Corrigendum: Risk Factors for Maternal and Fetal Mortality in Acute Fatty Liver of Pregnancy and New Predictive Models
- 1Department of Critical Care Medicine, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 3Department of Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Acute fatty liver of pregnancy (AFLP) is a rare but potentially life-threatening hepatic disorder that leads to considerable maternal and fetal mortality. To explore the risk factors for maternal and fetal mortality in AFLP and develop new predictive models, through this retrospective study, we analyzed the demographic characteristics, clinical symptoms, and laboratory findings of 106 patients with AFLP who were admitted to Shandong Provincial Hospital. Risk factors for maternal and fetal mortality were analyzed by univariate and multivariate logistic regression analysis. The new models based on the multivariate logistic regression analysis and the model for end-stage liver disease (MELD) were tested in AFLP. The receiver operating characteristic curve (ROC) was applied to compare the predictive efficiency, sensitivity, and specificity of the two models. Prenatal nausea (p = 0.037), prolonged prothrombin time (p = 0.003), and elevated serum creatinine (p = 0.003) were independent risk factors for maternal mortality. The ROC curve showed that the area under the curve (AUC) of the MELD was 0.948, with a sensitivity of 100% and a specificity of 83.3%. The AUC of the new model for maternal mortality was 0.926, with a sensitivity of 90% and a specificity of 94.8%. Hepatic encephalopathy (p = 0.016) and thrombocytopenia (p = 0.001) were independent risk factors for fetal mortality. Using the ROC curve, the AUC of the MELD was 0.694, yielding a sensitivity of 68.8% and a specificity of 64.4%. The AUC of the new model for fetal mortality was 0.893, yielding a sensitivity of 100% and a specificity of 73.3%. Both the new predictive model for maternal mortality and the MELD showed good predictive efficacy for maternal mortality in patients with AFLP (AUC = 0.926 and 0.948, respectively), and the new predictive model for fetal mortality was superior to the MELD in predicting fetal mortality (AUC = 0.893 and 0.694, respectively). The two new predictive models were more readily available, less expensive, and easier to implement clinically, especially in low-income countries.
Introduction
Acute fatty liver of pregnancy (AFLP) is a rare but potentially life-threatening hepatic disorder that occurs during the third trimester or early postpartum period. It is defined as severe hepatic synthetic dysfunction due to microvascular steatosis. Although the reported incidence of AFLP was 1 in 7,000 to 1 in 15,000 pregnancies (1), it could progress rapidly to serious complications such as disseminated intravascular coagulation (DIC), postpartum hemorrhage, multiple organ dysfunction syndrome (MODS), acute hepatic failure (AHF), and maternal or fetal death. The pathogenesis of AFLP remains unclear, and most of the literature supports that it is secondary to mitochondrial defects in the fetal long-chain 3-hydroxyacyl-coenzyme A dehydrogenase, as well as other enzymes potentially involved in fatty oxidation, leading to excessive accumulation of fatty acids in maternal hepatocytes, which, in turn, leads to lipotoxicity, oxidative damage, inflammation, and hepatocyte necrosis (2). Early recognition and diagnosis of AFLP with prompt termination of pregnancy and intensive supportive care are essential for both maternal and fetal survival. With advances in multidisciplinary supportive management of patients with AFLP, maternal and fetal mortality rates have decreased significantly to 7–18% and 9–23%, respectively (3).
Early assessment of the prognosis of patients with AFLP may play an important role in improving maternal and fetal survival (4), and predictive analytics for risk stratification can help to improve patients management in critical care setting and add some reference for this statement (5–7). Previous clinical studies on AFLP, largely based on a small number of patients owing to its low prevalence, have found significant differences in its epidemiology (1, 8), symptoms (9), complications (9), and outcomes (1, 10, 11). An excellent prediction model must be objective, easy to operate, and possess satisfactory sensitivity and specificity. The MELD, founded in 2000 by Malinchoc et al. of Mayo Clinic, the largest liver disease center in the United States, was a grading method for assessing the severity of end-stage liver disease. It was originally created to predict the survival of 231 patients with cirrhosis and portal hypertension after transjugular intrahepatic portosystemic shunt. The statistical model obtained by Cox proportional hazard regression identified four laboratory and clinical indicators that can be used to better assess the three-month survival of patients (12). Thereafter, Kamath et al. improved the scoring system to R = 3.8 ln (bilirubin) + 11.2 ln (INR) + 9.6 ln (creatinine) + 6.4 (13), which made the MELD one of the most widely used scoring systems for evaluating the prognosis of liver disease. Thus far, many studies have reported the ability of the MELD to predict the short-term prognosis of patients with acute liver failure and pregnancy-specific liver diseases (14, 15). However, large-sample studies have rarely investigated the efficacy of the MELD in predicting maternal and fetal outcome of AFLP, owing to the rarity of this condition. The existing literature predominantly consists of small hospital-based case series or historical cohorts identified retrospectively over a number of years. Therefore, there is an urgent need for clinical studies on AFLP, especially large-sample and multicenter prospective studies, to help clinicians make prognostic judgments.
Our study included 106 patients with AFLP who were admitted to our hospital during the past 10 years. We aimed to explore the independent risk factors for maternal and fetal mortality, and develop new models for predicting the poor prognosis of patients with AFLP.
