
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 11 August 2021
Sec. Intensive Care Medicine and Anesthesiology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.711717
Background: The relationship between urine output (UO) and severe-stage progression in the early phase of acute kidney injury (AKI) remains unclear. This study aimed to investigate the relationship between early-phase UO6−12h [UO within 6 h after diagnosis of stage 1 AKI by Kidney Disease: Improving Global Outcomes (KDIGO) UO criteria] and severe-stage progression of AKI and to identify a reference value of early-phase UO6−12h for guiding initial therapy in critical care.
Methods: Adult patients with UO < 0.5 ml/kg/h for the first 6 h after intensive care unit (ICU) admission (meeting stage 1 AKI by UO) and UO6−12h ≥ 0.5 ml/kg/h were identified from the Medical Information Mart for Intensive Care (MIMIC) III database. The primary outcome was progression to stage 2/3 AKI by UO. After other variables were adjusted through multivariate analysis, generalized additive model (GAM) was used to visualize the relationship between early-phase UO6−12h and progression to stage 2/3 AKI by UO. A two-piecewise linear regression model was employed to identify the inflection point of early-phase UO6−12h above which progression risk significantly leveled off. Sensitivity and subgroup analyses were performed to assess the robustness of our findings.
Results: Of 2,984 individuals, 1,870 (62.7%) with KDIGO stage 1 UO criteria progressed to stage 2/3 AKI. In the multivariate analysis, early-phase UO6−12h showed a significant association with progression to stage 2/3 AKI by UO (odds ratio, 0.40; 95% confidence interval, 0.34–0.46; p < 0.001). There was a non-linear relationship between early-phase UO6−12h and progression of AKI. Early-phase UO6−12h of 1.1 ml/kg/h was identified as the inflection point, above which progression risk significantly leveled off (p = 0.780). Patients with early-phase UO6−12h ≥ 1.1 ml/kg/h had significantly shorter length of ICU stay (3.82 vs. 4.17 days, p < 0.001) and hospital stay (9.28 vs. 10.43 days, p < 0.001) and lower 30-day mortality (11.05 vs. 18.42%, p < 0.001). The robustness of our findings was confirmed by sensitivity and subgroup analyses.
Conclusions: Among early-stage AKI patients in critical care, there was a non-linear relationship between early-phase UO6−12h and progression of AKI. Early-phase UO6−12h of 1.1 ml/kg/h was the inflection point above which progression risk significantly leveled off.
Acute kidney injury (AKI) is common in the intensive care unit (ICU), and mortality dramatically increases as the severer-stage progression of AKI in critical patients (1–3). According to the Kidney Disease: Improving Global Outcomes (KDIGO) guideline, AKI is defined by plasma creatinine criteria or urine output (UO) criteria (4). AKI by KDIGO UO criteria accounts for a large proportion of AKI population (5). In addition, compared with the traditional markers of renal function (e.g., urea nitrogen and creatinine), oliguria is widely regarded as an early marker for early-stage AKI (6, 7).
The therapeutic window of AKI could become narrower as kidney function worsens, and therefore, early improvement in the early phase of AKI is of importance for improving outcome (8–10). However, it has been shown that a number of early-stage AKI patients still suffered from progression to severer stage, even though their UO was above 0.5 ml/kg/h in the early phase of therapy (1). In addition, the latest update of the Surviving Sepsis Campaign guideline does not mention a specific initial therapy goal for UO (11), which was once recommended to be ≥0.5 ml/kg/h in the previous version of the guideline (12). This change indicates that 0.5 ml/kg/h might not be the optimal UO target for therapy in critical patients. Thus, in order to guide the initial therapy for early-stage AKI patients, it is necessary to understand the relationship between UO and progression of AKI in the early phase of AKI. However, there is no study focusing on the relationship between UO and progression of AKI.
The aims of this study were (1) to investigate the relationship between early-phase UO6−12h (defined as UO within 6 h after diagnosis of stage 1 AKI by KDIGO UO criteria) and progression of AKI and (2) to identify whether there was an inflection point of early-phase UO6−12h, above which progression risk significantly leveled off.
