytical approaches, and the timing of measurements impeded the comprehensive evaluation of the potential therapies. To address this, it is necessary to distinguish the measurement scale and operation procedure for dyspnea assessment. The dyspnea severity and variation could be recorded by unified unidimensional scales, such as the Likert scale and VAS. While the condition and timing of measurement could follow the standardized operation protocol that was established based on the understanding of the disease and experimental therapies (27, 64). With advancements in information technology, we could also record and manage in a timely manner the unstructured data (descriptive text, images, video, and audio material) to understand the provocation condition of dyspnea, its accompanying symptoms, and its impact on the quality of life. This will provide a more general measurement tool to assess patient-reported outcomes like dyspnea.
To more precisely evaluate the effect of experimental therapies on dyspnea in patients with AHF, the screening time from presentation to randomization is one of the factors that should be considered. As is reported in the ASCEND-HF trial, earlier administration of study medication was associated with modestly better dyspnea relief (65). For agents targeting symptom improvements, patients should be enrolled when symptoms are at the peak to minimize concomitant therapy if the effect of the novel agent is to be determined (66). However, the association between earlier administration and better dyspnea relief was not observed on the evidence map and requires further research. In addition, the results of dyspnea assessment can be quite different owing to its subjective nature. In the URGENT study, of the patients with AHF managed conventionally and enrolled within 1 h of first hospital medical evaluation, 58.4% reported moderate or marked dyspnea improvement at 6 h (67). While in the AQUAMARINE study, with a similar screening period, only 13% of the patients with AHF receiving conventional treatment experienced moderate or marked dyspnea improvement at 6 h (4). Therefore, for comparison of treatment effects, it is necessary to conduct a subjective assessment of dyspnea under blind conditions.
Limitations
This study has some limitations. The recognition of positive results was based on the original reports of the statistically significant difference (p < 0.05) which should be interpreted with discretion. Moreover, we only synthesized the results of more than three RCTs with the same intervention, dyspnea measurement scale, and timing of measurement, considering the clinical homogeneity.
Conclusions
This review and evidence map discusses the current evidence of dyspnea measurement in RCTs with patients with AHF. The applied scales, analytical approaches, and timing of measurement are in diversity, which has impeded the comprehensive evaluation of clinical efficacy of potential therapies managing dyspnea in patients with AHF. A more general measurement tool is warranted, which could be established on the unified unidimensional scales and standardized operation protocol to record the continuation and clinically significant difference of dyspnea variation. With advancements in information technology, we can manage the unstructured data to understand the provocation condition of dyspnea, its accompanying symptoms, and its impact on the quality of life. In addition, to more precisely evaluate the effect of experimental therapies on dyspnea in patients with AHF, the screening time and blinded assessment of dyspnea are factors that should be considered.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
HS, YL, JC, and XZ: conception and design. HS and JC: administrative support. XZ, CZ, HZ, WL, and JZ: collection and assembly of data. XZ, YL, CZ, KZ, and LZ: data analysis and interpretation. ZC, LY, and YW: provision of study materials or patients. All authors: manuscript writing and final approval of manuscript.