Patients and Methods
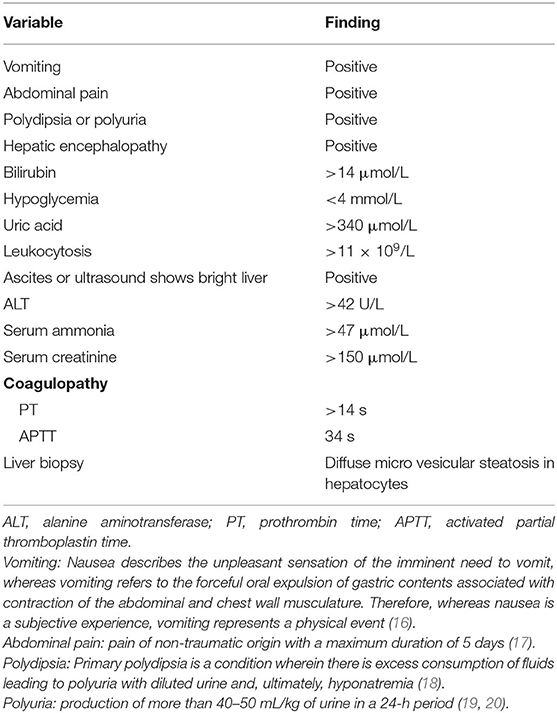
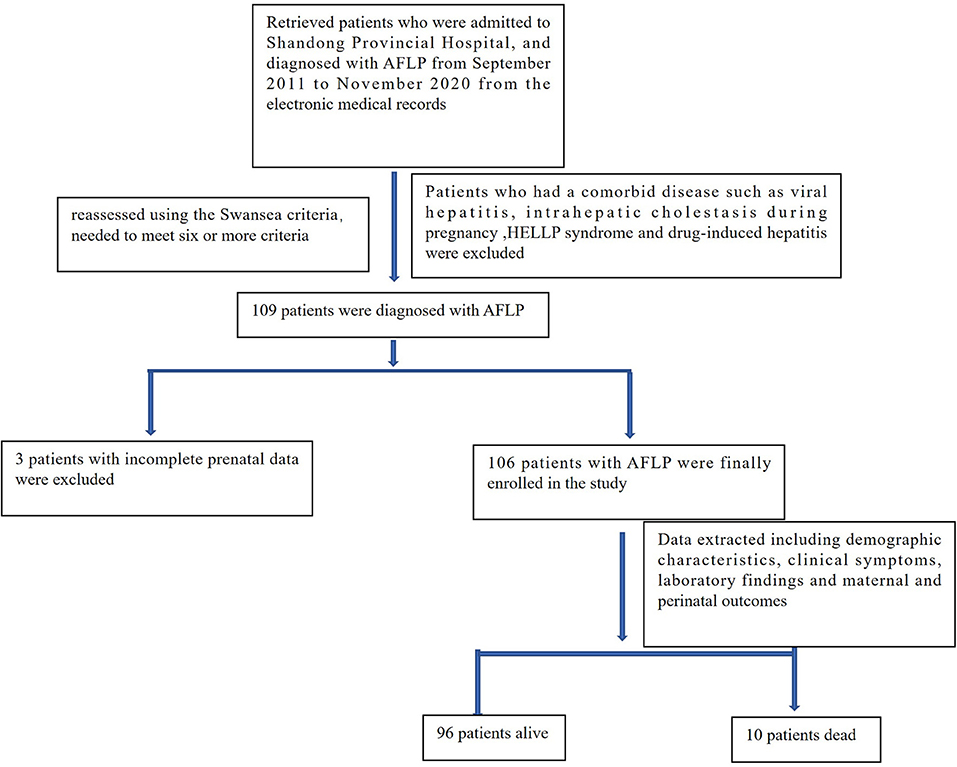
We retrospectively analyzed the data of 119 patients who were admitted to Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University and diagnosed with AFLP from September 2011 to November 2020. The diagnosis of all selected patients was reassessed using the Swansea criteria (wherein the diagnosis of AFLP requires the satisfaction of six or more criteria), as detailed in Table 1. Ten patients who also had a comorbid disease such as viral hepatitis, intrahepatic cholestasis during pregnancy; hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome; and drug-induced hepatitis, and three patients with incomplete prenatal data were excluded. A total of 106 patients with AFLP were finally enrolled in the study. The flowchart for patient enrollment is provided in Figure 1.
All patients were prenatally diagnosed with AFLP. Information regarding their laboratory findings, imaging data, and clinical symptoms was collected from the electronic medical records, and they were followed up within 1 month after discharge. The study was approved by the Biomedical Research Committee of Shandong Provincial Hospital (approval no. SWYX: NO.2021-052). The ethics committee waived the need for obtaining informed consent from the patients, because the study was an observational, retrospective study using a database from which the patients' identification information had been removed. Data extracted from these medical records included demographic characteristics, clinical symptoms, laboratory findings, clinical course, and maternal and perinatal outcomes.
Demographic characteristics included age, gestational weeks, parity, mode of delivery, single or twin fetus, fetal sex, admission to the intensive care unit (ICU) or not, and days from the first symptom to delivery. Clinical symptoms included abdominal pain, anorexia, nausea, vomiting, polyuria, jaundice, encephalopathy, and high blood pressure. Laboratory findings included prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), fibrinogen, white blood cell count, hemoglobin, percentage of neutrophils, neutrophils (N), platelet count (PLT), procalcitonin, blood urea nitrogen (BUN), blood creatinine (Cr), blood glucose, uric acid, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), total bilirubin (TBIL), direct bilirubin (DBIL), and albumin (Alb). Primary prognostic outcomes included maternal death and fetal death.
Swansea Criteria for Diagnosis of AFLP
Owing to the rarity of AFLP, currently no internationally recognized diagnostic standard for this condition exists, except for liver biopsy. However, many studies have mentioned that although the gold standard for diagnosis is liver biopsy, this procedure is not always necessary, and the Swansea diagnostic criteria are regarded as an alternative to liver biopsy, as shown in Table 1 (11, 21, 22). Six or more criteria are required to diagnose AFLP. The exclusion criteria were as follows: viral hepatitis, intrahepatic cholestasis of pregnancy, HELLP syndrome, drug-induced hepatitis, autoimmune hepatitis, and other diseases.
Statistical Analysis
GraphPad 8 and SPSS 21.0 software were used for statistical analysis. Continuous variables were expressed as mean and standard deviation, and categorical variables were expressed as count and percentages. Continuous variables were tested using Student's t-test, t-test with Welch correction, or Mann–Whitey U-test, depending on normal distribution and homogeneity in variance. The counting data were tested using the chi-square test. Univariate analysis was used to determine the factors related to maternal and fetal mortality. In order to avoid the omission of important factors, the significant variables obtained by univariate analysis and the variables with p-values between 0.05 and 0.1 or other clinically significant variables were included into the multiple logistic regression analysis performed by the forward selection method to determine the independent risk factors for different outcomes. Patients with severely incomplete data were excluded from the research. A small amount of missing data was not included in the univariate analysis; the results of univariate analysis showed that these variables with missing data were not related to maternal and fetal mortality. The data of the variables included in the multivariate analysis were complete. Independent risk factors, B-value, and constant obtained from the multiple logistic regression analysis were used to build prognostic prediction models. The new models and the MELD were used to assess all patients with AFLP. The receiver operating characteristic (ROC) curve was applied to compare the prediction efficiency, sensitivity, and specificity of the two models in evaluating the prognosis of patients with AFLP.
