
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 18 November 2021
Sec. Ophthalmology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.710595
 Alan G. Palestine1*
Alan G. Palestine1* Brandie D. Wagner1,2
Brandie D. Wagner1,2 Jennifer L. Patnaik1
Jennifer L. Patnaik1 Rebecca Baldermann3
Rebecca Baldermann3 Marc T. Mathias1
Marc T. Mathias1 Naresh Mandava1
Naresh Mandava1 Anne M. Lynch1 for the University of Colorado Retina Research Group
Anne M. Lynch1 for the University of Colorado Retina Research GroupPurpose: To determine the relationship between plasma concentrations of the C-C chemokines CCL2, CCL3, CCL4, and CCL5 and intermediate age-related macular degeneration (iAMD) patients compared with control inidividuals to further define the inflammatory pathways associated with age-related macular degeneration.
Methods: The concentrations of CCL2, CCL3, CCL4, and CCL5 were measured using multiplex assays in plasma collected from 210 patients with iAMD and 102 control individuals with no macular degeneration as defined by multi-modal imaging. Non-inflammatory data included in the analysis were: age, sex, family history of AMD, history of smoking, body mass index, presence of reticular pseudo-drusen and cardiovascular disease. Median concentrations as well as a cutoff value for each chemokine were compared between the two groups.
Results: The median concentrations of CCL2 and CCL4 did not differ between control and iAMD groups, however, CCL2 was elevated in iAMD when a cutoff comparison was used (p < 0.05). Median CCL3 and CCL5 concentrations were significantly decreased in the macular degeneration group compared with controls (p < 0.001) as well as when a cutoff value comparison was used. CCL3 and CCL5 were negatively correlated in cases and positively correlated in controls.
Conclusions: Plasma CCL3 and CCL5 concentrations were significantly decreased and CCL2 concentrations were increased in patients with iAMD compared with controls, suggesting a role for C-C chemokines in the systemic inflammatory processes associated with disease development.
Inflammation mediated by the innate immune system has a prominent role in the pathophysiology of age-related macular degeneration (AMD) and has been identified as a driving and potentially modifiable factor in the progression of this ocular disease (1). AMD is a progressive degenerative disease of the aging retina which accounts for significant visual loss in older patients. The early (eAMD) and intermediate (iAMD) stages of AMD are characterized by the presence of drusen and the development of pigmentary alterations in the retinal pigment epithelium (RPE) whereas advanced AMD can be divided into neovascular AMD (NVAMD) and geographic atrophy (GA) (2). It is the advanced forms of this disease that lead to visual loss, but intervention while the patient has iAMD has the potential to prevent future visual damage. Furthermore, the risk of developing AMD is associated with a number of systemic conditions including cardiovascular disease, obesity and smoking and these conditions are associated with systemic inflammatory markers (3). It is therefore important to determine how inflammation affects the risk of iAMD development and which inflammatory pathways may be involved.
Much of the emphasis of the role of inflammation in AMD has been directed toward the complement system, which is a pathway of soluble mediators involved in both cellular damage and chemotaxis. Specifically, mutations in complement factor H (CFH) have been shown to increase the risk of both AMD development and progression by increasing activation of the alternative pathway of complement (4). The most common variant in CFH is the Y402H mutation, however alterations in plasma complement levels in AMD are not correlated with the Y402H CFH mutation (5). The primary mediator of damage by complement activation has focused on the generation of C3b and the eventual production of the C5b-9 membrane attack complex (MAC) which can directly damage cells. Less emphasis has been placed on studying the cellular components of the innate immune system and the cytokines which regulate these cells, even though C3a and C5a are potent chemotactic factors which are increased during complement activation. We have previously shown that C3a and C5a are elevated in the plasma of patients with iAMD compared to controls (6) and hence chemotaxis of the cellular components of the innate immune system may also be important in AMD pathogenesis.