Funding
This study was supported by the National Key R&D Program of China (2017YFC1700400 to HS) and the National Natural Science Foundation of China (82004219 to XZ and 81803963 to CZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.728772/full#supplementary-material
References
1. Filippatos G, Angermann CE, Cleland JGF, Lam CSP, Dahlström U, Dickstein K, et al. Global differences in characteristics, precipitants, and initial management of patients presenting with acute heart failure. JAMA Cardiol. (2020) 5:401–10. doi: 10.1001/jamacardio.2019.5108
2. Metra M, O'Connor CM, Davison BA, Cleland JG, Ponikowski P, Teerlink JR, et al. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J. (2011) 32:1519–34. doi: 10.1093/eurheartj/ehr042
3. Felker GM, Mentz RJ, Cole RT, Adams KF, Egnaczyk GF, Fiuzat M, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. (2017) 69:1399–406. doi: 10.1016/j.jacc.2016.09.004
4. Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, et al. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction [published correction appears in J Card Fail. 2016 22:941]. J Card Fail. (2016). 22:423–32. doi: 10.1016/j.cardfail.2016.02.007
5. Gheorghiade M, Ruschitzka F. Beyond dyspnoea as an endpoint in acute heart failure trials. Eur Heart J. (2011) 32:1442–5. doi: 10.1093/eurheartj/ehr044
6. Mentz RJ, Hernandez AF, Stebbins A, Ezekowitz JA, Felker GM, Heizer GM, et al. Predictors of early dyspnoea relief in acute heart failure and the association with 30-day outcomes: findings from ASCEND-HF. Eur J Heart Fail. (2013) 15:456–64. doi: 10.1093/eurjhf/hfs188
7. Torii S, Matsue Y, Suzuki M, Yamaguchi S, Fukamizu S, Ono Y, et al. The relationship between dyspnea relief and prognosis in patients with acute heart failure-insights from AQUAMARINE study. Eur Heart J. (2016) 37:1325. doi: 10.1093/eurheartj/ehw434
8. West RL, Hernandez AF, O'Connor CM, Starling RC, Califf RM. A review of dyspnea in acute heart failure syndromes. Am Heart J. (2010) 160:209–14. doi: 10.1016/j.ahj.2010.05.020
9. Zannad F, Garcia AA, Anker SD, Armstrong PW, Calvo G, Cleland JG, et al. Clinical outcome endpoints in heart failure trials: a European Society of Cardiology Heart Failure Association consensus document. Eur J Heart Fail. (2013) 15:1082–94. doi: 10.1093/eurjhf/hft095
10. Goenka L, George M, Selvarajan S. End points in heart failure-are we doing it right? Eur J Clin Pharmacol. (2017) 73:651–9. doi: 10.1007/s00228-017-2228-0
11. Cullen DL, Rodak B. Clinical utility of measures of breathlessness. Respir Care. (2002) 47:986–93.
12. Burdon JG, Juniper EF, Killian KJ, Hargreave FE, Campbell EJ. The perception of breathlessness in asthma. Am Rev Respir Dis. (1982) 126:825–8.
13. Pang PS, Cleland JG, Teerlink JR, Collins SP, Lindsell CJ, Sopko G, et al. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J. (2008) 29:816–24. doi: 10.1093/eurheartj/ehn048
14. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure [published correction appears in N Engl J Med. 2011 365:773. Wilson, W H [corrected to Tang, W H W]]. N Engl J Med. (2011) 365:32–43. doi: 10.1056/NEJMx110061
15. Pang PS, Lane KA, Tavares M, Storrow AB, Shen C, Peacock WF, et al. Is there a clinically meaningful difference in patient reported dyspnea in acute heart failure? An analysis from URGENT Dyspnea. Heart Lung. (2017) 46:300–7. doi: 10.1016/j.hrtlng.2017.03.003
16. Pang PS, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, et al. Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur Heart J. (2017) 38:2364–73. doi: 10.1093/eurheartj/ehx196
17. Cotter G, Kaluski E, Stangl K, Pacher R, Richter C, Milo-Cotter O, et al. The hemodynamic and neurohormonal effects of low doses of tezosentan (an endothelin A/B receptor antagonist) in patients with acute heart failure. Eur J Heart Fail. (2004) 6:601–9. doi: 10.1016/j.ejheart.2004.05.004
18. Placido R, Gigaud C, Gayat E, Ferry A, Cohen-Solal A, Plaisance P, et al. Assessment of dyspnoea in the emergency department by numeric and visual scales: a pilot study. Anaesth Crit Care Pain Med. (2015) 34:95–9. doi: 10.1016/j.accpm.2014.09.001
