
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Med. , 08 September 2021
Sec. Geriatric Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.717168
This article is part of the Research Topic Reducing the Burden of Age-related Disease in relation to Osteoporosis, Sarcopenia and Osteosarcopenia View all 13 articles
 Chia-Che Lee1
Chia-Che Lee1 Chen-Yu Wang2,3,4
Chen-Yu Wang2,3,4 Chih-Chien Hung5
Chih-Chien Hung5 Chuan-Ching Huang1
Chuan-Ching Huang1 Chung-Yi Li6,7,8
Chung-Yi Li6,7,8 Hsuan-Yu Chen1
Hsuan-Yu Chen1 Yun-Liang Chang1
Yun-Liang Chang1 Wo-Jan Tseng9,10
Wo-Jan Tseng9,10 Ting-Ming Wang1
Ting-Ming Wang1 Rong-Sen Yang1
Rong-Sen Yang1 Tze-Hong Wong9*†
Tze-Hong Wong9*† Shau-Huai Fu1,5,6*†
Shau-Huai Fu1,5,6*†Background: Though denosumab is an effective treatment for osteoporosis, the rebound effect after discontinuation has drawn investigators' attention. It includes a dramatic loss of gained bone mineral density (BMD) and an increased risk of vertebral fractures. This prospective multi-institutional randomized controlled trial aims to investigate whether zoledronate prevents loss of BMD after discontinuation of denosumab. The trial was registered as Denosumab Sequential Therapy (DST) trial in March 2019 at clinicaltrials.gov, with the identifier NCT03868033.
Methods: The study is conducted at National Taiwan University Hospital and its branches. Patients who have continuously received denosumab treatment for two or more years are surveyed for eligibility. Baseline characteristics and questionnaires of life quality are recorded after recruitment. BMD, circulating levels of bone turnover markers (BTMs), including serum N-terminal propeptide of type 1 collagen (P1NP) and C-terminal telopeptide (CTX), are checked before the stratified randomization to 4 groups. Biological sex and the T-scores are used to create 4 strata. The participants in group 1 adhere to regular denosumab therapy for another 2 years. All the other patients receive on-time zoledronate treatment in the first year. The participants in group 2, 3, and 4 have on-time denosumab, on-time zoledronate and drug holiday in the second year, respectively. BMDs are checked annually. Pre-scheduled checkpoints of BTMs are also arranged. For patient safety, rescue treatment with another injection of zoledronate will be applied to the patients on drug holiday if the CTX levels raise above the pre-specified threshold, 0.573 ng/mL for women and 0.584 ng/mL for men. The primary outcomes are the percentage changes of BMDs in lumbar spine, total hip and femoral neck. The secondary outcomes include the changes of serum level of the BTMs, new osteoporotic fractures, extra zoledronate injections needed in group 4 and the differences of quality of life.
Discussion: We aim to provide evidence whether zoledronate prevents bone loss after denosumab cessation. To our knowledge, the study has the largest sample size. No other randomized controlled study included all the three different treatment strategies and a positive control. It is also the first associated randomized controlled trial outside Europe.
Denosumab (Dmab), a monoclonal antibody against the receptor activator of nuclear factor kappa-light-chain-enhancer of activated B cells ligand (RANKL), is an effective anti-resorptive agent to treat patients with osteoporosis (1, 2). The rebound effect after discontinuation of Dmab treatment has drawn investigators' attention in recent years. The rebound effect includes a complete or near-complete loss of gained bone mineral density (BMD), and an increased risk of vertebral fractures (3–6). After cessation of Dmab treatment, the serum levels of bone turnover markers (BTM) raise rapidly in 3 months and return to baseline about 24 months later (7). The BMD loss may occur with the increased rate of bone turnover. Bone et al. reported total hip BMD would lose about 4% within 1 year after the withdrawal from 2-year Dmab treatment (7). For the patients who were treated with 1-year zoledronate (ZOL) and discontinued the treatment in the second year, the total hip BMD loss would be about 1.7% (8).
