
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 23 September 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.727706
This article is part of the Research Topic Diagnosis, Treatment and Prognosis of Viral Hepatitis View all 21 articles
 Rongrong Ding1
Rongrong Ding1 Wei Lu1
Wei Lu1 Xinlan Zhou1
Xinlan Zhou1 Dan Huang1
Dan Huang1 Yanbing Wang1
Yanbing Wang1 Xiufen Li1
Xiufen Li1 Li Yan1
Li Yan1 Weijia Lin1
Weijia Lin1 Shu Song2*
Shu Song2* Zhanqing Zhang1*
Zhanqing Zhang1* Liang Chen3*
Liang Chen3*Background: Some controversy remains regarding conventional serum indices for the evaluation of liver fibrosis. Therefore, we aimed to combine the existing index with other serum parameters to discriminate liver fibrosis stages in patients with chronic hepatitis B (CHB).
Methods: A total of 1,622 treatment-naïve CHB patients were divided into training (n = 1,211) and validation (n = 451) cohorts. Liver histology was assessed according to the Scheuer scoring scheme. All common demographic and clinical parameters were analyzed.
Results: By utilizing the results of the logistic regression analysis, we developed a novel index, the product of GPR, international normalized ratio (INR), and type IV collagen (GIVPR), to discriminate liver fibrosis. In the training group, the areas under the ROCs (AUROCs) of GIVPR, APRI, FIB-4, and GPR for significant fibrosis were 0.81, 0.75, 0.72, and 0.77, respectively; the AUROCs of GIVPR, APRI, FIB-4, and GPR for advanced fibrosis were 0.82, 0.74, 0.74, and 0.78, respectively; and the AUROCs of GIVPR, APRI, FIB-4, and GPR for cirrhosis were 0.87, 0.78, 0.78, and 0.83, respectively. Similar results were also obtained in the validation group. Furthermore, the decision curve analysis suggested that GIVPR represented superior clinical benefits in both independent cohorts.
Conclusion: The GIVPR constructed on GPR represents a superior predictive model for discriminating liver fibrosis in CHB patients.
Hepatitis B virus (HBV) infection is a serious public health problem. It is estimated that more than 350 million people are chronically infected worldwide (1). From 1990 to 2013, the mortality rate of liver cirrhosis and hepatocellular carcinoma caused by HBV infection increased by 33% worldwide (2). Based on the outcomes of patients who receive early diagnosis and effective antiviral therapy, the prognosis of CHB can be significantly improved even if the case is histologically advanced fibrosis or cirrhosis (3). Therefore, it is of great importance to assess the risk of early liver fibrosis in CHB patients.
Currently, the gold standard for the assessment of liver fibrosis is still liver biopsy. However, its limitations, such as its invasiveness, sampling errors, cost, intra- and inter-observer discrepancies, and the risk of potentially life-threatening complications, restrict its clinical application (4). Clinical practice requires simple operations or non-invasive and easy methods to diagnose liver inflammation, injury or fibrosis (5). The World Health Organization (WHO) guidelines recommend serologic biomarkers and FibroScan as useful non-invasive methods for evaluating CHB patients (6). However, several factors, including necroinflammatory activity, ascites, cost, and lack of skilled operators, may diminish the clinical use of FibroScan (6, 7). Serum biomarkers are particularly important in these methods because they do not require qualified staff and expensive equipment for evaluation (8). The WHO has recommended the aspartate aminotransferase (AST)-platelet ratio index (APRI) and fibrosis-4 (FIB-4) as non-invasive indices for CHB patients (6). The diagnostic value of these two indices in liver fibrosis has been widely studied, but their sensitivity and specificity are still controversial (9). Recently, a study by Lemonie et al. (10) suggested that the γ-glutamyl transpeptidase to platelet ratio (GPR) was more accurate than APRI or FIB-4, and this study was supported by several studies on Chinese subjects (11, 12). However, there were still a few inconsistent conclusions (13). Therefore, novel non-invasive serum calculations are still needed because the current biochemical markers do not have enough diagnostic accuracy to replace liver biopsy.
Serum collagen, especially type IV collagen, has been confirmed to be a useful, non-invasive marker for measuring the activity of this pathway at a single time point and has been shown to reflect prognosis and responses to a variety of chronic liver diseases (14). INR is a routine serological marker associated with liver function and essentially reflects the progression of liver diseases. Wu et al. reported that the INR was an independent factor for the prediction of significant fibrosis in patients with CHB (6, 15).
More efforts should be dedicated to pursuing simple, safe and reliable non-invasive diagnostic measures to stage liver fibrosis. In this study, we aimed to construct and validate a predictive index consisting of GPR, INR, and type IV collagen to reflect liver fibrosis simply and effectively in CHB patients.
