- 1Department of Respiratory Medicine and Clinical Immunology, Graduate School of Medicine, Osaka University, Suita, Japan
- 2Department of Immunopathology, WPI, Immunology Frontier Research Center (iFReC), Osaka University, Suita, Japan
- 3Integrated Frontier Research for Medical Science Division, Institute for Open and Transdisciplinary Research Initiatives (OTRI), Osaka University, Suita, Japan
- 4Center for Infectious Diseases for Education and Research (CiDER), Osaka University, Suita, Japan
Purpose: There is no clear consensus on the clinical course of critical COVID-19 patients. We examined the clinical course among intubated survivors, non-survivors, and extracorporeal membrane oxygenation (ECMO) patients to reveal the standard clinical course and the difference among critical COVID-19 patients.
Methods: In this systematic review and meta-analysis, we searched PubMed, Web of Science, and Scopus for original studies published until December 11, 2020, including case accumulation and clinical course reporting. Pregnant patients and children were excluded. We followed PRISMA guidelines and registered them with PROSPERO (CRD42021235534).
Results: Of the 11,716 studies identified, 94 met the selection criteria, and 2,549 cases were included in this meta-analysis. The times from intubation to extubation and death were 12.07 days (95% confidence interval 9.80–14.33 days) and 10.14 days (8.18–12.10 days), respectively, and the ECMO duration was 14.72 days (10.57–18.87 days). The time from symptom onset to hospitalization (prehospitalization period) of intubated survivors, non-survivors, and ECMO patients was 6.15 (4.61–7.69 days), 6.45 (4.55–8.34 days), and 7.15 days (6.48–7.81 days), and that from symptom onset to intubation (preintubation period) was 8.58 (7.36–9.80 days), 9.14 (7.26–11.01 days), and 10.54 days (9.18–11.90 days), respectively. Sensitivity analysis showed that the time from intubation to extubation and death was longer in the US and Europe than in East Asia.
Conclusion: For COVID-19, we hypothesize that prehospitalization and preintubation periods are longer in intubated non-survivors and ECMO patients than in intubated survivors. These periods may serve as a predictor of disease severity or death and support therapeutic strategy determination.
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and was first reported in Wuhan, China, in December 2019 (1). As of August 2021, COVID-19 had spread to 223 countries, areas, or territories, and the global cumulative case numbers have reached 197 million. Over 4.2 million COVID-19 patients have died since the start of the pandemic (2), even though every government has taken aggressive preventive measures such as lockdown (3), universal masking (4), and social distancing (4). The hospitalization rate of COVID-19 is reportedly 14% (almost 10 times higher than influenza) (5–7). Moreover, up to 26.1% of hospitalized COVID-19 patients are admitted to the intensive care unit (ICU) (8). Therefore, COVID-19 has placed an unprecedented burden on the ICU, and in some regions, ICU capacity exceeds 100% with only COVID-19 patients because of the astonishing number, high rate of ICU admission, and long clinical course (9). Furthermore, 71–88% of COVID-19 patients in the ICU need intubation (2.45–4.01 times higher than influenza) (10–14), and 3–27.2% of intubated COVID-19 patients require ECMO (10, 15). Overall, the high occupancy rate of hospital beds and ICUs by COVID-19 patients is a serious problem worldwide.
The clinical course of patients with severe COVID-19 from symptom onset to clinical events is highly informative when considering prognosis, therapeutic strategy, ICU bed management, and medical economy. Nevertheless, comparing each patient's clinical course with the standard clinical course of COVID-19 is difficult because there is no consensus to date regarding the standard clinical course. For example, the duration of intubation has been reported to be 10–16 days (16, 17), yet both the patients' backgrounds and regions where the studies were conducted differed in these reports. Moreover, known risk factors for COVID-19 mortality include age (18), sex (19), comorbidities (19), and blood counts (absolute lymphocyte number and CRP) (20); however, few articles have assessed differences in the clinical course between intubated survivors, non-survivors, and ECMO patients.
In this study, we conducted a systematic review and meta-analysis of the clinical course, i.e., time (days) from symptom onset, hospitalization, intubation, and ECMO initiation to each clinical event in critical COVID-19 patients. We also assessed the difference in the clinical course between intubated survivors, non-survivors, and ECMO patients with COVID-19 to reveal whether the clinical course is a prognostic factor. Finally, we conducted sensitivity analysis to assess factors (patient background and region) that may influence the time from intubation to extubation or death.
Methods
Search Strategy and Selection Criteria
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (Supplementary Table 1) (21). This study searched for articles documenting the clinical course in critical COVID-19 patients: the time (days) from symptom onset to hospitalization (prehospitalization period) to intubation (preintubation period) and to ECMO initiation (pre-ECMO period); the time from hospitalization to intubation (hospitalization-intubation period) and to ECMO initiation (hospitalization-ECMO period), discharge (hospitalization-discharge period), and death (hospitalization-death period); the time from hospitalization to death (hospitalization-death period); the time from intubation to extubation (intubation period), to ECMO (intubation-ECMO period), and to death (intubation-death period); and the time from ECMO initiation to decannulation (ECMO period) and to death (ECMO-death period). Three sources, namely, PubMed, Web of Science, and Scopus, were searched [(COVID-19) OR (SARS-CoV-2) AND (intensive care unit) OR (acute respiratory distress syndrome) OR (mechanical ventilation) OR (extracorporeal membrane oxygenation)], with no language restriction. The searches were performed to identify articles published until December 11th, 2020, when the SARS-CoV-2 vaccine was first approved in the world, including “online first” articles, published until December 11, 2020, when the SARS-CoV-2 vaccine was first approved. The last searches were performed on June 26, 2021.
The inclusion criteria were studies of human subjects, case accumulations, a title or abstract consisting of the clinical course of intubated survivors, non-survivors, and/or ECMO patients with COVID-19, and a link from the search site to the full text (PDF or website) of the article. In this study, “survivors” referred to extubated patients who had not died during the study period. This study excluded studies involving children (under 18 years old) and pregnant women and non-English articles; a case report was also excluded because properly calculating the average value and standard deviation (SD) was difficult. Redundancies between the search sites were eliminated, i.e., individual studies were counted only once in this analysis.
Data Extraction and Quality Assessment
Data were extracted from all studies included in this analysis (author, year of publication, country where the study was conducted, number of patients, age, percentage of males, comorbidities, and treatment); the details are provided in Table 1. The average number of days and SD showing each clinical course or the median number of days and interquartile range (IQR) and/or range were extracted. The average number of days and SD were calculated from the median and IQR or range data using the reported methods if only the median and IQR or range were given in the study (22).
Two authors (K.F. and T.M.) independently assessed and selected references. In cases of inconsistent results, a third author (A.K.) provided an opinion to resolve the issue. The quality of the selected studies was evaluated according to the study quality assessment tools (Quality Assessment Tool for Case Series Studies) from the National Heart, Lung, and Blood Institute (NHLBI) (23). The evidence level was assessed based on the Oxford Centre for Evidence-Based Medicine 2011 (24). Asymmetry in a funnel plot was employed to determine publication bias.
Data Analysis
A meta-analysis was performed to estimate the clinical course of intubated survivors, non-survivors, and ECMO patients with COVID-19. Clinical data were analyzed using the metamean package. Outcomes are described as the mean number of days at each event, such as admission, intubation, or death from the onset of COVID-19 (baseline) and 95% confidence intervals (CIs) for each clinical course. For all outcomes, mean differences were calculated using the random-effects model (DerSimonian and Laird method) (25). I2 values of 25, 50, and 75% were defined as low, moderate, and high, respectively (26). All analyses were conducted using R version 4.0.3 (R Project for Statistical Computing) (27). Sensitivity analyses were carried out with regard to the intubation period and intubation-death period based on region (East Asia, the US, and Europe), age, sex, and comorbidities (hypertension and diabetes mellitus). Spearman's correlation coefficient was calculated in R version 4.0.3. P values ≤ 0.05 were considered statistically significant. This study was registered with PROSPERO (CRD42021235534).