Meanwhile, vertebral fractures after discontinuation of Dmab were observed in patients receiving two or more doses of Dmab. The vertebral fractures tended to be multi-level around the thoracolumbar junction (4, 6). Ferrari reported 1–10% of the patients with Dmab cessation may have vertebral fractures (9). Compared with patients who received on-time Dmab injection therapy, those delayed a dose by more than 16 weeks were associated with increased risks for vertebral fractures (10). Our nationwide population-based cohort study also showed discontinuation of Dmab resulted in an increased risk of major osteoporotic and vertebral fractures. The increased risk tended to reveal within 1 year after discontinuation and the risk was greater among the patients with longer duration of Dmab treatment (11). In addition to vertebral fractures, higher incidences of major osteoporotic fractures and hip fractures were also observed in the following years of Dmab withdrawal (12).
The open-label multi-institutional randomized controlled trial aims to investigate whether ZOL treatment at 6 months after previous Dmab administration prevents bone loss in patients who have received Dmab for two or more years. Moreover, three different treatment strategies over 2 years for BMD preservation are also investigated with a positive control group adherent to continuous Dmab treatment every 6 months.
Post-menopausal women and men aged 50 years or older, regularly treated with Dmab every 6 months for two or more years, are evaluated for eligibility. The criteria are listed in Table 1. The patients are recruited at NTUH, NTUH Hsin-Chu Branch, and NTUH Yunlin Branch. Under the condition of 90% power and a two-sided error α probability of 0.05 with a 3.27% standard deviation (SD) (8), at least 19 patients are considered necessary in each group. Take the potential dropouts into account, the estimated sample size is around 25 in each group. Totally 100 participants are estimated to be adequate to complete the study.
After the acquirement of written informed consents, the baseline demographic characteristics of the recruited participants are recorded, including age, sex, body height, body weight, body mass index (BMI), history of previous doses of Dmab administration, adverse effects of Dmab, past histories of fractures, comorbidities, fracture risk assessed by Fracture Risk Assessment Tool (FRAX), histories of falls and dental conditions. Baseline BMD in spine, total hip and femoral neck regions are checked as well as baseline laboratory tests, including serum level of creatinine, serum N-terminal propeptide of type 1 collagen (P1NP), C-terminal telopeptide (CTX). The participants are also interviewed for baseline health-related quality of life through the 5-level EQ-5D version (EQ-5D-5L) (13) and World Health Organization Quality of Life–BREF (WHOQOL-BREF) questionnaires (14, 15). Study data are collected and managed using the Research Electronic Data Capture (REDCap) tools hosted at National Taiwan University Hospital and its branches (16). The participants are stratified by biological sex and the lowest T-score in total hip, femoral neck and spine region, into 4 strata. Then the stratified participants are randomly allocated via a computer-generated sequence hidden from investigators. The distribution of the enrolled cases to the four groups from the four strata are shown in Table 2. The accesses to the data recorded on the Redcap tools are allowed only for groups members in charge of data analysis.
It is a 2-year prospective, multi-institutional, randomized controlled clinical trial. The study flowchart is shown in Figure 1. During the 2-year study period, the patients in group 1 continuously receive Dmab treatment once every 6 months for 2 years. Group 1 is regarded as the positive control group. The patients in the other three groups receive on-time ZOL, 6 months after last Dmab treatment, at the 1st year. At the 2nd year of the study, patients in group 2 switch back to have on-time Dmab treatment once every 6 months, 1 year after previous ZOL treatment. Patients in group 3 have on-time ZOL therapy in the 2nd year, while the patients in group 4 start to have drug holiday in the 2nd year. Spine, total hip and femoral neck BMDs are checked annually. Serum levels of P1NP and CTX are checked at baseline, 6, 12, 15, 18, and 24 months after the randomized allocation. Once the CTX level elevates above the pre-defined level in group 4 patients, an extra dose of ZOL will be given. For the safety of the participants, we use relatively strict and low threshold level of CTX, 0.573 ng/mL for post-menopausal women and 0.584 ng/mL in men, respectively (17–23). The events of morphologic vertebral fractures, clinical vertebral fractures and other osteoporotic fractures are confirmed by radiograph annually and whenever necessary by physician's decision. The adverse drug reactions, observed by research members or reported by the patients, are recorded. Life quality questionnaires are acquired every 6 months. We provide instruction for all participants to acquire at least 800 international unit of vitamin D3 and 1,000 mg of calcium daily.
