
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 19 July 2021
Sec. Intensive Care Medicine and Anesthesiology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.699969
Background: Postoperative elevation of plasma creatinine is a frequent complication to major surgery. A rise by 50% fulfills the criterion for Acute Kidney Injury. We studied the relationship between concentrated urine before surgery, which is usually a sign of chronically low intake of water, and the perioperative change in plasma creatinine.
Methods: The creatinine concentration was measured in plasma and urine just before and at 6 h, 1 day, and 2 days after major abdominal surgery in a consecutive series of 181 patients. Receiver operating curve analysis was used to find the optimal cut-off to separate concentrated from diluted urine.
Results: Urine creatinine of 11.3 mmol/L before the surgery started was exceeded in one third of the patients and associated with greater increase in plasma creatinine at 6 h (median 21 vs. 10%) and at 1 day postoperatively (21 vs. 7%; P < 0.0001). Elevation of plasma creatinine of >25% occurred in 41% and 19% in those with high and low urine creatinine, respectively (P < 0.001) and an increase by >50% in 16% and 10% (P = 0.27). Patients with high urine creatinine before surgery failed to further concentrate their urine during the perioperative period, which is normally associated with intensified renal fluid conservation.
Conclusion: High urinary concentration of creatinine before surgery should be considered as a risk factor for postoperative elevation of plasma creatinine. The mechanism is probably that the renal threshold is then more easily reached.
Postoperative acute kidney injury (AKI) is a complication to major surgery that is most commonly diagnosed by measuring plasma creatinine. The consensus criteria for Stage 1 AKI are fulfilled if the increase in plasma creatinine amounts to >26.5 μmol/L within 48 h after surgery, or an increase by 50% compared to baseline, known or presumed to have occurred within the prior 7 days. Alternatively, the urine flow is below 0.5 mL/kg/h for 6 h (KDIGO criteria) (1). Chronic kidney injury occasionally ensues, and follow-up studies show an increased overall morbidity and mortality (2, 3).
The background is probably multifactorial. Factors that are statistically associated with increasing plasma creatinine include arterial hypotension (4–6), restrictive fluid therapy (3, 7) and a high body mass index (8), while opinions differ regarding the role of intraoperative oliguria (9, 10).
Plasma creatinine increases permanently when 50% of the renal function is lost, but the fact that transient elevation of plasma creatinine is common after major surgery raises the question whether other mechanisms than injury to kidney cells may be involved.
In the present study, we propose an alternative explanation to these events that does not involve injury to kidney cells. The question is why plasma creatinine increases at all, since the dilution effects of the perioperative fluid therapy would rather decrease the concentration. Specifically, we examined whether concentrated urine before surgery predisposes to elevation of plasma creatinine after major surgery. Concentrated urine implies that the urinary creatinine is high, whereby less surgery-induced renal water conservation would be required to surpass the renal threshold for concentrating creatinine.
Biomarkers of concentrated urine have been used to study dehydration in sports medicine (11–13) and include creatinine, osmolality, and urine-specific weight. These three biomarkers are frequently elevated in the general population (14), where concentrated urine reflects a chronically low daily intake of water (15, 16). All three were measured by us to study whether creatinine undergoes concentration or dilution changes during surgery that are specific to this biomarker.
Our hypothesis was that concentrated urine before surgery increases the risk of occurrence of a postoperative elevation of plasma creatinine.
Ethical approval for this study was provided by the Ethical Committee of the Canton Bern, Switzerland (KEK Bern, Project-ID 2018-01804, Chairperson Professor C. Seiler on December 3rd, 2018) and registered at ClinicalTrials.gov as NCT03788070. Written informed consent was obtained from all patients before initiation of the study. This single-center prospective observational study accorded with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations and the Declaration of Helsinki.
Our purpose was to study the influence of concentrated urine on the risk of having a postoperative elevation of plasma creatinine. Data from a consecutive series of patients at the Department of Urology of the University Hospital Bern who were undergoing major abdominal urologic surgery were used. Other research questions included fluid balance, postoperative nausea and gastrointestinal function, and will be reported elsewhere. Inclusion criteria were age >18 years, elective major urologic laparotomy or robotic assisted laparoscopy. Patients with preoperative intravenous administration of fluid and end-stage kidney disease were excluded.