The data of this study were extracted from a large US-based critical care database named Medical Information Mart for Intensive Care (MIMIC-III) (13). The MIMIC-III database contains all the ICU data of patients who were admitted to Beth Israel Deaconess Medical Center between 2001 and 2012. The database was approved by the institutional review board (IRB) of Beth Israel Deaconess Medical Center (Boston, MA, USA) and the Massachusetts Institute of Technology (Cambridge, MA, USA). After completing the training course and the Protecting Human Research Participants examination, we have gained access to MIMIC-III (ID: 9786716).
All consecutive adult patients (aged ≥ 18 years) who had UO < 0.5 ml/kg/h for the first 6 h (meeting diagnostic criteria of stage 1 AKI by KDIGO UO criteria) (4) and had UO ≥ 0.5 ml/kg/h during the 6-12-h period after ICU admission were screened for possible inclusion in the study (Figure 1). For patients admitted to ICU more than once, only the first ICU admission was included. The exclusion criteria were (1) using any dialysis in the first 24 h after ICU admission and (2) length of ICU stay < 48 h.
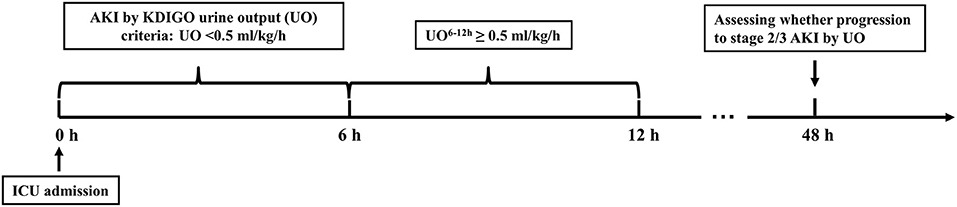
Figure 1. Schematic illustration of the time windows. AKI by KDIGO UO criteria was defined as urine output < 0.5 ml/kg/h for the first 6 h after ICU admission. UO, urine output; UO6−12h, urine output within 6 h after diagnosis of AKI by KDIGO UO criteria; ICU, intensive care unit; AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes.
Data including age, gender, weight, ethnicity, comorbidities, sepsis at ICU admission, serum creatinine (Scr) at ICU admission, and the Simplified Acute Physiology Score II (SAPS II) score of the first 24 h were collected from MIMIC-III database. Mean arterial pressure (MAP), vasopressor use, the amount of fluid intake, and UO during the 6-h period after diagnosis of AKI by KDIGO UO criteria were also extracted for analyses. The other fluid losses were initially explored but not included in the final analysis due to their high frequency (>50%) of missing data, included fluid losses in gastrointestinal decompression tube, thoracic drainage tube, and abdominal drainage tube during the 6-h period. Therefore, the fluid output in our study was equal to the amount of UO, and the fluid balance (ml) was defined as the amount of fluid intake (ml) minus the amount of UO (ml) during the 6-h period.
The primary outcome was progression to stage 2 or 3 AKI defined by the KDIGO UO criteria (stage 2: UO < 0.5 ml/kg/h for ≥12 h; stage 3: UO < 0.3 ml/kg/h for ≥24 h or anuria for ≥12 h) at any point in the first 48 h after ICU admission (4).
The secondary outcomes were (1) receiving any dialysis 24 h after ICU admission; (2) length of ICU stay; (3) length of hospital stay; and (4) 30-day mortality since ICU admission.
While continuous variables were reported as median and interquartile range (IQR), categorical variables were described as whole numbers and percentages. Student's t-test or Mann–Whitney U-test were used to analyze continuous variables according to their distribution. Fisher's exact test or chi-squared test was used to analyze categorical variables according to their frequencies. Multivariate logistic regression analyses were applied to investigate the effects of UO6−12h and the other variables on the occurrence of progression to stage 2/3 AKI by UO. Multicollinearity in regression analyses was detected by variance inflation factor (VIF), with a reference value of 5. Then, multivariate-adjusted model was used to assess the relationship between UO6−12h and progression to stage 2/3 AKI by UO. To visualize the relationship between UO6−12h and progression to stage 2/3 AKI by UO, generalized additive model (GAM) was used to identify the non-linear relationship. Once the non-linear correlation was observed, a two-piecewise linear regression model was performed to calculate the threshold effect of UO6−12h on progression to stage 2/3 AKI by UO in terms of the smoothing plot. When the ratio between progression to stage 2/3 AKI by UO and UO6−12h appears obvious in smoothed curve, recursive method automatically calculates the inflection point, where the maximum model likelihood will be used (14, 15). Then the inflection point was selected to dichotomize UO6−12h.