Results
Clinical Characteristics
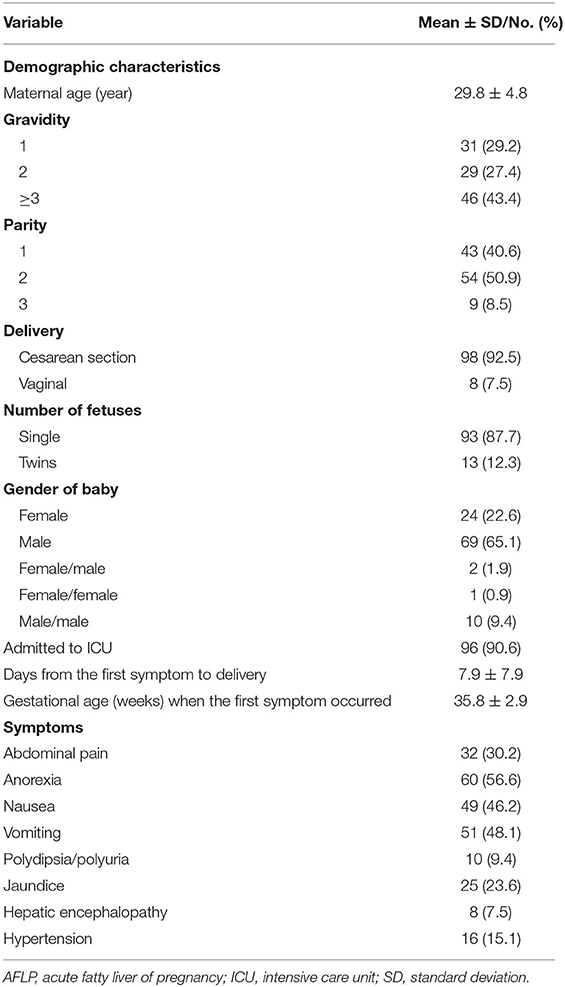
A total of 106 patients with AFLP were enrolled in this study. Their demographic characteristics and clinical symptoms are shown in Table 2. Laboratory findings and prognostic outcomes are shown in Tables 3, 4, respectively. The average maternal age was 29.8 ± 4.8 years, and the average gestational age was 35.8 ± 2.9 weeks. The median duration from the first symptom to delivery was 7.9 ± 7.9 days. In total, 43 (40.6%) patients were primigravida and 63 (59.4%) were multigravida; 98 (92.5%) patients delivered by cesarean section and 8 (7.5%) patients delivered vaginally. A total of 91 (76.5%) male and 28 (23.5%) female infants were born. Among all patients with AFLP, 96 (90.6%) were admitted to the intensive care unit (ICU) after delivery. The common clinical symptoms included anorexia (56.6%), vomiting (48.1%), nausea (46.2%), abdominal pain (30.2%), jaundice (23.6%), hypertension (15.1%), polydipsia and polyuria (9.4%), and hepatic encephalopathy/disturbance of consciousness (7.5%).
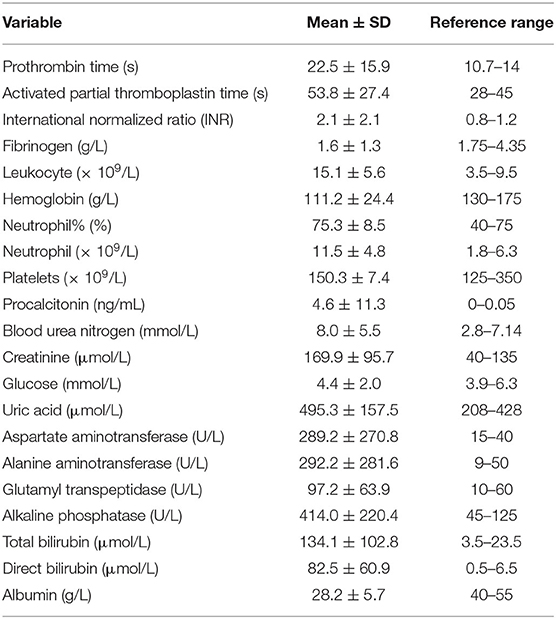
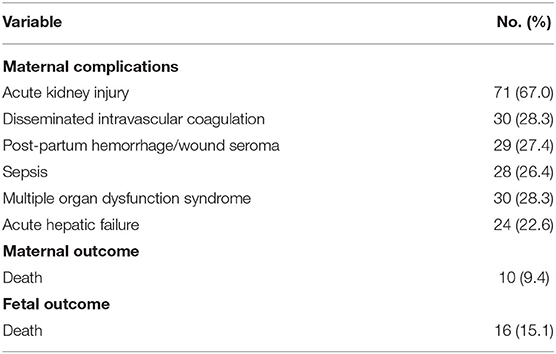
Coagulation tests yielded obviously abnormal results, including prolonged PT (22.5 ± 15.9 s), APTT (53.8 ± 27.4 s), INR (2.1 ± 2.1), and decreased fibrinogen levels (1.6 ± 1.3 g/L). The results of blood routine tests showed increased leukocyte (15.1 ± 5.6 × 109/L) and neutrophil (11.5 ± 4.8 × 109/L) counts, decreased hemoglobin (111.2 ± 24.4 g/L), and normal PLT count (150.3 ± 7.4 × 109/L). Procalcitonin was significantly increased (4.6 ± 11.3 ng/mL). Liver function tests revealed increased levels of ALT (292.2 ± 281.6 U/L), AST (289.2 ± 270.8 U/L), GGT (97.2 ± 63.9 U/L), ALP (414.0 ± 220.4 U/L), TBIL (134.1 ± 102.8 μmol/L), and DBIL (82.5 ± 60.9 μmol/L). Renal function was impaired, as evident from increased BUN (8.0 ± 5.5 mmol/L) and Cr (169.9 ± 95.7 μmol/L) levels. Abdominal ultrasound was performed for 71 patients, out of which 42 (59.2%) patients showed positive results. However, 35 patients did not undergo prenatal abdominal ultrasound. The maternal mortality rate was 9.4% (10/106) and the fetal mortality rate was 15.1% (16/106). Nine patients died from multiple organ dysfunction syndrome and one patient died from postpartum hemorrhage. The common severe complications included acute kidney injury (AKI; 67.0%), DIC (28.3%), MODS (28.3%), postpartum hemorrhage (27.4%), sepsis (26.4%), and AHF (22.6%).