The C-C chemokines are additional soluble protein mediators of chemotaxis for the innate immune system. C-C chemokines (CCLx) are 8–10 kD proteins characterized by two cysteine di-sulfide bonds and attract a variety of inflammatory cells depending on the specific chemokine and the chemokine receptors expressed on a specific cell including monocytes, NK cells, eosinophils and lymphocytes (7). CCL2 has been shown to be of possible importance in an experimental model of AMD where CCL2 deficient mice develop features of AMD including drusen-like structures at the level of the RPE, RPE disruption and photoreceptor degeneration (8). These mice show recruited macrophages and microglia and it is hypothesized that these macrophages in the absence of CCL2 cannot phagocytize C5a, leading to retinal degeneration (9). In addition, the RPE of these CCL2 deficient mice have decreased ability to remove waste products in the sub-retinal space and there is increased expression of CCL5 in CCL2 deficient mice (9). Furthermore, patients who have single nucleotide polymorphisms in CCL2 and its receptor CCR2 are at higher risk of developing advanced AMD (10). Hence, C-C chemokines may play a significant role in the development of AMD and specifically be important in development of drusen and RPE abnormalities. We studied plasma concentrations of CCL2, CCL3, CCL4, and CCL5 [also referred to as macrophage chemo-attractant protein-1 (MCP-1), macrophage inflammatory protein-1 alpha (MIP1α), macrophage inflammatory protein-1 beta (MIPβ), and RANTES, respectively] in patients with iAMD to improve our understanding of the systemic inflammatory milieu that exists in patients who are at-risk to develop AMD. We hypothesized that C-C chemokine concentrations would differ in patients with iAMD compared to controls.
We conducted this study on patients with iAMD who were recruited into the Colorado AMD registry (described in detail elsewhere) (6, 11). This registry is approved by the Colorado Multiple Institutional Review Board, utilizes a signed consent form and conforms to the Declarations of Helsinki. Patients included in the registry are recruited from the retina clinics at the UCHealth Sue Anschutz-Rodgers Eye Center. Informed consent is obtained from subjects after explanation of the nature and possible consequences of the study.
The recruitment, exclusion/inclusion criteria, and informed consent of each participant are described in detail elsewhere (6, 11). Briefly, patients are consented for: medical history review including family history, collection of serum and plasma samples for future biomarker studies, and review of multi-modal image data including fundus photography fundus autofluorescence and spectral domain ophthalmic coherence tomography (6, 11). Ocular exclusion criteria for the registry include: pan-retinal photocoagulation or anti-VEGF injections for diabetic retinopathy, branch, and central retinal vein occlusion (with severe macular damage), any active ocular inflammatory disease, or a severe decrease in visual acuity secondary to a preexisting severe retinal disease other than AMD. Control patients are cataract surgery patients over the age of 70 enrolled 1 month after cataract surgery who are confirmed to have no evidence of AMD by review of multimodal imaging.
The multimodal imaging described above is reviewed by two vitreo-retinal specialists, focusing on an examination of the anatomic macula, including the entire area between the retinal vascular arcades. All images are categorized into early, intermediate (iAMD), and advanced AMD (6, 11) using the classification described by Ferris et al. (12). Discrepancies are resolved by a third vitreo-retinal specialist. The iAMD cohort used for this study is a sub-cohort from the Colorado AMD registry. Intermediate AMD was defined as large drusen (>125 um) or pigmentary abnormalities associated with at least medium drusen (12) in either eye with no evidence of advanced AMD in either eye as assessed by fundus photography, spectral domain ophthalmic computerized tomography and fundus autofluorescence. Non-inflammatory risk factors included in the analysis were: age, sex, reported family history of AMD, body mass index, presence of reticular pseudo-drusen (RPD) on optical coherence tomography and select vascular co-morbidities.
Following phlebotomy, the Ethylenediaminetetraacetic acid tube was spun at 3,000 revolutions per minute (rpm) in a cooled (4° Celsius) centrifuge for 10 min to isolate plasma. The average time from phlebotomy to spin was low at 2.6 min ± 1.7 SD, range 0–10 min. All samples were pipetted into aliquots and stored at −80°C.
CCL2 (MCP-1), CCL3 (MIP1α), CCL4 (MIPβ), and CCL5 (RANTES) were measured at the Clinical Translational Core (CTRC) laboratory, located at Childrens' Hospital Colorado. Multiplex assays were performed using multiplex kits manufactured by R&D Systems that utilize color-coded microparticles coated with analyte-specific antibodies that are analyzed on dual-laser suspension array platforms. Specifically 150 microliters of plasma were analyzed using a magnetic bead-based multiplex method and read on the Luminex® FlexMap platform. All samples were performed in duplicate and had an acceptance threshold coefficient of variance of <15%.