19. Wang G, Wang P, Li Y, Liu W, Bai S, Zhen Y, et al. Efficacy and safety of 1-hour infusion of recombinant human atrial natriuretic peptide in patients with acute decompensated heart failure: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Medicine (Baltimore). (2016) 95:e2947. doi: 10.1097/MD.0000000000002947
20. AbouEzzeddine OF, Lala A, Khazanie PP, Shah R, Ho JE, Chen HH. Evaluation of a provocative dyspnea severity score in acute heart failure. Am Heart J. (2016) 172:34–41. doi: 10.1016/j.ahj.2015.10.009
21. Smithline HA, Donnino M, Blank FSJ, Barus R, Coute RA, Knee AB, et al. Supplemental thiamine for the treatment of acute heart failure syndrome: a randomized controlled trial. BMC Complement Altern Med. (2019) 19:96. (2019). doi: 10.1186/s12906-019-2506-8
22. Fu S, Yi S, Zhu B, Wang L, Wang H, Bai Y, et al. Efficacy and safety of a modified dosage regimen of nesiritide in patients older than 75 years with acute heart failure. Aging Clin Exp Res. (2012) 24:524–9. doi: 10.3275/8295
23. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. (2007) 297:1319–31. doi: 10.1001/jama.297.12.1319
24. Recombinant Human Brain Natriuretic Peptide Multicenter Clinical Study Group, Hu DY. Efficacy and safety of intravenous recombinant human brain natriuretic peptide in patients with decompensated acute heart failure: a multicenter, randomized, open label, controlled study. Zhonghua Xin Xue Guan Bing Za Zhi. (2011) 39:305–8. doi: 10.3760/cma.j.issn.0253-3758.2011.04.005
25. Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. (2010) 363:1419–28. doi: 10.1056/NEJMoa0912613
26. McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. (2007) 298:2009–19. doi: 10.1001/jama.298.17.2009
27. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. (2013) 381:29–39. doi: 10.1016/S0140-6736(12)61855-8
28. Kozhuharov N, Goudev A, Flores D, Maeder MT, Walter J, Shrestha S, et al. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA. (2019) 322:2292–302. doi: 10.1001/jama.2019.18598
29. Teerlink JR, Felker GM, McMurray JJV, Ponikowski P, Metra M, Filippatos GS, et al. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC-AHF study. J Am Coll Cardiol. (2016) 67:1444–55. doi: 10.1016/j.jacc.2016.01.031
30. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial [published correction appears in JAMA 2002 288:577]. JAMA. (2002). 287:1531–40. doi: 10.1001/jama.287.12.1531
31. Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol. (2017) 2:950–8. doi: 10.1001/jamacardio.2017.2198
32. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. (2013) 310:2533–43. doi: 10.1001/jama.2013.282190
33. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. (2011) 364:797–805. doi: 10.1056/NEJMoa1005419
34. Cotter G, Dittrich HC, Weatherley BD, Bloomfield DM, O'Connor CM, Metra M, et al. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail. (2008) 14:631–40. doi: 10.1016/j.cardfail.2008.08.010
35. Konstam MA, Kiernan M, Chandler A, Dhingra R, Mody FV, Eisen H, et al. Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol. (2017) 69:1409–19. doi: 10.1016/j.jacc.2016.12.035
36. Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. (2009) 373:1429–39. doi: 10.1016/S0140-6736(09)60622-X
37. Recombinant Human Brain Natriuretic Peptide Study Group. Comparison of the effects of intravenous recombinant human brain natriuretic peptide and nitroglycerin in patients with decompensated acute heart failure: a multicenter, randomized, open-label, parallel-design study. Zhonghua Xin Xue Guan Bing Za Zhi. (2006) 34:222–6 [in Chinese].
38. Triposkiadis FK, Butler J, Karayannis G, Starling RC, Filippatos G, Wolski K, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol. (2014) 172:115–21. doi: 10.1016/j.ijcard.2013.12.276
39. Harada K, Yamamoto T, Okumura T, Shigekiyo M, Terada N, Okada A, et al. Intravenous nicorandil for treatment of the urgent phase acute heart failure syndromes: a randomized, controlled trial. Eur Heart J Acute Cardiovasc Care. (2017) 6:329–38. doi: 10.1177/2048872616633837