No enteral bowel preparation was performed preoperatively. Pre-operative oral hydration was allowed up to 2 h before starting the anesthesia. Surgery was performed under general anesthesia, as described elsewhere (5). Fluid administration consisted of Ringer's lactate infused at a rate of 2 mL/kg/h. If hypotension was observed (mean arterial pressure <60 mmHg), norepinephrine was titrated to a maximum of 8 μg/kg/h after an initial bolus of 5–10 μg, or a 250 mL fluid bolus of Ringer's lactate was administered in cases where the patient was judged to be hypovolemic. Blood loss of up to 500 mL was replaced with an equal amount of Ringer's lactate. Packed red blood cells were transfused if the blood hemoglobin (Hb) concentration fell below 80 g/L (<100 g/L in patients with severe coronary artery disease). Additional boluses of Ringer's lactate (250 mL) were administered as a rescue medication if the mean arterial pressure was persistently low or if acidosis developed. Patients were allowed to drink clear fluids while in the intermediate care unit. No diuretics were administered during surgery or during the follow-up period.
Urine and plasma samples were collected the day before surgery, directly after induction of anesthesia, at 6 h postoperatively, and at 6 a.m. of the 1st and 2nd postoperative days. Whole blood was analyzed for the Hb concentration and plasma/serum/urine for creatinine and osmolality.
Measurements of osmolality were performed on a Station 6060 TT instrument (Array Global Business Inc., Japan) and all other biochemical analyses on a Cobas 8000/ISE Modul system (Roche Diagnostics, Basel, Switzerland) in the certified clinical chemistry laboratory at University Hospital Bern. Urine specific gravity was determined with a digital photometric refractometer in steps of 0.005 based on the reflection coefficient of light directed to strips dipped in urine (Uricon-Ne, Atago CO, LTD, Tokyo, Japan).
The results are presented as the median and 25–75th percentile range. Receiver operating curves (ROC) were used to search for cut-off values in urinary creatinine that distinguished patients who showed a postoperative rise in plasma creatinine of 25 and 50% from the others. Continuous data measured in these groups were compared with the Mann-Whitney's test and percentages by using contingency table analysis. Changes of continuous data were evaluated by the Wilcoxon's matched pair-test. P < 0.05 was considered statistically significant.
In total, 193 patients were recruited between February 2019 and March 2020. One withdrew consent, five patients dropped out, and complete data were missing from six patients, making 181 evaluable data sets. The original plan was to include about 200 patients, but the study had to be terminated due to the Covid-19 crisis.
The presentation is focused on postoperative elevation plasma creatinine as a complication of the surgery and investigates the relationship of this elevation to concentrated urine before the operation as the index of low habitual intake of water or dehydration. Three biomarkers of concentrated urine (osmolality, creatinine and specific weight) were measured, but we used urine creatinine as the key variable because this biomarker is used to diagnose AKI.
ROC curves were used to find if any preoperative urine creatinine concentration could distinguish between patients who would later have an increase in plasma creatinine of >25 and >50% from before surgery to the 1st postoperative day. The optimal cut-off was 11.3 μmol/L, and this value was used for further evaluations (Figures 1A,B).
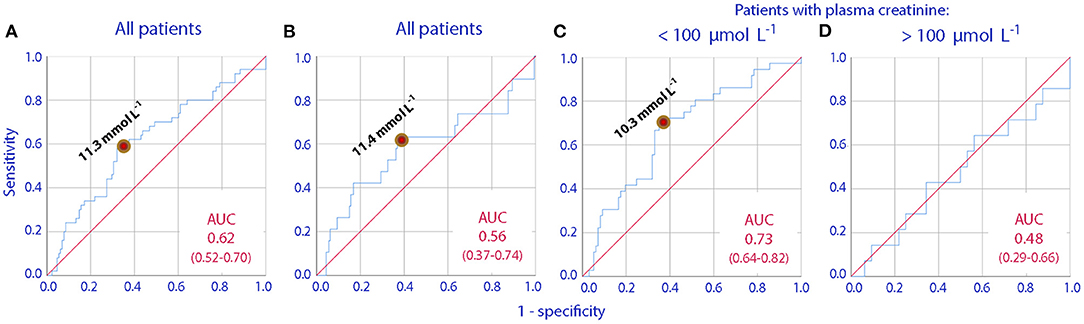
Figure 1. Receiver operating curves (ROC) showing how increasing urinary creatinine concentrations can predict a postoperative increase in plasma creatinine by (A) >25% in all patients, (B) >50% in all patients, (C,D) >25% in patients who have plasma creatinine concentrations lower or higher than 100 μmol/L before the initiation of surgery. The area under the curve (AUC) and its 95% confidence interval are given in each subplot.