Patient characteristics were compared between groups using either a chi-square test or Fisher's exact test, as appropriate, for categorical variables and a two-sample t-test for continuous variables. For CCL3, values below the lower limit of detection (LLOD) were randomly imputed using a uniform distribution between 0 and the LLOD. Analytes were log transformed (base 10 and anchored at 1). Chemokine values were compared between groups using a Wilcoxon ranked-sum test. Odds ratios (OR) and corresponding 95% confidence intervals (CI) were estimated using univariate logistic regressions. Cutoff values for the chemokines were determined using the Receiver Operator Curve (ROC) and the Youden index (13). Associations between chemokines were tested using Spearman rank-based correlation coefficients (Spr). All analyses were performed using SAS version 9.4 (The SAS Institute, Cary, NC).
Plasma samples were analyzed from 312 subjects in the registry, 210 with iAMD and 102 controls. Table 1 delineates the demographic characteristics of the iAMD and control cohorts. As expected, patients with iAMD were significantly more likely to have a family history of AMD than controls. The iAMD cases also had a higher incidence of stroke and were slightly older.
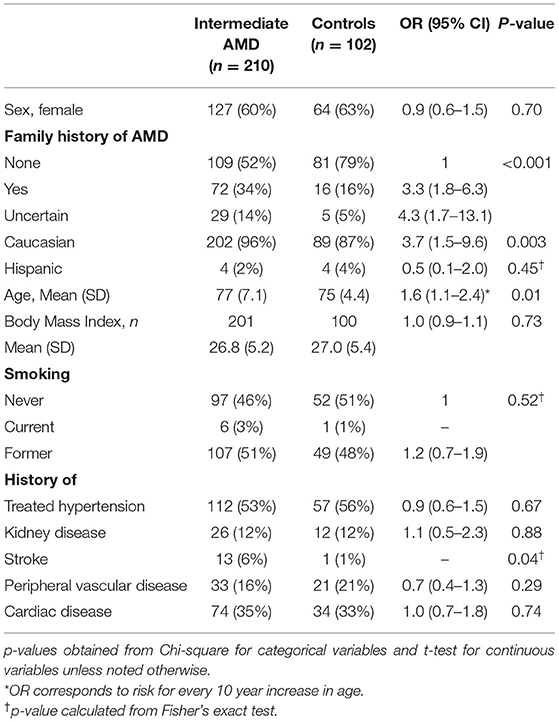
Table 1. Differences in clinical characteristics between subjects with intermediate AMD and controls.