Sensitivity analyses for the association of UO6−12h with AKI stage progression were conducted through the following settings: (1) progression to stage 2 or 3 AKI by KDIGO UO criteria within 7 days after ICU admission as the outcome, (2) progression to stage 3 AKI by KDIGO UO criteria within 48 h or 7 days, and (3) progression to stage 3 AKI by KDIGO Scr criteria within 48 h were employed as the outcomes, separately.
In order to assess the effect size of UO6−12h on progression to stage 2/3 AKI by UO in different subgroups, subgroup analyses were carried out in the subgroups as listed: (1) heart failure (yes/no); (2) SAPS II score (<38.95/≥38.95); (3) sepsis at ICU admission (yes/no); and (4) vasopressor use within 6 h after diagnosis of AKI by KDIGO UO criteria (Vasopressor6−12h) (yes/no). The adjusted odds ratio of UO6−12h for progression to stage 2/3 AKI by UO was calculated in each subgroup, and the interaction between UO6−12h and subgroups was assessed.
All statistical analyses were performed through R software version 3.4.2 (Institute for Statistics and Mathematics, Vienna, Austria; https://www.r-project.org/). For variables with ≥15% missing values, they were excluded for further analyses. For variables with <15% missing values, they were analyzed using multiple imputation method with “MICE” R package (16). A two-tailed p-value < 0.05 was considered to be statistically significant.
Figure 2 shows the selection process for the study patients. Among the 10,642 adult patients with UO < 0.5 ml/kg/h for the first 6 h after ICU admission, 5,033 patients had UO ≥ 0.5 ml/kg/h during the 6-12-h period after ICU admission. A number of 2,049 patients were excluded because of using any dialysis (N = 44) in the first 24 h, or the length of ICU stay <48 h (N = 2,005). After selection, 2,984 patients were included for analyses. A total of 1,870 patients had progression to stage 2/3 AKI by UO, and 1,114 patients had no progression.
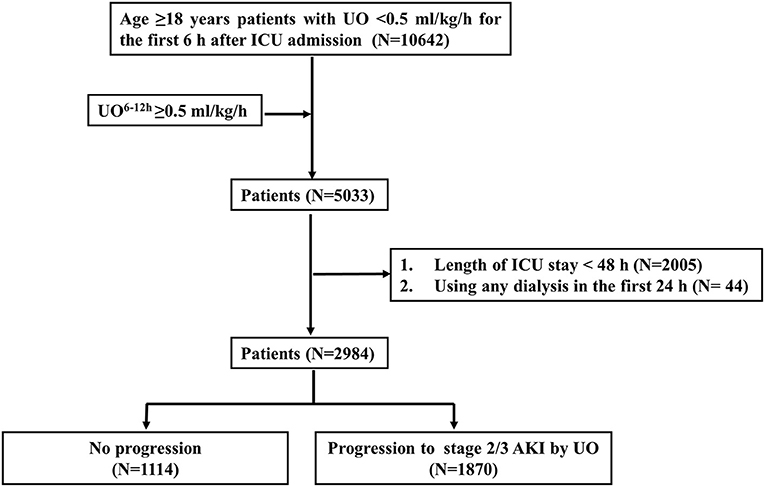
Figure 2. Flowchart of patient selection. UO, urine output; UO6−12h, urine output within 6 h after diagnosis of AKI by KDIGO UO criteria; ICU, intensive care unit; AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes.