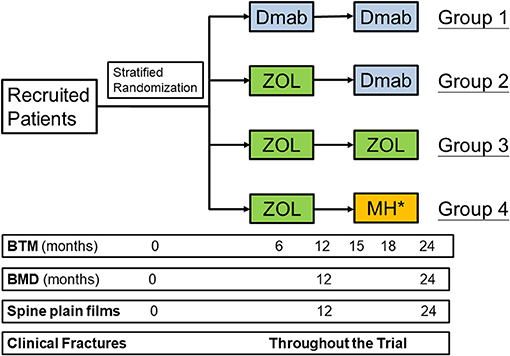
Figure 1. The flowchart of the study. Dmab, denosumab; ZOL, zoledronate; MH, monitored drug holiday; BTM, bone turnover markers; BMD, bone mineral density. *An extra ZOL injection will be given once the C-terminal telopeptide levels raise above the pre-specified threshold, 0.573 ng/mL in post-menopausal women and 0.584 ng/mL in men, respectively.
The primary outcomes are the percentage changes of BMDs in lumbar spine (LS), total hip (TH) and femoral neck (FN) among the study groups. The secondary outcomes include morphological and clinical osteoporotic vertebral fractures, other osteoporotic fractures, differences of the BTMs, extra zoledronate injections needed in group 4 and the longitudinal changes of the questionnaires-based life quality.
In the first year, we try to explore the extent of bone loss after drug switch. The percentage changes of the BMDs of the participants in group 1 are compared with those of the participants in the other groups who are treatment with ZOL in the first year. We will also investigate the factors related to significant bone loss after transition from Dmab to ZOL.
After final follow-up of the second year, we will compare the percentage changes of BMDs in Group 4 with historical negative control. Furthermore, the comparison of percentage changes of the BMDs among these four groups will be completed. The differences of circulating BTM changes among the four groups and the changes of life quality will also be investigated.
Intention-to-treat analysis will be performed. For the primary outcomes, Shapiro-Wilk test will be used to exam the normality. Normally distributed continuous data will be evaluated via one-way analysis of variance (ANOVA). Otherwise, the Kruskal-Wallis test will be applied. To detect factors related to significant bone loss after drug switch, we define significant bone loss as more than 5 % BMD loss in LS or more than 4% BMD loss in TH region according to the literature (24). We will also evaluate the associations between significant bone loss and potentially important prognostic factors, including age, sex, BMI, previous fracture history, FRAX, Dmab duration, baseline CTX level, baseline P1NP level, institution, baseline BMD in LS, FN and TH regions by univariate logistic analysis. Relevant covariates will be further included into the multivariate logistic regression analysis to identify the factors accounting for significant bone loss.
For the secondary outcomes, Fisher's exact test and chi-squared test are used to determine whether categorical data from different groups are independent. Depending on normality, one-way ANOVA or Kruskal-Wallis test will be applied for numerical data. Events of osteoporotic fractures, vertebral fractures and adverse reactions will be reported.
Real-world data showed that the compliance of continuous use, or the “persistence,” of Dmab ranged from 65.8 to 88% in the first year and decreased to be around 41.2–75% in the second year (25–28). During the era of COVID-19 pandemic, the persistence may drop further. Solid evidence for effective sequential therapy of osteoporosis to prevent bone loss after Dmab discontinuation is required.
Bisphoshonates (BPs) may, at least partially, preserve the gained bone mass and decrease the risk of vertebral fractures (29). There were two associated single-institutional randomized controlled trials. The Greek study group compared the treatment effect of single dose of zoledronic acid (ZOL) with two doses of Dmab, followed by direct drug holiday in women who reached non-osteoporotic BMD level. A single dose of ZOL was effective to prevent bone loss in most patients with low vertebral fracture risk at 2 years following drug switch. But three out of 27 participants still experienced BMD decrease greater than the least significant change. The bone loss was deemed to be caused by not-yet-defined intrinsic factors (30). The BTM levels elevated within 1 year after drug switch, suggesting that further cautious survey was necessary since further bone loss was possible (31). The randomized trial by the Danish group showed inevitable BMD loss in post-menopausal women and men above 50 years with osteopenia after ZOL treatment following Dmab cessation from long-term denosumab treatment for 4.6 ± 1.6 years. The bone loss corresponded to 0.25 to 0.5 standard deviation of gained bone mass, irrespective of the 6-, 9-month, or observational treatment strategy. On-time treatment with ZOL seemed to be the most attractive strategy among the investigated options in the study (32). Although a single dose of ZOL may be helpful, the individual setting for sequential therapy may vary widely regarding the baseline fracture risk, bone turnover rate, duration of Dmab treatment and other factors. Further randomized controlled trial was deemed to be particularly necessary (9).