Table 2 shows the results of the plasma C-C chemokine analysis for the four measured proteins, demonstrating significantly lower median concentrations of CCL3 and CCL5 in iAMD cases compared to controls and Figure 1 illustrates this data in boxplot form. For CCL3, 39% of controls and 44% of iAMD patients were below the LLOD for this chemokine. CCL3 and CCL5 levels were both significantly lower in iAMD cases compared to controls (p < 0.001).
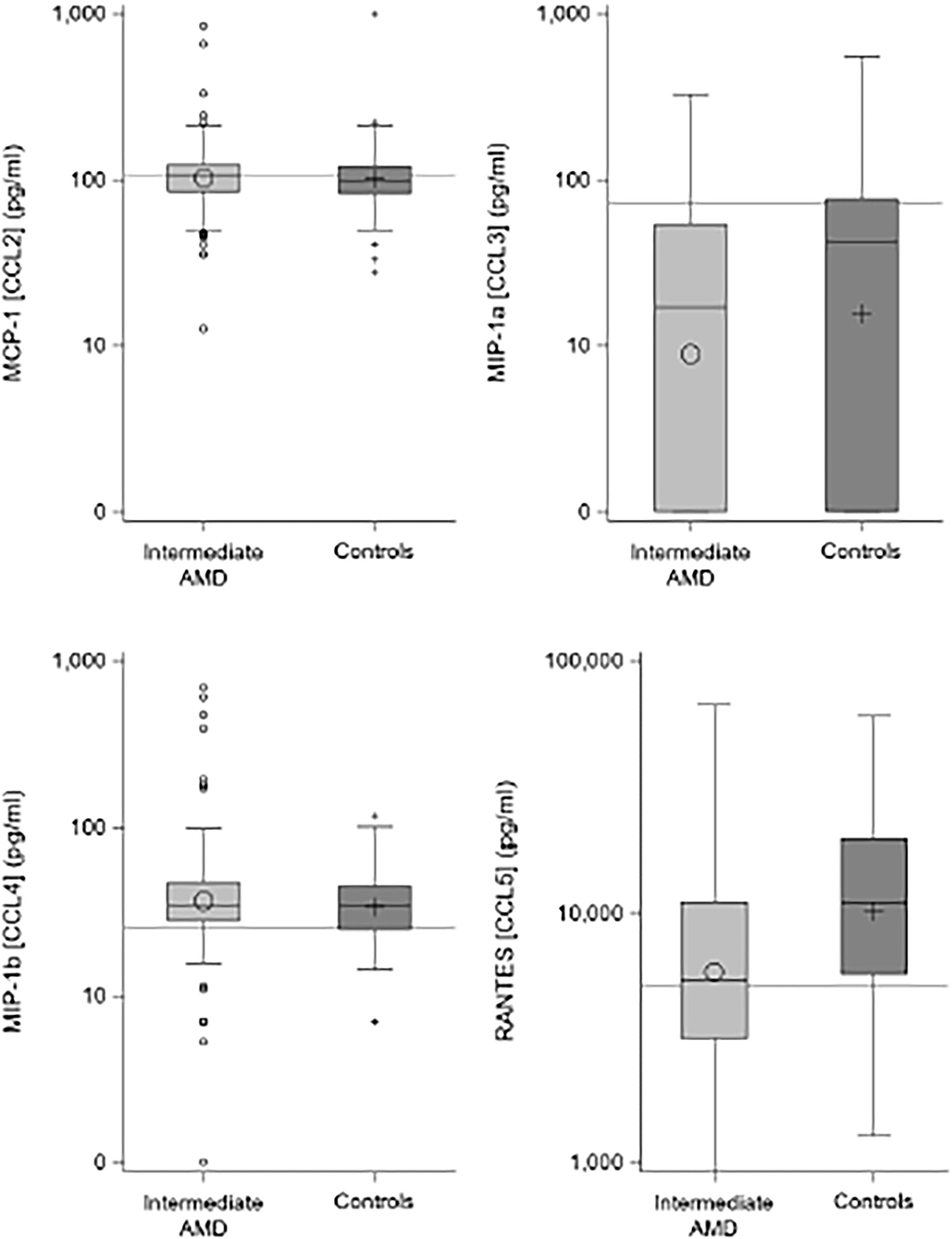
Figure 1. Boxplots comparing chemokine levels between iAMD and controls. Horizontal lines are the cutoff values from Table 3.
There were no differences seen in median CCL2 and CCL4 levels. Proposed optimal thresholds were selected for each chemokine using the Youden index (Table 3). There was again a significant difference between controls and iAMD in CCL3 and CCL5 levels (p < 0.001) using the cutoff values generated by the Youden index, but there was also a borderline significant difference in CCL2 between iAMD cases and controls (p = 0.05). The correlation between CCL3 and CCL5 was significant in both iAMD cases and controls, and the correlations differed between groups [Spr (95%CI) = −0.20 (−0.33, −0.07) in iAMD and Spr = 0.37 (0.19, 0.53)] (Figure 2).
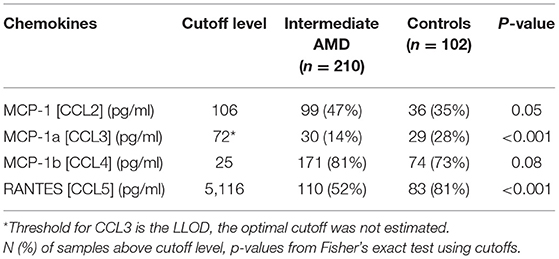
Table 3. Chemokine cutoff levels from ROC curve analysis for discriminating between cases with intermediate AMD and controls.