Risk Factors for Maternal Mortality, and the New Predictive Model
The distinction in demographic and clinical characteristics and laboratory findings between survivors and non-survivors is summarized in Table 5. Univariate analyses showed that maternal mortality was significantly related to nausea (p = 0.042), hepatic encephalopathy (p = 0.027), prolonged PT (p < 0.0001), prolonged APTT (p = 0.0009), increased INR (p < 0.0001), decreased fibrinogen (p = 0.004), increased leukocytes (p = 0.018), increased neutrophils (p = 0.012), thrombocytopenia (p = 0.0003), increased Cr (p = 0.002), increased TBIL (p = 0.006), increased DBIL (p = 0.024), and decreased Alb (p = 0.017).
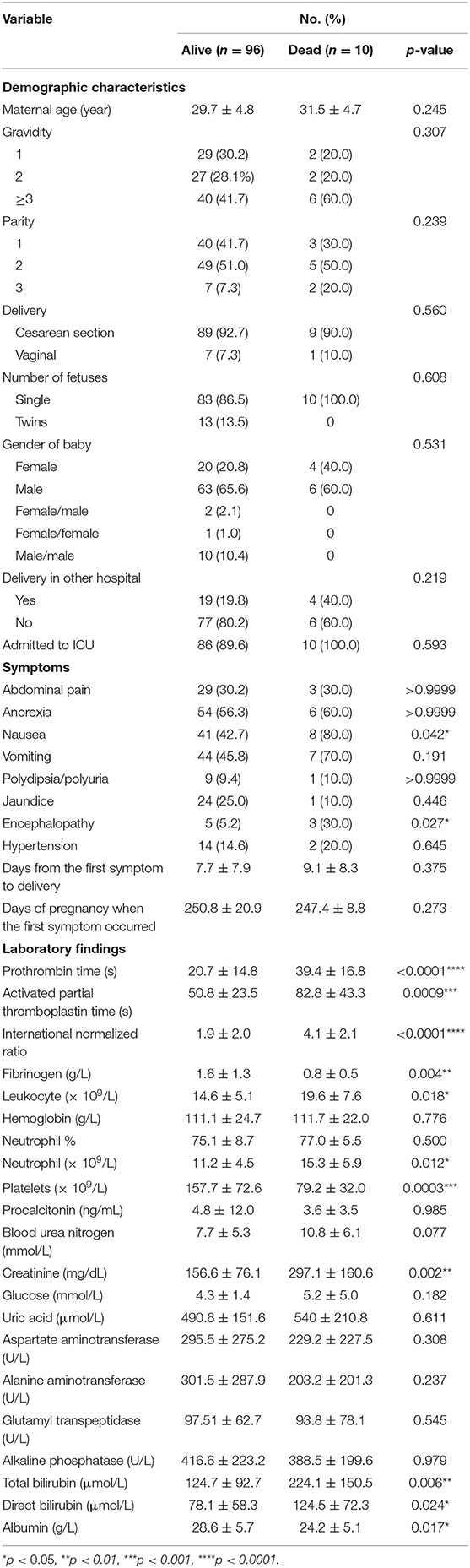
Table 5. Comparison of demographic, clinical, and laboratory characteristics between maternal survivors and non-survivors.
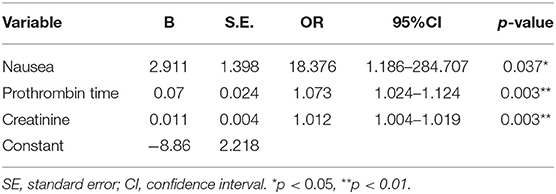
The above significant variables and BUN (p = 0.077) were included in the logistic regression analysis performed using the forward selection approach, in order to avoid missing important risk factors. The results of logistic regression analysis showed that nausea (p = 0.037), prolonged PT (p = 0.003), and increased Cr (p = 0.003) were independent risk factors for maternal mortality, as shown in Table 6. Based on these three variables, a new predictive model for maternal mortality was established using the following formula:
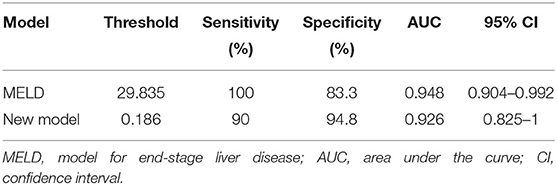
The ROC curve was used to evaluate the predictive efficiency of the new model and the MELD with regard to the prognosis of maternal mortality (Figure 2, Table 7). The threshold of the MELD was 29.835 and the AUC was 0.948, with a sensitivity of 100% and a specificity of 83.3%. The threshold of the new model was 0.186 and the AUC was 0.926, with a sensitivity of 90% and a specificity of 94.8%. Both the new model and the MELD could predict the prognosis of patients with AFLP. The new model was superior to the MELD in terms of specificity.
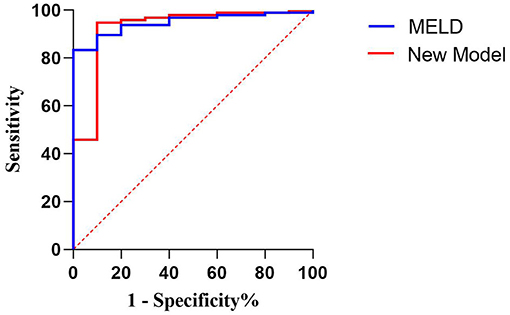
Figure 2. Receiver operating characteristic curve of the model for end-stage liver disease scoring system and the new model for predicting maternal death.
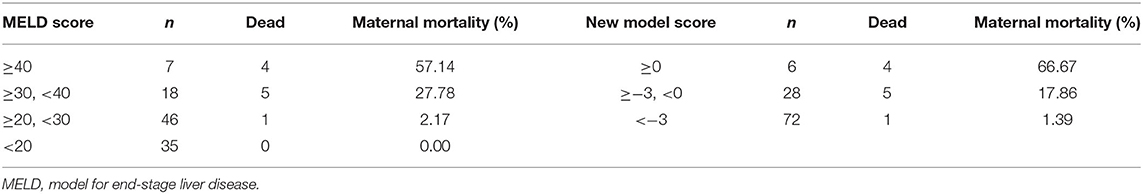
According to the threshold of the ROC curve, the MELD scores were divided into four groups and the new model scores were divided into three groups (Table 8). The mortality rate of the patients with AFLP gradually increased with the increase in the scores of the two models. When the MELD score was ≥40, the mortality rate was 57.14%; when the new model score was ≥0, the mortality rate was 66.67%. Therefore, the new model could more acutely predict maternal mortality. Moreover, the threshold, which was nearly 0, indicated that the new model was more convenient for clinical application.