Overall, between January 2014 and January 2021, we retrospectively screened 2,193 consecutive Chinese individuals with chronic hepatitis B who underwent liver biopsy and clinical examination at Shanghai Public Health Clinical Center, Fudan University. CHB was diagnosed when serum hepatitis B surface antigen (HBsAg) was persistently positive for more than 6 months (16). All the patients were >18 years old. Non-alcoholic fatty liver disease (NAFLD) was diagnosed as at least 5% biopsy-proven hepatic steatosis without significant alcohol consumption (17). The exclusion criteria were as follows: antiviral treatment history, coinfection with hepatitis C virus (HCV), hepatitis D virus (HDV), hepatitis E virus (HEV), or human immunodeficiency virus (HIV), significant alcohol consumption (>20 g/d), autoimmune hepatitis, hepatocellular carcinoma, decompensated cirrhosis, inadequate liver biopsy samples (<1.5 cm), and the use of warfarin.
We summarized the flow diagram of the study population in Figure 1. After excluding patients with coinfection with HCV, HDV, HEV, or HIV (n = 113), alcohol consumption (>20 g/d) (n = 104), autoimmune hepatitis (n = 51), history of antiviral treatment (n = 128), and incomplete clinical data (n = 79), 1,662 treatment-naïve patients with CHB were included. The population was randomly divided into a training set (n = 1,211) and a validation set (n = 451) for model development and validation using SPSS software.
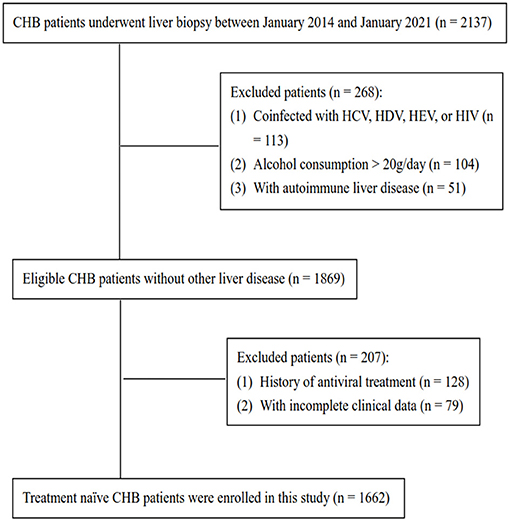
Figure 1. Flow diagram of this study population. CHB, chronic hepatitis B; HCV, hepatitis C virus; HDV, hepatitis D virus; HEV, hepatitis E virus; HIV, human immunodeficiency virus.
Percutaneous liver biopsy was performed using a 16 G needle under ultrasound guidance. Liver samples with a minimum length of 1.5 cm and at least 7 complete portal tracts were fixed in 10% formalin, embedded in paraffin, and stained with HE Masson's trichrome and reticulin for histological analysis. Liver histology was analyzed by two experienced pathologists who were blinded to other clinical and laboratory data and classified according to the Scheuer scoring system (18) as follows: S0 (no fibrosis), S1 (mild fibrosis without septa), S2 (moderate fibrosis with few septa), S3 (severe fibrosis with numerous septa without cirrhosis), and S4 (cirrhosis). In this study, liver fibrosis stage ≥S2 was defined as significant fibrosis, ≥S3 was defined as advanced fibrosis, and S4 was defined as cirrhosis. These definitions represent at minimum significant fibrosis and affect the management of patients in terms of treatment indications (16, 19).
Fasting blood samples were obtained within a week of liver biopsy. Platelets and other blood cells were counted using a Sysmex-XT 4000i automated hematology analyzer. The international normalized ratio (INR) and other coagulation indices were measured using a STAR Max automatic coagulation analyzer. Alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), hyaluronic acid, laminin, N-terminal propeptide of type III procollagen (PIIINP), type IV collagen, and other serum biochemical parameters were measured using an Architect C16000 automatic biochemical analysis system.
The formulas for APRI, FIB-4, and GPR are as follows: APRI = (AST (U/L)/ULN of AST)/platelet count (109/L) × 100 (20); FIB-4 = (age (years) × AST (U/L))/(platelet count (109/L) × (ALT (U/L))1/2) (21); GPR = (GGT (U/L)/ULN of GGT)/platelet count (109/L) × 100 (10).
Statistical analysis was performed using IBM SPSS Statistics version 26.0 (SPSS Inc., Chicago, USA) and R 4.0.3 (http://www.R-project.org). Continuous variables are expressed as the mean ± standard deviation or median (interquartile range, IQR) and were compared using Student's t-test (for normally distributed continuous variables) or the independent Mann–Whitney U-test (for non-normally distributed continuous variables). Categorical variables are expressed as proportions and were compared by the chi-square test. Logistic regression models were used to assess the correlations between variables and liver fibrosis. The performances of the non-invasive markers for predicting liver fibrosis were assessed by receiver operating characteristic (ROC) curve analyses. The Delong Z-test was used to compare the AUROCs of the serum models. Decision curve analysis (DCA) was used to further evaluate the predictive performances. A two-sided P < 0.05 was considered statistically significant.
A total of 1,662 treatment-naïve CHB patients who had undergone a liver biopsy were enrolled in the study, with median ages of 37 (31–45) and 37 (31–45) years in the training and validation sets, respectively. The clinical data of the studied groups are summarized in Table 1. Except for ALT, there were no statistically significant differences in other parameters between the training and validation sets. Additionally, 293 (24.2%) patients were in fibrosis stage S2, 138 (11.4%) were in S3, and 255 (21.1%) were in S4 in the training set, while 113 (25.1%) patients were in S2, 43 (9.5%) were in S3, and 77 (17.1%) were in S4 in the validation set.