Table 1 shows demographic and basic laboratory data separately for patients with low (<11.3 μmol/L) vs. high (≥11.3 μmol/L) urine creatinine before surgery.
Patients with high urine creatinine had a higher body weight (mean, 89 vs. 78 kg, P < 0.001) and were more often smokers (64 vs. 20%; P < 0.01).
The laboratory data show that those with high urine creatinine also had higher urine specific weight, 1.015 vs. 1.012, P < 0.001) and higher urine osmolality (650 vs. 512 mosmol/kg, P < 0.001) than the other patients. By contrast, serum osmolality was the same regardless of urine osmolality.
Overall, 50 patients (28%) had an increase in plasma creatinine by >25% and 22 patients (12%) an increase in plasma creatinine exceeding 50%, whereby the latter fulfilled the criterion for postoperative AKI.
The degree of the elevation differed depending on the urine creatinine concentration measured before the operation. Patients with high (≥11.3 μmol/L) urine creatinine before surgery showed a median rise in plasma creatinine of 21%, both at 6 h after surgery and on the next day, while those with low urine creatinine <11.3 μmol/L had a much smaller rise (Table 2, middle).
Table 3 shows the preoperative urine and plasma creatinine concentrations in terms of the increasing degrees of perioperative elevations of plasma creatinine.
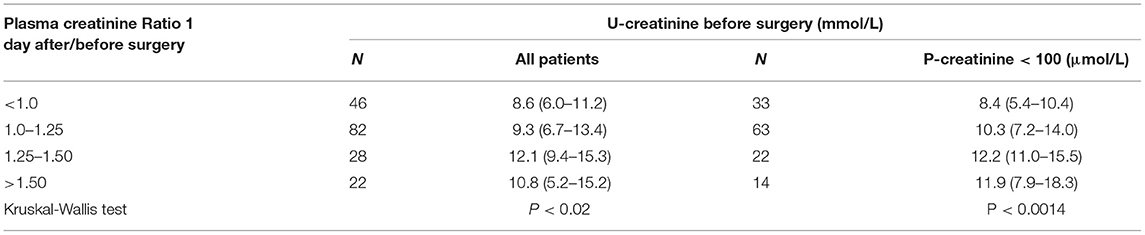
Table 3. The preoperative urine concentrations of creatinine for increasing degrees of perioperative elevations of plasma creatinine.
Patients with low urine creatinine (<11.3 μmol/L) before surgery concentrated their urine during the surgery; all three biomarkers of urine dilution increased (creatinine, osmolality, and urine-specific weight).
By contrast, no similar concentration process occurred among the patients with a high urine creatinine prior to surgery (Table 2, bottom).
The association between postoperative elevations of plasma creatinine and concentrated urine was valid only for those who had a normal plasma creatinine preoperatively (cf. Figures 1C,D).
The overall statistics did not change upon removal of the 49 patients with plasma creatinine >100 μmol/l before surgery started.
An exploratory analysis was also made after excluding the 56 patients in whom the surgery in any way involved the kidneys. The main result remained; the increase in plasma creatinine on the 1st postoperative day increased by 7 (1–15)% in those with a low urine creatinine as compared to 15 (4–24)% in those with high urine creatinine before surgery (P < 0.04).
The over-arching goal of our research is to increase knowledge about why elevation of plasma creatinine is common in the perioperative period. Approaches should be developed that can separate innocuous elevations from kidney injury of relevance to long-term prognosis. In the present study, we suggest a new mechanism that might promote elevation of plasma creatinine without necessarily involving harm to the kidney cells. The evidence can be summarized in the following four points.
1. Concentrated urine before initiation of surgery was associated with a 2–3 times greater increase in plasma creatinine during the postoperative follow-up after major surgery. This is a novel finding.
2. The preoperative urine osmolality and the urine specific weight values confirmed that patients with high urine creatinine had more intense renal water conservation before surgery than those with urinary creatinine of <11.3 μmol/L, which was our criterion for regarding the urine as diluted or concentrated. Their Hb level was also 7% higher in the high urine creatinine patients, which is compatible with kidney retention of fluid due to a low daily intake of water (16).