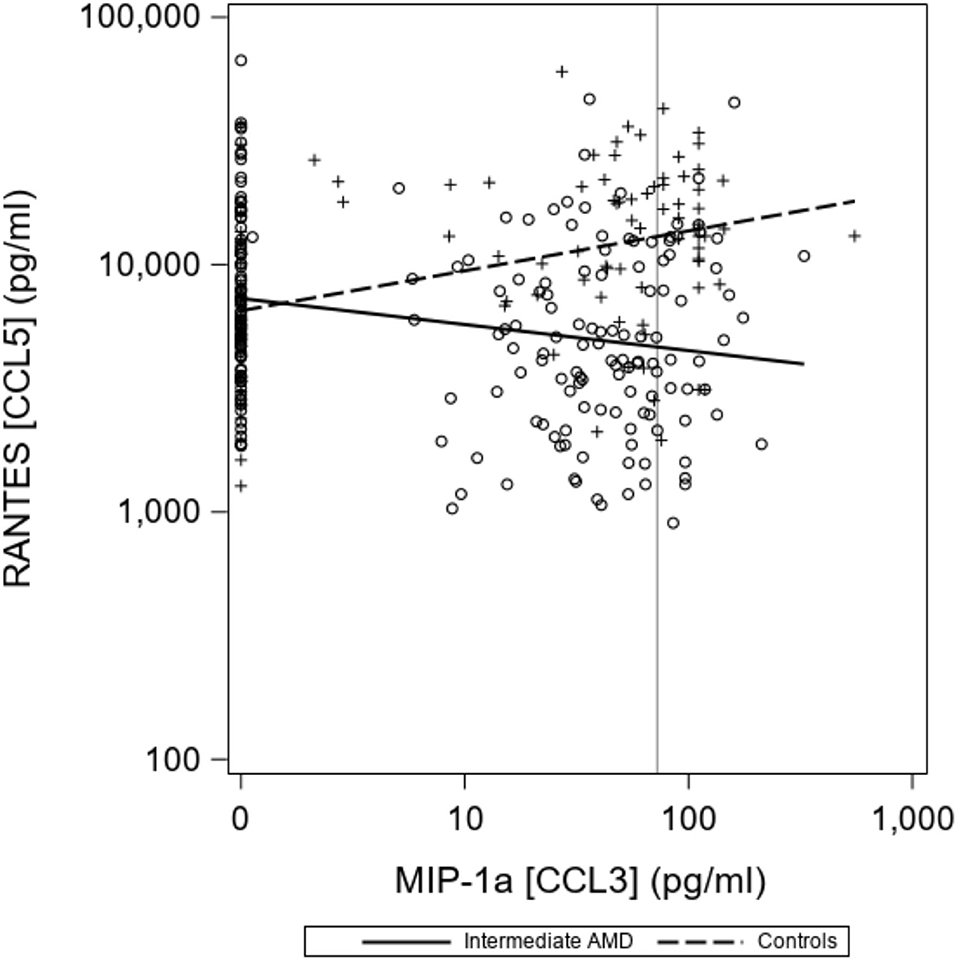
Figure 2. Correlations between chemokines CCL3 and CCL5 differ in controls and iAMD. The vertical line corresponds to the lower limit of detection (LLOD) for CCL3. Spearman Coefficient (95%CI) = −0.20 (−0.33, −0.07) in iAMD and = 0.37 (0.19, 0.53) for controls.
In the last two decades, it has become increasingly clear the inflammation as mediated by the innate immune system is important in the development of AMD. Although much of the understanding of this process has been focused on complement and specifically on CFH mutations, we believe that complement does not function in isolation in immune-mediated responses and that other soluble mediators as well as cellular components of inflammation may be important. For example, C reactive protein (CRP) has also been shown to be related to AMD development and we have shown that increased serum CRP is correlated with decreased choroidal thickness in iAMD (14). The final pathology in AMD is local damage to the RPE and outer retina, however the systemic inflammatory milieu may alter the local inflammatory responses. Obesity, smoking and cardiovascular disease are risk factors for AMD, suggesting that systemic factors can influence the local ocular environment.
Chemokines target and direct cellular migration of many of the cells of the innate immune system including macrophages, dendritic cells and natural killer cells, but also target lymphocytes of the adaptive immune system. There is evidence that alterations in C-C chemokines, and specifically CCL2, can produce many of the pathologic changes found in AMD in experimental animals (8, 9). CCL2 deficiency produces a pathology that resembles iAMD in mice, however our data did not show a strong relationship between median CCL2 concentrations and the risk for iAMD. The evidence suggesting that CCL2 deficiency produces retinal lesions that resemble AMD has been called into question because of the presence of the rd8 mutation in C57BL/6N strain as this mutation can also produce retinal degeneration (15). When we defined a cutoff value for CCL2, however, we were able to show a borderline relationship between CCL2 and the risk for iAMD in patients compared to controls. Further study of CCL2 is warranted based on the experimental model data as polymorphisms in CCL2 may be more important than absolute plasma concentration in defining the role of CCL2 as a risk factor for iAMD.