Results
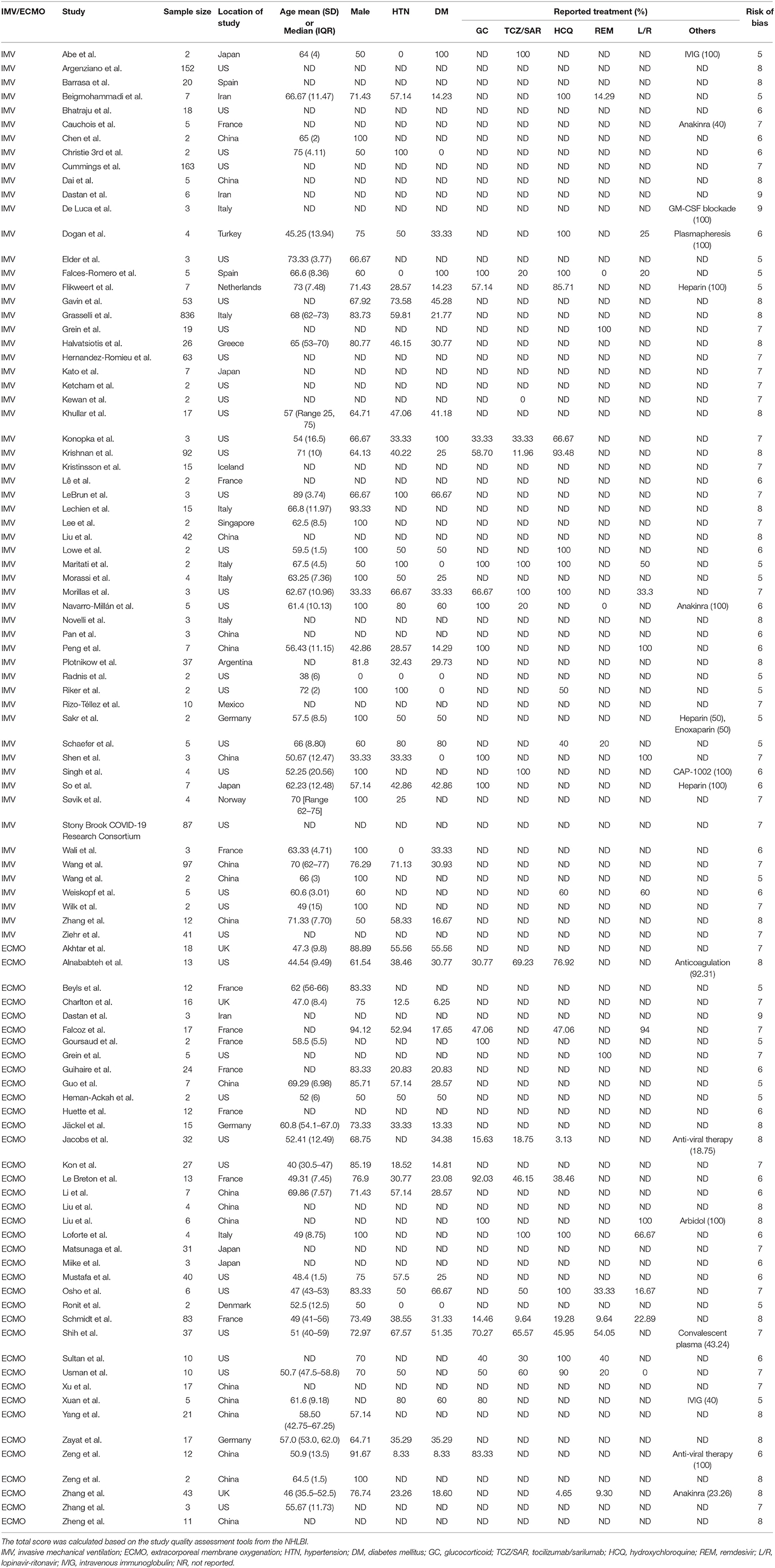
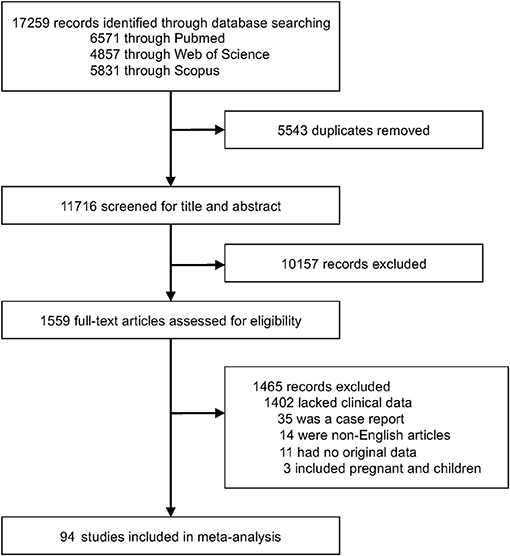
We identified 17,259 articles and excluded 5,543 due to duplication. We also screened 11,716 publications and identified 94 articles (15–17, 28–118), with 2,549 cases, from among 1,559 articles that underwent full-text assessment (Figure 1). Each article is summarized in Supplementary Table 1. The mean age ranged from 38 to 75 years, and the rate of male patients ranged from 0% to 100%. COVID-19 patients were reportedly treated with glucocorticoids, tocilizumab/sarilumab, remdesivir, and hydroxychloroquine; however, treatment was not described in more than 70% of the articles. There were 36 articles from the US, 19 from China, ten from France, seven from Italy, five from Japan, and a few from other countries. Despite several cohort studies, there were few intubated survivors and non-survivors, and most were case accumulations. Therefore, the risk of bias was calculated based on case accumulation. The risk of bias was more than 5 points, with 6.71 points as the average, i.e., moderate risk (Supplementary Table 2).
Moreover, we conducted a meta-analysis on the clinical course of intubated survivors, non-survivors, and ECMO patients with COVID-19. First, we analyzed the intubation period and the intubation-death period of intubated survivors and non-survivors. Thirty-three reports with 325 survivors and 24 reports with 1,225 non-survivors were identified and analyzed (Figure 2). The average intubation period among intubated survivors was 12.07 days (95% CIs 9.80–14.33 days), and the average intubation-death period was 10.14 days (8.18–12.10 days). The prehospitalization periods for intubated survivors and non-survivors were 6.15 (4.61–7.69 days) and 6.45 (4.55–8.34 days) days, respectively, and the preintubation periods were 8.58 days (7.36–9.80 days) and 9.64 days (7.75–11.53 days), respectively. A symptom-death period of 17.86 days (13.02–22.69 days) was calculated (Figure 3). Additionally, the hospitalization-intubation period among intubated survivors and non-survivors was 2.62 days (1.66–3.58 days) and 3.28 days (2.15–4.41 days), respectively; the hospitalization-discharge and hospitalization-death periods were 24.48 days (12.54–36.41 days) and 12.47 days (10.56–14.39 days), respectively (Figure 4). Funnel plots are illustrated in Supplementary Figure 1.

Figure 2. Forrest plot: a meta-analysis of the intubation period and the intubation-death period. The intubation period of intubated COVID-19 survivors (A) and the intubation-death period of intubated COVID-19 non-survivors (B) were calculated using the random effects model. MRAW, the raw data of mean; 95% CI, 95% confidence interval.