3. The fact that plasma creatinine had increased even by 6 h after the end of surgery further suggests that the changes in urine variables were occurring during the actual operation.
4. Patients with high a urinary creatinine before surgery did not further concentrate of their urine during perioperative period. By contrast, those who had low urine creatinine showed intensified renal water concentration.
The urine normally undergoes a gradual concentration process during anesthesia and surgery, in particular when the urine flow rate falls below 1 mL/min (17). The reason seems to be that the low arterial pressure induced by general anesthesia inhibits the diuretic response to infused crystalloid fluid (18).
In the present study, a concentration process was evident only in patients with low urine creatinine before surgery. Those patients showed the expected pattern of changes, which consist of increases of the urine specific weight, osmolality and creatinine concentration. By contrast, very small increases in urine osmolality and urine specific weight were recorded in the patients who entered the surgery with concentrated urine, and their urine creatinine even decreased by ~20%. Thus, the kidneys concentrated the urine poorly and, in particular, could not further concentrate creatinine. This combination had a statistically significant relationship with the fact that the urine was already concentrated before the surgery started.
In everyday life, the kidneys concentrate the urine by a factor of ~100, which is fairly close to the maximum capacity even for the normal kidney. How much further the kidneys are able to concentrate the urine is likely to depend on the individual, but the renal threshold is probably 15–25 mmol/L. The fluid retention that occurs during surgery could then be sufficient to exceed the maximum capacity to excrete creatinine, whereby creatinine accumulates in the blood. Patients with preoperatively concentrated urine may even reach the renal threshold more easily if the fluid administration program is overly restrictive; this is also a known downside of restrictive fluid therapy (3, 7).
The perioperative elevations of plasma creatinine we recorded are sufficient to make the diagnosis of AKI in 12% of the patients (1). The alternative KDIGO criterion, based on an absolute instead of a relative increase of plasma creatinine, identified virtually the same patients. Concentrated urine before surgery is a relevant factor for these elevations, although the influence seems to be moderately strong or unimportant in patients with already impaired kidney function. Inter-individual differences in the renal threshold for creatinine excretion probably confound its statistical significance. Therefore, concentrated urine and a slightly reduced renal tubular concentrating capacity before surgery may be factors that contribute to the frequent transient and harmless elevation of plasma creatinine that frequently occurs after surgery. This is a hypothesis that needs to be validated in clinical studies, as the demonstrated statistical associations we present do not infer causal relationships. However, a challenging aspect is that the hypothesis gives no reason to believe that damage to kidney cells occurs during the perioperative period in patients with transient elevation of plasma creatinine.
A retrospective analysis of 27,860 colorectal surgery patients found an association between the preoperative blood urea nitrogen to creatinine ratio and AKI (19). Ellis et al. measured urine conductivity and found an increased risk of AKI after nephrectomy (20). Small prospective studies have suggested that concentrated urine measured as high urine osmolality, specific weight and urine creatinine may increase the risk of plasma creatinine elevation after surgery (21, 22). The largest study comprised 88 patients with abdominal cancer and reported that the 15 patients with a minor postoperative increase in plasma creatinine also had a higher body weight and more concentrated urine than the others. They had lower urine flow but a higher creatinine clearance than the other patients (mean 155 vs. 80 mL/min) during the 1st postoperative h (21). The fact that the kidneys seem to work at the peak of their ability to excrete the accumulated creatinine is compatible with the view that moderately large elevations of plasma creatinine after surgery do not necessarily imply that kidney cells have been injured.
There is very little mechanistic knowledge the serves to explain why plasma creatinine frequently becomes elevated in the perioperative setting. Statistical links have usually been found only to personal traits. In the present study, a high body mass index and smoking were identified as such personal risk factors.
The main part of our study focuses on short-term perioperative changes in filtering capacity of the kidneys. The focus was on why plasma creatinine increases rather than on the AKI diagnosis per se. Limitations include that health conditions that may cause long-lasting elevations of the urine creatinine concentration were not studied. Moreover, specific biomarkers of kidney injury were not measured. Several types of urologic operations were included, although our exploratory analysis suggested the contribution of urological operations did not affect the main conclusions. No long-term follow-up of the patients was made.