To the best of our knowledge, this is the first “multi-institutional” study among the randomized controlled trials about subsequent treatment after Dmab discontinuation. The current trial may also include the largest sample size among the randomized controlled trials. The other strengths of the study are as the following. Firstly, we include both men and women with osteoporosis or osteopenia to evaluate therapeutic effects in different disease status. By these means we may improve the external validity of the study. Through stratified randomization, the biological characteristics, and the severity of osteoporosis along with possible confounding factors are expectantly to be equally distributed among four groups.
Secondly, we have four study groups with different treatment strategies. Group 1 stands as the positive control. In the first year, we may illuminate the extent of bone loss in LS, TH and FN regions after drug switch by comparing the percentage changes of the BMDs between the patients treated with Dmab and those with ZOL. The factors associated with significant bone loss after drug switch may provide important clinical implications. Group 2 will show the percentage changes of BMD after double drug switch, which has not yet been investigated previously. Group 3 will exhibit the effect of two consecutive ZOL injection on BMD and BTM levels. We will assess BMD and BTM levels changes after 1-year drug holiday following drug switch in group 4 participants. Comparison between group 3 and 4 may provide crucial information. Presumably two injections of ZOL may preserve more bone than one injection. The changes of BTM levels 1 year after drug transition and how it responds to the second injection of ZOL, will be observed. The percentage changes of BMDs in Group 4 will be compared with historical negative control due to ethical concerns. Thirdly, we have regular BTM checkpoints to show the chronological changes with different treatment strategies. Finally, we are the first Asian RCT study concerning Dmab sequential therapy.
To begin with, we will not be able to evaluate the therapeutic responses of patients in different timelines of ZOL treatment, as done by the Danish group. However, according to Sølling et al., on-time treatment may be the most effective and attractive option (32). Secondly, according to the database survey by the investigators, we have fewer male patients having long-term Dmab treatment. The male population in the recruited participants may drop even further, as shown in Table 2. This corresponds to the real-world situation in osteoporosis treatment (33). As mentioned above, the potential confounding factors may be reduced by the stratified randomization. Furthermore, due to ethical concerns, we do not design a group with direct drug holiday after Dmab cessation as the negative control. Historical control is applied instead. Thirdly, the dual-energy x-ray absorptiometry (DXA) devices are not unified among the institutions. This is commonly seen among the multi-institutional studies like, for example, the FREEDOM trial (34). We use General Electric Lunar Prodigy (General Electric Healthcare), Stratos DR (Diagnostic Medical Systems-Imaging) in NTUH, Stratos DR in NTUH Hsin-Chu Branch and Hologic (Hologic Inc.) in NTUH Yunlin Branch, respectively. Each participant is evaluated by only one specific type of device throughout the study. We use percentage changes of the BMDs as the outcome measures to eliminate the potential bias from the absolute values of the BMDs generated from different devices.
In summary, we aim to provide evidence to determine whether ZOL treatment prevents bone loss after Dmab cessation. We also try to determine the effectiveness of three sequential therapeutic strategies. The potential for extension of the study is preserved.
The studies involving human participants were reviewed and approved by National Taiwan University Hospital Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
S-HF designed the study with the help from C-YL and C-YW. C-CL, S-HF, and T-HW are the project instructor in NTUH, NTUH Yun-lin Branch, and NTUH Hsin-Chu Branch, respectively. S-HF, C-CL, and H-YC handled the research ethics. C-CL, Chi-CH, Chu-CH, H-YC, Y-LC, T-MW, W-JT, and S-HF contributed to the execution of the study. C-CL wrote this manuscript as checked by C-YW and S-HF. Supervision is provided by C-YW, C-YL, T-MW, R-SY, T-HW, and S-HF. Statistics are managed by C-YL, C-YW, and S-HF. All authors have given their final approval of the version to be published and agree to be accountable for all aspects of the work.