In the training cohort, a significantly increased odds ratio of stage S2–4 was associated with age, NAFLD, HBV DNA, ALT, AST, ALP, GGT, total bilirubin (TBil), direct bilirubin (DBil), albumin, total cholesterol (TC), triglyceride (TG), INR, activated partial thromboplastin time (APTT), white blood cells (WBC), neutrophils, platelets, hyaluronic, laminin, PIIINP, and type IV collagen. Multivariable analysis identified TBil, INR, platelets, and type IV collagen as independent predictors of significant liver fibrosis. Similarly, a significantly increased odds ratio of stage S4 was associated with sex, age, NAFLD, ALT, AST, ALP, GGT, TBil, DBil, albumin, TC, high-density lipoprotein (HDL), low-density lipoprotein (LDL), INR, APTT, WBC, neutrophils, lymphocytes, platelets, hyaluronic, laminin, PIIINP, and type IV collagen. Multivariable analysis identified ALP, INR, platelets, and type IV collagen as independent predictors of cirrhosis (Table 2). Thus, in addition to platelets, both INR and type IV collagen were independent predictors of significant fibrosis and cirrhosis (all P < 0.01).
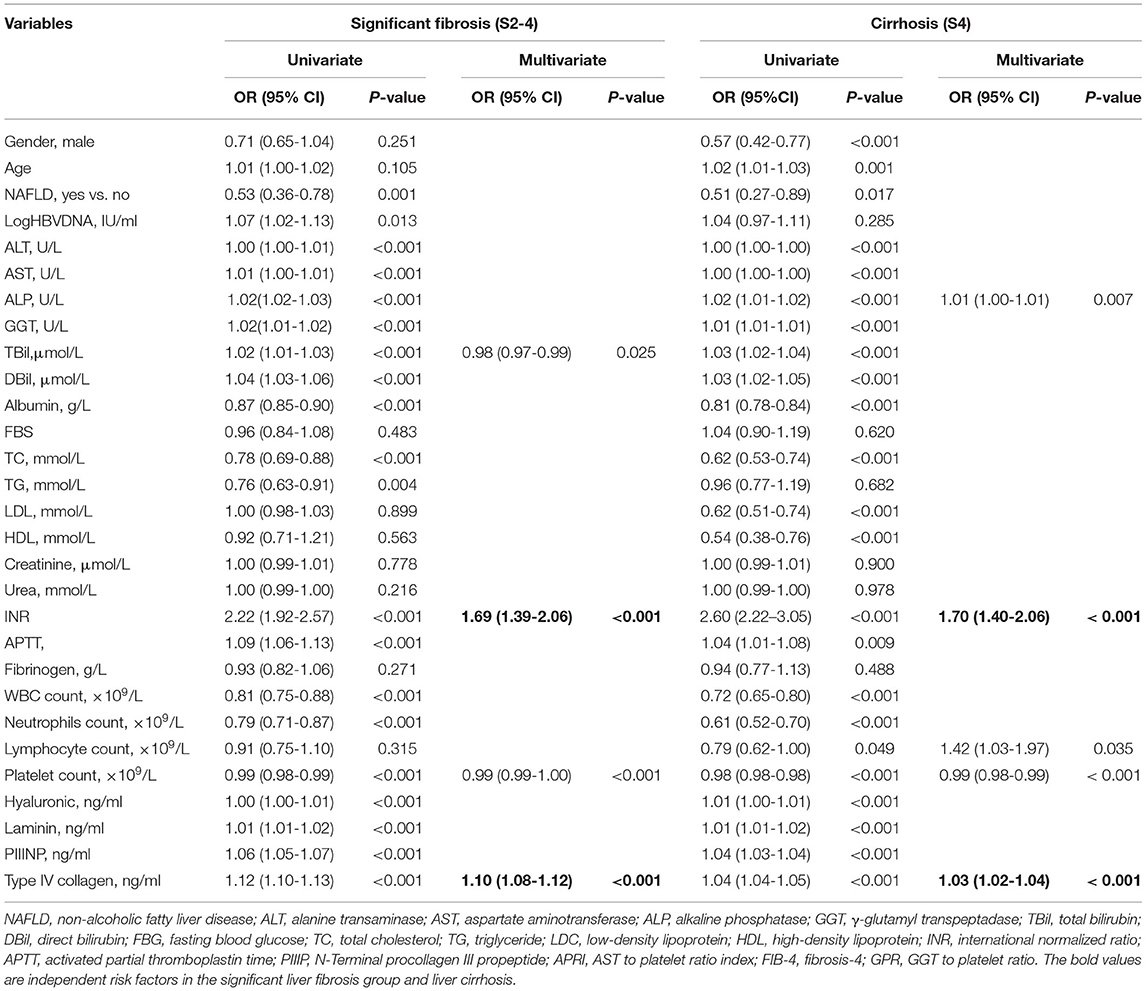
Table 2. Variables associated with significant fibrosis and cirrhosis by logistic analysis in training cohort.
