
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 04 October 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.713981
This article is part of the Research Topic Diagnosis, Treatment and Prognosis of Viral Hepatitis View all 21 articles
Hepatitis B virus (HBV) infection is a common contributor to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Approximately 10% of people with human immunodeficiency virus (HIV) also have chronic HBV co-infection, owing to shared transmission routes. HIV/HBV coinfection accelerates the progression of chronic HBV to cirrhosis, end-stage liver disease, or hepatocellular carcinoma compared to chronic HBV mono-infection. HBV/HIV coinfection alters the natural history of hepatitis B and renders the antiviral treatment more complex. In this report, we conducted a critical review on the epidemiology, natural history, and pathogenesis of liver diseases related to HBV/HIV coinfection. We summarized the novel therapeutic options for these coinfected patients.
Chronic hepatitis B virus (HBV) infection affects ~250 million people worldwide and can cause progressive liver fibrosis and hepatocellular carcinoma (HCC) (1, 2). Over 50% of HCC cases globally have been attributed to HBV infection (3, 4). Owing to their similar transmission patterns, HBV/HIV coinfection is relatively common in endemic areas (5). About 10% of HIV-infected individuals have been found to be chronically infected with HBV (6, 7). Compared with HBV mono-infection, HBV/HIV coinfection complicates the natural course and increases the risk of deterioration of diseases (8). Although some current therapeutic strategies are considered effective options in treating single virus infections, HBV/HIV coinfection has altered the natural history of the virus, requiring novel individualized therapeutic forms. This paper reviews the epidemiology, natural history, and pathogenesis of liver diseases in HBV/HIV coinfection. We also highlighted the individualized therapeutic options in these patients.
We performed a comprehensive literatures search in Pubmed, Embase, and Web of Science with the key words of “hepatitis B virus,” “HBV,” “human immunodeficiency virus,” “HIV,” “coinfection,” “liver disease,” “epidemiology,” “pathogenesis,” “treatment,” and “clinical treatment.” Clinical trials (http://clinicaltrials.gov/) was also searched for the important clinical trials about the HBV/HIV coinfection therapies. Literatures with no full text, describing only protocol design or preliminary results were excluded.
HBV, HIV, and HBV/HIV coinfection are caused by several means including unsafe drug injection, inappropriate medical practices, unsafe therapeutic injections, and high-risk unprotected heterosexual and man-man sexual acts. Overlapping transmission routes contribute to the prevalence of HBV, HIV, and HBV/HIV coinfection (9). Nevertheless, the prevalence varies in different geographic regions, ranging from 10 to 28% (10–13). Based on the prevalence of chronic HBV infection, it can classify the endemicity to high, intermediate, and low endemicity areas in geography (14). In the high endemicity area, such as sub-Saharan Africa and east Asia, ~10% of HIV-infected individuals have been observed to be concurrently infected with HBV (15). In Vietnam, the rate could be as high as 28% in the unsafe drug injection populations (11). In these regions, perinatal transmission, close household contact during childhood, or cultural procedures are the most common transmission routes (10, 16). However, in areas of low endemicity, such as North America, Western Europe, and Australia, HBV/HIV coinfection is usually recorded in adolescents or adults via unsafe drug injection or sexual transmission (15) with the estimated infection rate 6–14% (17). In the highest risk group of unsafe male homosexuals, the estimated rate ranges from 9 to 17% (14).
Nevertheless, prevalence of HBV/HIV infection showed a slightly decreased trend based on several studies published recently. An analysis developed by the North American Cohort Collaboration on Research and Design collected information covering 12 clinical sites from 1996 to 2010 and revealed that the prevalence of chronic HBV infection in HIV cohort was only 7% (18). Another research from the US Military HIV Natural History Study found that the overall incidence of chronic HBV infection was 4.3% (19). The most prevalence was appeared in 1995 with an obvious decrease in 2008, according to the evaluation of cross-sectional incidence (19). Even so, the health threat posed by HBV/HIV coinfection still cannot be ignored.
Acute HBV infection in adults is difficult to clinically detect. Spontaneously recovery is commonly observed among the most immunocompetent adults whose antibodies against hepatitis B surface antigen (anti-HBs) can be detected (20). Approximately 5–10% of immunocompetent adults will progress to chronic infection (21), 20% of individuals with chronic infection are likely to develop cirrhosis within 1–13 years (21). HCC and decompensated liver diseases occur in 6 and 23% of patients with cirrhosis, respectively (22).
The natural history of HBV factors are associated to the characteristics of virus, host, and environment. HBV/HIV coinfection accelerates the progression of HBV infection by impacting the immune response of the host (23). People infected with HIV have a high risk of contracting chronic HBV infection (24). Lower rates of HBeAg and/or HBcAg clearance and anti-HBe and anti-HBs seroconversion with higher rates of HBV replication were observed in HBV/HIV coinfected persons (25, 26). The acceleration of the process of cirrhosis and HCC is the most serious consequence in the liver-related damages (27). HBV/HIV-coinfected individuals have approximately five- to six-fold higher risk of HCC incidence with the presence of cirrhosis (28–30). Additionally, HIV/HBV-coinfected accelerate the progress to liver cirrhosis (31). Host CD4+ T cells are vital to the recognition of viral antigens presented by Kupffer cells and the regulation of the activities of CD8+ cytotoxicity T cells, antibody producing B cells, and secreting cytokines cells. Host immunosuppression as manifested by the depletion of CD4+ T cells may be the key for HIV to alter the natural course of HBV which associated to an increase in liver-related mortality (5, 32–34).
The mechanism by which HIV facilitates liver-related damage has not been completely delineated. HIV-induced immunodeficiency seems to enhance HBV-related hepatotoxicity, which is mediated by the immune response (15). Depletion of CD4+T cells is an important feature of HIV infection which suppress the antigen presentation of liver resident macrophages (Kupffer cells) and the cytokine secretion of lymphocytes, in resulting the host immunosuppression (32). The inhibition of the host immune response enhances HBV replication substantially to further cause severe liver damage (35, 36). HBV infected hepatocytes is found non-cytopathic, without distinct cellular damage and viral cytopathic effects. However, HBV/HIV coinfection persons shows fibrosing cholestatic hepatitis (37, 38). HIV/HBV coinfection causes changes in the hepatic cytokine environment (15, 39, 40). It has been reported that HIV glycoproteins stimulate the hepatocyte to express the tumor necrosis factor related apoptosis inducing ligand (TRAIL) to induce hepatocyte apoptosis (41, 42). HIV envelope protein activates the caspase-independent apoptosis in Huh7 cells (43). HIV infection induces hepatocyte apoptosis through phagocytosed by macrophages or hepatic stellate cells and contributes to the inflammation and fibrosis of the liver (44). The increase of hepatocyte apoptosis has been observed among HBV/HIV coinfected patients compared with HBV mono-infected patients (45). HIV gp120 has been demonstrated to stimulate the hepatic expression of IL-8 to mediate the hepatic inflammation (46). Elevation of HBV load increases the X protein of HBV (HBx), which can also transactivate the expression of IL-8 via NF-κB and C/EBP-like cis-elements (47). As a leukocyte chemotactic molecule, IL-8 plays a crucial part in maintaining the inflammatory environment and HCC development (48). Furthermore, the content of IL-8 is positively correlated with the degree of liver damage (49).
HBx can also stimulate the expression of cyclooxygenase-2 (COX-2), which is overexpressed in liver cirrhosis (50, 51). Moreover, COX-2 expression can be activated by IL-8 through CREB and C/EBP (47). Accumulating evidence shows that HBV proteins activate IL-8 and COX-2 to maintain the inflammatory environment (47). The inflammatory hepatocytes secrete C-X-C motif chemokine 10 (CXCL10) which linked to the severity of liver damage involving viral hepatitis (52, 53). Once CXCL10 binds to its receptor, chemokine receptor 3 (CXCR3), immunocytes such as natural killer cells, and activated T cells and B cells are attracted to the inflammatory sites (54). Elevated CXCL10 was found in HBV/HIV coinfected patients but not in HBV mono-infected patients. This observation indicates that CXCL10/CXCR3 in the liver contributes to the acceleration of liver diseases (55–57).
In another hypothesis of mechanisms of the pathogenesis of liver diseases in coinfected patients, the depleted CD4+ T cells in the gastrointestinal tract contribute to the increase in microbial translocation and enhance the levels of circulating lipopolysaccharides (LPS) (58). When LPS binds to Toll-like receptor 4 and stimulates the NF-κB pathway or other pathways, it induces the secretion of pro-inflammatory cytokines (56). Although the relationship between microbial translocation and liver cirrhosis is reportedly closed, a similar evidence has never been found in HBV/HIV coinfected patients, including the direct relationship between circulating LPS and liver cirrhosis (56, 59). However, studies on simian immunodeficiency virus-infected rhesus macaques indicated that microbial load is capable of triggering the secretion of chemokines and enhancing the infiltration of CXCR6+-activated NK cells, thereby resulting in liver fibrosis (60). Further research is warranted to confirm this theory.
Antiviral therapies should be initiated for HBV/HIV coinfected patients as soon as possible regardless of the clinical stage of the disease and the count of CD4+ cells (61, 62). This recommendation is based on the evidence that the effects of anti-HBV treatment might be reduced following the deterioration of immunodeficiency (63). Moreover, utilization of agents against HBV only can lead to drug resistance to HIV. Therefore, the optimal therapeutic options should include agents possessing dual anti-HBV and anti-HIV activity (64, 65). Current dual antiviral choices can be classified into virus-based agents and host-based agents. The former includes nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and cyclophilin inhibitors. The latter consists of immunomodulators and monoclonal antibodies. Here, we provide an overview of the antiviral mechanism of these drugs.