Risk Factors for Fetal Mortality, and the New Predictive Model
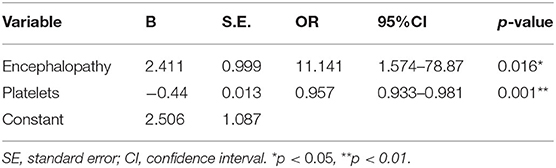
As shown in Table 9, univariate analysis showed that fetal mortality was significantly related to encephalopathy (p = 0.017), prolonged PT (p = 0.0005), prolonged APTT (p < 0.0001), increased INR (p = 0.0008), decreased fibrinogen (p = 0.016), elevated leukocyte (p = 0.043), thrombocytopenia (p < 0.0001), decreased GGT (p = 0.019), increased TBIL (p = 0.007), increased DBIL (p = 0.018), and decreased Alb (p = 0.006).
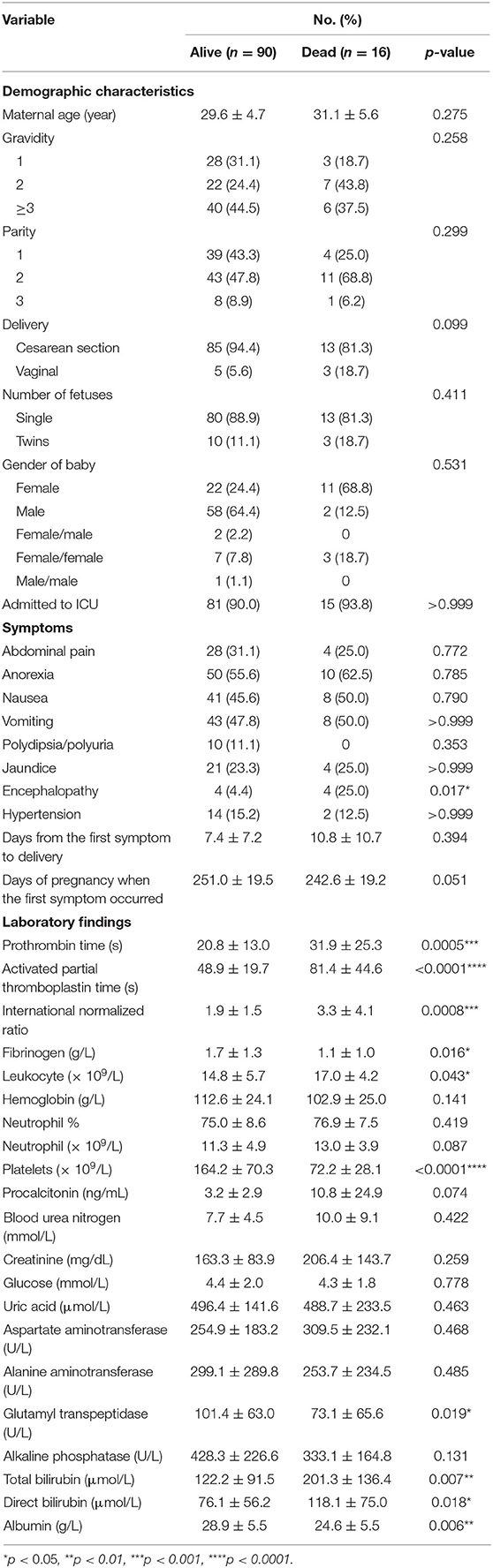
Table 9. Comparison of demographic, clinical, and laboratory characteristics between fetal survivors and non-survivors.
Multivariate logistic regression analysis showed that encephalopathy (p = 0.016) and thrombocytopenia (p = 0.001) were independent risk factors for fetal mortality (Table 10). Thereafter, a new predictive model for fetal mortality was established using the following formula:
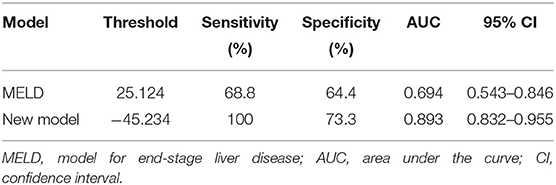
In predicting fetal mortality, the threshold of the MELD was 25.124 and the AUC was 0.694, with a sensitivity of 68.8% and a specificity of 64.4%. The threshold of the new model was −45.234 and the AUC was 0.893, with a sensitivity of 100% and a specificity of 73.3%. Thus, compared with the MELD, the new model could more accurately predict fetal mortality, with a higher sensitivity and specificity (Table 11, Figure 3).
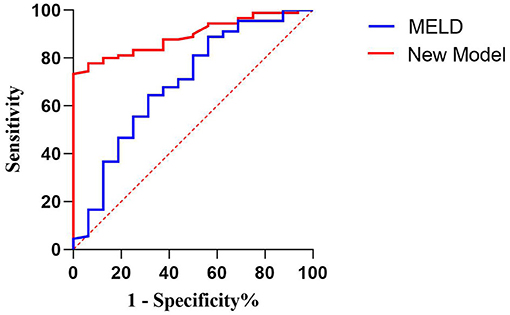
Figure 3. Receiver operating characteristic curve of the model for end-stage liver disease scoring system and the new model for predicting fetal death.
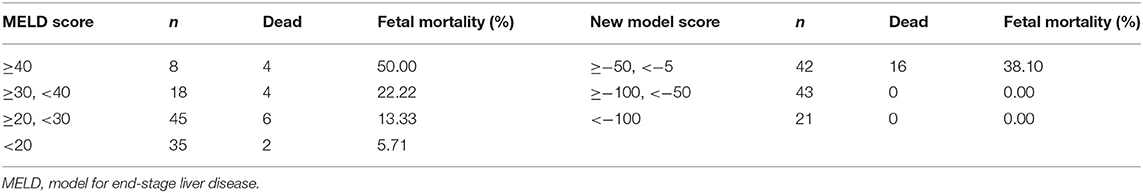
Both models were grouped according to the previous method (Table 12). The fetal mortality of patients with AFLP also gradually increased with the increase in the scores of the two models. When the MELD score was ≥40, the fetal mortality rate was 50%; when the new model score was ≥−50 and < −5„ the fetal mortality rate was 38.1%. Although the fetal mortality rate was lower when the new model score exceeded the threshold, it could predict the adverse outcomes of all fetuses, which indicated the high sensitivity and clinical application value of this model.