With regard to the preoperative situation, concentrating the urine is the body's “first line” defense to maintain the body fluid volumes when the daily intake of water is low. Volunteers with concentrated urine have stable hemodynamics when performing the “passive leg raising test” (16) and hypovolemia of clinical importance is likely to develop only very late in a chronic dehydration process.
We cannot rule out that hypovolemia during the surgery elevated plasma creatinine, but we find it unlikely due to the meticulous protocol for the clinical management of hemodynamics and fluid balance used. Invasive arterial pressure was monitored, and all patients underwent the same standard procedure with additional fluid administration and vasopressor treatment to combat hypovolemia and hypotension whenever needed. Moreover, hypovolemia and fluid deficiency would stimulate release of vasopressin that concentrates the urine, but further concentration of the urine did not occur during the perioperative period in the patients who had urinary creatinine ≥11.3 mmol/L before surgery started.
Our original study plan was to use the urine specific weight measured on the day before the surgery as the reference. However, recent research shows that a spot sample taken mid-day is an uncertain measure of the fluid balance; the optimal approach is to sample urine in the early morning or else to make a 24 h collection (6, 23). Therefore, we decided to use the urine sample collected in the early morning on the day of surgery as the reference. Urine creatinine was chosen as the key biomarker of interest because this molecule is used to diagnose AKI.
A high urine concentration of creatinine (≥11.3 mmol/L) before surgery increased the incidence of a postoperative rise in plasma creatinine of 25% or more from 19% to 41%. Preoperative concentrated urine should therefore be considered a risk factor for postoperative elevation of plasma creatinine.
The original contributions generated for this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethical Committee of the Canton Bern, Switzerland (KEK Bern, Project-ID 2018-01804, Chairperson Professor C. Seiler on December 3rd, 2018). The patients/participants provided their written informed consent to participate in this study.
PW planned the study after a concept designed jointly with RH. DE, LL, and PW collected the data. RH made the calculations and wrote the manuscript, which was approved by all authors. All authors contributed to the article and approved the submitted version.
This study was supported by internal institutional research funds of the Department of Anaesthesiology and Pain Medicine, University Hospital Bern, Bern, Switzerland; from the Burgergemeinde Bern (Reference number 2019-1055) and from the Stiftung für die Forschung in Anästhesiologie und Intensivmedizin (Foundation for Research in Anesthesiology and Intensive Care, 3010 Bern, [grant number 31/2019]). None of the three agencies had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We were grateful to the staff of the Department of Urology, Inselspital, University Hospital Bern, Switzerland, for their help with the collection of data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.699969/full#supplementary-material
AKI, acute kidney injury; KDIGO, Kidney Disease Improving Global Outcome; ROC, Receiver Operating Characteristics.
1. Zarbock A, Koyner JL, Hoste EAJ, Kellum JA. Update on perioperative acute kidney injury. Anesth Analg. (2018) 127:1236–45. doi: 10.1213/ANE.0000000000003741
2. Bernardi MH, Ristl R, Neugebauer T, Hiesmayr MJ, Druml W, Lassnigg A. Very early changes in serum creatinine are associated with 30-day mortality after cardiac surgery. Eur J Anaesthesiol. (2020) 37:898–907. doi: 10.1097/EJA.0000000000001214
3. Furrer MA, Schneider MP, Löffel LM, Buckhard FC, Wuethrich PY. Impact of intra-operative fluid and noradrenaline administration on early postoperative renal function after cystectomy and urinary diversion: a retrospective observational cohort study. Eur J Anaesthesiol. (2018) 35:41–9. doi: 10.1097/EJA.0000000000000808
4. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. (2015) 23:515–23. doi: 10.1097/ALN.0000000000000765
5. Salmasi V, Maheshwari K, Yang D, Mascha EJ, Singh A, Sessler DI, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. (2017) 126:47–65. doi: 10.1097/ALN.0000000000001432
6. Löffel LM, Bachmann KF, Furrer MA, Wuethrich PY. Impact of intraoperative hypotension on early postoperative acute kidney injury in cystectomy patients - a retrospective cohort analysis. J Clin Anesth. (2020) 66:32615512. doi: 10.1016/j.jclinane.2020.109906
7. Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. New Engl J Med. (2018) 378:2263–74. doi: 10.1056/NEJMoa1801601
8. Argalious MY, Makarova N, Leone A, Cywinski J, Faraq E. Association of body mass index and postoperative acute kidney injury in patients undergoing laparoscopic surgery. Ochsner J. (2017) 17:224–32.