The life cycle of a virus can be a potential target for antiviral agents. HIV is an RNA virus with the ability of reverse transcribing into DNA, which could be integrated into the host genome (66). By contrast, HBV is an enveloped DNA virus (67). Given that they belong to different types, HBV and HIV undergo different life cycles (Figure 1). Nevertheless, similarities in their life cycles are important in the development of dual antiviral drugs for HBV and HIV. According to a recent study, the polymerase of HBV and the reverse transcriptase of HIV have similar structures and functions, indicating that agents targeting these proteins have the ability to interrupt the life cycle of both HBV and HIV (68, 69). NRTIs are prodrugs that must be phosphorylated into active forms by cellular kinases (69, 70). Activated NRTIs are capable of disturbing the functions of both HIV reverse transcriptase and HBV polymerase by competing with natural nucleotide substrates for joining into DNA chains (69). In general, owing to the lack of 3′-OH, NRTIs work as chain-terminators, thereby interrupting DNA synthesis (69, 71, 72). Furthermore, protein priming activity, which is absent in HIV reverse transcriptase, is considered as another target for NRTIs (69). Inhibiting protein priming also substantially interferes with HBV replication (69). Cyclophilin A (Cyp A) belongs to the cyclophilin family with a peptidyl–prolyl isomerase activity (73, 74). Regarded as an acceleration factor for protein folding and assembly, Cyp A plays a crucial role in the replication of various viruses, including HBV and HIV. Moreover, it is linked to the pathogenesis of virus infection (75, 76). By interacting with the Gap protein of HIV or the small surface protein of HBV, Cyp A facilitates the replication and infectivity of viruses, suggesting that blocking Cyp A could be a potential anti-HIV and anti-HBV strategy (77, 78).
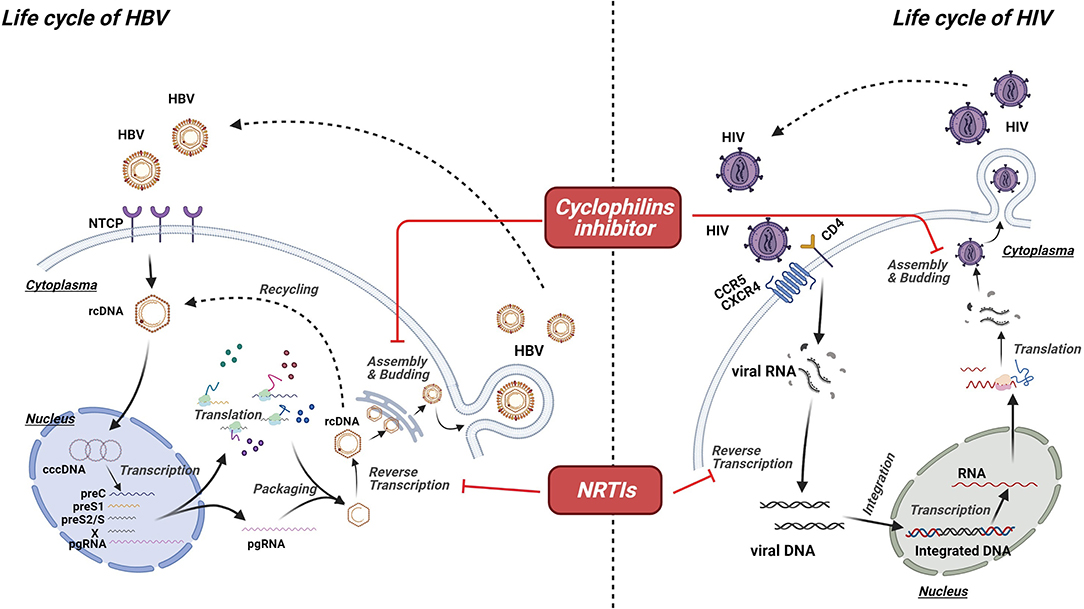
Figure 1. Mechanism of virus-targeted agents. Though undergoing different life cycles, HBV and HIV share some similarities in their life cycles, which are important for the development of dual antiviral drugs for HBV and HIV. Activated nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) are capable of disturbing the functions of both HIV reverse transcriptase and HBV polymerase by competing with natural nucleotide substrates for joining into DNA chains, resulting in the chain termination and viral replication. Cyclophilin A (Cyp A) plays a crucial role in the replication of various viruses, including HBV and HIV. Thus, cyclophilins inhibitor could be a potential option for anti-HIV and anti-HBV strategy.
In response to viral invasion, pattern-recognition receptors, including Toll-like receptors (TLRs), are activated, leading to the production of interferon (IFN) (79). Detection of viral DNA or RNA is crucial in triggering the innate immune response, culminating in the activation of transcription factors and release of antiviral cytokines, such as IFN (80, 81). When secreted, IFN interacts with its cognate receptors (i.e., IFNAR2) and activates receptor-associated kinases (i.e., JAK1 and Tyk 2), thereby contributing to the activation of the STAT family to form a transcription factor complex or a homo-/heterodimer (82–84). The transcription factor complex and the homo-/heterodimer bind to the ISRE and GAS promoter elements, respectively, and encode numerous viral restriction factors with potent inhibition potential on viral replication (Figure 2) (84–86). Consequently, immunomodulators (i.e., IFN), which can enhance the host's innate immune response, offer a rational option for treating viral infection, including HBV and HIV. The adaptive immune response is also a promising target for novel therapeutic interventions owing to the key position of T cells in viral infection control (66, 87). Regardless of HBV or HIV infection, virus-specific T cells have been found to have a distinct dysfunction, which is supposed to be linked to the expression disorder of programmed cell death protein (PD-1) and its ligand (PD-L1) (88). PD-1 and PD-L1 have been observed to be upregulated during viral infection, including HIV, HBV, and HCV, confirming that the PD-1/PD-L1 axis plays an important role in the pathogenic process of viruses (89). Considering that high expression levels of PD-1 and PD-L1 are usually related to unsatisfactory immune response, agents based on the PD-1/PD-L1 axis might be able to reverse this immune suppression consequence and exert corresponding antiviral effects, including on HBV and HIV (90, 91). Hence, immune checkpoint inhibitor targeting the PD-1/PD-L1 axis can be another option for treating HBV/HIV coinfection.
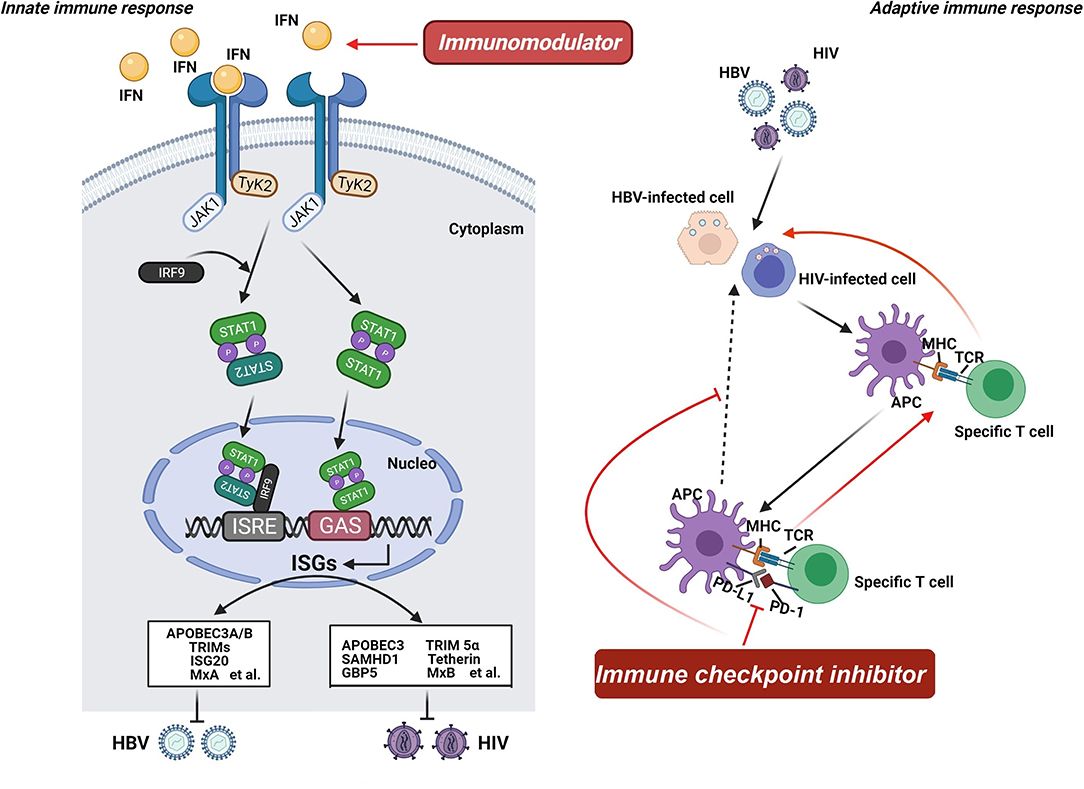
Figure 2. Mechanism of host-targeted agents. Pattern-recognition receptors are activated when the virus invades, leading to the production of interferon (IFN). IFN interacts with its cognate receptors and activates receptor-associated kinases, contributing to the activation of the STAT family to form a transcription factor complex or a homo-/heterodimer. The transcription factor complex and the homo-/heterodimer bind to the ISRE and GAS promoter elements, respectively, and encode numerous viral restriction factors with potent inhibition potential on viral replication. Consequently, immunomodulators offer a rational option for treating HBV and HIV infection. Additionally, the adaptive immune response is also a promising target for novel therapeutic interventions owing to the key position of T cells in viral infection control. Regardless of HBV or HIV infection, virus-specific T cells have been found to have a distinct dysfunction. And the PD-1/PD-L1 axis plays an important role in the pathogenic process of viruses. Considering that high expression levels of PD-1 and PD-L1 are usually related to unsatisfactory immune response, agents based on the PD-1/PD-L1 axis might be able to reverse this immune suppression consequence and exert corresponding antiviral effects. Hence, immune checkpoint inhibitor targeting the PD-1/PD-L1 axis can be another option for treating HBV/HIV coinfection.
Specialized treatment and management of coinfected patients demand multidisciplinary cooperation. Although the life expectancy of HIV-infected individuals has been prolonged due to antiviral therapy (ART), liver injury induced by HBV has become the main cause of death in coinfected people. The primary objective of anti-HBV therapy is to suppress the replication of HBV, reduce the activity of inflammation, and halt the progression of liver damage. Several antiviral drugs have been approved for the clinical treatment of HBV, some of which are described as dual antiviral agents against HBV and HIV (Table 1). Combination antiviral therapies (cART) are commonly adopted in treating coinfected cases, and clinical trials have been conducted to explore their effectiveness in the treatment of coinfected populations (Table 2).
