- Department of Ophthalmology and Visual Sciences, Illinois Eye and Ear Infirmary, University of Illinois at Chicago College of Medicine, Chicago, IL, United States
Purpose: Non-infectious uveitis is a leading cause of vision loss in the developed world. The purpose of this systematic review is to investigate the epidemiology and risk factors of non-infectious uveitis over the last 50 years.
Methods: A systematic literature search of Pubmed/MEDLINE database was performed in the 50-year period from January 1971 to January 2021, according to the PRISMA guidelines. Studies that assessed the epidemiology and risk factors for non-infectious uveitis were included.
Results: Few epidemiologic studies focus specifically on non-infectious uveitis. In the Unites States, the estimated prevalence of non-infectious uveitis is 121/100,000. The incidence and prevalence varies considerably worldwide. Females and the working age group (20–50 years) appear to be the most affected. Smoking and vitamin D deficiency are the biggest risk factors for non-infectious uveitis, while pregnancy appears to be protective. Additional risk factors include presence of other autoimmune diseases (thyroid disease, diabetes, celiac), pre-eclampsia/eclampsia, psychological stress, and certain medications (bisphosphonates, immune checkpoint inhibitors, female hormone therapy, and etanercept).
Discussion: Our systematic review summarizes the incidence and prevalence of non-infectious uveitis and associated modifiable and non-modifiable risk factors.
Introduction
Uveitis refers to inflammation of the uveal tissues of the eye, including the iris, ciliary body, and choroid. Other intraocular structures can also be involved in uveitis, including the sclera (termed scleritis), retina, retinal blood vessels, and the optic nerve. Uveitis can be associated with significant visual morbidity, with over one-third of patients with uveitis having visual impairment (1). In the developed world, it is the 5th or 6th leading cause of blindness, accounting for about 10–15% of all cases of blindness (2, 3). Unlike other ocular diseases, such as glaucoma or age-related macular degeneration, which generally affect elderly populations, uveitis can occur in all age groups and often affects young adults (4, 5).
Uveitis is categorized as infectious or non-infectious. Non-infectious uveitis can occur with systemic autoimmune disease and autoimmune diseases localized to the eye. Etiologies of non-infectious uveitis include HLA-B27 associated anterior uveitis, Fuchs uveitis syndrome, sarcoidosis, Vogt-Koyanagi-Harada (VKH), sympathetic ophthalmia, birdshot chorioretinopathy, multifocal choroiditis, serpiginous choroiditis, and Behçet disease. Non-infectious uveitis represents the majority of uveitis cases (67–90%) in the developed world (6–8). Most previous epidemiologic studies combine infectious and non-infectious etiologies together. Although risk factors for infectious and non-infectious uveitis may overlap, the causes of the inflammation are inherently different and require different management approaches. For this review we chose to include only non-infectious causes of uveitis, to avoid obscuring any epidemiologic data and risk factors specific to non-infectious causes.
Multiple factors influence the development of non-infectious uveitis, including age, sex, race and ethnicity, environmental and social factors, genetics, systemic conditions, and certain medications. The purpose of this systematic review is to investigate the epidemiology and risk factors of non-infectious uveitis in adults over the last 50 years.
Materials and Methods
A systematic literature search, data extraction, statistical analyses and assessment of the quality of evidence were performed according to a pre-specified protocol using the PRISMA guidelines (9).
Search Strategy
For this systematic review, the authors conducted an electronic database search of Pubmed/MEDLINE using a combination of keywords related to uveitis (non-infectious/non-infectious uveitis, ocular inflammation, HLA-B27 uveitis, sarcoid uveitis, VKH, and Behçet uveitis) and epidemiology (prevalence, incidence, population, risk factors, and survey). The search period was from January 1971 to January 2021. The articles deemed relevant were cross-referenced for additional manuscripts which were not directly found through the above search.
Eligibility Criteria
Studies published between January 1971 and January 2021 were included in this systematic review if they met the following inclusion criteria: (1) population-based cross-sectional or cohort studies or large case series [with at least 20 patients] (2) uveitis clearly defined as non-infectious (3) articles written in English, (4) studies performed in humans, (5) full-text available. Exclusion criteria included: (1) self-reported diagnosis of uveitis, (2) studies in persons <18 years of age.