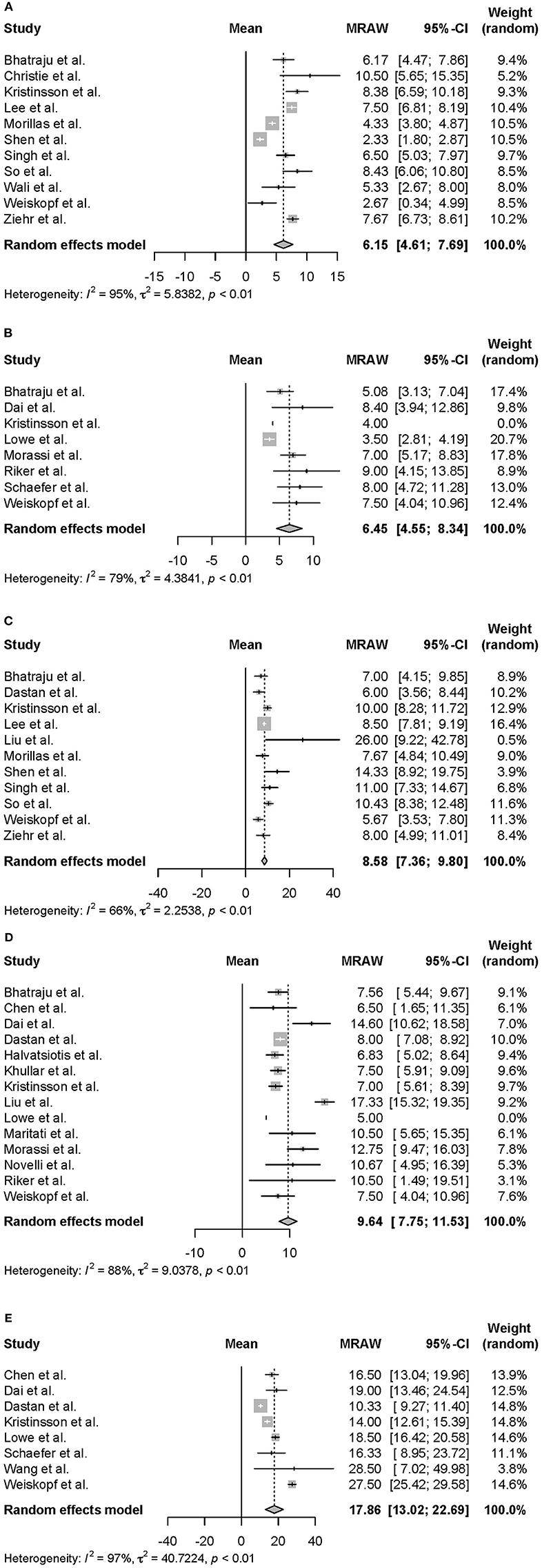
Figure 3. Forrest plot: a meta-analysis of the time from symptom onset to each clinical endpoint in intubated COVID-19 patients. The prehospitalization period of intubated survivors (A), the prehospitalization period of intubated non-survivors (B), the preintubation period of intubated survivors (C), the preintubation period of intubated non-survivors (D), and the symptom-death period (E) were calculated using the random effects model. MRAW, the raw data of mean; 95% CI, 95% confidence interval.
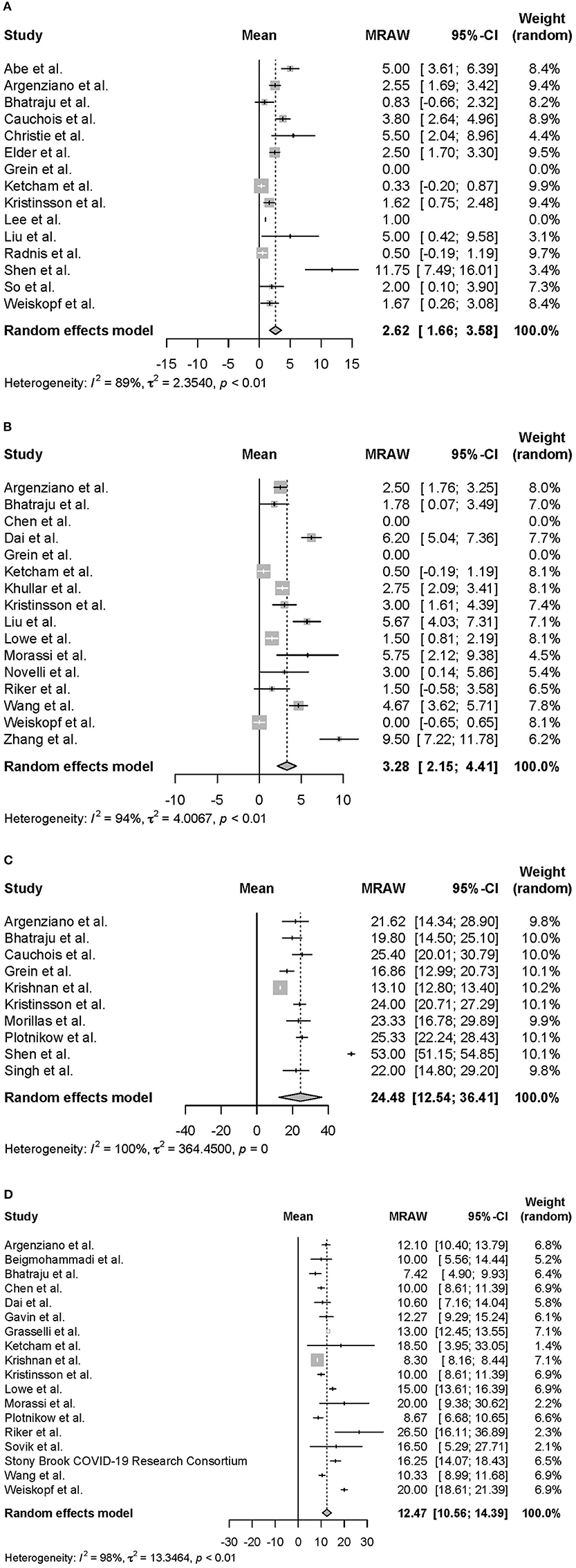
Figure 4. Forrest plot: a meta-analysis of the time from hospitalization onset to each clinical endpoint in intubated COVID-19 patients. The hospitalization-intubation period of intubated survivors (A), the hospitalization-intubation period of intubated non-survivors (B), the hospitalization-discharge period of intubated survivors (C), and the hospitalization-death period of intubated non-survivors (D) were calculated using the random effects model. MRAW, the raw data of mean; 95% CI, 95% confidence interval.
Regarding the clinical course of those treated with ECMO, the ECMO period of both survivors and non-survivors and the ECMO-death period were 14.72 days (10.57–18.87 days) and 21.05 days (12.04–30.07 days), respectively (Supplementary Figure 2). For ECMO patients, the prehospitalization, preintubation, and pre-ECMO periods were 7.15 (6.48–7.81 days), 10.54 (9.18–11.90 days), and 14.80 (13.29–16.31 days) days, respectively, and the hospitalization-intubation, hospitalization-ECMO, and intubation-ECMO periods were 3.39 (2.08–4.69 days), 5.97 (3.91–8.02 days), and 4.57 (3.59–5.54 days) days, respectively (data not shown).
The results provided above are summarized in Figure 5. The prehospitalization and preintubation periods of intubated non-survivors and ECMO patients appeared to be longer than those of intubated survivors (no direct comparison).
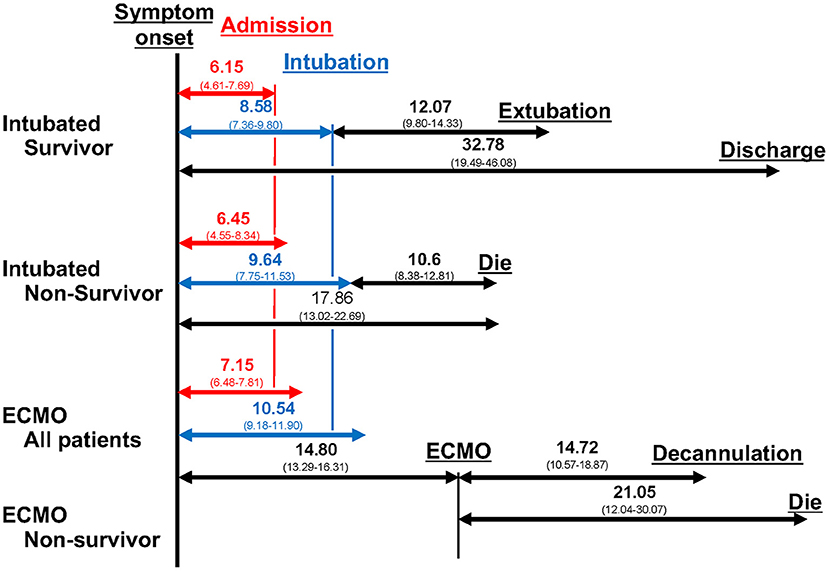
Figure 5. Clinical course. The duration from symptoms to each clinical event, from intubation to each clinical event, and from ECMO to each clinical event are shown. The number is the mean number of days, and the number in parentheses is the 95% confidence interval.