The differences of patient characteristics are presented in Table 1. Patients with progression to stage 2/3 AKI by UO were more likely to be older (68.37 vs. 65.35 years, p < 0.001), have diabetes (32.83 vs. 28.10%, p = 0.007), have heart failure (40.53 vs. 28.19%, p < 0.001), have hypertension (58.72 vs. 54.58%, p = 0.027), have sepsis (54.60 vs. 44.34%, p < 0.001), and have higher Scr level at ICU admission (1.00 vs. 0.90 mg/dl, p < 0.001) and higher SAPS II score (40.75 vs. 35.93, p < 0.001). During the 6-h period after diagnosis of AKI by KDIGO UO criteria, patients with progression to stage 2/3 AKI by UO have higher fluid balance (400.00 vs. 250.00 ml, p< 0.001) and lower UO6−12h (0.72 vs. 1.08 ml/kg/h, p < 0.001).
Multivariate logistic regression analysis for progression to stage 2/3 AKI by UO is displayed in Table 2. The fluid output (ml) was excluded from multivariate analysis because its VIF was >5. The remaining variables were selected into the multivariate analysis, including age, gender, ethnicity, hypertension, metastatic cancer, liver failure, respiratory failure, heart failure, diabetes, Vasopressor6−12h, Diuretics0−24h, MAP0−24h, SAPS II score, Scr at ICU admission, sepsis at ICU admission, fluid intake, and fluid balance. It was shown that heart failure, sepsis at ICU admission, SAPS II score, Vasopressor6−12h, and UO6−12h were independently associated with the progression to stage 2/3 AKI by UO.
GAM was used to visualize the relationship between UO6−12h and progression to stage 2/3 AKI by UO (Figure 3). When UO6−12h was between 0.5 and 1.0 ml/k/h, the probability of progression to stage 2/3 AKI by UO decreased rapidly as UO6−12h increased. When UO6−12h was above 1.0 ml/kg/h, the probability almost plateaued with little fluctuation. Subsequently, piecewise linear regression was applied to select the optimal threshold for UO6−12h. The result showed that 1.1 ml/kg/h was the inflection point for UO6−12h after adjusted-multivariate analysis (Table 3), and it was selected to dichotomize UO6−12h in the following analyses. Table 3 demonstrates that when UO6−12h was ≥0.5–1.1 ml/kg/h, the risk of progression to stage 2/3 AKI by UO reduced significantly by 98% (p < 0.001) as UO6−12h increased per one unit (ml/kg/h). However, when UO6−12h was ≥1.1 ml/kg/h, as UO6−12h increased per one unit (ml/kg/h), the risk of progression reduced by 10%, which did not reach statistical significance (p = 0.780).
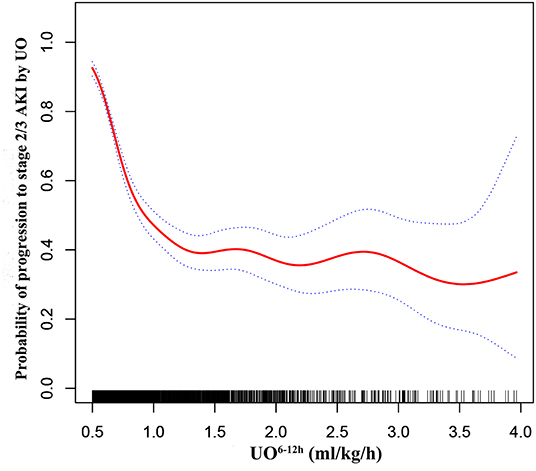
Figure 3. Adjusted association between UO6−12h and progression to stage 2/3 AKI by UO. A threshold, non-linear association between UO6−12h and progression to stage 2/3 AKI by UO was found in a generalized additive model (GAM). Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit. All adjusted for age, gender, ethnicity, hypertension, metastatic cancer, liver failure, respiratory failure, heart failure, diabetes, Vasopressor6−12h, Diuretics0−24h, MAP0−24h, SAPS II score, Scr at ICU admission, sepsis at ICU admission, fluid intake, and fluid balance. UO6−12h, urine output within 6 h after diagnosis of AKI by KDIGO UO criteria; MAP0−24h, mean arterial pressure within 24 h after diagnosis of AKI by KDIGO UO criteria; Vasopressor6−12h, vasopressor use within 6 h after diagnosis of AKI by KDIGO UO criteria; Scr, serum creatinine; KDIGO, Kidney Disease: Improving Global Outcomes.