Spearman's correlation analysis showed that type IV collagen (r = 0.58), INR (r = 0.43), ALP (r = 0.35), and TBil (r = 0.25) were significantly correlated with liver fibrosis scores (Figure 2). Based on these independent predictors, we devised two simple models to amplify the predictive performances of the established non-invasive indices and serum parameters for the progression of liver fibrosis. The models are as follows: GIVPR = GPR × INR × type IV collagen; GIVPTAR = GPR × INR × type IV collagen × TBil × ALP. GIVPR (r = 0.61) was significantly positively correlated with the Scheure fibrosis score with a higher correlation coefficient than APRI, FIB-4, GPR, and GIVPTAR (r = 0.47, 0.45, 0.53, and 0.59, respectively) (Figure 2).
Using ROC curve analysis, GIVPR was compared to GIVPTAR, APRI, FIB-4, and GPR for staging liver fibrosis. GIVPR displayed better accuracy in predicting significant fibrosis, advanced fibrosis, and cirrhosis. The ROC curves for the fourth non-invasive serum marker are shown in the training set (Figure 3) and the validation set (Figure 4). In the training set, for the discrimination of significant fibrosis, GIVPR had the highest AUC (0.81, sensitivity 68.95% and specificity 79.23%) compared with GIVPTAR (0.80, sensitivity 69.53% and specificity 78.67%), APRI (0.75, sensitivity 68.37% and specificity 70.10%), FIB-4 (0.72, sensitivity 56.20% and specificity 77.86%), and GPR (0.77, sensitivity 71.37% and specificity 70.86%). When discriminating advanced fibrosis, GIVPR had the highest AUC (0.82, sensitivity 74.81% and specificity 74.57%) compared with GIVPTAR (0.81, sensitivity 75.06% and specificity 75.06%), APRI (0.74, sensitivity 65.14% and specificity 72.00%), FIB-4 (0.74, sensitivity 66.07% and specificity 70.38%), and GPR (0.78, sensitivity 73.03% and specificity 72.62%). For predicting cirrhosis, GIVPR also had the best AUC (0.87, sensitivity 73.33% and specificity 84.21%) compared with GIVPTAR (0.86, sensitivity 75.69% and specificity 81.80%), APRI (0.78 sensitivity 72.94% and specificity 71.86%), FIB-4 (0.78, sensitivity 70.59% and specificity 73.90%), and GPR (0.78, sensitivity 80.00% and specificity 71.44%). The cutoffs of GIVPR for the assessment of significant fibrosis, advanced fibrosis, and cirrhosis were 11.57, 15.45, and 29.07, respectively (Table 3).

Figure 3. Area under receiver operating characteristic (ROC) comparison of GIVPR, GIVPTAR, APRI, FIB-4, and GPR in training set. (A) ROC comparison for predicting significant fibrosis; (B) ROC comparison for predicting advanced fibrosis; (C) ROC comparison for predicting cirrhosis.
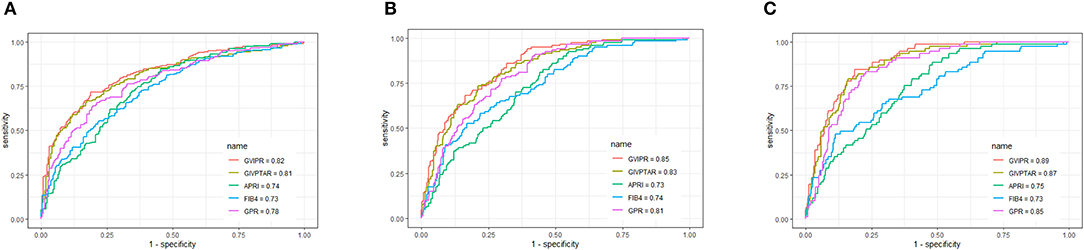
Figure 4. Area under receiver operating characteristic (ROC) comparison of GIVPR, GIVPTAR, APRI, FIB-4, and GPR in validation set. (A) ROC comparison for predicting significant fibrosis; (B) ROC comparison for predicting advanced fibrosis; (C) ROC comparison for predicting cirrhosis.

Table 3. Predictive performances of GIVPR, GIVPTAR, APRI, FIB-4, and GPR for liver fibrosis in CHB patients (Training cohort).
Similarly, in the validation set, compared to the other four serum indices, GIVPR had the highest AUCs of 0.82 (sensitivity 73.82% and specificity 75.23%) for predicting significant fibrosis, 0.85 (sensitivity 81.67% and specificity 70.09%) for predicting advanced fibrosis, and 0.80 (sensitivity 84.42% and specificity 78.88%) for predicting cirrhosis (Table 4). These results suggest that GIVPR is an excellent predictor of liver fibrosis in CHB patients.