Finally, sensitivity analysis focusing on regional differences and patient backgrounds was performed. The regions where the studies were conducted were classified into three groups: Europe, the US, and East Asia. The intubation period was 14.87 days (10.99–18.76 days), 12.61 days (10.50–14.72 days), and 9.66 days (5.07–14.25 days) in Europe, the US, and East Asia (Supplementary Figure 3), and the intubation-death period was 13.05 days (9.53–16.58 days), 11.34 days (8.06–14.61 days), and 5.39 days (-1.14–11.91 days), respectively. Both the intubation period and intubation-death period tended to be longer in the US and Europe than in East Asia. Nevertheless, the mean age of intubated survivors did not differ much among Europe [64.85 (60.84–68.85)], the US [58.09 (49.32–66.87)], and Asia [61.64 (57.20–66.07)]; the mean age of intubated non-survivors was 67.86 (65.86–69.86) in Europe and 70.67 (61.88–79.46) in the US [one paper reported that the mean age of non-survivors in China was 65 (±4)]. We also analyzed age, sex, and comorbidities (diabetes mellitus and hypertension) but found no significant differences (data not shown).
Discussion
This study demonstrated the clinical course and differences among the clinical courses of intubated survivors, non-survivors, and ECMO patients with COVID-19. In this meta-analysis, intubation, intubation-death, and ECMO periods were 12.07 days (9.80–14.33 days), 10.14 days (8.18–12.10 days), and 14.72 days (10.57–18.87 days), respectively. The prehospitalization periods of intubated survivors, non-survivors, and ECMO patients were 6.15 days (4.61–7.69 days), 6.45 days (4.55–8.34 days), and 7.15 days (6.48–7.81 days), respectively, and the preintubation periods were 8.58 days (7.36–9.80 days), 9.14 days (7.26–11.01 days), and 10.54 days (9.18–11.90 days), respectively. According to sensitivity analysis, the intubation period in survivors and the intubation-death period were longer in the US and Europe than in East Asia.
For COVID-19, the intubation period in survivors and the intubation-death period appear to be more prolonged than those in patients intubated for other diseases or reasons. In addition, the intubation periods in survivors and non-survivors were 12.1 days and 10 days, respectively. In contrast, previously reported intubation periods in ICU patients, including postoperative patients, chronic obstructive pulmonary disease (COPD) patients, pneumocystis pneumonia survivors, acute respiratory distress syndrome (ARDS) patients, community-acquired pneumonia patients, and SARS-CoV-1 pneumonia survivors, were 3.3 ± 3 days (mortality 24.3%) (119), 6.8 ± 4.9 days (mortality 13%) (120), 7.7 ± 8.2 days (121), 8.8 (± 6) days (122), 10–11 days (123), 12.1 ± 6.1 days (124), and 7.3–15 days (mean days are calculated from each original datum) (125–127), respectively. One study comparing COVID-19 with influenza found that the intubation period in COVID-19 patients was longer than that in influenza patients (16.2 vs. 7.3 days) (127). Thus, the intubation period in COVID-19 survivors is prolonged compared with that in patients intubated due to COPD, HIV-PCP, and influenza. However, approximately the same duration has been observed for patients intubated due to community-acquired pneumonia and SARS-CoV-1 or COVID-19.
Moreover, the ECMO period in COVID-19 patients was not longer than that in patients with ARDS for other reasons. Although ECMO is commonly used during cardiac surgery, severe ARDS patients (aPaO2:FiO2 of <80 mmHg, a Murray score >3.0 and pH <7.20) have been treated with ECMO, improving the survival rate to more than 50% (128, 129). Accordingly, critical COVID-19 patients also receive ECMO. In our meta-analysis, the ECMO period with COVID-19 was 14.72 days (10.57–18.87 days); in previous reports, the ECMO period in severe ARDS patients was 10.3 ± 7.5 (mean days were calculated from the data) days (128) and 15 ± 13 days (129), and that in severe ARDS patients with influenza was 10 days (130). These data indicate that the ECMO period in COVID-19 patients is not as long as we expected. We presume that time is needed to improve both ARDS caused by COVID-19 and ARDS caused by other reasons, as recovery in patients with critical ARDS is difficult.
In this study, the preintubation period was longer in intubated survivors than in intubated non-survivors or ECMO patients. This tendency was also observed when assessing data for the prehospitalization and hospitalization-intubation periods, despite no direct comparison. Indeed, the time from symptom onset to pneumonia was longer in COVID-19 patients with severe disease than in those without severe disease (131), the time from symptom onset to dyspnea and hospitalization in ICU COVID-19 patients was longer than that in non-ICU COVID-19 patients, and the time from symptom onset to ICU admission tended to be longer in COVID-19 non-survivors than in COVID-19 survivors (10).
There are two possibilities for these findings. First, hospitalization delay and lack of medical resources may contribute to the result. COVID-19 pandemic forces people to self-restraint, and it leads to hospitalization delay. Moreover, a shortage of ventilators also leads to intubation delay and poor prognosis (132–134). In fact, the hospitalization-intubation period among non-survivors, and ECMO patients tended to be longer than that among intubated survivors; the hospitalization-intubation period among intubated survivors, non-survivors, and ECMO patients with COVID-19 was 2.62 days (1.66–3.58 days), 3.28 days (2.15–4.41 days), and 3.39 (2.08–4.69 days), respectively. Second, a critical condition itself leads to a long prehospitalization period. Although the mechanism has yet to be elucidated, some of the COVID-19 patients experience asymptomatic hypoxia, which is also called “silent hypoxia.” In COVID-19 patients, 28.1% of hospitalized patients are estimated to have “silent hypoxia;” 33% of hospitalized patients with “silent hypoxia” are admitted to the ICU, and 25.9% of hospitalized patients with “silent hypoxia” die (135). Hence, “silent hypoxia” itself is a critical condition that leads to a long prehospitalization period. In this situation, monitoring blood oxygen, early hospitalization with oxygen supplementation, and systemic management arguably lead to a better prognosis.
The reasons why the intubation period was shorter in East Asia than in the US and Europe may include selection bias, information bias, differences in treatment, ventilator and ICU bed availability, race, and genetics. We detected bias concerning the East Asia data, which showed minor variations in regions and faculties compared to those from the US and Europe because East Asia's data were mainly from China, especially Wuhan. The shortage of ventilators and ICU beds has been highlighted in the US and Europe (132, 136), and it arguably contributed to a delay of intubation and a prolonged clinical course. Race and genetic background are also possible reasons for the observed clinical differences among regions. For example, data from the US show that Asians were hospitalized less but that Black and Hispanics were hospitalized more (137, 138). Sixteen percent of the genes were derived from Neanderthals, one of the prognostic factors maintained in Europe (almost 0% in East Asia) (139). GWASs have revealed that SNPs and blood type, the percentages of which differ among races and regions, are also prognostic factors. Thus, an international cohort study is needed to reveal the difference in clinical course between race and region.
Limitations
There were also some limitations in this meta-analysis. Although many studies were included, more studies and patients are needed. Furthermore, heterogeneity was high because it was difficult to standardize the patients' backgrounds. This study revealed the clinical course of survivors and non-survivors, but a direct comparison with survivors and non-survivors is still needed. Clinical information, for example, age, BMI, cardiac disease, kidney disease, and chronic obstructive disease, was not sufficiently described in the cases we included, and articles in some of the countries reporting were limited. Social information was also not described in the cases we included; however, whether social information, such as patient or national income level, affects the clinical course is of great interest. Moreover, differences in viral strain and treatment including anti-inflammatory treatment, because of the study period. In general, comparing clinical data with our data will reveal more knowledge about COVID-19.