Data Collection and Extraction
Two reviewers (KAJ and AMLC) conducted data extraction based on the inclusion and exclusion criteria above. Data extracted included the first author's name, year of publication, study location, sample size, data collection methods, prevalence and incidence of non-infectious uveitis, anatomic location of inflammation (anterior, intermediate, posterior, panuveitis), duration/chronicity of inflammatory process (acute, recurrent, chronic) and demographic and other factors including age, sex, race and ethnicity, environmental and social factors, genetics, systemic conditions, and medications.
Synthesis of Evidence
The same information was extracted, when available, from all included studies. A meta-analysis of all included studies was not able to be conducted because of study population heterogeneity and differences in the methodology of the included studies.
Results
Description of Studies
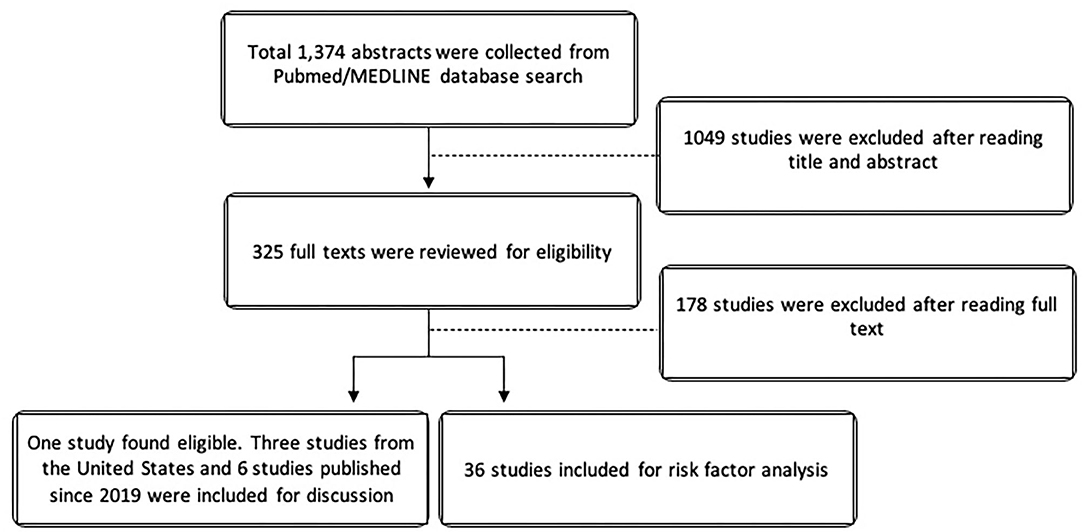
The search yielded 1,374 studies from Pubmed/MEDLINE databases. After cross-referencing for additional relevant studies, there was a total of 325 studies that were reviewed. For the epidemiologic analysis, only one study specifically evaluated non-infectious uveitis in terms of prevalence. Due to the paucity of epidemiologic data on non-infectious uveitis, we elected to also described the epidemiology of uveitis in the United Stated using the 3 large population-based studies from the United States in the last 50 years. Since Hsu et al. (10), Tsirouki et al. (5), and García-Aparicio et al. (11) have already extensively reviewed the incidence and prevalence of uveitis worldwide, we elected to minimize references and describe only the most recent international studies published since January 2019. For risk factor analysis, 36 studies were included. Figure 1 illustrates the PRISMA flow diagram for literature selection.