9. Shiba A, Uchino S, Fujii T, Takinami M, Uezono S. Association between intraoperative oliguria and acute kidney injury after major noncardiac surgery. Anesth Analg. (2018) 127:1229–35. doi: 10.1213/ANE.0000000000003576
10. Inácio R, Gameiro J, Amaro S, Duarte M. Intraoperative oliguria does not predict postoperative acute kidney injury in major abdominal surgery: a cohort analysis. Braz J Nephrol. (2021) 43:9–19. doi: 10.1590/2175-8239-jbn-2019-0244
11. Casa DJ, Armstrong LE, Hillman SK, Montain SJ, Reiff RV, Rich BS, et al. National athletic trainers' association position statement: fluid replacement for athletes. J Athl Train. (2000) 35:212–24.
12. Popowski LA, Oppliger RA, Patrick Lambert GP, Johnson RF, Johnson A, Gisolf CV. Blood and urinary measures of hydration status during progressive acute dehydration. Med Sci Sports Exerc. (2001) 33:747–53. doi: 10.1097/00005768-200105000-00011
13. Hahn RG, Waldréus N. An aggregate urine analysis tool to detect acute dehydration. Int J Sport Nutr Exerc Metab. (2013) 23:303–11. doi: 10.1123/ijsnem.23.4.303
14. Hahn RG, Grankvist N, Krizhanovskii C. Urinary analysis of fluid retention in the general population: a cross-sectional study. PLoS ONE. (2016) 11:e0164152. doi: 10.1371/journal.pone.0164152
15. Johnson EC, Munoz CX, Le Bellego L, Klein A, Casa DJ, Maresh CM, et al. Markers of the hydration process during volume modification with habitual high or low daily fluid intakes. Eur J Appl Physiol. (2015) 115:1067–74. doi: 10.1007/s00421-014-3088-2
16. Hahn RG. Effects of diet, habitual water intake and increased hydration on body fluid volumes and urinary analysis of renal fluid retention in healthy volunteers. Eur J Nutr. (2021) 60:691–702. doi: 10.1007/s00394-020-02275-4
17. Hahn RG, Nemme J. Volume kinetic analysis of fluid retention after induction of general anaesthesia. BMC Anaesthesiol. (2020) 20:95. doi: 10.1186/s12871-020-01001-1
18. Hahn RG. Arterial pressure and the rate of elimination of crystalloid fluid. Anesth Analg. (2017) 124:1824–33. doi: 10.1213/ANE.0000000000002075
19. Moghadamyeghaneh Z, Phelan MJ, Carmichael JC, Mills SD, Pigazzi A, Nguyen NT, et al. Preoperative dehydration increases risk of postoperative acute renal failure in colon and rectal surgery. J Gastrointest Surg. (2014) 12:2178–85. doi: 10.1007/s11605-014-2661-7
20. Ellis RJ, Del Vecchio SJ, Kalma B, Ng KL, Morias C, Francis RS, et al. Association between preoperative hydration status and acute kidney injury in patients managed surgically for kidney tumours. Int Urol Nephrol. (2018) 50:1211–7. doi: 10.1007/s11255-018-1901-2
21. Hahn RG, Li Y, He R. Fluid retention is alleviated by crystalloid but not by colloid fluid after induction of general anesthesia: an open-labelled clinical trial. J Anesth Clin Res. (2016) 7:1. doi: 10.4172/2155-6148.1000597
22. Hahn RG. Renal injury during hip fracture surgery: an exploratory study. Anaesth Intens Ther. (2015) 47:84–90. doi: 10.5603/AIT.a2015.0029
Keywords: creatinine plasma urine, acute kidney injury physiology, dehydration urine, general surgery, osmolality urine, fluid retention
Citation: Engel D, Löffel LM, Wuethrich PY and Hahn RG (2021) Preoperative Concentrated Urine Increases the Incidence of Plasma Creatinine Elevation After Major Surgery. Front. Med. 8:699969. doi: 10.3389/fmed.2021.699969
Received: 24 April 2021; Accepted: 25 June 2021;
Published: 19 July 2021.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaCopyright © 2021 Engel, Löffel, Wuethrich and Hahn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert G. Hahn, ci5oYWhuQHRlbGlhLmNvbQ==; cm9iZXJ0LmhhaG5Aa2kuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.