Discussion
AFLP is a rare and fatal obstetric emergency that occurs in the second and third trimester of pregnancy or in the early postpartum period. It can lead to acute liver failure, AKI, multiple organ failure, and even maternal and fetal death. Many studies have analyzed the high-risk factors for the morbidity associated with AFLP, fatal complications, and perinatal death. Recent studies have shown that being a primigravida, multiple pregnancies, carrying a male fetus, other liver diseases during pregnancy, previous history of AFLP, and pre-eclampsia are the potential risk factors for AFLP (1, 23–25). The recognition of high-risk factors is helpful for the prevention and treatment of AFLP, and can consequently improve the prognosis of the mother and the child. Early diagnosis; prompt delivery; and multidisciplinary supportive care from the departments of obstetrics, blood transfusion, and the ICU have resulted in improved maternal mortality (3). Although liver biopsy is the gold standard for the diagnosis of AFLP, it is rarely performed owing to its invasive nature and because it can cause complications in the presence of coagulopathy. In addition, liver biopsy is just a diagnostic method and does not contribute significantly to the treatment of AFLP. Therefore, none of the patients with AFLP in this study underwent liver biopsy.
The 106 patients with AFLP who were enrolled in this study delivered 119 fetuses, including 13 twin pregnancies and 93 single pregnancies. The incidence of twin pregnancy was 12.3% (13/106), which occurred only in the surviving group; however, there was no statistically significant difference in the incidence of twin pregnancy between the survivor and non-survivor groups (13.5% vs. 0, p = 0.608). This finding is similar to the results of another retrospective study conducted in China by Cheng et al. (26) that showed that the incidence of twin pregnancy among patients with AFLP was 28.1%; however, there was a statistically significant difference between the survivor and non-survivor groups (44.4 vs. 7.1%, p = 0.02). This indicated that twin pregnancy may be a potential protective factor for patients with AFLP; however, this is contrary to the results of the prospective study conducted by Knight et al. (1). Although our study enrolled the largest number of patients among the three studies, it was still not a sufficiently large sample. Because of the rarity of AFLP, our study does not have the power to determine whether this is a statistically significant relationship or just a chance finding. A previous study by Gao et al. showed that male fetus, intrauterine death, postpartum diagnosis of AFLP, DIC, and prolonged PT and APTT were potential risk factors for maternal mortality in AFLP, whereas a history of legal termination of pregnancy, and increased TBIL and serum Cr were independent risk factors (27). In this study, male fetuses (p = 0.580) and a history of legal termination of pregnancy (p = 0.239) showed no statistically significant difference between the two groups and were not included in the potential risk factors for maternal mortality in AFLP.
Previous studies have rarely included a predictive model for fetal mortality. In this study, a new model for predicting fetal mortality was established and the predictive value of the MELD was also verified. The results of multivariate logistic regression analysis indicated that hepatic encephalopathy (p = 0.016) and thrombocytopenia (p = 0.001) were independent risk factors for fetal mortality in patients with AFLP. Hepatic encephalopathy is a comprehensive disorder of central nervous system dysfunction caused by severe liver disease. As the most direct complication of liver damage in patients with acute liver failure, it is one of the causes of death in patients with liver disease. Its occurrence suggests that patients with AFLP have had acute liver failure before delivery and the fetus has a high incidence of intrauterine distress and stillbirth. In patients with pre-eclampsia and HELLP syndrome, thrombocytopenia is an independent risk factor for postpartum complications such as infection, thromboembolism, and DIC, and these complications are also common in patients with AFLP (28). The retrospective study by Cheng et al. showed that carrying a male fetus and vaginal delivery were risk factors for fetal mortality; however, these two variables did not show significant positive predictive value in our study (26). Gao et al. found that fetal distress and prolonged APTT were risk factors for fetal mortality (27). The univariate analysis in our study showed that prolonged APTT was a risk factor for fetal mortality (p < 0.0001), but multivariate analysis showed no positive predictive value. The new predictive model for fetal mortality based on hepatic encephalopathy and thrombocytopenia was compared with the MELD with regard to the prediction of fetal mortality. The results showed that, compared with the MELD, the new predictive model for fetal mortality could more accurately predict fetal mortality (AUC = 0.893 and 0.694, respectively), with a higher sensitivity and specificity, and the variables were fewer, more readily available, and less expensive.
In the present study, the common severe complications besides death were AKI (67.0%), DIC (28.3%), MODS (28.3%), postpartum hemorrhage (27.4%), sepsis (26.4%), and AHF (22.6%), which is consistent with the results of the study by Chen et al. (29). In their study, the most common maternal complication was acute renal dysfunction (79.5%), followed by DIC (47.7%) and MODS (38.6%).
Maternal and fetal mortality rates attributable to AFLP vary greatly among studies, with the maternal mortality rate ranging from 12 to 18% and the fetal mortality rate ranging from 7 to 58% (30). Our previous clinical study showed that the maternal and fetal mortality rates of 52 patients with AFLP admitted to our hospital from January 2001 to December 2011 were 8% and 23%, respectively (31). In this study, a total of 106 patients with AFLP were admitted to our hospital from September 2011 to November 2020, and 119 fetuses were delivered. The maternal and fetal mortality rates were 9.4% (10/106) and 15.1% (18/119) respectively, both of which were lower than those reported in other studies. Timely termination of pregnancy is crucial in the treatment and a basic principle, because AFLP is an idiopathic disease during pregnancy. In the last decade, in our hospital, 83 (78.3%) pregnancies were terminated within 24 h when AFLP was strongly suspected or diagnosed, and 96 (90.6%) patients were admitted to the ICU after delivery. We attributed the lower maternal and fetal mortality to the proactive policy of early diagnosis and prompt termination, and close cooperation between obstetricians and critical care physicians. Compared with the last decade, the maternal mortality rate has declined slightly and the fetal mortality rate has decreased significantly in our hospital. This may be related to the loosening of the two-child policy, leading to an increasing number of older mothers and, consequently, more complications during pregnancy, causing a slight increase in maternal mortality. However, with the development of multidisciplinary supportive management in our hospital, especially pediatric intensive care, the level of comprehensive treatment of the fetus has been greatly improved, leading to a significant decline in the fetal mortality rate.