Conclusions
Our data indicate that prehospitalization and intubation periods were longer in intubated non-survivors and ECMO patients than in intubated survivors with COVID-19. These periods might serve as predictors of disease severity or death and support therapeutic strategy determination. In the near future, viral strains and treatments should be taken into account when evaluating the clinical course of COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
KF and TM designed the study, carried out the literature search, independently acquired the data, screened the records, extracted the data, assessed the risk of bias, and performed the statistical analyses. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by research grants from the Center of Innovation program (COISTREAM) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (to AK), the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP18H05282 to AK), the Japan Agency for Medical Research and Development (AMED) (J200705023, J200705710, J200705049, JP18cm016335, JP18cm059042, and J210705582 to AK), a grant from the Kansai Economic Federation (KANKEIREN), and Grants from Mitsubishi Foundation (to AK). This work was also supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP21K16287 to TM).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank our colleagues from Osaka University Center of Medical Data Science and Advanced Clinical Epidemiology Investigator's Research Project for their providing insight and expertise for our research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.727101/full#supplementary-material
Abbreviations
CIs, confidence intervals; COVID-19, Coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ECMO-death period, duration from ECMO initiation to death; ECMO period, duration from ECMO initiation to decannulation; hospitalization-death period, duration from hospitalization to death; hospitalization-discharge period, duration from hospitalization to discharge; hospitalization-ECMO period, duration from hospitalization to ECMO initiation; hospitalization-intubation period, duration from hospitalization to intubation; ICU, intensive care unit; intubation-death period, duration from intubation to death; intubation-ECMO period, duration from intubation to ECMO initiation; intubation period, duration from intubation to extubation; IQR, interquartile range; preECMO period, duration from symptom onset to ECMO initiation; prehospitalization period, duration from symptom onset to hospitalization; preintubation period, duration from symptom onset to intubation; SARS-CoV-1, severe acute respiratory syndrome coronavirus 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; symptom-death period, duration from symptom onset to death.
References
1. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
2. Weekly Epidemiological Update on COVID-19,. (2021). Available online at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-−3-august-2021 (accessed August 5, 2021).
3. Flaxman S, Mishra S, Gandy A, Unwin HJT, Mellan TA, Coupland H, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. (2020) 584:257–61. doi: 10.1038/s41586-020-2405-7
4. Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. (2020) 395:1973–87. doi: 10.1016/S0140-6736(20)31142-9
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
6. Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:759–65. doi: 10.15585/mmwr.mm6924e2
7. CDC. Disease Burden of Influenza. (2020). Available online at: https://www.cdc.gov/flu/about/burden/index.html (accessed June 1, 2021).
8. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
9. Bravata DM, Perkins AJ, Myers LJ, Arling G, Zhang Y, Zillich AJ, et al. Association of intensive care unit patient load and demand with mortality rates in US department of veterans affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. (2021) 4:e2034266. doi: 10.1001/jamanetworkopen.2020.34266
10. Yang X, Yu Y, Xu J, Shu H, Xia J 'an, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Res Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
11. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. (2020) 323:1612–4. doi: 10.1001/jama.2020.4326
12. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with sars-cov-2 admitted to ICUs of the lombardy region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
13. Piroth L, Cottenet J, Mariet A-S, Bonniaud P, Blot M, Tubert-Bitter P, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. (2021) 9:251–9. doi: 10.1016/S2213-2600(20)30527-0
14. Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with covid-19 and seasonal influenza: cohort study. BMJ. (2020) 371:m4677. doi: 10.1136/bmj.m4677
15. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
16. Gavin W, Campbell E, Zaidi S-A, Gavin N, Dbeibo L, Beeler C, et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am J Infect Control. (2021) 49:158–65. doi: 10.1016/j.ajic.2020.07.005
17. Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. (2020) 201:1560–4. doi: 10.1164/rccm.202004-1163LE
18. Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. (2020) 158:97–105. doi: 10.1016/j.chest.2020.04.010
19. Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. (2020) 75:1788–95. doi: 10.1093/gerona/glaa089
20. Wu S, Du Z, Shen S, Zhang B, Yang H, Li X, et al. Identification and validation of a novel clinical signature to predict the prognosis in confirmed coronavirus disease 2019 patients. Clin Infect Dis. (2020) 71:3154–62. doi: 10.1093/cid/ciaa793
21. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
23. Study Quality Assessment Tools. Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed May 28, 2021).
24. OCEBM Levels of Evidence (2020). Available online at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (Accessed March 28, 2021).
25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
27. The R Project for Statistical Computing. Available online at: https://www.r-project.org/ (accessed January 4, 2021).
28. Abe T, Izumo T, Ueda A, Hayashi M, Ishibashi Y. Successful treatment of two Japanese ESRD cases with severe COVID-19 pneumonia. CEN Case Rep. (2021) 10:42–5. doi: 10.1007/s13730-020-00512-7
29. Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. (2020) 369:m1996. doi: 10.1136/bmj.m1996
30. Barrasa H, Rello J, Tejada S, Martín A, Balziskueta G, Vinuesa C, et al. SARS-CoV-2 in spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. (2020) 39:553–61. doi: 10.1016/j.accpm.2020.04.001
31. Beigmohammadi MT, Jahanbin B, Safaei M, Amoozadeh L, Khoshavi M, Mehrtash V, et al. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Pathol. (2021) 29:135–45. doi: 10.1177/1066896920935195
32. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. (2020) 382:2012–22. doi: 10.1056/NEJMoa2004500
33. Cauchois R, Koubi M, Delarbre D, Manet C, Carvelli J, Blasco VB, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci USA. (2020) 117:18951–3. doi: 10.1073/pnas.2009017117
34. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
35. Christie DB 3rd, Nemec HM, Scott AM, Buchanan JT, Franklin CM, Ahmed A, et al. Early outcomes with utilization of tissue plasminogen activator in COVID-19-associated respiratory distress: a series of five cases. J Trauma Acute Care Surg. (2020) 89:448–52. doi: 10.1097/TA.0000000000002787
36. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. (2020) 10:783–91. doi: 10.1158/2159-8290.CD-20-0422