This study was supported by the Research Assistantships funded by the Ministry of Science and Technology, Taiwan (grant number MOST 108-2314-B-002-100-MY3, to S-HF); National Taiwan University Hospital Yun-Lin Branch (grant number NTUHYL109.X009 & NTUHYL109.F005, to S-HF); National Taiwan University Hospital (grant number NTUH 109-031, to C-CL). The funders have no role in the study design, data collection, analysis, interpretation nor the writing of the report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the following members of the National Taiwan University Hospital Yun-Lin Branch: Syun-Ping Fu, MS; Wen-Yen Hsu, BS; Nai-Fen Chuang, MS, and the members of Taiwan University Hospital: Yi-Ling Chen, BS, for their efforts on data collection and validation of this research project.
1. Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. (2008) 43:222–9. doi: 10.1016/j.bone.2008.04.007
2. Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab. (2014) 99:2599–607. doi: 10.1210/jc.2013-4175
3. Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. (2017) 32:1291–6. doi: 10.1002/jbmr.3110
4. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. (2018) 33:190–8. doi: 10.1002/jbmr.3337
5. McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM. Observations following discontinuation of long-term denosumab therapy. Osteoporos Int. (2017) 28:1723–32. doi: 10.1007/s00198-017-3919-1
6. Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, et al. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. (2017) 105:11–7. doi: 10.1016/j.bone.2017.08.003
7. Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. (2011) 96:972–80. doi: 10.1210/jc.2010-1502
8. Lehmann T, Aeberli D. Possible protective effect of switching from denosumab to zoledronic acid on vertebral fractures. Osteoporos Int. (2017) 28:3067–8. doi: 10.1007/s00198-017-4108-y
9. Ferrari S. Zoledronate following denosumab discontinuation: partial reassurance but no confidence. J Bone Miner Res. (2020) 35:1205–6. doi: 10.1002/jbmr.4022
10. Lyu H, Yoshida K, Zhao SS, Wei J, Zeng C, Tedeschi SK, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis : a population-based cohort study. Ann Intern Med. (2020) 173:516–26. doi: 10.7326/M20-0882
11. Fu SH, Wang CY, Hung CC, Lee CC, Yang RS, Huang CC, et al. Increased fracture risk after discontinuation of anti-osteoporosis medications among hip fracture patients: a population-based cohort study. J Intern Med. (2021). doi: 10.1111/joim.13354. [Epub ahead of print].
12. Tripto-Shkolnik L, Fund N, Rouach V, Chodick G, Shalev V, Goldshtein I. Fracture incidence after denosumab discontinuation: real-world data from a large healthcare provider. Bone. (2020) 130:115150. doi: 10.1016/j.bone.2019.115150
13. Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. (2011) 20:1727–36. doi: 10.1007/s11136-011-9903-x
14. Saxena S, Carlson D, Billington R, Orley J. The WHO quality of life assessment instrument (WHOQOL-Bref): the importance of its items for cross-cultural research. Qual Life Res. (2001) 10:711–21. doi: 10.1023/A:1013867826835
15. Yao G, Chung CW, Yu CF, Wang JD. Development and verification of validity and reliability of the WHOQOL-BREF Taiwan version. J Formos Med Assoc. (2002) 101:342–51.
16. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Informat. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
17. Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. lancet Diabetes Endocrinol. (2017) 5:908–23. doi: 10.1016/S2213-8587(17)30184-5
18. Li M, Li Y, Deng W, Zhang Z, Deng Z, Hu Y, et al. Chinese bone turnover marker study: reference ranges for C-terminal telopeptide of type I collagen and procollagen I N-terminal peptide by age and gender. PLoS ONE. (2014) 9:e103841. doi: 10.1371/journal.pone.0103841
19. Michelsen J, Wallaschofski H, Friedrich N, Spielhagen C, Rettig R, Ittermann T, et al. Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone. (2013) 57:399–404. doi: 10.1016/j.bone.2013.09.010
20. Park SG, Jeong SU, Lee JH, Ryu SH, Jeong HJ, Sim YJ, et al. The changes of CTX, DPD, osteocalcin, and bone mineral density during the postmenopausal period. Ann Rehabil Medi. (2018) 42:441–8. doi: 10.5535/arm.2018.42.3.441
21. Hu WW, Zhang Z, He JW, Fu WZ, Wang C, Zhang H, et al. Establishing reference intervals for bone turnover markers in the healthy shanghai population and the relationship with bone mineral density in postmenopausal women. Int J Endocrinol. (2013) 2013:513925. doi: 10.1155/2013/513925
22. Jenkins N, Black M, Paul E, Pasco JA, Kotowicz MA, Schneider HG. Age-related reference intervals for bone turnover markers from an Australian reference population. Bone. (2013) 55:271–6. doi: 10.1016/j.bone.2013.04.003
23. Park SY, Ahn SH, Yoo JI, Chung YJ, Jeon YK, Yoon BH, et al. Clinical application of bone turnover markers in osteoporosis in Korea. J Bone Metab. (2019) 26:19–24. doi: 10.11005/jbm.2019.26.1.19
24. Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, et al. Treatment failure in osteoporosis. Osteoporos Int. (2012) 23:2769–74. doi: 10.1007/s00198-012-2093-8
25. Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos. (2017) 12:22. doi: 10.1007/s11657-017-0316-5
26. Morley J, Moayyeri A, Ali L, Taylor A, Feudjo-Tepie M, Hamilton L, et al. Persistence and compliance with osteoporosis therapies among postmenopausal women in the UK Clinical Practice Research Datalink. Osteoporos Int. (2020) 31:533–45. doi: 10.1007/s00198-019-05228-8
27. Reyes C, Tebe C, Martinez-Laguna D, Ali MS, Soria-Castro A, Carbonell C, et al. One and two-year persistence with different anti-osteoporosis medications: a retrospective cohort study. Osteoporos Int. (2017) 28:2997–3004. doi: 10.1007/s00198-017-4144-7
28. Silverman SL, Siris E, Belazi D, Recknor C, Papaioannou A, Brown JP, et al. Persistence at 24 months with denosumab among postmenopausal women with osteoporosis: results of a prospective cohort study. Arch Osteoporos. (2018) 13:85. doi: 10.1007/s11657-018-0491-z
29. Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T. A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res. (2020) 35:1207–15. doi: 10.1002/jbmr.3962
30. Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P. Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. a prospective 2-year clinical trial. J Bone Miner Res. (2019) 34:2220–8. doi: 10.1002/jbmr.3853
31. Johansson H, Odén A, Kanis JA, McCloskey EV, Morris HA, Cooper C, et al. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int. (2014) 94:560–7. doi: 10.1007/s00223-014-9842-y
32. Sølling AS, Harsløf T, Langdahl B. Treatment with Zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J Bone Miner Res. (2020) 35:1858–70. doi: 10.1002/jbmr.4098
33. Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. (2011) 469:1900–5. doi: 10.1007/s11999-011-1780-7
Keywords: denosumab, rebound effect, osteoporosis, zoledronate, bone loss, bone mineral density
Citation: Lee C-C, Wang C-Y, Hung C-C, Huang C-C, Li C-Y, Chen H-Y, Chang Y-L, Tseng W-J, Wang T-M, Yang R-S, Wong T-H and Fu S-H (2021) A Multi-Institutional Randomized Controlled Trial to Investigate Whether Zoledronate Prevents Bone Loss After Discontinuation of Denosumab: The Study Protocol of Denosumab Sequential Therapy (DST) Trial. Front. Med. 8:717168. doi: 10.3389/fmed.2021.717168
Received: 30 May 2021; Accepted: 12 August 2021;
Published: 08 September 2021.
Edited by:
Bagher Larijani, Tehran University of Medical Sciences, IranReviewed by:
Sayed Mahmoud Sajjadi Jazi, Tehran University of Medical Sciences, IranCopyright © 2021 Lee, Wang, Hung, Huang, Li, Chen, Chang, Tseng, Wang, Yang, Wong and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shau-Huai Fu, YjkwNDAxMDQ1QGdtYWlsLmNvbQ==; Tze-Hong Wong, dHplaG9uZ3dvbmdAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.