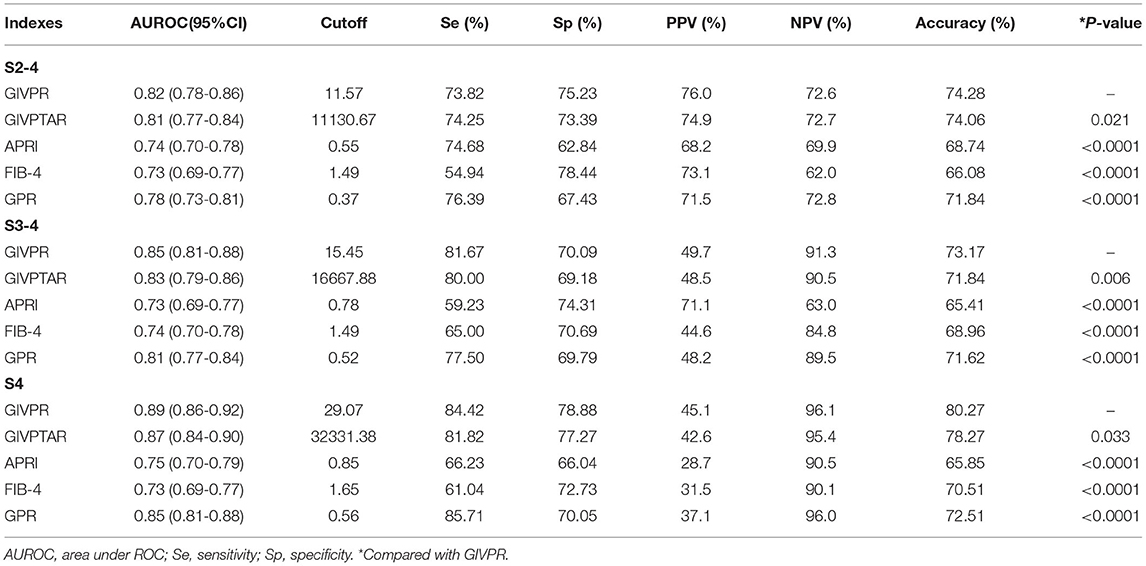
Table 4. Predictive performances of GIVPR, GIVPTAR, APRI, FIB-4, and GPR for liver fibrosis in CHB patients (Validation cohort).
Moreover, we conducted DCA to further investigate the clinical application value of GIVPR, GIVPTAR, APRI, FIB-4, and GPR for predicting liver fibrosis. In the training group, DCAs revealed that from a threshold probability of 20–80%, the application of GIVPR to predict liver fibrosis risk increased the benefit considerably more than the other four scores (Figure 5). Regarding the validation group, the DCAs of GIVPR also showed a better net benefit with a wide range of threshold probabilities and better performances for predicting liver fibrosis than GIVPTAR, APRI, FIB-4, and GPR (Figure 6).
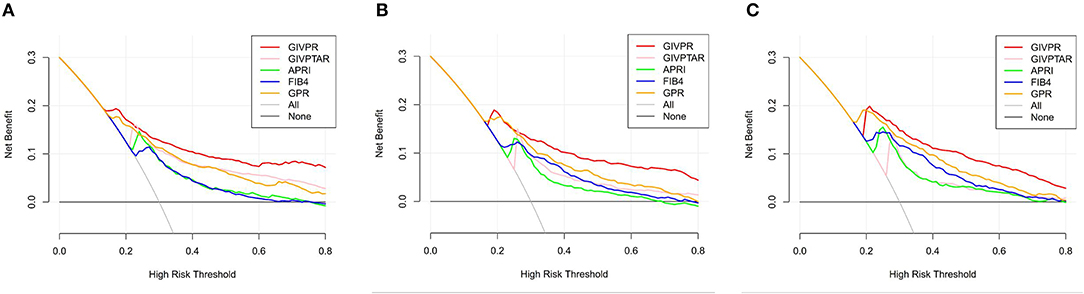
Figure 5. Liver fibrosis decision curve analysis in training set. Decision curve analysis depict the clinical net benefit. GIVPR is compared with GIVPTAR, APRI, FIB-4, and GPR for predicting significant fibrosis (A); GIVPR is compared with GIVPTAR, APRI, FIB-4, and GPR for predicting advanced fibrosis (B); GIVPR is compared with GIVPTAR, APRI, FIB-4, and GPR for predicting cirrhosis (C). Black line, net benefit when no patient will experience the event; gray line, net benefit when all patients will experience the event. The preferred markers is the marker with the highest net benefit at any given threshold.