A much stronger relationship was seen for CCL3 and the risk of iAMD, but not for CCL4. These two chemokines are similar as they are also referred to as macrophage inflammatory protein alpha and beta, respectively. For example, CD4+ T cells and dendritic cells produce CCL3 and CCL4 when activated (16) and both chemokines recruit CD8+ cells (17). Both have been shown to increase with age (18) and are secreted together by monocytes and lymphocytes (19). However, in our study, there was a definite difference in the relationship of CCL3 and CCL4 to iAMD with no correlation seen for CCL4 and lower leverls of CCL3 seen in iAMD. Our study is limited, however, in that many patients with and without iAMD had plasma CCL3 levels below the LLOD in our assay. Further investigations with more sensitive assays may show a role for CCL3 in the evolution of AMD to advanced disease.
The most interesting chemokine in our study was CCL5, also known as RANTES (Regulated on Activation, Normal T cell Expressed and Secreted), which was significantly decreased in iAMD patients in our study. Decreased plasma levels of CCL5 have been associated with worse morbidity and mortality from cardiovascular disease (20), but elevated CCL5 has been associated with increased short term mortality in patients with acute coronary disease (21). Furthermore, elevated levels of CCL5 have been reported in patients with GA AMD compared to controls (22) and also in Parkinson's disease (23). Hence, alterations in CCL5 plasma concentrations may not simply be associated with aging but may have a role in regulating inflammatory processes in diseases of aging. Our finding that CCL5 is actually decreased in iAMD in contrast to the general notion that CCL5 increases with age suggests that this may be a marker of altered macrophage or T cell migration in patients with iAMD. Our findings are disparate from the recent report of elevated CCL5 in GA (22), suggesting that longitudinal studies could demonstrate changes in plasma CCL5 as AMD progresses. As noted above, CCL2 deficient mice have increased ocular expression of CCL5 (9). Hence, CCL5 may have a role both in iAMD development and in advanced AMD. There are several possible pathways to explore including the fact the CCL5 increased expression of multiple immune modulators including Il6 and TNF alpha in dendritic cells (24). The expression of the CCL5 receptor (CCR5) on CD8+ cells is negatively correlated with GA progression (22). The interaction of CCL5 with its receptor has been shown to have a role in neuroinflammatory diseases such as multiple sclerosis (25). Furthermore, increased secretion in human RPE cells of CCL5 (and CCL7) in response to cytokines such as TNF-alpha, IL1-beta, and IFN-gamma and the expression of CCR3 in choroidal blood vessels in patients with AMD suggests potential mechanism for both local and plasma CCL5 to interact with choroidal blood vessels and macrophage migration in AMD (26). Our finding of decreased plasma CCL3 and CCL5 in iAMD needs to be reconciled with the elevated levels of CCL5 reported in GA in order to speculate further on the mechansisms. Since CCL5 levels are not uniformly increased or decreased in aging diseases, the interaction of this chemokine and its receptor with ocular cells deserves further study.
The negative correlation between CCL3 and CCL5 in our iAMD patients as opposed to the positive correlation of these two chemokines in controls demonstrates a possible interaction between chemokine pathways. This has not previously been described and may point toward a unique feature of AMD pathogenesis and evolution. These correlations may represent differences in the systemic inflammatory milieu in AMD, suggesting the possibility that, while AMD is one of many degenerative aging diseases, it develops in a specific context of inflammation in the aging patient. It will be important to correlate the expression of chemokine receptors in autopsy specimens of patients with AMD to understand the significance of plasma chemokine alterations in AMD.
Limitations of our study include a cross sectional measurement of chemokine plasma levels at a single time point of enrollment in the registry in the presence of varying severity of iAMD pathology in the evolution of AMD in these patients. Longitudinal and repeated measurements of these chemokines in future studies will be of interest. Furthermore, our patients are recruited from a retina service and may not represent the spectrum of iAMD seen in the overall aging population.