In this study, prenatal nausea (p = 0.037), prolonged PT (p = 0.003), and elevated serum Cr (p = 0.003) were independent risk factors for maternal mortality in patients with AFLP. Another study reported that ascites, thrombocytopenia, and serum Cr were independent risk factors for postpartum complications in pre-eclampsia and HELLP syndrome (32). The clinical symptoms of AFLP are similar to those of HELLP syndrome, and both belong to pregnancy-specific liver diseases. The predictive model for AFLP also included one clinical symptom and two laboratory findings, and elevated serum Cr was an independent risk factor for both AFLP and HELLP syndrome. However, the difference between the PLT count in AFLP was statistically significant in univariate analysis (p = 0.0003) and was eliminated in multivariate logistic regression analysis. This suggests that thrombocytopenia is a potential risk factor for maternal mortality in AFLP, which needs to be verified by a larger-sample study. Transaminase levels have not been shown to be important across most disease models in liver disease, including our model for AFLP (p > 0.05). A single-center retrospective study with 130 cases (AFLP = 32; HELLP = 81; pre-eclampsia and liver disease = 17) showed that both the MELD and the new model with two objective variables, namely serum TBIL and INR, were reliable for predicting the short-term mortality in patients with pregnancy-specific liver disease (followed up until 3 months after delivery or until death) (15). In the present study, TBIL and INR were statistically significant in univariate analysis (p = 0.006 and p < 0.0001, respectively), but they were eliminated in multivariate logistic regression analysis, which also suggested that increased TBIL and prolonged INR are potential risk factors for maternal mortality in AFLP; further prospective studies with larger sample sizes are warranted to explore the risk factors for maternal mortality in patients with AFLP.
Previous clinical studies have shown that the MELD, based on TBIL, Cr, and INR shows good predictive efficacy for acute liver failure and pregnancy-specific liver disease (14, 15). A study conducted in China showed that the MELD was a good predictor of all complications of AFLP, including ascites, hepatic encephalopathy, sepsis, and renal insufficiency (all AUCs > 0.8), and the optimal cut-off values were close to 30 (33). Our study also verified that both the MELD and the new model show good predictive efficacy in predicting maternal mortality in AFLP (AUC = 0.948 and 0.926, respectively).
Overall, compared with previous models based on laboratory findings alone, the new predictive model for maternal mortality included one clinical symptom and two laboratory findings, making it more readily available, less expensive, and easier to implement clinically. To the best of our knowledge, the symptom of nausea that we identified as an independent risk factor for AFLP has not been previously described.
This study had a long duration of almost 10 years, and is the largest single-center clinical study on AFLP so far. The number of patients with AFLP enrolled in this study is only second to that in the multicenter study by Gao et al. in which our hospital has participated in the past (27). As all patients with AFLP came from one single center, they received similar obstetric and multidisciplinary treatments after hospitalization, and some limitations of different medical levels were counter-balanced.
There are some limitations to our research. Firstly, we did not evaluate the morbidity of AFLP owing to the deficiency of data on total pregnant women during the study period. Secondly, this was a single-center and small-sample study because of the rarity of AFLP, which might reduce the general applicability of our findings, although we had extended the study period to almost one decade and our study was a retrospective study. Thirdly, as Shandong Provincial Hospital is a tertiary referral center for critical patients in China, some patients with AFLP were referred to our hospital after severe postpartum complications, and their condition was relatively critical. The manner and timing of medical intervention during their prenatal treatment differed, which directly affected the prognosis of the patients. Finally, it is noteworthy that the AUROC value is inflated in the internal data because external validation was not performed in the study.
In summary, both the new predictive model for maternal mortality and the MELD showed good predictive efficacy for maternal mortality in patients with AFLP (AUC = 0.926 and 0.948, respectively), and the new predictive model was superior to the MELD in predicting fetal mortality (AUC = 0.893 and 0.694, respectively). The two new predictive models were more readily available, less expensive, and easier to implement clinically, especially in low-income countries. However, as our study is a single-center, retrospective study with a small sample size, further prospective studies with large sample sizes are warranted to evaluate the efficiency of these two models for predicting maternal and fetal mortality in patients with AFLP.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Biomedical Research Committee of Shandong Provincial Hospital (approval no. SWYX: NO.2021-052). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CW, MC, and ZM contributed to the conception and design of the study. CW and WF organized the database. MC performed the statistical analysis. ZM wrote the first draft of the manuscript. MM, JZ, QW, and GQ revised the sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81903086) and Shandong Provincial Natural Science Foundation (Grant No. ZR2019QH014), which was received by MC, and by Shanghai Shenkang Hospital Development Center (Grant No. SHDC12019125), which was received by MM.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.719906/full#supplementary-material
References
1. Knight M, Nelson-Piercy C, Kurinczuk JJ, Spark P, Brocklehurst P. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. (2008) 57:951–6. doi: 10.1136/gut.2008.148676
2. Natarajan SK, Ibdah JA. Role of 3-Hydroxy fatty acid-induced hepatic lipotoxicity in acute fatty liver of pregnancy. Int J Mol Sci. (2018) 19:322. doi: 10.3390/ijms19010322