Lamivudine (3TC) is a dideoxynucleoside cytosine analog with an antiviral effect on both HBV and HIV (92). It exerts its antiviral ability by terminating the chain and suppressing the replication of virus, leading to the reduction in viral load and remission of disease symptoms (92, 93). Amelioration of liver fibrosis and suppression of liver disease progression can be achieved in patients with chronic HBV receiving lamivudine treatment (94–96). Furthermore, a remarkably improved virologic response was detected after 10 years of lamivudine treatment. HBV DNA was undetected among all patients, and 14 and 11% of patients achieved HBsAg seroconversion and loss, whereas 83 and 42% of patients achieved HBeAg seroconversion and loss, respectively (94).
As the first-line NRTI, lamivudine had been approved for HIV treatment in 1995 and had been proposed as part of fix-dose combinations in antiretroviral therapy (97, 98). Preclinical studies have revealed the potent antiviral efficacy of lamivudine; its half inhibitory concentration in infected cell lines of diverse HIV strains ranges from 0.002 to 1.14 μM (99, 100). Previous clinical trials also confirmed that single-tablet regimen (STR) containing lamivudine has persistent viral suppression and favorable safety in patients with HIV (93, 101). Dolutegravir is the most commonly used agent in combination with lamivudine for HIV clinical treatment. With regard to HBV/HIV coinfected individuals, lamivudine-based ART regimens achieved 30–60% HBV DNA suppression after 48 weeks of treatment (102–106).
Although lamivudine can be tolerated well and despite its outstanding antiviral effect, its application was restricted because of high rates of resistance, a character frequently observed in nucleoside analogs (107). Based on the guideline published in 2017 from the clinicalinfo.HIV.gov, lamivudine in combination with other antiviral agents, such as TAF or TDF, could be an alternative option for HIV-infected individuals with confirmed HBV infection (108). Nevertheless, regimens containing 3TC is hardly recommended to the treatment for coinfected patients, according to the latest guideline from British HIV association (BHIVA) and European AIDS Clinical Society (EACS) (109, 110).
Similar to lamivudine, emtricitabine (FTC) is a nucleoside with dual HBV/HIV inhibitory effects (111). Apart from HIV treatment, emtricitabine, as combined drug, has been approved by Food and Drug Administration (FDA) for the prevention of HIV infection (111). Although it is not an FDA-approved agent for HBV treatment, emtricitabine has an outstanding antiviral value against HBV; it can notably decrease HBV DNA in serum and achieve normal ALT in patients with HBV at the recommended dose of 200 mg/day (112). According to a preclinical study, emtricitabine is superior to lamivudine in terms of intracellular half-life (113). Nevertheless, both of them are considered clinically equivalent (114). A phase III clinical trial (NCT02607930) has demonstrated that emtricitabine-based STR was non-inferior in HIV virological suppression comparable to that of lamivudine-containing regimens (115). Moreover, this regimen involving emtricitabine, bictegravir, and tenofovir alafenamide affords guideline-recommended therapeutic strategy for HBV/HIV coinfected cohorts (115). Other clinical studies have also reached the same conclusion, confirming the clinical value of emtricitabine (114, 116).
Although limited studies are available to confirm the efficacy of emtricitabine in HIV/HBV coinfection cohorts, its combination with other antiviral agents are superior to emtricitabine monotherapy in reducing HBV DNA (117). A study also indicated that a combination of emtricitabine and tenofovir disoproxil fumarate (TDF) has excellent outcomes with 14% of patients achieved seroconversion to anti-HBe and 94% of them had undetected HBV DNA in the serum (118). Nevertheless, this study had a small sample size. Thus, multicenter and large-scale trials are needed to confirm the value of emtricitabine in HIV/HBV coinfection treatment. Currently, co-formulated FTC and TDF is recommended as part of a suppression combination regimen applicated in HIV-infected individuals with confirmed or presumed sensitive HBV (108, 109).
Tenofovir is an adenosine nucleotide analog that has been approved for treatment of HIV infection (119, 120). Owing to its poor bioavailability, it is usually available commercially as TDF and tenofovir alfenamide (TAF) (119, 121). The former releases tenofovir in the bloodstream, whereas the latter releases tenofovir after entering the cells. Together with emtricitabine, tenofovir is used in HIV treatment and pre-exposure prophylaxis (PrEP) (122, 123). Furthermore, tenofovir-containing PrEP strategies are applicable for HIV-negative nursing mothers. In HBV treatment, tenofovir has been discovered to be capable of overcoming resistance to lamivudine and adefovir dipivoxil in HBV treatment (124, 125). Patients with HBV were found to benefit from tenofovir therapy, including TDF and TAF (126). Results showed that 73 and 75% of the HBeAg-positive individuals who received TDF or TAF achieved HBV DNA levels of <29 IU/mL at 96 weeks, respectively (126). Furthermore, no distinct difference was observed between TAF and TDF regimens in terms of loss rate and seroconversion of HBeAg and HBsAg (126). Two landmark studies also confirmed the therapeutic effects of TDF and TAF on HBeAg-positive or HBeAg-negative cohorts. Moreover, a low dose of TAF (25 mg/day) achieved a response similar to that of TDF (300 mg/day) at 48 weeks (127, 128). Akin to HBV, TAF exhibits potent anti-HIV effects in vivo at the low dose of 10 mg, which is 30-fold lower than that of TDF (129, 130).
Owing to their dual antiviral ability, tenofovir-containing regimens are extensively used to treat concurrent HBV and HIV (125, 131, 132). A study that enrolled 110 patients coinfected with HBV and HIV revealed that regimens containing TDF are superior to lamivudine therapy in the seroconversion rate of HBeAg (133). Result of this study showed that the proportion of patients displayed seroconversion was 57% in the group of TDF combined with FTC, 50% in the TDF group and 21% in the lamivudine group, respectively (133). Moreover, suppression of HBV replication was observed in 91% of the individuals during the median observation period. According to a meta-analysis of 23 studies involving a total of 550 patients with concurrent HBV and HIV and receiving TDF treatment noted persistent viral suppression, and the ratio of patients who achieved suppression of viral replication was 57.4, 79, and 85.6% after 1, 2, and 3 years, respectively (134). In addition, virus rebound had been rarely reported in TDF treatment. Therefore, all of the patients with HBV/HIV coinfection should receive tenofovir-based antiretroviral treatment unless history of tenofovir intolerance, according to the guideline of EACS (110).
Although rare, renal impairments, including tubular dysfunction, increase in serum creatinine, and acute renal failure, could be substantially induced by TDF. Hence, renal functions should be regularly monitored during TDF treatment (135).
Not all NRTIs have potent antiviral effects on HBV and HIV. Apart from the agents discussed above, several other NRTIs, including adefovir, entecavir, and telbivudine, are active against HBV but display minimal activity against HIV (136–138). Consequently, their application in HIV treatment is rare. However, considering the potent suppressive effects of adefovir, entecavir, and telbivudine on HBV replication, experts proposed that these NRTIs might be of value in the treatment of HBV/HIV coinfection when combined with other antiviral agents.
As the first nucleotide analog approved for HBV treatment, adefovir strongly inhibits HBV replication with a low incidence of resistance (107). However, the dose of adefovir used in HIV treatment is usually linked to nephrotoxicity (31). According to a pilot study on HBV/HIV coinfection, adefovir can postpone the deterioration of liver diseases, enhance HBeAg seroconversion, and normalize ALT levels by suppressing HBV DNA (139). Several clinical trials have been conducted to estimate the value of adefovir in treating individuals with concurrent HBV and HIV (NCT00033163, NCT00013702, and NCT00023153). A prospective study (ACTG A5127) involving HBV/HIV coinfected patients revealed that either TDF or adefovir treatment results in evident decrease in serum HBV DNA; moreover, results showed that these NRTIs were well-tolerated (140). Benhamou (141) also indicated that treating with emtricitabine-containing regimen plus adefovir for 144 weeks decreased serum HBV DNA levels in 45% of HBV/HIV coinfected subjects, which was lower than that in HBV monoinfection (56%).
Entecavir, a guanosine analog, is superior to emtricitabine and adefovir in suppressing serum HBV DNA (142–144). Moreover, it is effective against not only wild-type HBV but also emtricitabine-resistant and adefovir-resistant HBV (31). Although entecavir was once considered as an inactive agent to HIV, a study uncovered a remarkable phenomenon showing that entecavir could result in evident reduction in serum HIV RNA in three HBV/HIV coinfection patients (145). However, such residual antiviral activity might be able to induce resistant changes in HIV (71). Hence, the FDA warned that entecavir should not be used in the absence of antiretroviral therapy in HBV/HIV coinfected cohorts (137). Numerous clinical trials have been conducted to explore the potential value of entecavir in treating patients coinfected with HBV and HIV (Table 2).
CRV431, which was previously called CPI-431-32, is a non-immunosuppressive cyclophilin inhibitor–cyclosporin A analog (146). Previous studies have confirmed the efficacy of cyclophilin inhibitors against HIV and HCV (146, 147). HBV DNA, HBsAg, and HBeAg could be effectively reduced by CRV 431 by interrupting the interaction of CypA with HBsAg or HBeAg, as well as by blocking the entry of HBV (148). In addition, an in vivo study of transgenic mice reported that CRV 431 can lower serum HBsAg levels and HBV DNA loads in the liver in a dose-dependent manner (149). Moreover, the viral inhibitory effect was enhanced when low CRV 431 dose (10 mg/kg/day) was added 5 mg/kg/day of tenofovir exalidex, a prodrug of tenofovir (149). Furthermore, liver fibrosis and tumor burden in a non-alcoholic steatohepatitis mouse model were ameliorated, highlighting the potential of CRV 431 as a novel therapy for liver disease (150). An ongoing clinical trial is assessing the safety and tolerability of CRV 431 in healthy volunteers (NCT 03596697).