Epidemiology of Non-infectious Uveitis
Incidence and Prevalence in the United States
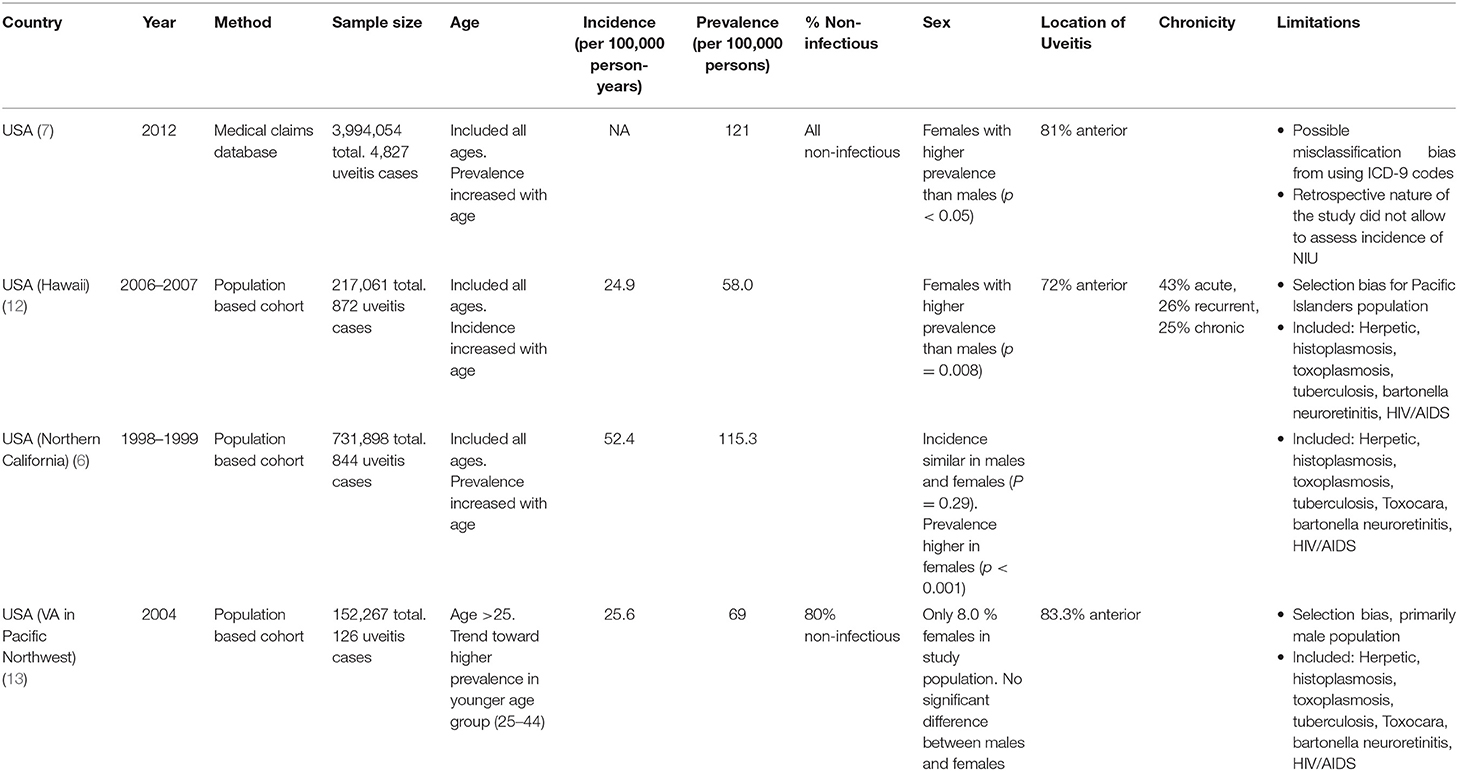
In the United States, only one study specifically met our search criteria for the prevalence of adult non-infectious uveitis. Thorne et al. used a medical claims database of almost 4 million patients throughout the United States and showed that the prevalence of adult non-infectious uveitis was 121 per 100,000 persons. Given the methodology of the study, Thorne et al. was not able to assess incidence of non-infectious uveitis (7).
Over the last 50 years, there have been 3 large population-based epidemiologic studies of uveitis in the United States, and they included infectious causes of uveitis, such as herpetic, histoplasmosis, toxoplasmosis, tuberculosis, bartonella neuroretinitis, and HIV/AIDS (6, 12, 13). Two studies came from the Kaiser Permanente Health system: one in Hawaii (Pacific Ocular Inflammation Study) and one in Northern California (Northern California Epidemiology of Uveitis Study). The Pacific Ocular Inflammation Study found an annual incidence of uveitis of 24.9, while the Northern California study found an incidence of 52.4 cases of uveitis per 100,000 person-years (6, 12). The third study, from the Pacific Northwest Veterans Administration (VA), found the annual incidence of uveitis to be 25.6 per 100,000 person years (13). There may be several reasons for large variability in uveitis incidence between these studies, including demographics of the study populations.