40. Carubelli V, Zhang Y, Metra M, Lombardi C, Felker GM, Filippatos G, et al. Treatment with 24 hour istaroxime infusion in patients hospitalised for acute heart failure: a randomised, placebo-controlled trial. Eur J Heart Fail. (2020) 22:1684–93. doi: 10.1002/ejhf.1743
41. Llorens P, Miró Ò, Herrero P, Martín-Sánchez FJ, Jacob J, Valero A, et al. Clinical effects and safety of different strategies for administering intravenous diuretics in acutely decompensated heart failure: a randomised clinical trial. Emerg Med J. (2014) 31:706–13. doi: 10.1136/emermed-2013-202526
42. Peacock WF, Chandra A, Char D, Collins S, Der Sahakian G, Ding L, et al. Clevidipine in acute heart failure: results of the a study of blood pressure control in acute heart failure-a pilot study (PRONTO). Am Heart J. (2014) 167:529–36. doi: 10.1016/j.ahj.2013.12.023
43. Liu C, Liu K; COPE-ADHF Study Group. Cardiac outcome prevention effectiveness of glucocorticoids in acute decompensated heart failure: COPE-ADHF study. J Cardiovasc Pharmacol. (2014) 63:333–8. doi: 10.1097/FJC.0000000000000048
44. Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure [published correction appears in J Am Coll Cardiol. (2007) 49:1136]. J Am Coll Cardiol. (2007) 49:675–83. doi: 10.1016/j.jacc.2006.07.073
45. Al-Hwiesh AK, Abdul-Rahman IS, Al-Audah N, Al-Hwiesh A, Al-Harbi M, Taha A. Tidal peritoneal dialysis versus ultrafiltration in type 1 cardiorenal syndrome: a prospective randomized study. Int J Artif Organs. (2019) 42:684–94. doi: 10.1177/0391398819860529
46. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. (2020) 22:713–22. doi: 10.1002/ejhf.1713
47. Zhang YH, Qing EM, Zhang J, Huang Y, Lü R, Zhou Q, et al. Hemodynamic and efficacies of domestic levosimendan versus dobutamine in patients with acute decompensated heart failure. Zhonghua Yi Xue Za Zhi. (2012) 92:555–8 [in Chinese].
48. Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, Parisis C, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. (2010) 16:922–30. doi: 10.1016/j.cardfail.2010.07.246
49. Bergh CH, Andersson B, Dahlström U, Forfang K, Kivikko M, Sarapohja T, et al. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur J Heart Fail. (2010) 12:404–10. doi: 10.1093/eurjhf/hfq032
50. Shanmugam E, Doss CR, George M, Jena A, Rajaram M, Ramaraj B, et al. Effect of tolvaptan on acute heart failure with hyponatremia–a randomized, double blind, controlled clinical trial. Indian Heart J. (2016) 68(Suppl 1):S15–S21. doi: 10.1016/j.ihj.2015.07.006
51. Sepehrvand N, Alemayehu W, Rowe BH, McAlister FA, van Diepen S, Stickland M, et al. High vs. low oxygen therapy in patients with acute heart failure: HiLo-HF pilot trial. ESC Heart Fail. (2019) 6:667–77. doi: 10.1002/ehf2.12448
52. Xie CL, Meng SR, Wang W, Chen SM, Li P, Feng XG. Therapeutic effect of recombinant human brain natriuretic peptide for treatment of decompensated heart failure: comparison with nitroglycerin. Nan Fang Yi Ke Da Xue Xue Bao. (2008) 28:839–42 [in Chinese].
53. Sato N, Takahashi W, Hirayama A, Ajioka M, Takahashi N, Okishige K, et al. Multicenter, randomized, double-blinded, placebo-controlled phase II study of serelaxin in Japanese patients with acute heart failure. Circ J. (2015) 79:1237–47. doi: 10.1253/circj.CJ-15-0227
54. Pang PS, Mehra M, Maggioni AP, Filippatos G, Middlebrooks J, Turlapaty P, et al. Rationale, design, and results from RENO-DEFEND 1: a randomized, dose-finding study of the selective A1 adenosine antagonist SLV320 in patients hospitalized with acute heart failure. Am Heart J. (2011) 161:1012–23.e3. doi: 10.1016/j.ahj.2011.03.004
55. Llorens P, Miro O, Roman F, Zapater P, Carbajosa-Dalmau J, Llanos L. Efficacy of early administration of levosimendan in emergency department in patients with acute heart failure: a randomized pilot clinical trial. Emergencias. (2012) 24:268–76.