CsA analogs have been confirmed to be effective against HIV by blocking cyclophilins and the HIV capsid to form complexes (151, 152). Hence, CRV 431, which belongs to CsA analogs, could be another promising agent with anti-HIV activity. According to its metabolization, CRV 431 is speculated to not interact with other NRTIs (153). Therefore, CRV431 might be a potential agent for the treatment of patients suffering from both liver diseases and HIV. Coformulation of CRV 431 and current drugs could achieve favorable outcomes in HBV/HIV coinfected patients.
Interferons (INFs), a cluster of signaling proteins, are secreted by host cells in response to pathogenic invasion (154, 155). Moreover, they are the first class of agents approved for the treatment of chronic hepatitis B. Interferon-α (INF-α) used to be the standard choice but was eventually replaced by PEGylated interferon-α because the latter has a longer half-life and a stronger potency than the former (31, 156). INF-α and PEGylated INF-α can effectively inhibit HBV replication in vitro via stimulated-INF genes and augment host immunity to defend against HBV infection. IFN therapy has shown remarkable efficacy among HBeAg+ HBV infection patients with the characteristic of elevated alanine aminotransferase and low serum HBV DNA (157–159). Nevertheless, limited benefits and increased toxicity were discovered in HIV/HBV coinfected patients probably because of abnormal immunity (25, 160). Hence, such agents might be applied to non-decompensated patients who have a good response to INFs (161). In general, the period of treatment lasts for 12 months. The guideline of BH via in 2013 gave the recommendation that PEG-IFN should be used only in HBsAg+ individuals with repeatedly raised ALT and low level of HBV DNA, regardless of the status of HBeAg (162). Nevertheless, the place of PEG-IFN therapies is not mentioned in neither the latest EACS nor BHIVA.
GS-9620, also known as vesatolimod, is an oral small-molecule antagonist of toll-like receptor 7 (TLR-7) with outstanding anti-HBV potency; moreover, it is considered in the clinical treatment of chronic HBV infection (163, 164). Preclinical studies reported sustained reduction of HBV DNA, HBV RNA, and HBsAg levels in HBV-infected cells administered with GS-9620 through an IFN-dependent manner (163, 165, 166). According to a study on a chimpanzee model of chronic HBV, GS-9620 participates in the accumulation of CD8+ T cells and B cells in the portal regions of liver, thereby playing a role in wiping out HBV-infected cells or restricting HBV infection (167). Another study on woodchucks with chronic HBV infection found reduced levels of cccDNA and risk of HCC due to GS-9620 (165). Having achieved favorable HBV suppressive effects in preclinical studies, GS-9620 is undergoing clinical trials to definitively establish its therapeutic efficacy in patients with chronic HBV. Currently, GS-9620 is considered to be safe in and well-tolerated by individuals with chronic HBV (168–170).
Existing HIV therapeutic strategies can achieve HBV suppression to undetectable levels rather than complete removal of viruses, which leads to lifelong treatment (171). To overcome this difficulty, experts have focused on the induction of latent HIV expression and the enhancement of the viral recognition ability of the immune system to eliminate latent HIV. On the basis of the viewpoint that TLR can induce HIV expression from infected cells, scientists have explored the value of GS-9620 in HIV treatment (171–173). Results were consistent with this assumption, confirming that GS-9620 has the ability to activate HIV from the peripheral blood mononuclear cells of HIV-infected patients receiving anti-HIV treatment, thereby improving immune functions and enhancing HIV clearance (171). HIV replication was also observed during GS-9620 treatment (174). A clinical trial (NCT02858401) reported that GS-9620 is safe in and well-tolerated by HIV-infected cohorts (175). Owing to its potential dual antiviral capability, GS-9620 is a promising novel agent that can be applied in the combination therapy of HBV/HIV coinfection.
Pembrolizumab, a checkpoint inhibitor that targets PD-1, has been approved for treatment of various carcinomas, including lung cancer (176). On account of the critical function of the PD-1/PD-L1 axis in the pathogenesis of chronic diseases, including HBV and HIV, PD-1 inhibitors have been speculated to be effective in disease treatment (90, 91, 177, 178). Several studies have evaluated the safety and feasibility of pembrolizumab in the therapy of patients suffering from various types of carcinoma concurrently with HIV (179–182). Treatment with pembrolizumab has an impact on HIV-specific T cell response and HIV load, showing as a transient increase of CD8+ T cell activation and a transient reduction of HIV DNA (180, 182). No serious adverse effects were observed during the treatment, indicating that pembrolizumab is safe and well-tolerated by the patients (181). Among patients with tumor and HBV infection, pembrolizumab was found to be safe (183–185). Moreover, several studies have been developed to explore the efficacy of pembrolizumab in HBV infection, suggesting that it might enhance the host immune status (182, 184, 185). However, further research is needed to assess the efficacy and safety of pembrolizumab in HBV treatment. On the basis of the application of pembrolizumab in the treatment of patients with cancer and HIV or HBV, experts assumed that it might be effective in HVB/HIV coinfected individuals. However, the evidence remains inadequate because patients with HIV or HBV are usually excluded from research on immune inhibitors because of the immune reconstitution inflammatory syndrome (186). Few studies have indicated that PD-1 inhibitors have proviral effects on HBV infection. However, they are regarded to be able to strengthen the immune function and may be a potential option for HIV treatment (88). Combination of PD-1 inhibitors with other agents might be a reasonable strategy for viral coinfection treatment.
Though current guidelines suggest treatment of HBV/HIV coinfected patients with dual antiviral regimen targeting HBV and HIV, immune reconstruction-related hepatic flare following the ART should be noted (187). IRIS is considered as a complication induced by the initiation of highly active antiretroviral therapy (ART) in HBV/HIV coinfected patients. It is an inflammatory disorder related to the worsening status of existing infection (188).
As to the coinfected patients receiving ART, elevation of liver enzymes is common, most of which are mild and do not require modification of treatment (189, 190). It is uncommon to develop into severe hepatotoxicity, manifesting as liver enzymes higher than 10 times the upper limit of normal (191). Moreover, the acute liver failure is also rare (192). Unfortunately, high proportion of mortality can be observed among acute liver failure patients, maybe owing to the potential impairment of liver (191, 193). According to a recent research, 20–25% of the coinfected patients might appear HBV flares (HF) after the start of ART (194). Currently, little is known about the impact of IRIS induced HF on the natural history of HBV infection. Patients undergoing HF presented an increase of CD4 T cell counts, a peak level of serum alanine aminotransferase (ALT) and a decrease of HBV DNA (195, 196). A recent study revealed that HBsAg loss was more common in patients developed IRIS induced HF compared with those who did not, suggesting that IRIS induced HF after ART was closely linked with the loss of HBsAg (197). The researchers also raised that younger age and higher HBV DNA titer at baseline were related with the development of IRIS induced HF (197). However, the occurrence mechanism of HF has never been illustrated clearly. It is speculated that the exploration of immune response of IRIS induced HF might be benefit to the treatment of HBV/HIV coinfection. Further studies on such aspect are warranted.
Accumulating evidence indicates that the coinfection of HBV and HIV place a heavy burden to the society (148, 198, 199). Coinfection is capable of accelerating the progression of liver diseases (200). Treatment with dual antiviral agents must be initiated as soon as possible (61, 62). However, several factors increase the difficulty of treatment. Agents with a single antiviral effect could induce drug resistance during the duration of therapy. For instance, agents against HBV only could lead to drug resistance to HIV. Hence, combination therapeutic strategies with dual antiviral effects are important (65). Drug-related side effects must also be considered when formulating therapeutic regimens. Renal dysfunction is the most common adverse effect, thus it should be considered before choosing drugs, especially tenofovir, for treatment (135). Damages to important organs might limit the application of existing regimens. Therefore, novel dual antiviral agents with less adverse effects must be developed. In-depth research on disease mechanisms has identified several critical pathogenic mechanisms, providing new approaches for disease treatment. PD-1/PD-L1 participates in the pathogenicity of viruses, including HBV and HIV (88–90). The inhibitors that antagonize the PD-1/PD-L1 axis might be a promising drug for HBV/HIV coinfection treatment. Moreover, immunoregulators with the ability to enhance the innate immune response against HBV and HIV are acceptable. Regardless of the type of agents applied for the treatment of HBV/HIV infection, drug-related adverse effects should be closely monitored.