37. Dastan F, Saffaei A, Haseli S, Marjani M, Moniri A, Abtahian Z, et al. Promising effects of tocilizumab in COVID-19: a non-controlled, prospective clinical trial. Int Immunopharmacol. (2020) 88:106869. doi: 10.1016/j.intimp.2020.106869
38. De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. (2020) 2:e465–73. doi: 10.1016/S2665-9913(20)30170-3
39. Dogan L, Kaya D, Sarikaya T, Zengin R, Dincer A, Akinci IO, et al. Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: case series. Brain Behav Immun. (2020) 87:155–8. doi: 10.1016/j.bbi.2020.05.022
40. Elder C, Bawa S, Anderson D, Atkinson S, Etzel J, Moritz T. Expectant management of pneumothorax in intubated COVID-19 positive patients: a case series. J Cardiothorac Surg. (2020) 15:263. doi: 10.1186/s13019-020-01297-7
41. Falces-Romero I, Ruiz-Bastián M, Díaz-Pollán B, Maseda E, García-Rodríguez J, SARS-CoV-2 Working Group. Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish Tertiary Care Hospital. Mycoses. (2020) 63:1144–8. doi: 10.1111/myc.13155
42. Flikweert AW, Grootenboers MJJH, Yick DCY, du Mée AWF, van der Meer NJM, Rettig TCD, et al. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. (2020) 59:149–55. doi: 10.1016/j.jcrc.2020.07.002
43. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in lombardy, Italy. JAMA Intern Med. (2020) 180:1345–55. doi: 10.1001/jamainternmed.2020.3539
44. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. (2020) 382:2327–36. doi: 10.1056/NEJMoa2007016
45. Halvatsiotis P, Kotanidou A, Tzannis K, Jahaj E, Magira E, Theodorakopoulou M, et al. Demographic and clinical features of critically ill patients with COVID-19 in Greece: the burden of diabetes and obesity. Diabetes Res Clin Pract. (2020) 166:108331. doi: 10.1016/j.diabres.2020.108331
46. Hernandez-Romieu AC, Adelman MW, Hockstein MA, Robichaux CJ, Edwards JA, Fazio JC, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. (2020) 48:e1045–53. doi: 10.1097/CCM.0000000000004600
47. Kato H, Shimizu H, Shibue Y, Hosoda T, Iwabuchi K, Nagamine K, et al. Clinical course of 2019 novel coronavirus disease (COVID-19) in individuals present during the outbreak on the Diamond Princess cruise ship. J Infect Chemother. (2020) 26:865–9. doi: 10.1016/j.jiac.2020.05.005
48. Ketcham SW, Adie SK, Malliett A, Abdul-Aziz AA, Bitar A, Grafton G, et al. Coronavirus disease-2019 in heart transplant recipients in southeastern michigan: a case series. J Card Fail. (2020) 26:457–61. doi: 10.1016/j.cardfail.2020.05.008
49. Kewan T, Covut F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClin Med. (2020) 24:100418. doi: 10.1016/j.eclinm.2020.100418
50. Khullar R, Shah S, Singh G, Bae J, Gattu R, Jain S, et al. Effects of prone ventilation on oxygenation, inflammation, and lung infiltrates in COVID-19 related acute respiratory distress syndrome: a retrospective cohort study. J Clin Med Res. (2020) 9:4129. doi: 10.3390/jcm9124129
51. Konopka KE, Nguyen T, Jentzen JM, Rayes O, Schmidt CJ, Wilson AM, et al. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology. (2020) 77:570–8. doi: 10.1111/his.14180
52. Krishnan S, Patel K, Desai R, Sule A, Paik P, Miller A, et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. (2020) 67:110005. doi: 10.1016/j.jclinane.2020.110005
53. Kristinsson B, Kristinsdottir LB, Blondal AT, Thormar KM, Kristjansson M, Karason S, et al. Nationwide incidence and outcomes of patients with coronavirus disease 2019 requiring intensive care in Iceland. Crit Care Med. (2020) 48:e1102–5. doi: 10.1097/CCM.0000000000004582
54. Lê MP, Jaquet P, Patrier J, Wicky P-H, Le Hingrat Q, Veyrier M, et al. Pharmacokinetics of lopinavir/ritonavir oral solution to treat COVID-19 in mechanically ventilated ICU patients. J Antimicrob Chemother. (2020) 75:2657–60. doi: 10.1093/jac/dkaa261
55. LeBrun DG, Konnaris MA, Ghahramani GC, Premkumar A, DeFrancesco CJ, Gruskay JA, et al. Hip fracture outcomes during the COVID-19 pandemic: early results from New York. J Orthop Trauma. (2020) 34:403–10. doi: 10.1097/BOT.0000000000001849
56. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. (2020) 277:2251–61. doi: 10.1007/s00405-020-05965-1
57. Lee AJY, Chung CLH, Young BE, Ling LM, Ho BCH, Puah SH, et al. Clinical course and physiotherapy intervention in 9 patients with COVID-19. Physiotherapy. (2020) 109:1–3. doi: 10.1016/j.physio.2020.06.002
58. Liu J, Zhang S, Wu Z, Shang Y, Dong X, Li G, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. (2020) 10:99. doi: 10.1186/s13613-020-00706-3
59. Lowe A, Chang DD, Creek G. Multiple fatalities in a family cluster of COVID-19 with acute respiratory distress syndrome. Ochsner J. (2020) 20:134–8. doi: 10.31486/toj.20.0056
60. Maritati F, Cerutti E, Zuccatosta L, Fiorentini A, Finale C, Ficosecco M, et al. SARS-CoV-2 infection in kidney transplant recipients: experience of the italian marche region. Transpl Infect Dis. (2020) 22:e13377. doi: 10.1111/tid.13377
61. Morassi M, Bagatto D, Cobelli M, D'Agostini S, Gigli GL, Bnà C, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. (2020) 267:2185–92. doi: 10.1007/s00415-020-09885-2
62. Morillas JA, Marco Canosa F, Srinivas P, Asadi T, Calabrese C, Rajendram P, et al. Tocilizumab therapy in 5 solid and composite tissue transplant recipients with early ARDS due to SARS-CoV-2. Am J Transplant. (2020) 20:3191–7. doi: 10.1111/ajt.16080
63. Navarro-Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol. (2020) 72:1990–7. doi: 10.1002/art.41422
64. Novelli L, Raimondi F, Ghirardi A, Pellegrini D, Capodanno D, Sotgiu G, et al. At the peak of COVID-19 age and disease severity but not comorbidities are predictors of mortality: COVID-19 burden in Bergamo, Italy. Panminerva Med. (2021) 63:51–61. doi: 10.23736/S0031-0808.20.04063-X
65. Pan C, Chen L, Lu C, Zhang W, Xia J-A, Sklar MC, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. (2020) 201:1294–7. doi: 10.1164/rccm.202003-0527LE
66. Peng M, Liu X, Li J, Ren D, Liu Y, Meng X, et al. Successful management of seven cases of critical COVID-19 with early noninvasive-invasive sequential ventilation algorithm and bundle pharmacotherapy. Front Med. (2020) 14:674–80. doi: 10.1007/s11684-020-0796-3
67. Plotnikow GA, Matesa A, Nadur JM, Alonso M, Nuñez II, Vergara G, et al. Characteristics and outcomes of patients infected with nCoV19 requiring invasive mechanical ventilation in Argentina. Rev Bras Ter Intensiva. (2020) 32:348–53. doi: 10.5935/0103-507X.20200062
68. Radnis C, Qiu S, Jhaveri M, Da Silva I, Szewka A, Koffman L. Radiographic and clinical neurologic manifestations of COVID-19 related hypoxemia. J Neurol Sci. (2020) 418:117119. doi: 10.1016/j.jns.2020.117119
69. Riker RR, May TL, Fraser GL, Gagnon DJ, Bandara M, Zemrak WR, et al. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res Pract Thromb Haemost. (2020) 4:936–41. doi: 10.1002/rth2.12390
70. Rizo-Téllez SA, Méndez-García LA, Flores-Rebollo C, Alba-Flores F, Alcántara-Suárez R, Manjarrez-Reyna AN, et al. The neutrophil-to-monocyte ratio and lymphocyte-to-neutrophil ratio at admission predict in-hospital mortality in mexican patients with severe SARS-CoV-2 infection (Covid-19). Microorganisms. (2020) 8:1560. doi: 10.3390/microorganisms8101560
71. Sakr Y, Giovini M, Leone M, Pizzilli G, Kortgen A, Bauer M, et al. The clinical spectrum of pulmonary thromboembolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a European case series. J Crit Care. (2021) 61:39–44. doi: 10.1016/j.jcrc.2020.09.021