While data on the incidence of uveitis is helpful in understanding the rate of new cases in a particular time period, prevalence of uveitis may be more meaningful since many patients with uveitis may develop a chronic and/or recurrent course. The population study from Northern California reported a prevalence of 114.5 per 100,000 adults (6), which is similar to the prevalence reported by Thorne et al. of 121 per 100,000 persons (7). However, comparing the two directly is not possible as the Northern California study included cases of infectious uveitis. In the Veterans Administration study, the prevalence was lower at 69 per 100,000 persons, likely due to a primarily male population compared to other studies (13). Likewise, the population studied in the Pacific Ocular Inflammation study out of the Kaiser Health system in Hawaii contributed to lower prevalence of uveitis of 58 per 100,000, likely because Pacific Islanders have a lower prevalence of uveitis compared to other racial/ethnic groups studied in other population-based studies (12). Table 1 summarizes the findings of these 4 epidemiologic studies from the United States.
Incidence and Prevalence Worldwide
Worldwide the incidence and prevalence rates of non-infectious uveitis varies widely. Like in the United States, literature specific to only non-infectious uveitis is scarce. We were not able to identify any international studies that met our search criteria, since many studies include both infectious and non-infectious, and idiopathic etiologies. In most cases, idiopathic etiology was non-infectious (10). In 2019 Hsu et al. described the epidemiology of non-infectious uveitis in the Asia Pacific region (10). The prevalence of uveitis ranged from 152 per 100,000 persons in China, to 173 per 100,000 persons in South Korea (14), to 194 per 100,000 persons in Taiwan (15). The prevalence from studies in India ranged from 317 per 100,000 (16) to 730 per 100,000 (17). Importantly, these large population based studies did not distinguish between infectious and non-infectious etiologies. In 2018, Tsirouki et al. described the heterogeneity of the incidence and prevalence of uveitis worldwide (5). In 2021, García-Aparicio et al. published a systematic review and meta-analysis of the prevalence and incidence of uveitis, including studies published up until January 2019 (11). To avoid reviewing already extensively reviewed manuscripts, Table 2 describes the most recent epidemiologic studies published since January 2019, not previously reviewed by Hsu et al. (10), Tsirouki et al. (5), and García-Aparicio et al. (11). Our goal of reviewing these studies, it to update the knowledge on the incidence and prevalence of uveitis since 2019. Table 2 shows that the prevalence of uveitis varies from 12.4 per 100,000 persons in Portugal (19) to 580 per 100,000 persons in Thailand (22). Once again, these recent studies did not specifically focus on non-infectious uveitis, although most of the cases were of non-infectious etiology (18–23).