The balance of up and down regulation of systemic chemokines, local chemokines, and chemokine receptors likely influences the development and progression of many neuro-degenerative diseases (27). It has been suggested that chemokine alterations prime the retina and choroid to develop degenerative aging changes (28), but it is also possible that plasma chemokines interact directly with choroidal blood vessels, altering macrophage migration into the eye. Our findings combined with observations of others suggest an important but not fully defined role of chemokines and their targeted control of inflammatory cellular traffic in the development of iAMD. The kinetics and longitudinal evolution of chemokine interactions with ocular tissue may prove to be useful in understanding the pathogenesis of AMD and provide new pathways for treatment and prevention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Colorado Multiple Institutional Review Board, University of Colorado. The patients/participants provided their written informed consent to participate in this study.
Rebecca Baldermann, Anne M. Lynch, Naresh Mandava, Marc T. Mathias, Scott C. N. Oliver, Jeffery L. Olson, Alan G. Palestine, Jennifer L. Patnaik, Paula E. Pecen, Frank S. Siringo, Jesse M. Smith, Brandie D. Wagner.
AP and AL: design, data acquisition, analysis, and manuscript writing. BW and RB: analysis and manuscript writing. JP: data acquisition, analysis, and manuscript writing. MM: data acquisition and manuscript writing. NM: design and manuscript writing. All authors contributed to the article and approved the submitted version.
This research was supported by the National Eye Institute of the National Institutes of Health under award number R01EY032456 (AL), a Research to Prevent Blindness grant to the Department of Ophthalmology, University of Colorado, the Frederic C. Hamilton Macular Degeneration Center, Sue Anschutz-Rogers Eye Center Research Fund, and by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rozing MP, Durhuus JA, Krogh Nielsen Nielsen M, Subhi Y, Kirkwood TB, Westendorp RG, et al. Age-related macular degeneration: a two-level model hypothesis. Prog Retin Eye Res. (2020) 76:100825. doi: 10.1016/j.preteyeres.2019.100825
2. Wong TY, Chakravarthy U, Klein R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. (2008) 115:116–26. doi: 10.1016/j.ophtha.2007.03.008
3. Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. (2012) 119:2282–9. doi: 10.1016/j.ophtha.2012.05.027
4. Seddon JM, George S, Rosner B, Klein ML. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Heredity. (2006) 61:157–65. doi: 10.1159/000094141
5. Silva AS, Teixeira AG, Bavia L, Lin F, Velletri R, Belfort R, Isaac L, et al. Plasma levels of complement proteins from the alternative pathway in patients with age-related macular degeneration are independent of Complement Factor H Tyr4°2His polymorphism. Mol Vis. (2012) 18:2288–99.
6. Lynch AM, Palestine AG, Wagner BD, Patnaik JL, Frazier-Abel AA, Mathias MT, et al. Complement factors and reticular pseudodrusen in intermediate age-related macular degeneration staged by multimodal imaging. BMJ Open Ophthalmol. (2020) 5:e000361. doi: 10.1136/bmjophth-2019-000361
7. Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. (1999) 10:61–86. doi: 10.1016/S1359-6101(99)00005-2
8. Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. (2007) 48:3827–36. doi: 10.1167/iovs.07-0051
9. Ross RJ, Zhou M, Shen D, Fariss RN, Ding X, Bojanowski CM, et al. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp Eye Res. (2008) 86:675–83. doi: 10.1016/j.exer.2008.01.014
10. Anand A, Sharma NK, Gupta A, Prabhakar S, Sharma SK, Singh R, et al. Single nucleotide polymorphisms in MCP-1 and its receptor are associated with the risk of age related macular degeneration. PLoS One. (2012) 7:e49905. doi: 10.1371/journal.pone.0049905
11. Lynch AM, Wagner BD, Weiss SJ, Wall KM, Palestine AG, Mathias MT, et al. Proteomic profiles in advanced age-related macular degeneration using an aptamer-based proteomic technology. Transl Vis Sci Technol. (2019) 8:14. doi: 10.1167/tvst.8.1.14
12. Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology. (2013) 120:844–51. doi: 10.1016/j.ophtha.2012.10.036
13. Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. (2006) 163:670–5. doi: 10.1093/aje/kwj063
14. Chen RC, Palestine AG, Lynch AM, Patnaik JL, Wagner BD, Mathias MT, et al. Increased systemic C-reactive protein is associated with choroidal thinning in intermediate age-related macular degeneration. Transl Vis Sci Technol. (2021) 10:7. doi: 10.1167/tvst.10.12.7
15. Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. (2012) 53:2921–7. doi: 10.1167/iovs.12-9662
16. Honey K. CCL3 and CCL4 actively recruit CD8+ T cells. Nat Rev Immunol. (2006) 6:427. doi: 10.1038/nri1862
17. Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. (2006) 440:890–5. doi: 10.1038/nature04651
18. Thorsen SU, Eising S, Mortensen HB, Skogstrand K, Pociot F, Johannesen J, et al. Systemic levels of CCL2, CCL3, CCL4 and CXCL8 differ according to age, time period and season among children newly diagnosed with type 1 diabetes and their healthy siblings. Scand J Immunol. (2014) 80:452–61. doi: 10.1111/sji.12240
19. Guan E, Wang J, Norcross MA. Identification of human macrophage inflammatory proteins 1alpha and 1beta as a native secreted heterodimer. J Biol Chem. (2001) 276:12404–9. doi: 10.1074/jbc.M006327200
20. Badacz R, Podolec J, Przewlocki T, Siedlinski M, Jozefczuk E, Oleksy H, et al. The role of chemokine CCL5/RANTES and metalloproteinase-9 as inflammatory modulators in symptomatic internal carotid artery stenosis. J Physiol Pharmacol. (2019) 70. doi: 10.26402/jpp.2019.4.06
21. de Jager SC, Bongaerts BW, Weber M, Kraaijeveld AO, Rousch M, Dimmeler S, et al. Chemokines CCL3/MIP1α, CCL5/RANTES and CCL18/PARC are independent risk predictors of short-term mortality in patients with acute coronary syndromes. PloS one. (2012) 7:e45804. doi: 10.1371/journal.pone.0045804
22. Krogh Nielsen M, Subhi Y, Molbech CR, Falk MK, Nissen MH, Sørensen TL. Chemokine profile and the alterations in CCR5-CCL5 axis in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. (2020) 61:28. doi: 10.1167/iovs.61.4.28
23. Tang P, Chong L, Li X, Liu Y, Liu P, Hou C, et al. Correlation between serum RANTES levels and the severity of Parkinson's disease. Oxid Med Cell Long. (2014) 2014:208408. doi: 10.1155/2014/208408
24. Fischer FR, Luo Y, Luo M, Santambrogio L, Dorf ME. RANTES-induced chemokine cascade in dendritic cells. J Immunol. (2001) 167:1637–43. doi: 10.4049/jimmunol.167.3.1637
25. Tomioka R, Matsui M. Biomarkers for multiple sclerosis. Intern Med. (2014) 53:361–5. doi: 10.2169/internalmedicine.53.1246
26. Nagineni CN, Kommineni VK, Ganjbaksh N, Nagineni KK, Hooks JJ, Detrick B. Inflammatory cytokines induce expression of chemokines by human retinal cells: role in chemokine receptor mediated age-related macular degeneration. Aging Dis. (2015) 6:444–55. doi: 10.14336/AD.2015.0323
27. Cardona SM, Garcia JA, Cardona AE. The fine balance of chemokines during disease: trafficking, inflammation, and homeostasis. Methods Mol Biol. (2013) 1013:1–16. doi: 10.1007/978-1-62703-426-5_1
Keywords: age-related macular degeneration, inflammation, chemokines, CCL2, CCL3, CCL4, CCL5, RANTES
Citation: Palestine AG, Wagner BD, Patnaik JL, Baldermann R, Mathias MT, Mandava N and Lynch AM (2021) Plasma C-C Chemokine Concentrations in Intermediate Age-Related Macular Degeneration. Front. Med. 8:710595. doi: 10.3389/fmed.2021.710595
Received: 19 June 2021; Accepted: 21 October 2021;
Published: 18 November 2021.
Edited by:
Masaru Takeuchi, National Defense Medical College, JapanReviewed by:
Hu Huang, University of Missouri, United StatesCopyright © 2021 Palestine, Wagner, Patnaik, Baldermann, Mathias, Mandava and Lynch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alan G. Palestine, YWxhbi5wYWxlc3RpbmVAY3VhbnNjaHV0ei5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.