The efficacy and safety of many strategies for the treatment of HBV/HIV coinfection are being assessed in clinical trials. Several agents remain at the preclinical phase and are not yet available for the clinical treatment of HBV/HIV coinfection. More research and clinical trials are required to definitively establish the value of such agents for the therapy of HBV/HIV coinfection. Finally, novel agents with potent antiviral effects on both HBV and HIV are the ideal approaches.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Potthoff A, Manns MP, Wedemeyer H. Treatment of HBV/HCV coinfection. Expert Opin Pharmacother. (2010) 11:919–28. doi: 10.1517/14656561003637659
2. Mavilia MG, Wu GY. HBV-HCV coinfection: viral interactions, management, and viral reactivation. J Clin Transl Hepato. (2018) 6:296–305. doi: 10.14218/JCTH.2018.00016
3. Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. (2010) 15(Suppl. 4):5–13. doi: 10.1634/theoncologist.2010-S4-05
4. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. (2007) 132:2557–76. doi: 10.1053/j.gastro.2007.04.061
5. Shire NJ, Sherman KE. Management of HBV/HIV-coinfected patients. Semin Liver Dis. (2005) 25(Suppl. 1):57–57. doi: 10.1055/s-2005-915646
6. Utsumi T, Lusida MI. Viral hepatitis and human immunodeficiency virus co-infections in Asia. World J Virol. (2015) 4:96–104. doi: 10.5501/wjv.v4.i2.96
7. Kaspar MB, Sterling RK. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol. (2017) 4:e000166. doi: 10.1136/bmjgast-2017-000166
8. Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet. (2002) 360:1921–6. doi: 10.1016/S0140-6736(02)11913-1
9. Wyles DL. Antiretroviral effects on HBV/HIV co-infection and the natural history of liver disease. Clin Liver Dis. (2019) 23:473–86. doi: 10.1016/j.cld.2019.04.004
10. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection–a global challenge. N Engl J Med. (2012) 366:1749–52. doi: 10.1056/NEJMp1201796
11. Dunford L, Carr MJ, Dean J, Nguyen LT, Ta Thi TH, Nguyen BT, et al. A multicentre molecular analysis of hepatitis B and blood-borne virus coinfections in Viet Nam. PLoS ONE. (2012) 7:e39027. doi: 10.1371/journal.pone.0039027
12. Templeton DJ, Wright ST, McManus H, Lawrence C, Russell DB, Law MG, et al. Antiretroviral treatment use, co-morbidities and clinical outcomes among Aboriginal participants in the Australian HIV Observational Database (AHOD). BMC Infect Dis. (2015) 15:326. doi: 10.1186/s12879-015-1051-4
13. Bell TG, Makondo E, Martinson NA, Kramvis A. Hepatitis B virus infection in human immunodeficiency virus infected southern African adults: occult or overt–that is the question. PLoS ONE. (2012) 7:e45750. doi: 10.1371/journal.pone.0045750
14. Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review. World J Gastroenterol. (2014) 20:14598–614. doi: 10.3748/wjg.v20.i40.14598
15. Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. (2009) 49(5 Suppl):S45–45. doi: 10.1002/hep.22883
17. Phung BC, Sogni P, Launay O. Hepatitis B and human immunodeficiency virus co-infection. World J Gastroenterol. (2014) 20:17360–7. doi: 10.3748/wjg.v20.i46.17360
18. Klein MB, Althoff KN, Jing Y, Lau B, Kitahata M, Lo Re V, et al. Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in north america from the early to modern antiretroviral therapy eras. Clin Infect Dis. (2016) 63:1160–7. doi: 10.1093/cid/ciw531
19. Chun HM, Fieberg AM, Hullsiek KH, Lifson AR, Crum-Cianflone NF, Weintrob AC, et al. Epidemiology of Hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis. (2010) 50:426–36. doi: 10.1086/649885
20. Thimme R, Spangenberg HC, Blum HE. Hepatitis B or hepatitis C and human immunodeficiency virus infection. J Hepatol. (2005) 42(Suppl. 1):S44–44. doi: 10.1016/j.jhep.2005.01.002
21. Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, et al. Natural history and prognostic factors for chronic hepatitis type B. Gut. (1991) 32:294–8. doi: 10.1136/gut.32.3.294
22. Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. (1995) 21:77–82. doi: 10.1002/hep.1840210114
23. McGovern BH. The epidemiology, natural history and prevention of hepatitis B: implications of HIV coinfection. Antiviral Ther. (2007) 12(Suppl. 3):H3–13.
24. Koblin BA, Taylor PE, Rubinstein P, Stevens CE. Effect of duration of hepatitis B virus infection on the association between human immunodeficiency virus type-1 and hepatitis B viral replication. Hepatology. (1992) 15:590–2. doi: 10.1002/hep.1840150406
25. Di Martino V, Thevenot T, Colin JF, Boyer N, Martinot M, Degos F, et al. Influence of HIV infection on the response to interferon therapy and the long-term outcome of chronic hepatitis B. Gastroenterology. (2002) 123:1812–22. doi: 10.1053/gast.2002.37061
26. Thio CL, Netski DM, Myung J, Seaberg EC, Thomas DL. Changes in hepatitis B virus DNA levels with acute HIV infection. Clin Infect Dis. (2004) 38:1024–9. doi: 10.1086/382534
27. Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, et al. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: the French national Mortalité 2005 study. J Hepatol. (2009) 50:736–45. doi: 10.1016/j.jhep.2008.11.018
28. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. (2017) 12:6–11. doi: 10.1097/COH.0000000000000327
29. Hleyhel M, Hleyhel M, Bouvier AM, Belot A, Tattevin P, Pacanowski J, et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS. (2014) 28:2109–18. doi: 10.1097/QAD.0000000000000382
30. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. (2014) 28:881–90. doi: 10.1097/QAD.0000000000000163
31. Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. (2010) 85:303–15. doi: 10.1016/j.antiviral.2009.10.021
33. Park JS, Saraf N, Dieterich DT. HBV plus HCV, HCV plus HIV, HBV plus HIV. Curr Gastroenterol Rep. (2006) 8:67–74. doi: 10.1007/s11894-006-0066-9
34. Joshi D, O'Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. (2011) 377:1198–209. doi: 10.1016/S0140-6736(10)62001-6
35. Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. (1999) 29:1306–10. doi: 10.1002/hep.510290447
36. Gürtler LG. Effect of antiretroviral HIV therapy on hepatitis B virus replication and pathogenicity. Intervirology. (2014) 57:212–7. doi: 10.1159/000360942
37. Revill PA, Littlejohn M, Ayres A, Yuen L, Colledge D, Bartholomeusz A, et al. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus co-infected individuals. AIDS. (2007) 21:1701–10. doi: 10.1097/QAD.0b013e32826fb305
38. Warner N, Locarnini S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology. (2008) 48:88–98. doi: 10.1002/hep.22295
39. Svegliati-Baroni G, De Minicis S. HIV protein gp120 and chemokines receptor for liver fibrosis. Gut. (2010) 59:428–9. doi: 10.1136/gut.2009.195024
40. Bruno R, Galastri S, Sacchi P, Cima S, Caligiuri A, DeFranco R, et al. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. (2010) 59:513–20. doi: 10.1136/gut.2008.163287
41. Babu CK, Suwansrinon K, Bren GD, Badley AD, Rizza SA. HIV induces TRAIL sensitivity in hepatocytes. PLoS ONE. (2009) 4:e4623. doi: 10.1371/journal.pone.0004623
42. Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, Dolan MJ, et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. (2005) 105:2458–64. doi: 10.1182/blood-2004-08-3058
43. Vlahakis SR, Villasis-Keever A, Gomez TS, Bren GD, Paya CV. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. (2003) 188:1455–60. doi: 10.1086/379738
44. Jeyarajan AJ, Chung RT. Insights into the pathophysiology of liver disease in HCV/HIV: does it end with HCV cure? J Infect Dis. (2020) 222(Suppl. 9):S13–13. doi: 10.1093/infdis/jiaa279
45. Iser DM, Avihingsanon A, Wisedopas N, Thompson AJ, Boyd A, Matthews GV, et al. Increased intrahepatic apoptosis but reduced immune activation in HIV-HBV co-infected patients with advanced immunosuppression. AIDS. (2011) 25:197–205. doi: 10.1097/QAD.0b013e3283410ccb
46. Crane M, Iser D, Lewin SR. Human immunodeficiency virus infection and the liver. World J Hepatol. (2012) 4:91–8. doi: 10.4254/wjh.v4.i3.91
47. Yu Y, Gong R, Mu Y, Chen Y, Zhu C, Sun Z, et al. Hepatitis B virus induces a novel inflammation network involving three inflammatory factors, IL-29, IL-8, and cyclooxygenase-2. J Immunol. (2011) 187:4844–60. doi: 10.4049/jimmunol.1100998
48. Cougot D, Wu Y, Cairo S, Caramel J, Renard CA, Lévy L, et al. The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J Biol Chem. (2007) 282:4277–87. doi: 10.1074/jbc.M606774200
49. Wang JY, Wang XL, Liu P. Detection of serum TNF-alpha, IFN-beta, IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. (1999) 5:38–40. doi: 10.3748/wjg.v5.i1.38
50. Lara-Pezzi E, Gómez-Gaviro MV, Gálvez BG, Mira E, Iñiguez MA, Fresno M, et al. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J Clin Investig. (2002) 110:1831–8. doi: 10.1172/JCI200215887
51. Mohammed NA, Abd El-Aleem SA, El-Hafiz HA, McMahon RF. Distribution of constitutive (COX-1) and inducible (COX-2) cyclooxygenase in postviral human liver cirrhosis: a possible role for COX-2 in the pathogenesis of liver cirrhosis. J Clin Pathol. (2004) 57:350–4. doi: 10.1136/jcp.2003.012120
52. Zhang X, Shen J, Man K, Chu ES, Yau TO, Sung JC, et al. CXCL10 plays a key role as an inflammatory mediator and a non-invasive biomarker of non-alcoholic steatohepatitis. J Hepatol. (2014) 61:1365–75. doi: 10.1016/j.jhep.2014.07.006
53. Chen LJ, Lv J, Wen XY, Niu JQ. CXC chemokine IP-10: a key actor in liver disease? Hepatol Int. (2013) 7:798–804. doi: 10.1007/s12072-013-9445-0
54. Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. (1993) 177:1809–14. doi: 10.1084/jem.177.6.1809
55. Singh KP, Zerbato JM, Zhao W, Braat S, Deleage C, Tennakoon GS, et al. Intrahepatic CXCL10 is strongly associated with liver fibrosis in HIV-Hepatitis B co-infection. PLoS Pathog. (2020) 16:e1008744. doi: 10.1371/journal.ppat.1008744
56. Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. (2017) 31:2035–52. doi: 10.1097/QAD.0000000000001574
57. Crane M, Avihingsanon A, Rajasuriar R, Velayudham P, Iser D, Solomon A, et al. Lipopolysaccharide, immune activation, and liver abnormalities in HIV/hepatitis B virus (HBV)-coinfected individuals receiving HBV-active combination antiretroviral therapy. J Infect Dis. (2014) 210:745–51. doi: 10.1093/infdis/jiu119
58. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. (2006) 12:1365–71. doi: 10.1038/nm1511
59. Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. (2008) 135:226–33. doi: 10.1053/j.gastro.2008.03.022
60. Evans TI, Li H, Schafer JL, Klatt NR, Hao XP, Traslavina RP, et al. SIV-induced translocation of bacterial products in the liver mobilizes myeloid dendritic and natural killer cells associated with liver damage. J Infect Dis. (2016) 213:361–9. doi: 10.1093/infdis/jiv404
61. Zhou K, Terrault N. Management of hepatitis B in special populations. Best Prac Res Clin Gastroenterol. (2017) 31:311–20. doi: 10.1016/j.bpg.2017.06.002
62. Ganesan M, Poluektova LY, Kharbanda KK, Osna NA. Human immunodeficiency virus and hepatotropic viruses co-morbidities as the inducers of liver injury progression. World J Gastroenterol. (2019) 25:398–410. doi: 10.3748/wjg.v25.i4.398
63. Núñez M, Puoti M, Camino N, Soriano V. Treatment of chronic hepatitis B in the human immunodeficiency virus-infected patient: present and future. Clin Infect Dis. (2003) 37:1678–85. doi: 10.1086/379774
64. Mendes-Corrêa M, Núñez M. Management of HIV and hepatitis virus coinfection. Expert Opin Pharmacother. (2010) 11:2497–516. doi: 10.1517/14656566.2010.500615
65. Lin JJ, Lin KY, Tang HJ, Lin SP, Lee YC, Liu CE, et al. Hepatitis B virus seroprevalence among HIV-infected patients receiving combination antiretroviral therapy three decades after universal neonatal hepatitis B immunization program in Taiwan. J Microbiol Immunol Infect. (2021) 54:228–37. doi: 10.1016/j.jmii.2019.10.005
66. Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. (2015) 1:15035. doi: 10.1038/nrdp.2015.35
67. Seitz S, Urban S, Antoni C, Böttcher B. Cryo-electron microscopy of hepatitis B virions reveals variability in envelope capsid interactions. EMBO J. (2007) 26:4160–7. doi: 10.1038/sj.emboj.7601841
68. Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Res. (2008) 134:235–49. doi: 10.1016/j.virusres.2007.12.024
69. Menéndez-Arias L, Álvarez M, Pacheco B. Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Curr Opin Virol. (2014) 8:1–9. doi: 10.1016/j.coviro.2014.04.005
70. Bazzoli C, Jullien V, Le Tiec C, Rey E, Mentré F, Taburet AM. Intracellular pharmacokinetics of antiretroviral drugs in HIV-infected patients, and their correlation with drug action. Clin Pharmacokinetics. (2010) 49:17–45. doi: 10.2165/11318110-000000000-00000
71. Tchesnokov EP, Obikhod A, Schinazi RF, Götte M. Delayed chain termination protects the anti-hepatitis B virus drug entecavir from excision by HIV-1 reverse transcriptase. J Biol Chem. (2008) 283:34218–28. doi: 10.1074/jbc.M806797200
72. Langley DR, Walsh AW, Baldick CJ, Eggers BJ, Rose RE, Levine SM, et al. Inhibition of hepatitis B virus polymerase by entecavir. J Virol. (2007) 81:3992–4001. doi: 10.1128/JVI.02395-06
73. Castro AP, Carvalho TM, Moussatché N, Damaso CR. Redistribution of cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles. J Virol. (2003) 77:9052–68. doi: 10.1128/JVI.77.16.9052-9068.2003
75. Liu X, Sun L, Yu M, Wang Z, Xu C, Xue Q, et al. Cyclophilin A interacts with influenza A virus M1 protein and impairs the early stage of the viral replication. Cell Microbiol. (2009) 11:730–41. doi: 10.1111/j.1462-5822.2009.01286.x
76. Liu X, Zhao Z, Xu C, Sun L, Chen J, Zhang L, et al. Cyclophilin A restricts influenza A virus replication through degradation of the M1 protein. PLoS ONE. (2012) 7:e31063. doi: 10.1371/journal.pone.0031063
77. An P, Wang LH, Hutcheson-Dilks H, Nelson G, Donfield S, Goedert JJ, et al. Regulatory polymorphisms in the cyclophilin A gene, PPIA, accelerate progression to AIDS. PLoS Pathog. (2007) 3:e88. doi: 10.1371/journal.ppat.0030088
78. Tian X, Zhao C, Zhu H, She W, Zhang J, Liu J, et al. Hepatitis B virus (HBV) surface antigen interacts with and promotes cyclophilin a secretion: possible link to pathogenesis of HBV infection. J Virol. (2010) 84:3373–81. doi: 10.1128/JVI.02555-09
79. Gürtler C, Bowie AG. Innate immune detection of microbial nucleic acids. Trends Microbiol. (2013) 21:413–20. doi: 10.1016/j.tim.2013.04.004
80. Shlomai A, Schwartz RE, Ramanan V, Bhatta A, de Jong YP, Bhatia SN, et al. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc Natl Acad Sci USA. (2014) 111:12193–8. doi: 10.1073/pnas.1412631111
81. Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. (2015) 42:123–32. doi: 10.1016/j.immuni.2014.12.016
82. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. (2014) 14:36–49. doi: 10.1038/nri3581
83. de Weerd NA, Vivian JP, Nguyen TK, Mangan NE, Gould JA, Braniff SJ, et al. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nat Immunol. (2013) 14:901–7. doi: 10.1038/ni.2667
84. Bourke NM, Napoletano S, Bannan C, Ahmed S, Bergin C, McKnight Á, et al. Control of HIV infection by IFN-α: implications for latency and a cure. Cell Mol Life Sci. (2018) 75:775–83. doi: 10.1007/s00018-017-2652-4
85. Liu SY, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. (2013) 38:92–105. doi: 10.1016/j.immuni.2012.11.005
86. Tan G, Song H, Xu F, Cheng G. When Hepatitis B Virus meets interferons. Front Microbiol. (2018) 9:1611. doi: 10.3389/fmicb.2018.01611
87. Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, et al. Hepatitis B virus infection. Nat Rev Dis Primers. (2018) 4:18035. doi: 10.1038/nrdp.2018.35
88. Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology. (2015) 12:14. doi: 10.1186/s12977-015-0144-x
89. Schönrich G, Raftery MJ. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. (2019) 9:207. doi: 10.3389/fcimb.2019.00207
90. Wang XF, Lei Y, Chen M, Chen CB, Ren H, Shi TD. PD-1/PDL1 and CD28/CD80 pathways modulate natural killer T cell function to inhibit hepatitis B virus replication. J Viral Hepatitis. (2013) 20(Suppl. 1):39–39. doi: 10.1111/jvh.12061
91. Féray C, López-Labrador FX. Is PD-1 blockade a potential therapy for HBV? JHEP Rep. (2019) 1:142–4. doi: 10.1016/j.jhepr.2019.07.007
92. Lamivudine. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
93. Scott LJ. Dolutegravir/lamivudine single-tablet regimen: a review in HIV-1 infection. Drugs. (2020) 80:61–72. doi: 10.1007/s40265-019-01247-1
94. Xu B, Lin L, Xu G, Zhuang Y, Guo Q, Liu Y, et al. Long-term lamivudine treatment achieves regression of advanced liver fibrosis/cirrhosis in patients with chronic hepatitis B. J Gastroenterol Hepatol. (2015) 30:372–8. doi: 10.1111/jgh.12718
95. Kobashi H, Miyake Y, Ikeda F, Yasunaka T, Nishino K, Moriya A, et al. Long-term outcome and hepatocellular carcinoma development in chronic hepatitis B or cirrhosis patients after nucleoside analog treatment with entecavir or lamivudine. Hepatol Res. (2011) 41:405–16. doi: 10.1111/j.1872-034X.2011.00785.x
96. Kurokawa M, Hiramatsu N, Oze T, Yakushijin T, Miyazaki M, Hosui A, et al. Long-term effect of lamivudine treatment on the incidence of hepatocellular carcinoma in patients with hepatitis B virus infection. J Gastroenterol. (2012) 47:577–85. doi: 10.1007/s00535-011-0522-7
97. Quercia R, Perno CF, Koteff J, Moore K, McCoig C, St Clair M, et al. Twenty-five years of lamivudine: current and future use for the treatment of HIV-1 infection. J Acquired Immune Deficiency Syndr. (2018) 78:125–35. doi: 10.1097/QAI.0000000000001660
98. WHO Guidelines Approved by the Guidelines Review Committee. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva: World Health Organization (2016).
99. Coates JA, Cammack N, Jenkinson HJ, Mutton IM, Pearson BA, Storer R, et al. The separated enantiomers of 2'-deoxy-3'-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrobial Agents Chemother. (1992) 36:202–5. doi: 10.1128/AAC.36.1.202
100. Schinazi RF, Chu CK, Peck A, McMillan A, Mathis R, Cannon D, et al. Activities of the four optical isomers of 2',3'-dideoxy-3'-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrobial Agents Chemother. (1992) 36:672–6. doi: 10.1128/AAC.36.3.672
101. Cahn P, Rolón MJ, Figueroa MI, Gun A, Patterson P, Sued O. Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. Journal of the International AIDS Society. (2017) 20:21678. doi: 10.7448/IAS.20.01.21678
102. Li Y, Xie J, Han Y, Wang H, Zhu T, Wang N, et al. Lamivudine monotherapy-based cART is efficacious for HBV treatment in HIV/HBV coinfection when baseline HBV DNA <20,000 IU/mL. J Acquired Immune Deficiency Syndr. (2016) 72:39–45. doi: 10.1097/QAI.0000000000000927
103. Idoko J, Meloni S, Muazu M, Nimzing L, Badung B, Hawkins C, et al. Impact of hepatitis B virus infection on human immunodeficiency virus response to antiretroviral therapy in Nigeria. Clin Infect Dis. (2009) 49:1268–73. doi: 10.1086/605675
104. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. (2009) 50:661–2. doi: 10.1002/hep.23190
105. Ive P, MacLeod W, Mkumla N, Orrell C, Jentsch U, Wallis CL, et al. Low prevalence of liver disease but regional differences in HBV treatment characteristics mark HIV/HBV co-infection in a South African HIV clinical trial. PLoS ONE. (2013) 8:e74900. doi: 10.1371/journal.pone.0074900
106. Gu L, Han Y, Li Y, Zhu T, Song X, Huang Y, et al. Emergence of lamivudine-resistant HBV during antiretroviral therapy including lamivudine for patients coinfected with HIV and HBV in China. PLoS ONE. (2015) 10:e0134539. doi: 10.1371/journal.pone.0134539
107. Tillmann HL. Antiviral therapy and resistance with hepatitis B virus infection. World J Gastroenterol. (2007) 13:125–40. doi: 10.3748/wjg.v13.i1.125
108. Considerations for Antiretroviral Use in Patients With Coinfections (2017). Available online at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/hepatitis-b-virushiv-coinfection
109. Chair LW, Ahmed N, Angus B, Boffito M, Bower M, Churchill D, et al. BHIVA guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update). Br HIV Assoc. (2016) 103. Available online at: https://www.bhiva.org/file/RVYKzFwyxpgiI/treatment-guidelines-2016-interim-update.pdf
110. Behrens G, Ryom L, Arribas J, Molina J-M, De Miguel Buckley R, d'Arminio Monforte A, et al. Update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.1. Euro AIDS Clin Soc. (2020) 96. Available online at: https://www.eacsociety.org/media/guidelines-10.1_30032021_1.pdf
111. Emtricitabine. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
112. Lim SG, Ng TM, Kung N, Krastev Z, Volfova M, Husa P, et al. A double-blind placebo-controlled study of emtricitabine in chronic hepatitis B. Arch Internal Med. (2006) 166:49–56. doi: 10.1001/archinte.166.1.49