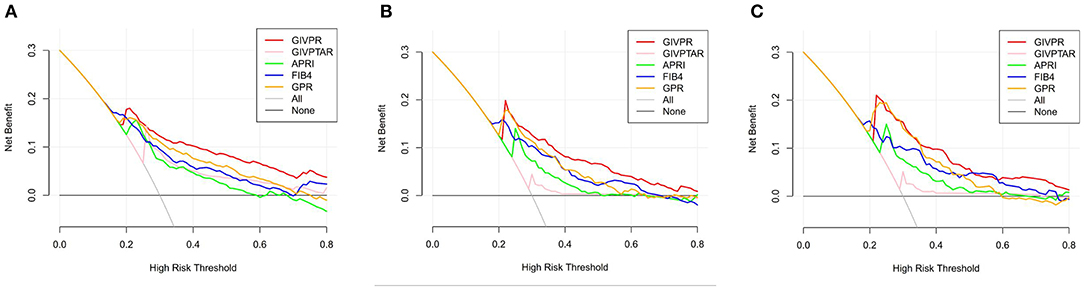
Figure 6. Liver fibrosis decision curve analysis in validation set. Decision curve analysis depict the clinical net benefit. GIVPR is compared with GIVPTAR, APRI, FIB-4, and GPR for predicting significant fibrosis (A); GIVPR is compared with GIVPTAR, APRI, FIB-4, and GPR for predicting advanced fibrosis (B); GIVPR is compared with GIVPTAR, APRI, FIB-4, and GPR for predicting cirrhosis (C). Black line, net benefit when no patient will experience the event; gray line, net benefit when all patients will experience the event. The preferred markers is the marker with the highest net benefit at any given threshold.
Early diagnosis and accuracy in evaluating liver fibrosis or cirrhosis may play important roles not only in controlling disease progression but also in the treatment of chronic HBV infection (22). Liver biopsy is the gold standard for evaluating liver fibrosis in chronic liver disease. However, although liver biopsy is usually a safe procedure, it has some technical limitations and risks (23). Thus, there is an increasing need for simple and reliable non-invasive predictors for liver fibrosis, some of which have been evaluated in multiple studies. However, how their sensitivity and accuracy are affected by various factors is still a matter of debate (24). By combining non-invasive indicators, the overall diagnostic coincidence rate can be improved.
In the present study, we assessed the relationships between serum parameters and non-invasive indices and liver fibrosis in CHB patients. GIVPR and GIVPTAR based on GPR all exhibited excellent capacities to predict the progression of liver fibrosis. However, GIVPTAR, which required more variables, did not obtain higher AUCs than GIVPR and did not improve the predictive performance for liver fibrosis. We also compared the predictive accuracy of GIVPR with APRI, FIB-4, and GPR. Our results showed that in both the training and validation cohorts, GIVPR had the best AUC value for staging significant fibrosis, advanced fibrosis, and cirrhosis. Thus, GIVPR, which requires only GPR, INR, and type IV collagen and is simple to calculate, has a more powerful predictive performance for liver fibrosis in CHB patients.
There were two kinds of serum biomarkers for liver fibrosis progression, indirect serum markers and direct serum markers (25). Indirect serum markers had no direct correlation with liver fibrosis but reflected liver dysfunction or other fibrosis-related symptoms. They are often calculated into mathematical formulas or may be used individually (26). APRI and FIB-4 are the two non-invasive procedures for evaluating liver fibrosis that receive the most attention. They were reported to have a high AUROC to detect significant fibrosis and cirrhosis in CHB patients in East Africa and Asia (27, 28). The WHO CHB guidelines also recommend APRI and FIB-4 for application in resource-limited health care regions (29). However, a meta-analysis suggested that their diagnostic performance was not good enough to discriminate liver fibrosis in CHB patients and could not be used as an ideal replacement for liver biopsy (30). GPR is a novel index to assess liver fibrosis in patients with CHB in West African cohorts. It was shown to be better than the classical models APRI and FIB-4 (10). Additionally, GPR was reported to diagnose significant liver fibrosis and cirrhosis well in a large cohort of HBV monoinfected Gambian patients using FibroScan measures as a reference (31). However, GPR showed a less clear advantage in a Brazilian cohort and other Chinese cohorts (13, 32). In this study, our GIVPR model showed acceptable distinguishing power for the prediction of significant live fibrosis, advanced liver fibrosis, and cirrhosis in the training set, with AUCs of 0.797, 0.815, and 0.844, respectively; similar results were obtained in the validation set. Furthermore, we confirmed significantly better performance for the assessment of liver histological scores compared to the biochemical marker panels APRI, FIB-4, and GPR. Due to the different inflammatory and clinical conditions of patients with chronic hepatitis B and chronic hepatitis C, the effect of etiology on fibrosis progression and clinical biomarkers can explain this result (33, 34).
Moreover, the indirect serum markers evaluated in this study included the measurement of coagulation parameters, which were found to increase with the progression of liver fibrosis. Among these routine markers, INR was identified as an independent factor for the prediction of significant fibrosis and cirrhosis in CHB patients. Sterling et al. (21) reported that the INR was an independent predictor of liver fibrosis, and its concentration was directly related to liver function. Another study demonstrated that the INR level was associated with liver fibrosis and used INR as a parameter in their King's score, which was closely related to the progression of liver fibrosis (35, 36).
Direct biomarkers of liver fibrosis are fragments of liver matrix components produced in the process of fibrosis. These markers represent the intensity of fibrogenesis or fibrinolysis, such as type IV collagen, laminin, hyaluronic acid and metalloproteinases (37). Serum collagen levels, especially type IV, have been shown to be a useful, non-invasive measure of the activity of this pathway at a single time point and have been shown to reflect prognosis and responses to a variety of chronic liver diseases (14). Type IV collagen is an important component of the normal extracellular matrix. Compared with type I and type III collagen, which are partially hydrolyzed, type IV collagen remains intact in the matrix; therefore, the serum composition of type IV collagen is considered to mainly reflect the degradation of the matrix (38). Serum type IV collagen has been confirmed to be associated with both the progression of liver inflammation and fibrosis, which is in line with our data (26, 39).