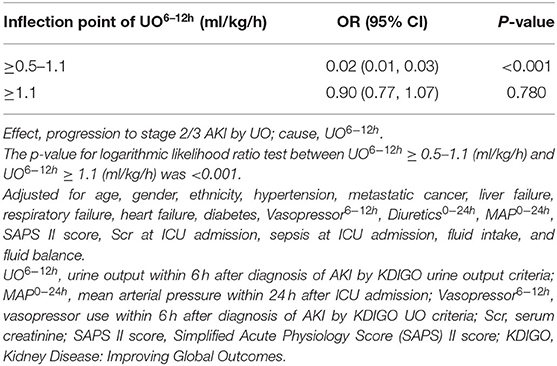
Table 3. Threshold effect analysis of UO6−12h and progression to stage 2/3 AKI by UO using piecewise linear regression.
In addition, patients with UO6−12h ≥1.1 ml/kg/h were prone to have significantly lower rate of receiving any dialysis 24 h after ICU admission (1.14 vs. 2.77%, p = 0.008), shorter length of ICU stay (3.82 vs. 4.17 days, p < 0.001), shorter length of hospital stay (9.28 vs. 10.43 days, p < 0.001), and lower 30-day mortality since ICU admission (11.05 vs. 18.42%, p < 0.001).
Sensitivity analyses showed that UO6−12h was a significantly independent factor for progression to severer AKI stage by different definitions (Supplementary Table 1). In addition, subgroup analyses showed that the significant impact of UO6−12h did not differ among subgroups based on heart failure, sepsis, SAPS II score (<38.95/≥38.95), and sepsis at ICU admission (data not shown).
In this retrospective study, we demonstrated that early-phase UO6−12h was a significantly independent variable associated with progression of AKI. As the early-phase UO6−12h increased per one unit (ml/kg/h), the risk of progression to stage 2/3 AKI by UO reduced significantly by 60% (p < 0.001). More importantly, we found that there was a non-linear relationship between early-phase UO6−12h and progression of AKI. Early-phase UO6−12h of 1.1 ml/kg/h was the inflection point, above which progression risk significantly leveled off. Those who had early-phase UO6−12h ≥ 1.1 ml/kg/h had significant shorter length of ICU stay and hospital stay, as well as lower 30-day mortality since ICU admission. These findings could provide information for guiding initial therapy among early-stage AKI patients in the ICU.
In our study, we focused on early-stage AKI defined by oliguria, because UO is an easy-to-find marker. In addition, previous studies demonstrated that outcomes were worse when patients had AKI by KDIGO UO criteria, even though the Scr level was not elevated (7, 17), indicating that UO is more sensitive than Scr for adverse outcomes. Furthermore, since the “therapeutic window” becomes narrower as kidney injury evolves, a continuous UO monitoring enables clinicians to implement early treatment. Hence, UO is a helpful and critical indicator for early diagnosis and timely treatment for AKI.
Previously, a number of therapies have been reported to attenuate kidney injury among early-stage AKI patients (2, 9, 18–20). However, there is no study focusing on the relationship between UO and progression of AKI in the early phase AKI. In order to carry out more effective treatments, it is necessary for clinicians to understand the relationship between UO and progression of AKI in the early phase of AKI. In this study, the smoothing plot showed that there was a non-linear relationship between early-phase UO6−12h and progression of AKI. In addition, early-phase UO6−12h of 1.1 ml/kg/h was selected as the inflection point by piecewise linear regression, above which progression risk significantly leveled off. This result indicated that 1.1 ml/kg/h could be a reference value of UO6−12h for guiding initial therapies among early-stage AKI patients. In addition, if a patient's UO6−12h did not reach 1.1 ml/kg/h, it could remind clinicians to pay more attention to the progression risk and implement more aggressive treatment to avoid progression in the following period.