113. Masho SW, Wang CL, Nixon DE. Review of tenofovir-emtricitabine. Ther Clin Risk Manage. (2007) 3:1097–104.
114. Ford N, Shubber Z, Hill A, Vitoria M, Doherty M, Mills EJ, et al. Comparative efficacy of Lamivudine and emtricitabine: a systematic review and meta-analysis of randomized trials. PLoS ONE. (2013) 8:e79981. doi: 10.1371/journal.pone.0079981
115. Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet. (2017) 390:2063–72. doi: 10.1016/S0140-6736(17)32299-7
116. Raffi F, Rachlis A, Brinson C, Arasteh K, Górgolas M, Brennan C, et al. Dolutegravir efficacy at 48 weeks in key subgroups of treatment-naive HIV-infected individuals in three randomized trials. AIDS. (2015) 29:167–74. doi: 10.1097/QAD.0000000000000519
117. Avihingsanon A, Lewin SR, Kerr S, Chang JJ, Piyawat K, Napissanant N, et al. Efficacy of tenofovir disoproxil fumarate/emtricitabine compared with emtricitabine alone in antiretroviral-naive HIV-HBV coinfection in Thailand. Antiviral Ther. (2010) 15:917–22. doi: 10.3851/IMP1645
118. Nüesch R, Ananworanich J, Srasuebkul P, Chetchotisakd P, Prasithsirikul W, Klinbuayam W, et al. Interruptions of tenofovir/emtricitabine-based antiretroviral therapy in patients with HIV/hepatitis B virus co-infection. AIDS. (2008) 22:152–4. doi: 10.1097/QAD.0b013e3282f303bf
119. Tenofovir. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
120. Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, et al. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrobial Agents Chemother. (1993) 37:332–8. doi: 10.1128/AAC.37.2.332
121. Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrobial Agents Chemother. (1998) 42:612–7. doi: 10.1128/AAC.42.3.612
122. Krakower DS, Mayer KH. Pre-exposure prophylaxis to prevent HIV infection: current status, future opportunities and challenges. Drugs. (2015) 75:243–51. doi: 10.1007/s40265-015-0355-4
123. Buchbinder SP. Maximizing the benefits of HIV preexposure prophylaxis. Topics Antiviral Med. (2018) 25:138–42.
124. Dore GJ, Cooper DA, Pozniak AL, DeJesus E, Zhong L, Miller MD, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. (2004) 189:1185–92. doi: 10.1086/380398
125. Nelson M, Portsmouth S, Stebbing J, Atkins M, Barr A, Matthews G, et al. An open-label study of tenofovir in HIV-1 and Hepatitis B virus co-infected individuals. AIDS. (2003) 17:F7–10. doi: 10.1097/00002030-200301030-00002
126. Scott LJ, Chan HLY. Tenofovir alafenamide: a review in chronic Hepatitis B. Drugs. (2017) 77:1017–28. doi: 10.1007/s40265-017-0754-9
127. Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. (2016) 1:185–95. doi: 10.1016/S2468-1253(16)30024-3
128. Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. (2016) 1:196–206. doi: 10.1016/S2468-1253(16)30107-8
129. Ruane PJ, DeJesus E, Berger D, Markowitz M, Bredeek UF, Callebaut C, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of tenofovir alafenamide as 10-day monotherapy in HIV-1-positive adults. J Acquired Immune Deficiency Syndr. (2013) 63:449–55. doi: 10.1097/QAI.0b013e3182965d45
130. De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharm. (2016) 119:1–7. doi: 10.1016/j.bcp.2016.04.015
131. Benhamou Y, Tubiana R, Thibault V. Tenofovir disoproxil fumarate in patients with HIV and lamivudine-resistant hepatitis B virus. N Engl J Med. (2003) 348:177–8. doi: 10.1056/NEJM200301093480218
132. Ristig MB, Crippin J, Aberg JA, Powderly WG, Lisker-Melman M, Kessels L, et al. Tenofovir disoproxil fumarate therapy for chronic hepatitis B in human immunodeficiency virus/hepatitis B virus-coinfected individuals for whom interferon-alpha and lamivudine therapy have failed. J Infect Dis. (2002) 186:1844–7. doi: 10.1086/345770
133. Kosi L, Reiberger T, Payer BA, Grabmeier-Pfistershammer K, Strassl R, Rieger A, et al. Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients. J Viral Hepatitis. (2012) 19:801–10. doi: 10.1111/j.1365-2893.2012.01601.x
134. Price H, Dunn D, Pillay D, Bani-Sadr F, de Vries-Sluijs T, Jain MK, et al. Suppression of HBV by tenofovir in HBV/HIV coinfected patients: a systematic review and meta-analysis. PLoS ONE. (2013) 8:e68152. doi: 10.1371/journal.pone.0068152
135. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. (2010) 51:496–505. doi: 10.1086/655681
136. Adefovir. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
137. Entecavir. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
138. Telbivudine. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
139. Benhamou Y, Bochet M, Thibault V, Calvez V, Fievet MH, Vig P, et al. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet. (2001) 358:718–23. doi: 10.1016/S0140-6736(01)05840-8
140. Peters MG, Andersen J, Lynch P, Liu T, Alston-Smith B, Brosgart CL, et al. Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127. Hepatology. (2006) 44:1110–6. doi: 10.1002/hep.21388
141. Benhamou Y. Treatment algorithm for chronic hepatitis B in HIV-infected patients. J Hepatol. (2006) 44(1 Suppl):S4–4. doi: 10.1016/j.jhep.2005.11.019
142. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. (2006) 354:1001–10. doi: 10.1056/NEJMoa051285
143. Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. (2006) 354:1011–20. doi: 10.1056/NEJMoa051287
144. Soriano V, Vispo E, Barreiro P. New 2011 updated DHHS antiretroviral treatment guidelines and chronic hepatitis B. AIDS. (2011) 25:1013–4. doi: 10.1097/QAD.0b013e328344fe96
145. McMahon MA, Jilek BL, Brennan TP, Shen L, Zhou Y, Wind-Rotolo M, et al. The HBV drug entecavir - effects on HIV-1 replication and resistance. N Engl J Med. (2007) 356:2614–21. doi: 10.1056/NEJMoa067710
146. Bobardt M, Hansson MJ, Mayo P, Ure D, Foster R, Gallay P. Structurally distinct cyclosporin and sanglifehrin analogs CRV431 and NV556 suppress established HCV infection in humanized-liver mice. PLoS ONE. (2020) 15:e0237236. doi: 10.1371/journal.pone.0237236
147. Gallay PA, Bobardt MD, Chatterji U, Trepanier DJ, Ure D, Ordonez C, et al. The novel cyclophilin inhibitor CPI-431-32 concurrently blocks HCV and HIV-1 infections via a similar mechanism of action. PLoS ONE. (2015) 10:e0134707. doi: 10.1371/journal.pone.0134707
148. Sun S, Yang Q, Sheng Y, Fu Y, Sun C, Deng C. Investigational drugs with dual activity against HBV and HIV (Review). Exp Ther Med. (2021) 21:35. doi: 10.3892/etm.2020.9467
149. Gallay P, Ure D, Bobardt M, Chatterji U, Ou J, Trepanier D, et al. The cyclophilin inhibitor CRV431 inhibits liver HBV DNA and HBsAg in transgenic mice. PLoS ONE. (2019) 14:e0217433. doi: 10.1371/journal.pone.0217433
150. Kuo J, Bobardt M, Chatterji U, Mayo PR, Trepanier DJ, Foster RT, et al. A pan-cyclophilin inhibitor, CRV431, decreases fibrosis and tumor development in chronic liver disease models. J Pharmacol Exp Ther. (2019) 371:231–41. doi: 10.1124/jpet.119.261099
151. Franke EK, Yuan HE, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. (1994) 372:359–62. doi: 10.1038/372359a0
152. Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, et al. Functional association of cyclophilin A with HIV-1 virions. Nature. (1994) 372:363–5. doi: 10.1038/372363a0
153. Trepanier DJ, Ure DR, Foster RT. In vitro phase I metabolism of CRV431, a novel oral drug candidate for chronic Hepatitis B. Pharmaceutics. (2017) 9:51. doi: 10.3390/pharmaceutics9040051
154. De Andrea M, Ravera R, Gioia D, Gariglio M, Landolfo S. The interferon system: an overview. Euro J Paediatric Neurol. (2002) 6(Suppl. A):A6–6; discussion: A8–8. doi: 10.1053/ejpn.2002.0573
155. Lee S, Baldridge MT. Interferon-lambda: a potent regulator of intestinal viral infections. Front Immunol. (2017) 8:749. doi: 10.3389/fimmu.2017.00749
156. Cooksley WG, Piratvisuth T, Lee SD, Mahachai V, Chao YC, Tanwandee T, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepatitis. (2003) 10:298–305. doi: 10.1046/j.1365-2893.2003.00450.x
158. Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: an update. Clin Gastroenterol Hepatol. (2006) 4:936–62. doi: 10.1016/j.cgh.2006.05.016
159. Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. (2007) 45:1056–75. doi: 10.1002/hep.21627
160. Cooksley WG. Treatment with interferons (including pegylated interferons) in patients with hepatitis B. Semin Liver Dis. (2004) 24(Suppl. 1):53–53. doi: 10.1055/s-2004-828678
161. Soriano V, Puoti M, Peters M, Benhamou Y, Sulkowski M, Zoulim F, et al. Care of HIV patients with chronic hepatitis B: updated recommendations from the HIV-Hepatitis B virus international panel. AIDS. (2008) 22:1399–410. doi: 10.1097/QAD.0b013e3282f8b46f
162. Wilkins E, Nelson M, Agarwal K, Awoyemi D, Barnes E, Bhagani S, et al. British HIV Association guidelines for the management of hepatitis viruses in adults infected with HIV 2013. HIV Med. (2013) 14(Suppl. 4):1–71. doi: 10.1111/hiv.12106