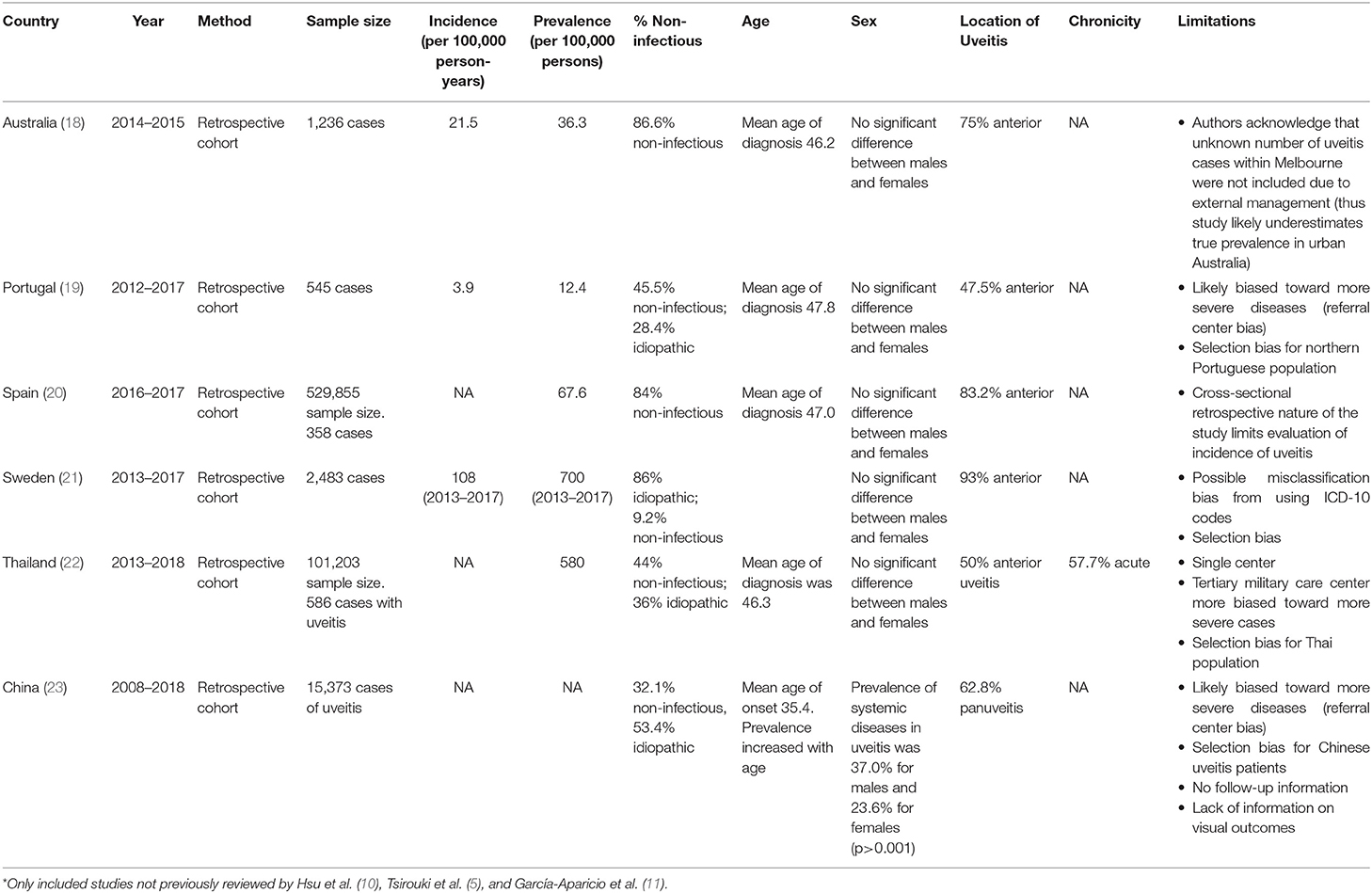
Table 2. Summary of large population-based epidemiologic studies worldwide published since Jan 2019*.
Location of Uveitis and Chronicity
Uveitis is classified according to anatomic location in the eye: anterior, intermediate, posterior, or panuveitis. Location of uveitis is important as it can portend visual compromise and development of ocular complications, such as cataract, macular edema, and glaucoma. Additionally, uveitis can be classified based on duration and chronicity of disease, including acute onset uveitis, recurrent uveitis, and chronic uveitis (24).
As can be seen from Tables 1, 2, anterior location was the most common location of non-infectious uveitis, representing 47.5 to 93% of cases. After anterior uveitis, panuveitis and posterior uveitis have similar frequency of about 20% of cases of uveitis and intermediate uveitis is the least common form of uveitis at ~10–15% of cases (5–7, 10, 12–15, 19, 21, 22, 25, 26). From the study by Throne et al. it was estimated that 10% of anterior uveitis can be classified as severe, requiring more advanced therapies than topical corticosteroids (7). Interestingly, McCannel et al. demonstrated that there were significantly more cases of unilateral acute anterior uveitis seen in a community-based practice (90.6%) compared to those seen in a tertiary care hospital (60.6%), which saw more cases of chronic anterior uveitis (27). This study suggested that most studies on uveitis patients from tertiary referral centers were subject to referral bias as these centers treated the most severe cases (27).
In terms of chronicity, the Pacific Ocular Inflammation study demonstrated that most cases of uveitis (43%) were acute. It is unclear how many of these are non-infectious. From the recent Thailand study (22), acute uveitis represented more than half of cases (57.7%), which included 44% non-infectious and 36% idiopathic cases. Additional studies are needed to elucidate further epidemiologic trends in chronicity of non-infectious uveitis.
Demographics of Non-infectious Uveitis
Age
Uveitis can occur in all age groups. Many studies have previously demonstrated a high incidence of uveitis in the working age groups (20–50 years) (5, 10, 18, 19, 21, 25). Interestingly, Thorne et al. showed that non-infectious uveitis increases with age (7). Similarly, the Pacific Northwest VA study, which had 80% non-infectious cases, showed increased incidence with increasing age, however it did have limited data on younger age groups (13). These studies suggest that while uveitis can affect individuals throughout their lifetime, there may be a higher burden of disease in the elderly than originally suspected from earlier studies. Possible reasons for increased incidence of disease in older adults include higher likelihood of prior ocular surgery which can contribute to inflammation (28) and increased incidence of underlying autoimmune disease with age (29).