Sepsis is the leading cause of AKI in the ICU, and septic AKI is associated with poorer outcomes (21). Besides, the most recent Surviving Sepsis Campaign guideline does not mention a UO improvement goal, indicating that the optimal UO recovery value may vary in different sepsis patients. In this study, UO6−12h ≥ 1.1 ml/kg/h had significant impacts on progression to stage 2/3 AKI by UO for sepsis group and non-sepsis group (data not shown). Additionally, the adjusted OR for UO6−12h was significantly lower (0.25 vs. 0.35, p = 0.027) for the sepsis group, indicating that septic AKI patients could have more benefits from the early-phase UO improvement (≥1.1 ml/kg/h) against progression to severer-stage AKI, compared with those without sepsis.
Our study has several strengths. Firstly, the data were extracted from a large critical care database MIMIC-III. Secondly, we employed smoothing plot to directly observe the relationship between UO and AKI progression and to find out the inflection point with clinical implication. Thirdly, our results were robust after subgroup analyses and sensitivity analyses.
There are also some limitations in our study. Firstly, this study is a retrospective study. It is necessary to carry out prospective study to verify the results. Secondly, since the critical patients' conditions are complex, the results could still be confounded by unknown variables, although the confounding factors have been adjusted by multivariate analyses in the study. Thirdly, since there is a lack of information for urinary catheter in MIMIC-III, the urinary catheter type was not included in this study. This could cause bias for the amount of UO collection, because using Foley catheter is more precise than the ordinary catheter. Fourthly, the fluid balance (ml) in our study was defined as the amount of fluid intake (ml) minus the amount of UO (ml) during the 6-h period, since other fluid losses during 6-h period were excluded due to their high frequency of missing data. However, Table 2 shows that neither fluid intake nor fluid balance was independently associated with the progression to stage 2/3 AKI by UO. In addition, we have performed multivariate analysis without adjusting for fluid intake and fluid balance; the main results of our study (i.e., inflection point of UO6−12h was 1.1 ml/kg/h) remained robust (data not shown). These results indicated that fluid intake and fluid balance did not affect the main results of our study.
Among early-stage AKI patients in critical care, early-phase UO6−12h was a significantly independent variable associated with progression of AKI, and there was a non-linear relationship between early-phase UO6−12h and progression of AKI. Early-phase UO6−12h of 1.1 ml/kg/h was the inflection point above which progression risk significantly leveled off; and it could be a reference value for guiding initial therapy among early-stage AKI patients, pending further prospective study.
Requests to access the datasets should be directed to Y2FvbWhAbWFpbC5zeXN1LmVkdS5jbg==.
The study was an analysis of a third-party anonymized publicly available database with pre-existing institutional review board (IRB) approval. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HH designed the study, extracted and analyzed the data, and wrote the first draft of the manuscript. XB reviewed all statistical analyses and critically revised the manuscript. FJ interpreted the data and critically revised the manuscript. HX critically revised the manuscript. YF supervised the analysis of the data and critically revised the manuscript. MC supervised the analysis of the data, critically revised the manuscript, and offered administrative support. All authors have read and approved the final manuscript.
This work was supported by the Guangzhou Science and Technology Project (No. 202002020002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.711717/full#supplementary-material
UO6−12h, urine output within 6 h after diagnosis of stage 1 AKI by KDIGO UO criteria; MAP0−24h, mean arterial pressure within 24 h after diagnosis of AKI by KDIGO UO criteria; Vasopressor6−12h, vasopressor use within 6 h after diagnosis of AKI by KDIGO UO criteria; Scr, serum creatinine; AKI, acute kidney injury; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes.
1. Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. (2015) 41:1411-23. doi: 10.1007/s00134-015-3934-7
2. Himmelfarb J, Joannidis M, Molitoris B, Schietz M, Okusa MD, Warnock D, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. (2008) 3:962-7. doi: 10.2215/CJN.04971107
3. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: a cohort analysis. JAMA. (1996) 275:1489-94. doi: 10.1001/jama.1996.03530430033035
4. John A, Kellum Norbert, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. (2013) 17:204. doi: 10.1186/cc11454
5. Jin K, Murugan R, Sileanu FE, Foldes E, Priyanka P, Clermont G, et al. Intensive monitoring of urine output is associated with increased detection of acute kidney injury and improved outcomes. Chest. (2017) 152:972–9. doi: 10.1016/j.chest.2017.05.011
6. Izawa J, Kitamura T, Iwami T, Uchino S, Takinami M, Kellum JA, et al. Early-phase cumulative hypotension duration and severe-stage progression in oliguric acute kidney injury with and without sepsis: an observational study. Crit Care. (2016) 20:405. doi: 10.1186/s13054-016-1564-2
7. Kaddourah A, Basu RK, Goldstein SL, Sutherland SM, Assessment of Worldwide Acute Kidney Injury RAaEI. Oliguria and acute kidney injury in critically ill children: implications for diagnosis and outcomes. Pediatr Crit Care Med. (2019) 20:332–9. doi: 10.1097/PCC.0000000000001866
8. Levy MM, Macias WL, Vincent JL, Russell JA, Williams MDJCCM. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. (2005) 33:2194–201. doi: 10.1097/01.CCM.0000182798.39709.84
9. Miller TE, Bunke M, Nisbet P, Brudney CSJPM. Fluid resuscitation practice patterns in intensive care units of the USA: a cross-sectional survey of critical care physicians. Perioper Med. (2016) 5:15. doi: 10.1186/s13741-016-0035-2
10. Raimundo M, Crichton S, Martin JR, Syed Y, Varrier M, Wyncoll D, et al. Increased fluid administration after early acute kidney injury is associated with less renal recovery. Shock. (2015) 44:431–7. doi: 10.1097/SHK.0000000000000453
11. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. (2017) 45:486–552. doi: 10.1097/CCM.0000000000002255
12. Dellinger R, Levy M, Rhodes A, Annane D, Gerlach H, Opal S, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. (2013) 39:165–228.13. doi: 10.1007/s00134-012-2769-8
13. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
14. Pincus D, Ravi B, Wasserstein D, Huang A, Paterson JM, Nathens AB, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. (2017) 318:1994–2003. doi: 10.1001/jama.2017.17606
15. Chen C, Dai JL. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. (2018) 17:130. doi: 10.1186/s12944-018-0776-7
16. Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. (2016) 4:30. doi: 10.3978/j.issn.2305-5839.2015.12.63
17. Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. (2015) 2015:2231–8. doi: 10.1681/ASN.2014070724
18. Rivers EP. Fluid-management strategies in acute lung injury–liberal, conservative, or both? N Engl J Med. (2006) 354:2598–600. doi: 10.1056/NEJMe068105
19. Zhang J, Crichton S, Dixon A, Seylanova N, Peng Z, Ostermann MJCc. Cumulative fluid accumulation is associated with the development of acute kidney injury and non-recovery of renal function: a retrospective analysis. Crit Care. (2019) 23:392. doi: 10.1186/s13054-019-2673-5
20. Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med. (2015) 41:1529–37. doi: 10.1007/s00134-015-3850-x
21. Pereira M, Rodrigues N, Godinho I, Gameiro J, Neves M, Gouveia J, et al. Acute kidney injury in patients with severe sepsis or septic shock: a comparison between the 'risk, injury, failure, loss of kidney function, end-stage kidney disease' (RIFLE), acute kidney injury network (AKIN) and kidney disease: improving global outcomes (KDIGO) classifications. Clin Kidney J. (2017) 10:332–40. doi: 10.1093/ckj/sfw107
Keywords: acute kidney injury, intensive care unit, oliguria, urine output, sepsis
Citation: Huang H, Bai X, Ji F, Xu H, Fu Y and Cao M (2021) Early-Phase Urine Output and Severe-Stage Progression of Oliguric Acute Kidney Injury in Critical Care. Front. Med. 8:711717. doi: 10.3389/fmed.2021.711717
Received: 19 May 2021; Accepted: 19 July 2021;
Published: 11 August 2021.
Edited by:
Marcelo Arruda Nakazone, Faculdade de Medicina de São José do Rio Preto, BrazilReviewed by:
Vojko Kanic, Maribor University Medical Centre, SloveniaCopyright © 2021 Huang, Bai, Ji, Xu, Fu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanni Fu, ZnV5YW5uaUBtYWlsLnN5c3UuZWR1LmNu; Minghui Cao, Y2FvbWhAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.