This study has several limitations worth considering. First, this was a retrospective study in a single center and should be further confirmed in more patients from other centers. Second, GIVPR was not dynamically observed. We recommend further investigation into the efficacy of GIVPR compared to other non-invasive indices in evaluating fibrosis progression and in predicting liver-related end-stage disease after long-term antiviral inhibition of HBV.
In summary, a novel non-invasive calculation, GIVPR, was established from GPR, INR, and type IV collagen. GIVPR demonstrates superior diagnostic accuracy and clinical usefulness compared to conventional serum indices. Although the clinical usefulness of GIVPR warrants future investigation, our findings showing that GIVPR is non-invasive and easily administered indicate that it could be a promising tool for the discrimination of liver fibrosis, especially in resource-limited regions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study protocol and informed consent documents were reviewed and approved by the Ethics Committee of Shanghai Public Health Clinical Center, Fudan University. The patients/participants provided their written informed consent to participate in this study.
RD designed the study and wrote the manuscript. WL, XZ, and DH collected and analyzed data. YW and LY reviewed the statistical data. XL and WL were involved in critical revision of the paper. SS, ZZ, and LC approved the final manuscript. All authors have read and approved the manuscript.
This work was supported by the 13th Five-year National Science and Technology Major Project of China (2017ZX10203202). This fund was acquired by ZZ. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. (2015) 386:1546–55. doi: 10.1016/S0140-6736(15)61412-X
2. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. (2016) 388:1081–8. doi: 10.1016/S0140-6736(16)30579-7
3. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. (2004) 351:1521–31. doi: 10.1056/NEJMoa033364
4. D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. (2006) 44:217–31. doi: 10.1016/j.jhep.2005.10.013
5. Zhang Z, Wang G, Kang K, Wu G, Wang P. The diagnostic accuracy and clinical utility of three noninvasive models for predicting liver fibrosis in patients with HBV infection. PLoS ONE. (2016) 11:e0152757. doi: 10.1371/journal.pone.0152757
6. Chen YP, Hu XM, Liang XE, Huang LW, Zhu YF, Hou JL. Stepwise application of fibrosis index based on four factors, red cell distribution width-platelet ratio, and aspartate aminotransferase-platelet ratio for compensated hepatitis B fibrosis detection. J Gastroenterol Hepatol. (2018) 33:256–63. doi: 10.1111/jgh.13811
7. Fung J, Lai CL, Cheng C, Wu R, Wong DK, Yuen MF. Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am J Gastroenterol. (2011) 106:492–6. doi: 10.1038/ajg.2010.463
8. Gozdas HT, Ince N. Elevated mean platelet volume to platelet ratio predicts advanced fibrosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. (2020) 32:524-7. doi: 10.1097/MEG.0000000000001599
9. Li Q, Ren X, Lu C, Li W, Huang Y, Chen L. Evaluation of APRI and FIB-4 for noninvasive assessment of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT </= 2 ULN: a retrospective cohort study. Medicine. (2017) 96:e6336. doi: 10.1097/MD.0000000000006336
10. Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. (2016) 65:1369-76. doi: 10.1136/gutjnl-2015-309260
11. Li Q, Lu C, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase to platelet ratio for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B and non-alcoholic fatty liver disease. Oncotarget. (2017) 8:28641-9. doi: 10.18632/oncotarget.16162
12. Wang RQ, Zhang QS, Zhao SX, Niu XM, Du JH, Du HJ, et al. Gamma-glutamyl transpeptidase to platelet ratio index is a good noninvasive biomarker for predicting liver fibrosis in Chinese chronic hepatitis B patients. J Int Med Res. (2016) 44:1302–13. doi: 10.1177/0300060516664638
13. Huang R, Wang G, Tian C, Liu Y, Jia B, Wang J, et al. Gamma-glutamyl-transpeptidase to platelet ratio is not superior to APRI,FIB-4 and RPR for diagnosing liver fibrosis in CHB patients in China. Sci Rep. (2017) 7:8543. doi: 10.1038/s41598-017-09234-w
14. Kooreman K, Babbs C, Fessler J. Effect of ischemia and reperfusion on oxidative processes in the large colon and jejunum of horses. Am J Vet Res. (1998) 59:340-6.