56. Okuhara Y, Hirotani S, Naito Y, Nakabo A, Iwasaku T, Eguchi A, et al. Intravenous salt supplementation with low-dose furosemide for treatment of acute decompensated heart failure. J Card Fail. (2014) 20:295–301. doi: 10.1016/j.cardfail.2014.01.012
57. Ng DL, Chai CS, Tan KL, Chee KH, Tung YZ, Wai SY, et al. The efficacy of a single session of 20-minute mindful breathing in reducing dyspnea among patients with acute decompensated heart failure: a randomized controlled trial. Am J Hosp Palliat Care. (2021) 38:246–52. doi: 10.1177/1049909120934743
58. Jamieson S. Likert scales: how to (ab)use them. Med Educ. (2004) 38:1217–8. doi: 10.1111/j.1365-2929.2004.02012.x
59. Smithline HA, Caglar S, Blank FS. Physician vs patient assessment of dyspnea during acute decompensated heart failure. Congest Heart Fail. (2010) 16:60–4. doi: 10.1111/j.1751-7133.2009.00127.x
60. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. (2006) 119(12 Suppl. 1):S3–S10. doi: 10.1016/j.amjmed.2006.09.011
61. Johnson MJ, Oxberry SG, Cleland JG, Clark AL. Measurement of breathlessness in clinical trials in patients with chronic heart failure: the need for a standardized approach: a systematic review. Eur J Heart Fail. (2010) 12:137–47. doi: 10.1093/eurjhf/hfp194
62. Allen LA, Metra M, Milo-Cotter O, Filippatos G, Reisin LH, Bensimhon DR, et al. Improvements in signs and symptoms during hospitalization for acute heart failure follow different patterns and depend on the measurement scales used: an international, prospective registry to evaluate the evolution of measures of disease severity in acute heart failure (MEASURE-AHF). J Card Fail. (2008) 14:777–84. doi: 10.1016/j.cardfail.2008.07.188
63. Pang PS, Collins SP, Sauser K, Andrei AC, Storrow AB, Hollander JE, et al. Assessment of dyspnea early in acute heart failure: patient characteristics and response differences between likert and visual analog scales. Acad Emerg Med. (2014) 21:659–66. doi: 10.1111/acem.12390
64. Zhang J, Sun Y, Zhou K, Zhang X, Chen Y, Hu J, et al. Rationale and design of the AUGUST-AHF Study. ESC Heart Fail. (2020) 7:3124–3133. doi: 10.1002/ehf2.12787
65. Wong YW, Mentz RJ, Felker GM, Ezekowitz J, Pieper K, Heizer G, et al. Nesiritide in patients hospitalized for acute heart failure: does timing matter? Implication for future acute heart failure trials. Eur J Heart Fail. (2016) 18:684–92. doi: 10.1002/ejhf.487
66. Collins SP, Levy PD, Lindsell CJ, Pang PS, Storrow AB, Miller CD, et al. The rationale for an acute heart failure syndromes clinical trials network. J Card Fail. (2009) 15:467–74. doi: 10.1016/j.cardfail.2008.12.013
Keywords: evidence map, systematic review, measurement, acute heart failure, dyspnea
Citation: Zhang X, Zhao C, Zhang H, Liu W, Zhang J, Chen Z, You L, Wu Y, Zhou K, Zhang L, Liu Y, Chen J and Shang H (2021) Dyspnea Measurement in Acute Heart Failure: A Systematic Review and Evidence Map of Randomized Controlled Trials. Front. Med. 8:728772. doi: 10.3389/fmed.2021.728772
Received: 22 June 2021; Accepted: 31 August 2021;
Published: 06 October 2021.
Edited by:
Kevin Lu, University of South Carolina, United StatesReviewed by:
Wei Jiang, Guangdong Provincial Hospital of Chinese Medicine, ChinaYue Liu, Xiyuan Hospital, China
Sam Li, University of Tennessee Health Science Center (UTHSC), United States
Copyright © 2021 Zhang, Zhao, Zhang, Liu, Zhang, Chen, You, Wu, Zhou, Zhang, Liu, Chen and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongcai Shang, c2hhbmdob25nY2FpQGZveG1haWwuY29t; Jianxin Chen, Y2p4QGJ1Y20uZWR1LmNu; Yan Liu, c2FzbGl1QHllYWgubmV0
†These authors share first authorship
 Xiaoyu Zhang
Xiaoyu Zhang Chen Zhao
Chen Zhao Houjun Zhang1†
Houjun Zhang1† Wenjing Liu
Wenjing Liu Zhao Chen
Zhao Chen Yan Liu
Yan Liu Jianxin Chen
Jianxin Chen Hongcai Shang
Hongcai Shang