4. Knox TA, Olans LB. Liver disease in pregnancy. N Engl J Med. (1996) 335:569–76. doi: 10.1056/NEJM199608223350807
5. Zhang Z, Zheng B, Liu N, Ge H, Hong Y. Mechanical power normalized to predicted body weight as a predictor of mortality in patients with acute respiratory distress syndrome. Intensive Care Med. (2019) 45:856–64. doi: 10.1007/s00134-019-05627-9
6. Zhang Z, Navarese EP, Zheng B, Meng Q, Liu N, Ge H, et al. Analytics with artificial intelligence to advance the treatment of acute respiratory distress syndrome. J Evid Based Med. (2020) 13:301–12. doi: 10.1111/jebm.12418
7. Zhang Z, Liu J, Xi J, Gong Y, Zeng L, Ma P. Derivation and validation of an ensemble model for the prediction of agitation in mechanically ventilated patients maintained under light sedation. Crit Care Med. (2021) 49:e279–90. doi: 10.1097/CCM.0000000000004821
8. Nelson DB, Yost NP, Cunningham FG. Acute fatty liver of pregnancy: clinical outcomes and expected duration of recovery. Am J Obstet Gynecol. (2013) 209:456.e1–7. doi: 10.1016/j.ajog.2013.07.006
9. Minakami H, Morikawa M, Yamada T, Yamada T, Akaishi R, Nishida R. Differentiation of acute fatty liver of pregnancy from syndrome of hemolysis, elevated liver enzymes and low platelet counts. J Obstet Gynaecol Res. (2014) 40:641–9. doi: 10.1111/jog.12282
10. Xiong HF, Liu JY, Guo LM, Li XW. Acute fatty liver of pregnancy: over six months follow-up study of twenty-five patients. World J Gastroenterol. (2015) 21:1927–31. doi: 10.3748/wjg.v21.i6.1927
11. Goel A, Ramakrishna B, Zachariah U, Ramachandran J, Eapen CE, Kurian G, et al. How accurate are the swansea criteria to diagnose acute fatty liver of pregnancy in predicting hepatic microvesicular steatosis? Gut. (2011) 60:138–9; author reply 39–40. doi: 10.1136/gut.2009.198465
12. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. (2000) 31:864–71. doi: 10.1053/he.2000.5852
13. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. (2001) 33:464–70. doi: 10.1053/jhep.2001.22172
14. McPhail MJ. Improving MELD for use in acute liver failure. J Hepatol. (2011) 54:1320; author reply 20–1. doi: 10.1016/j.jhep.2010.11.017
15. Murali AR, Devarbhavi H, Venkatachala PR, Singh R, Sheth KA. Factors that predict 1-month mortality in patients with pregnancy-specific liver disease. Clin Gastroenterol Hepatol. (2014) 12:109–13. doi: 10.1016/j.cgh.2013.06.018
16. Quigley EM, Hasler WL, Parkman HP. AGA technical review on nausea and vomiting. Gastroenterology. (2001) 120:263–86. doi: 10.1053/gast.2001.20516
17. Gans SL, Pols MA, Stoker J, Boermeester MA. Guideline for the diagnostic pathway in patients with acute abdominal pain. Dig Surg. (2015) 32:23–31. doi: 10.1159/000371583
18. Christ-Crain M, Bichet DG, Fenske WK, Goldman MB, Rittig S, Verbalis JG, et al. Diabetes insipidus. Nat Rev Dis Primers. (2019) 5:54. doi: 10.1038/s41572-019-0103-2
19. Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. (2017) 147:w14514. doi: 10.4414/smw.2017.14514
20. Nigro N, Grossmann M, Chiang C, Inder WJ. Polyuria-polydipsia syndrome: a diagnostic challenge. Intern Med J. (2018) 48:244–53. doi: 10.1111/imj.13627
21. Ch'ng CL, Morgan M, Hainsworth I, Kingham JG. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. (2002) 51:876–80. doi: 10.1136/gut.51.6.876
22. Joshi D, James A, Quaglia A, Westbrook RH, Heneghan MA. Liver disease in pregnancy. Lancet. (2010) 375:594–605. doi: 10.1016/S0140-6736(09)61495-1
23. Davidson KM, Simpson LL, Knox TA, D'Alton ME. Acute fatty liver of pregnancy in triplet gestation. Obstet Gynecol. (1998) 91 (5 Pt. 2):806–8. doi: 10.1016/S0029-7844(97)00477-8
24. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. (2010) 36:751–6. doi: 10.1111/j.1447-0756.2010.01242.x
25. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. (2009) 15:897–906. doi: 10.3748/wjg.15.897
26. Cheng N, Xiang T, Wu X, Li M, Xie Y, Zhang L. Acute fatty liver of pregnancy: a retrospective study of 32 cases in South China. J Matern Fetal Neonatal Med. (2014) 27:1693–7. doi: 10.3109/14767058.2013.871704
27. Gao Q, Qu X, Chen X, Zhang J, Liu F, Tian S, et al. Outcomes and risk factors of patients with acute fatty liver of pregnancy: a multicentre retrospective study. Singapore Med J. (2018) 59:425–30. doi: 10.11622/smedj.2018001
28. Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. (2007) 46:1844–52. doi: 10.1002/hep.21838
29. Chen G, Huang K, Ji B, Chen C, Liu C, Wang X, et al. Acute fatty liver of pregnancy in a Chinese tertiary care center: a retrospective study. Arch Gynecol Obstet. (2019) 300:897–901. doi: 10.1007/s00404-019-05259-w
30. Rajasri AG, Srestha R, Mitchell J. Acute fatty liver of pregnancy (AFLP)–an overview. J Obstet Gynaecol. (2007) 27:237–40. doi: 10.1080/01443610701194705
31. Wang S, Li SL, Cao YX, Li YP, Meng JL, Wang XT. Noninvasive swansea criteria are valuable alternatives for diagnosing acute fatty liver of pregnancy in a Chinese population. J Matern Fetal Neonatal Med. (2017) 30:2951–55. doi: 10.1080/14767058.2016.1269316
32. Deruelle P, Coudoux E, Ego A, Houfflin-Debarge V, Codaccioni X, Subtil D. Risk factors for post-partum complications occurring after preeclampsia and HELLP syndrome. A study in 453 consecutive pregnancies. Eur J Obstet Gynecol Reprod Biol. (2006) 125:59–65. doi: 10.1016/j.ejogrb.2005.07.011
Keywords: AFLP, maternal mortality, fetal mortality, risk factor, prognostic model
Citation: Meng Z, Fang W, Meng M, Zhang J, Wang Q, Qie G, Chen M and Wang C (2021) Risk Factors for Maternal and Fetal Mortality in Acute Fatty Liver of Pregnancy and New Predictive Models. Front. Med. 8:719906. doi: 10.3389/fmed.2021.719906
Received: 03 June 2021; Accepted: 12 July 2021;
Published: 05 August 2021.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Dawei Wang, Wuhan University, ChinaJianfeng Wu, The First Affiliated Hospital of Sun Yat-sen University, China
Fen Liu, The First Affiliated Hospital of Nanchang University, China
Camelia Cojocariu, Grigore T. Popa University of Medicine and Pharmacy, Romania
Yu Huang, Qingdao Women and Children's Hospital, China
Copyright © 2021 Meng, Fang, Meng, Zhang, Wang, Qie, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunting Wang, wcteicu@126.com; Man Chen, chenman_521@126.com
 Zhaoli Meng
Zhaoli Meng Wei Fang
Wei Fang Mei Meng
Mei Meng Jicheng Zhang1,2
Jicheng Zhang1,2 Man Chen
Man Chen