163. Niu C, Li L, Daffis S, Lucifora J, Bonnin M, Maadadi S, et al. Toll-like receptor 7 agonist GS-9620 induces prolonged inhibition of HBV via a type I interferon-dependent mechanism. J Hepatol. (2018) 68:922–31. doi: 10.1016/j.jhep.2017.12.007
164. Roethle PA, McFadden RM, Yang H, Hrvatin P, Hui H, Graupe M, et al. Identification and optimization of pteridinone Toll-like receptor 7 (TLR7) agonists for the oral treatment of viral hepatitis. J Med Chem. (2013) 56:7324–33. doi: 10.1021/jm400815m
165. Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol. (2015) 62:1237–45. doi: 10.1016/j.jhep.2014.12.026
166. Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. (2013) 144:1508–17. doi: 10.1053/j.gastro.2013.02.003
167. Li L, Barry V, Daffis S, Niu C, Huntzicker E, French DM, et al. Anti-HBV response to toll-like receptor 7 agonist GS-9620 is associated with intrahepatic aggregates of T cells and B cells. J Hepatol. (2018) 68:912–21. doi: 10.1016/j.jhep.2017.12.008
168. Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. (2018) 68:431–40. doi: 10.1016/j.jhep.2017.10.027
169. Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, et al. TLR7 agonist increases responses of Hepatitis B virus-specific t cells and natural killer cells in patients with chronic Hepatitis B treated with Nucleos(T)Ide analogues. Gastroenterology. (2018) 154:1764–77.e7. doi: 10.1053/j.gastro.2018.01.030
170. Agarwal K, Ahn SH, Elkhashab M, Lau AH, Gaggar A, Bulusu A, et al. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepatitis. (2018) 25:1331–40. doi: 10.1111/jvh.12942
171. Tsai A, Irrinki A, Kaur J, Cihlar T, Kukolj G, Sloan DD, et al. Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J Virol. (2017) 91:e02166–16. doi: 10.1128/JVI.02166-16
172. Martinsen JT, Gunst JD, Højen JF, Tolstrup M, Søgaard OS. The use of Toll-like receptor agonists in HIV-1 cure strategies. Front Immunol. (2020) 11:1112. doi: 10.3389/fimmu.2020.01112
173. Offersen R, Nissen SK, Rasmussen TA, Østergaard L, Denton PW, Søgaard OS, et al. A novel Toll-like receptor 9 agonist, MGN1703, enhances HIV-1 transcription and NK cell-mediated inhibition of HIV-1-infected autologous CD4+ T cells. J Virol. (2016) 90:4441–53. doi: 10.1128/JVI.00222-16
174. Bam RA, Hansen D, Irrinki A, Mulato A, Jones GS, Hesselgesser J, et al. TLR7 agonist GS-9620 is a potent inhibitor of acute HIV-1 infection in human peripheral blood mononuclear cells. Antimicrobial Agents Chemother. (2017) 61:e01369–16. doi: 10.1128/AAC.01369-16
175. Riddler SA, Para M, Benson CA, Mills A, Ramgopal M, DeJesus E, et al. Agonist, Induces Immune Activation in Virally Suppressed Adults Living With Human Immunodeficiency Virus-1. Clin Infect Dis. (2021) 72:e815–24. doi: 10.1093/cid/ciaa1534
176. Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Human Vaccines Immunotherapeutics. (2016) 12:2777–89. doi: 10.1080/21645515.2016.1199310
177. Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. (2016) 22:754–61. doi: 10.1038/nm.4113
178. Evans VA, van der Sluis RM, Solomon A, Dantanarayana A, McNeil C, Garsia R, et al. Programmed cell death-1 contributes to the establishment and maintenance of HIV-1 latency. AIDS. (2018) 32:1491–7. doi: 10.1097/QAD.0000000000001849
179. Uldrick TS, Gonçalves PH, Abdul-Hay M, Claeys AJ, Emu B, Ernstoff MS, et al. Assessment of the safety of pembrolizumab in patients with HIV and advanced cancer-a phase 1 study. JAMA Oncol. (2019) 5:1332–9. doi: 10.1001/jamaoncol.2019.2244
180. Spano JP, Veyri M, Gobert A, Guihot A, Perré P, Kerjouan M, et al. Immunotherapy for cancer in people living with HIV: safety with an efficacy signal from the series in real life experience. AIDS. (2019) 33:F13–9. doi: 10.1097/QAD.0000000000002298
181. Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. (2019) 5:1049–54. doi: 10.1001/jamaoncol.2018.6737
182. Blanch-Lombarte O, Gálvez C, Revollo B, Jiménez-Moyano E, Llibre JM, Manzano JL, et al. Enhancement of antiviral CD8(+) T-cell responses and complete remission of metastatic melanoma in an HIV-1-Infected subject treated with pembrolizumab. J Clin Med. (2019) 8:2089. doi: 10.3390/jcm8122089
183. Wen X, Wang Y, Ding Y, Li D, Li J, Guo Y, et al. Safety of immune checkpoint inhibitors in Chinese patients with melanoma. Melanoma Res. (2016) 26:284–9. doi: 10.1097/CMR.0000000000000256
184. Pandey A, Ezemenari S, Liaukovich M, Richard I, Boris A. A rare case of pembrolizumab-induced reactivation of Hepatitis B. Case Rep Oncol Med. (2018) 2018:5985131. doi: 10.1155/2018/5985131
185. Zhang X, Zhou Y, Chen C, Fang W, Cai X, Zhang X, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer. (2019) 7:322. doi: 10.1186/s40425-019-0808-5
186. Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: a case series. Melanoma Res. (2018) 28:155–8. doi: 10.1097/CMR.0000000000000434
187. Rowley MW, Patel A, Zhou W, Wong M, Seetharam AB. Immune reconstitution syndrome with initiation of treatment of HBV/HIV co-infection: activity flare associated with E antigen seroconversion. Ann Hepatol. (2019) 18:220–4. doi: 10.5604/01.3001.0012.7918
188. French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. (2009) 48:101–7. doi: 10.1086/595006
189. Ofotokun I, Smithson SE, Lu C, Easley KA, Lennox JL. Liver enzymes elevation and immune reconstitution among treatment-naïve HIV-infected patients instituting antiretroviral therapy. Am J Med Sci. (2007) 334:334–41. doi: 10.1097/MAJ.0b013e31811ec780
190. den Brinker M, Wit FW, Wertheim-van Dillen PM, Jurriaans S, Weel J, van Leeuwen R, et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. (2000) 14:2895–902. doi: 10.1097/00002030-200012220-00011
191. Anderson AM, Mosunjac MB, Palmore MP, Osborn MK, Muir AJ. Development of fatal acute liver failure in HIV-HBV coinfected patients. World J Gastroenterol. (2010) 16:4107–11. doi: 10.3748/wjg.v16.i32.4107
192. Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. (2002) 186:23–31. doi: 10.1086/341084
193. Puoti M, Torti C, Ripamonti D, Castelli F, Zaltron S, Zanini B, et al. Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, and outcome. J Acquired Immune Deficiency Syndr. (2003) 32:259–67. doi: 10.1097/00126334-200303010-00004
194. Avihingsanon A, Matthews GV, Lewin SR, Marks P, Sasadeusz J, Cooper DA, et al. Assessment of HBV flare in a randomized clinical trial in HIV/HBV coinfected subjects initiating HBV-active antiretroviral therapy in Thailand. AIDS Res Ther. (2012) 9:6. doi: 10.1186/1742-6405-9-6
195. Wong D, Littlejohn M, Edwards R, Jackson K, Revill P, Gaggar A, et al. ALT flares during nucleotide analogue therapy are associated with HBsAg loss in genotype A HBeAg-positive chronic hepatitis B. Liver Int. (2018) 38:1760–9. doi: 10.1111/liv.13716
196. Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Clearance of hepatitis B surface antigen during long-term nucleot(s)ide analog treatment in chronic hepatitis B: results from a nine-year longitudinal study. J Gastroenterol. (2013) 48:930–41. doi: 10.1007/s00535-012-0688-7
197. Yoshikawa S, Yoshio S, Yoshida Y, Tsutsui Y, Kawai H, Yamazoe T, et al. Impact of immune reconstitution-induced hepatic flare on Hepatitis B surface antigen loss in Hepatitis B virus/human immunodeficiency virus-1 coinfected patients. J Infect Dis. (2021) 223:2080–9. doi: 10.1093/infdis/jiaa662
198. Platt L, French CE, McGowan CR, Sabin K, Gower E, Trickey A, et al. Prevalence and burden of HBV co-infection among people living with HIV: a global systematic review and meta-analysis. J Viral Hepatitis. (2020) 27:294–315. doi: 10.1111/jvh.13217
199. Bollinger RC, Thio CL, Sulkowski MS, McKenzie-White J, Thomas DL, Flexner C. Addressing the global burden of hepatitis B virus while developing long-acting injectables for the prevention and treatment of HIV. Lancet HIV. (2020) 7:e443–e8. doi: 10.1016/S2352-3018(19)30342-X
Keywords: hepatitis B virus, human immunodeficiency virus, coinfection, liver disease, clinical treatment
Citation: Cheng Z, Lin P and Cheng N (2021) HBV/HIV Coinfection: Impact on the Development and Clinical Treatment of Liver Diseases. Front. Med. 8:713981. doi: 10.3389/fmed.2021.713981
Received: 24 May 2021; Accepted: 23 August 2021;
Published: 04 October 2021.
Edited by:
Jian Wu, Zhejiang University, ChinaReviewed by:
Thi Thanh Hang Pham, Stanford University, United StatesCopyright © 2021 Cheng, Lin and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nansheng Cheng, Y2hlbmduYW5zaGVuZ2h4QHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.