72. Schaefer I-M, Padera RF, Solomon IH, Kanjilal S, Hammer MM, Hornick JL, et al. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. (2020) 33:2104–14. doi: 10.1038/s41379-020-0595-z
73. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. (2020) 323:1582–9. doi: 10.1001/jama.2020.4783
74. Singh S, Chakravarty T, Chen P, Akhmerov A, Falk J, Friedman O, et al. Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: compassionate-use case series. Basic Res Cardiol. (2020) 115:36. doi: 10.1007/s00395-020-0795-1
75. So C, Ro S, Murakami M, Imai R, Jinta T. High-dose, short-term corticosteroids for ARDS caused by COVID-19: a case series. Respirol Case Rep. (2020) 8:e00596. doi: 10.1002/rcr2.596
76. Søvik S, Bådstøløkken PM, Sørensen V, Myhre PL, Prebensen C, Omland T, et al. A single-centre, prospective cohort study of COVID-19 patients admitted to ICU for mechanical ventilatory support. Acta Anaesthesiol Scand. (2021) 65:351–9. doi: 10.1111/aas.13726
77. Stony Brook COVID-19 Research Consortium. Geospatial distribution and predictors of mortality in hospitalized patients with COVID-19: a cohort study. Open Forum Infect Dis. (2020) 7:ofaa436. doi: 10.1093/ofid/ofaa436
78. Wali A, Rizzo V, Bille A, Routledge T, Chambers AJ. Pneumomediastinum following intubation in COVID-19 patients: a case series. Anaesthesia. (2020) 75:1076–81. doi: 10.1111/anae.15113
79. Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. (2020) 201:1430–4. doi: 10.1164/rccm.202003-0736LE
80. Wang X-H, Duan J, Han X, Liu X, Zhou J, Wang X, et al. High incidence and mortality of pneumothorax in critically Ill patients with COVID-19. Heart Lung. (2021) 50:37–43. doi: 10.1016/j.hrtlng.2020.10.002
81. Weiskopf D, Schmitz KS, Raadsen MP, Grifoni A, Okba NMA, Endeman H, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. (2020) 5:eabd2071. doi: 10.1126/sciimmunol.abd2071
82. Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. (2020) 26:1070–6. doi: 10.1038/s41591-020-0944-y
83. Zhang L, Li J, Zhou M, Chen Z. Summary of 20 tracheal intubation by anesthesiologists for patients with severe COVID-19 pneumonia: retrospective case series. J Anesth. (2020) 34:599–606. doi: 10.1007/s00540-020-02778-8
84. Akhtar W, Olusanya O, Baladia MM, Young H, Shah S. SARS-CoV-2 and ECMO: early results and experience. Indian J Thorac Cardiovasc Surg. (2020) 37:53–60. doi: 10.1007/s12055-020-01084-y
85. Alnababteh M, Hashmi MD, Vedantam K, Chopra R, Kohli A, Hayat F, et al. Extracorporeal membrane oxygenation for COVID-19 induced hypoxia: single-center study. Perfusion. (2021) 36:564–72. doi: 10.1177/0267659120963885
86. Beyls C, Huette P, Abou-Arab O, Berna P, Mahjoub Y. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome and risk of thrombosis. Br J Anaesth. (2020) 125:e260–2. doi: 10.1016/j.bja.2020.04.079
87. Charlton M, Dashey S, Stubbs A, Lai FY, Bird PW, Badhwar V, et al. Comparing SARS-CoV-2 and influenza A(H1N1)pdm09-infected patients requiring ECMO - a single-centre, retrospective observational cohort experience. J Infect. (2021) 82:84–123. doi: 10.1016/j.jinf.2020.11.003
88. Falcoz P-E, Monnier A, Puyraveau M, Perrier S, Ludes P-O, Olland A, et al. Extracorporeal membrane oxygenation for critically ill patients with COVID-19-related acute respiratory distress syndrome: worth the effort? Am J Respir Crit Care Med. (2020) 202:460–3. doi: 10.1164/rccm.202004-1370LE
89. Goursaud S, Descamps R, Daubin C, du Cheyron D, Valette X. Corticosteroid use in selected patients with severe acute respiratory distress syndrome related to COVID-19. J Infect. (2020) 81:e89–90. doi: 10.1016/j.jinf.2020.05.023
90. Guihaire J, Owyang CG, Madhok J, Laverdure F, Gaillard M, Girault A, et al. Specific considerations for venovenous extracorporeal membrane oxygenation during coronavirus disease 2019 pandemic. ASAIO J. (2020) 66:1069–72. doi: 10.1097/MAT.0000000000001251
91. Guo Z, Sun L, Li B, Tian R, Zhang X, Zhang Z, et al. Anticoagulation management in severe coronavirus disease 2019 patients on extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. (2021) 35:389–97. doi: 10.1053/j.jvca.2020.08.067
92. Heman-Ackah SM, Su YS, Spadola M, Petrov D, Chen HI, Schuster J, et al. Neurologically devastating intraparenchymal hemorrhage in COVID-19 patients on extracorporeal membrane oxygenation: a case series. Neurosurgery. (2020) 87:E147–51. doi: 10.1093/neuros/nyaa198
93. Huette P, Beyls C, Guilbart M, Coquet A, Berna P, Haye G, et al. Extracorporeal membrane oxygenation for respiratory failure in COVID-19 patients: outcome and time-course of clinical and biological parameters. Can J Anaesth. (2020) 67:1486–8. doi: 10.1007/s12630-020-01727-z
94. Jäckel M, Rilinger J, Lang CN, Zotzmann V, Kaier K, Stachon P, et al. Outcome of acute respiratory distress syndrome requiring extracorporeal membrane oxygenation in Covid-19 or influenza: a single-center registry study. Artif Organs. (2021) 45:593–601. doi: 10.1111/aor.13865
95. Jacobs JP, Stammers AH, St Louis J, Hayanga JWA, Firstenberg MS, Mongero LB, et al. Extracorporeal membrane oxygenation in the treatment of severe pulmonary and cardiac compromise in coronavirus disease 2019: experience with 32 patients. ASAIO J. (2020) 66:722–30. doi: 10.1097/MAT.0000000000001185
96. Kon ZN, Smith DE, Chang SH, Goldenberg RM, Angel LF, Carillo JA, et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. (2021) 111:537–43. doi: 10.1016/j.athoracsur.2020.07.002
97. Le Breton C, Besset S, Freita-Ramos S, Amouretti M, Billiet PA, Dao M, et al. Extracorporeal membrane oxygenation for refractory COVID-19 acute respiratory distress syndrome. J Crit Care. (2020) 60:10–12. doi: 10.1016/j.jcrc.2020.07.013
98. Li X, Guo Z, Li B, Zhang X, Tian R, Wu W, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. ASAIO J. (2020) 66:475–81. doi: 10.1097/MAT.0000000000001172
99. Liu J, Dong Y-Q, Yin J, He G, Wu X, Li J, et al. Critically ill patients with COVID-19 with ECMO and artificial liver plasma exchange: a retrospective study. Medicine. (2020) 99:e21012. doi: 10.1097/MD.0000000000021012
100. Loforte A, Dal Checco E, Gliozzi G, Benedetto M, Cavalli GG, Mariani C, et al. Veno-venous extracorporeal membrane oxygenation support in COVID-19 respiratory distress syndrome: initial experience. ASAIO J. (2020) 66:734–8. doi: 10.1097/MAT.0000000000001198
101. Matsunaga N, Hayakawa K, Terada M, Ohtsu H, Asai Y, Tsuzuki S, et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 REGISTRY JAPAN. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa1470
102. Miike S, Sakamoto N, Washino T, Kosaka A, Kuwahara Y, Ishida T, et al. Critically ill patients with COVID-19 in Tokyo, Japan: a single-center case series. J Infect Chemother. (2021) 27:291–5. doi: 10.1016/j.jiac.2020.10.019
103. Mustafa AK, Alexander PJ, Joshi DJ, Tabachnick DR, Cross CA, Pappas PS, et al. Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg. (2020) 155:990–2. doi: 10.1001/jamasurg.2020.3950
104. Osho AA, Moonsamy P, Hibbert KA, Shelton KT, Trahanas JM, Attia RQ, et al. Veno-venous extracorporeal membrane oxygenation for respiratory failure in COVID-19 patients: early experience from a major academic medical center in North America. Ann Surg. (2020) 272:e75–8. doi: 10.1097/SLA.0000000000004084
105. Ronit A, Berg RMG, Bay JT, Haugaard AK, Ahlström MG, Burgdorf KS, et al. Compartmental immunophenotyping in COVID-19 ARDS: a case series. J Allergy Clin Immunol. (2021) 147:81–91. doi: 10.1016/j.jaci.2020.09.009
106. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. (2020) 8:1121–31. doi: 10.1016/S2213-2600(20)30328-3
107. Shih E, DiMaio JM, Squiers JJ, Banwait JK, Meyer DM, George TJ, et al. Venovenous extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019 (COVID-19): multicenter experience of referral hospitals in a large health care system. J Thorac Cardiovasc Surg. (2020) (inpress). doi: 10.1016/j.jtcvs.2020.11.073
108. Sultan I, Habertheuer A, Usman AA, Kilic A, Gnall E, Friscia ME, et al. The role of extracorporeal life support for patients with COVID-19: preliminary results from a statewide experience. J Card Surg. (2020) 35:1410–3. doi: 10.1111/jocs.14583
109. Usman AA, Han J, Acker A, Olia SE, Bermudez C, Cucchiara B, et al. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. (2020) 34:3006–12. doi: 10.1053/j.jvca.2020.07.063
110. Xuan W, Chen C, Jiang X, Zhang X, Zhu H, Zhang S, et al. Clinical characteristics and outcomes of five critical COVID-19 patients treated with extracorporeal membrane oxygenation in Leishenshan Hospital in Wuhan. J Clin Anesth. (2020) 67:110033. doi: 10.1016/j.jclinane.2020.110033
111. Xu J, Xie J, Du B, Tong Z, Qiu H, Bagshaw SM. Clinical characteristics and outcomes of patients with severe COVID-19 induced acute kidney injury. J Intensive Care Med. (2021) 36:319–26. doi: 10.1177/0885066620970858
112. Yang X, Cai S, Luo Y, Zhu F, Hu M, Zhao Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019-induced acute respiratory distress syndrome: a multicenter descriptive study. Crit Care Med. (2020) 48:1289–95. doi: 10.1097/CCM.0000000000004447
113. Zayat R, Kalverkamp S, Grottke O, Durak K, Dreher M, Autschbach R, et al. Role of extracorporeal membrane oxygenation in critically Ill COVID-19 patients and predictors of mortality. Artif Organs. (2021) 45:E158–70. doi: 10.1111/aor.13873
114. Zeng Y, Cai Z, Xianyu Y, Yang BX, Song T, Yan Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: a retrospective case series. Crit Care. (2020) 24:148. doi: 10.1186/s13054-020-2840-8
115. Zeng J-H, Wu W-B, Qu J-X, Wang Y, Dong C-F, Luo Y-F, et al. Cardiac manifestations of COVID-19 in Shenzhen, China. Infection. (2020) 48:861–70. doi: 10.1007/s15010-020-01473-w
116. Zhang J, Merrick B, Correa GL, Camporota L, Retter A, Doyle A, et al. Veno-venous extracorporeal membrane oxygenation in coronavirus disease 2019: a case series. ERJ Open Res. (2020) 6:00463. doi: 10.1183/23120541.00463-2020
117. Zhang Q, Shen J, Chen L, Li S, Zhang W, Jiang C, et al. Timing of invasive mechanic ventilation in critically ill patients with coronavirus disease 2019. J Trauma Acute Care Surg. (2020) 89:1092–8. doi: 10.1097/TA.0000000000002939
118. Zheng Y, Sun L-J, Xu M, Pan J, Zhang Y-T, Fang X-L, et al. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B. (2020) 21:378–87. doi: 10.1631/jzus.B2000174
119. Feng Y, Amoateng-Adjepong Y, Kaufman D, Gheorghe C, Manthous CA. Age, duration of mechanical ventilation, and outcomes of patients who are critically ill. Chest. (2009) 136:759–64. doi: 10.1378/chest.09-0515
120. Faisy C, Meziani F, Planquette B, Clavel M, Gacouin A, Bornstain C, et al. Effect of acetazolamide vs. placebo on duration of invasive mechanical ventilation among patients with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. (2016) 315:480–8. doi: 10.1001/jama.2016.0019
121. Barbier F, Coquet I, Legriel S, Pavie J, Darmon M, Mayaux J, et al. Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Med. (2009) 35:1678–86. doi: 10.1007/s00134-009-1559-4
122. Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. (2002) 287:345–55. doi: 10.1001/jama.287.3.345
123. Shorr AF, Bodi M, Rodriguez A, Sole-Violan J, Garnacho-Montero J, Rello J, et al. Impact of antibiotic guideline compliance on duration of mechanical ventilation in critically ill patients with community-acquired pneumonia. Chest. (2006) 130:93–100. doi: 10.1016/S0012-3692(15)50958-6
124. Wang J-T, Sheng W-H, Fang C-T, Chen Y-C, Wang J-L, Yu C-J, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. (2004) 10:818–24. doi: 10.3201/eid1005.030640
125. Tsai M-J, Yang K-Y, Chan M-C, Kao K-C, Wang H-C, Perng W-C, et al. Impact of corticosteroid treatment on clinical outcomes of influenza-associated ARDS: a nationwide multicenter study. Ann Intensive Care. (2020) 10:26. doi: 10.1186/s13613-020-0642-4
126. Kovacevic P, Matijasevic J, Dragic S, Zlojutro B, Gavrilovic S, Jandric M, et al. Characteristics and outcomes of critically ill patients with influenza A (H1N1) in the Western Balkans during the 2019 post-pandemic season. Indian J Med Microbiol. (2020) 38:415–20. doi: 10.4103/ijmm.IJMM_20_169
127. Ludwig M, Jacob J, Basedow F, Andersohn F, Walker J. Clinical outcomes and characteristics of patients hospitalized for Influenza or COVID-19 in Germany. Int J Infect Dis. (2021) 103:316–22. doi: 10.1016/j.ijid.2020.11.204
128. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. (2009) 374:1351–63. doi: 10.1016/S0140-6736(09)61069-2
129. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. (2018) 378:1965–75. doi: 10.1056/NEJMoa1800385
130. Sukhal S, Sethi J, Ganesh M, Villablanca PA, Malhotra AK, Ramakrishna H. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: a systematic review and meta-analysis. Ann Card Anaesth. (2017) 20:14–21. doi: 10.4103/0971-9784.197820
131. Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
132. Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. (2020) 382:e41. doi: 10.1056/NEJMp2006141
133. Carrillo A, Gonzalez-Diaz G, Ferrer M, Martinez-Quintana ME, Lopez-Martinez A, Llamas N, et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. (2012) 38:458–66. doi: 10.1007/s00134-012-2475-6
134. Kang BJ, Koh Y, Lim C-M, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. (2015) 41:623–32. doi: 10.1007/s00134-015-3693-5
135. Brouqui P, Amrane S, Million M, Cortaredona S, Parola P, Lagier J-C, et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis. (2021) 102:233–8. doi: 10.1016/j.ijid.2020.10.067
136. Ramachandran P, Swamy L, Kaul V, Agrawal A. A national strategy for ventilator and ICU resource allocation during the coronavirus disease 2019 pandemic. Chest. (2020) 158:887–9. doi: 10.1016/j.chest.2020.04.050
137. Cunningham JW, Vaduganathan M, Claggett BL, Jering KS, Bhatt AS, Rosenthal N, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. (2021) 181:379–81. doi: 10.1001/jamainternmed.2020.5313
138. Karaca-Mandic P, Georgiou A, Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 States. JAMA Intern Med. (2021) 181:131–4. doi: 10.1001/jamainternmed.2020.3857
Keywords: COVID-19, clinical course, invasive mechanical ventilation, extracorporeal membrane oxygenation, meta-analysis
Citation: Funakoshi K, Morita T and Kumanogoh A (2021) Longer Prehospitalization and Preintubation Periods in Intubated Non-survivors and ECMO Patients With COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 8:727101. doi: 10.3389/fmed.2021.727101
Received: 18 June 2021; Accepted: 20 September 2021;
Published: 15 October 2021.
Edited by:
Reza Lashgari, Shahid Beheshti University, IranReviewed by:
Shivank Singh, Southern Medical University, ChinaHeidi J. Dalton, Inova Health System, United States
Copyright © 2021 Funakoshi, Morita and Kumanogoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takayoshi Morita, dC1tb3JpdGFAaW1lZDMubWVkLm9zYWthLXUuYWMuanA=
 Kenji Funakoshi
Kenji Funakoshi Takayoshi Morita
Takayoshi Morita Atsushi Kumanogoh1,2,3,4
Atsushi Kumanogoh1,2,3,4