15. Wu SD, Ni YJ, Liu LL, Li H, Lu LG, Wang JY. Establishment and validation of a simple noninvasive model to predict significant liver fibrosis in patients with chronic hepatitis B. Hepatol Int. (2012) 6:360–8. doi: 10.1007/s12072-011-9328-1
16. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. (2016) 10:1–98. doi: 10.1007/s12072-015-9675-4
17. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
18. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. (1991) 13:372–4. doi: 10.1016/0168-8278(91)90084-O
19. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. (2016) 63:261–83. doi: 10.1002/hep.28156
20. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. (2003) 38:518–26. doi: 10.1053/jhep.2003.50346
21. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. (2006) 43:1317–25. doi: 10.1002/hep.21178
22. Shiha G, Ibrahim A, Helmy A, Sarin SK, Omata M, Kumar A, et al. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int. (2017) 11:1–30. doi: 10.1007/s12072-016-9760-3
23. Guha IN, Rosenberg WM. Noninvasive assessment of liver fibrosis: serum markers, imaging, and other modalities. Clin Liver Dis. (2008) 12:883-900, x. doi: 10.1016/j.cld.2008.07.010
24. Ponomarenko Y, Leo MA, Kroll W, Lieber CS. Effects of alcohol consumption on eight circulating markers of liver fibrosis. Alcohol Alcohol. (2002) 37:252–5. doi: 10.1093/alcalc/37.3.252
25. Martinez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. (2011) 53:325–35. doi: 10.1002/hep.24013
26. El-Mezayen HA, Habib S, Marzok HF, Saad MH. Diagnostic performance of collagen IV and laminin for the prediction of fibrosis and cirrhosis in chronic hepatitis C patients: a multicenter study. Eur J Gastroenterol Hepatol. (2015) 27:378–85. doi: 10.1097/MEG.0000000000000298
27. Desalegn H, Aberra H, Berhe N, Gundersen SG, Johannessen A. Are non-invasive fibrosis markers for chronic hepatitis B reliable in sub-Saharan Africa? Liver Int. (2017) 37:1461-7. doi: 10.1111/liv.13393
28. Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. (2008) 40:267–74. doi: 10.1016/j.dld.2007.10.011
29. Guidelines for the Prevention Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization (2015).
30. Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. (2015) 61:292–302. doi: 10.1002/hep.27382
31. Lemoine M, Thursz M, Mallet V, Shimakawa Y. Diagnostic accuracy of the gamma-glutamyl transpeptidase to platelet ratio (GPR) using transient elastography as a reference. Gut. (2017) 66:195-6. doi: 10.1136/gutjnl-2016-311554
32. Schiavon LL, Narciso-Schiavon JL, Ferraz MLG, Silva AEB, Carvalho-Filho RJ. The gamma-glutamyl transpeptidase to platelet ratio (GPR) in HBV patients: just adding up? Gut. (2017) 66:1169–70. doi: 10.1136/gutjnl-2016-312658
33. Li J, Gordon SC, Rupp LB, Zhang T, Boscarino JA, Vijayadeva V, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. (2014) 21:930–7. doi: 10.1111/jvh.12224
34. Kayadibi H, Yilmaz B, Ozgur Yeniova A, Koseoglu H, Simsek Z. Development and evaluation of a novel noninvasive index for predicting significant fibrosis, advanced fibrosis, and cirrhosis in patients with chronic hepatitis B infection. Eur J Gastroenterol Hepatol. (2020). doi: 10.1097/MEG.0000000000001973. [Epub ahead of print].
35. Cross TJ, Rizzi P, Berry PA, Bruce M, Portmann B, Harrison PM. King's Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. (2009) 21:730–8. doi: 10.1097/MEG.0b013e32830dfcb3
36. Wu J, Guo N, Zhang X, Xiong C, Liu J, Xu Y, et al. HEV-LFS: a novel scoring model for patients with hepatitis E virus-related liver failure. J Viral Hepat. (2019) 26:1334–43. doi: 10.1111/jvh.13174
37. Jarcuska P, Janicko M, Veseliny E, Jarcuska P, Skladany L. Circulating markers of liver fibrosis progression. Clin Chim Acta. (2010) 411:1009–17. doi: 10.1016/j.cca.2010.04.009
38. Murawaki Y, Ikuta Y, Nishimura Y, Koda M, Kawasaki H. Serum markers for connective tissue turnover in patients with chronic hepatitis B and chronic hepatitis C: a comparative analysis. J Hepatol. (1995) 23:145–52. doi: 10.1016/0168-8278(95)80328-9
Keywords: CHB, liver fibrosis, type IV collagen, INR, GPR
Citation: Ding R, Lu W, Zhou X, Huang D, Wang Y, Li X, Yan L, Lin W, Song S, Zhang Z and Chen L (2021) A Novel Non-invasive Model Based on GPR for the Prediction of Liver Fibrosis in Patients With Chronic Hepatitis B. Front. Med. 8:727706. doi: 10.3389/fmed.2021.727706
Received: 19 June 2021; Accepted: 30 August 2021;
Published: 23 September 2021.
Edited by:
Chuanlong Zhu, Nanjing Medical University, ChinaCopyright © 2021 Ding, Lu, Zhou, Huang, Wang, Li, Yan, Lin, Song, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Song, eWNzczE5NzFAMTYzLmNvbQ==; orcid.org/0000-0002-5373-5583; Zhanqing Zhang, ZG9jdG9yenpxc3BoY0AxNjMuY29t; orcid.org/0000-0001-7709-9027; Liang Chen, Y2hlbmxpYW5nQHNocGhjLm9yZy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.