Sex
In the United States, there is a higher frequency of female adult patients with uveitis than male adult patients (6, 7, 12). Both the Pacific Ocular Inflammation Study and the Northern California Epidemiology of Uveitis Study demonstrated a higher prevalence of uveitis in female compared to male patients, and the Northern California Epidemiology of Uveitis Study demonstrated a higher rate of uveitis in females among all age groups (6, 12). In the study by Thorne et al, 56.8% of adult patients with non-infectious uveitis were female (7). Since the primary cause of non-infectious uveitis is related to autoimmune disease and autoimmune diseases are more prevalent in adult females, it is reasonable to anticipate that autoimmune uveitis may be more frequent in adult female patients. Sex hormones, including estrogen, likely contribute to the differing immune response and susceptibility to development of autoimmune diseases, including uveitis, in women (30). Many studies worldwide found that males and females are equally affected by uveitis, however these studies included both infectious and non-infectious causes of uveitis (5, 10, 18–22).
Race/Ethnicity
Among the many subtypes of uveitis, including uveitis associated with systemic diseases, there can be certain racial predilections. For example, Behcet disease, which can be associated with severe anterior uveitis and occlusive retinal vasculitis, is more commonly seen in racial/ethnic groups along the Silk Road, including the Middle East and Asia (5, 10, 31). Vogt Koyanagi Harada syndrome, which is associated with a bilateral granulomatous panuveitis, is more commonly seen in races/ethnicities with more skin pigmentation, including Asian, Native American, and Hispanic individuals (10, 32, 33). In the Pacific Ocular Inflammation Study, there was a higher incidence of uveitis in the Caucasian/white and black racial groups compared to Pacific Islanders (12).
Association With Systemic Disease
The most common known cause of non-infectious uveitis in the developed world is HLA-B27 associated uveitis (5). The overall prevalence of HLA-B27 in the United States is 6.1%, with non-Hispanic whites having the highest prevalence of all other races/ethnicities at 7.5% (34). Prevalence of HLA-B27 varies widely, with 15.9% reported in Norway (35) and <1% reported in Japan (36). It is estimated that 30–80% of patients with seronegative spondyloarthropathies are HLA-B27 positive (37, 38). Patients may be tested for HLA typing after presenting with a recurrent acute anterior uveitis in order to screen for risk of seronegative spondyloarthropathies. In a large nationwide cohort study in Korea, the incidence rate ratio for development of ankylosing spondylitis (AS) increased with every episode of recurrent acute anterior uveitis, with a rate of 277.3 (95% CI 171.6–423.8) for more than 2 episodes of uveitis (39).
Sarcoidosis represents another systemic disease that can be associated with the development of eye disease in 30–60% of patients, including 20–30% of cases with non-infectious uveitis (40). Sarcoidosis is more common in African American and Asian patients, but can be seen in all populations (5, 10). Uveitis in sarcoidosis can be acute or chronic and can precede any systemic or extraocular manifestations; in one recent study, one-third of patients with ocular sarcoidosis developed symptomatic systemic disease within 16 months of when the uveitis started (41). The manifestations of ocular sarcoidosis may differ based on race, with black patients more likely to develop chronic anterior uveitis and white patients more likely to develop posterior uveitis (40).
Behçet disease is a multisystem inflammatory disease that is diagnosed based on clinical criteria including ocular inflammatory disease and recurrent oral and genital ulcers. The prevalence of Behçet disease has significant geographic variation with pooled global prevalence of 10.3 per 100,000 inhabitants, but with prevalence as high as 119 per 100,000 in Turkey to 2.1 per 100,000 for Northern Europe (31). Behçet disease can be associated with severe uveitis, with panuveitis being the most commonly observed location for uveitis (42). Occlusive retinal vasculitis is another common manifestation. A recent study by Sota et al. compared 64 juvenile-onset and 332 adult-onset Behçet disease patients, and showed that those with juvenile-onset Behçet disease (first manifestation of disease before age 16) have a lower prevalence of uveitis than adult-onset Behcet disease (42). Male patients may have higher risk for severe ocular involvement than female patients (43).
Risk Factors Associated With Non-infectious Uveitis
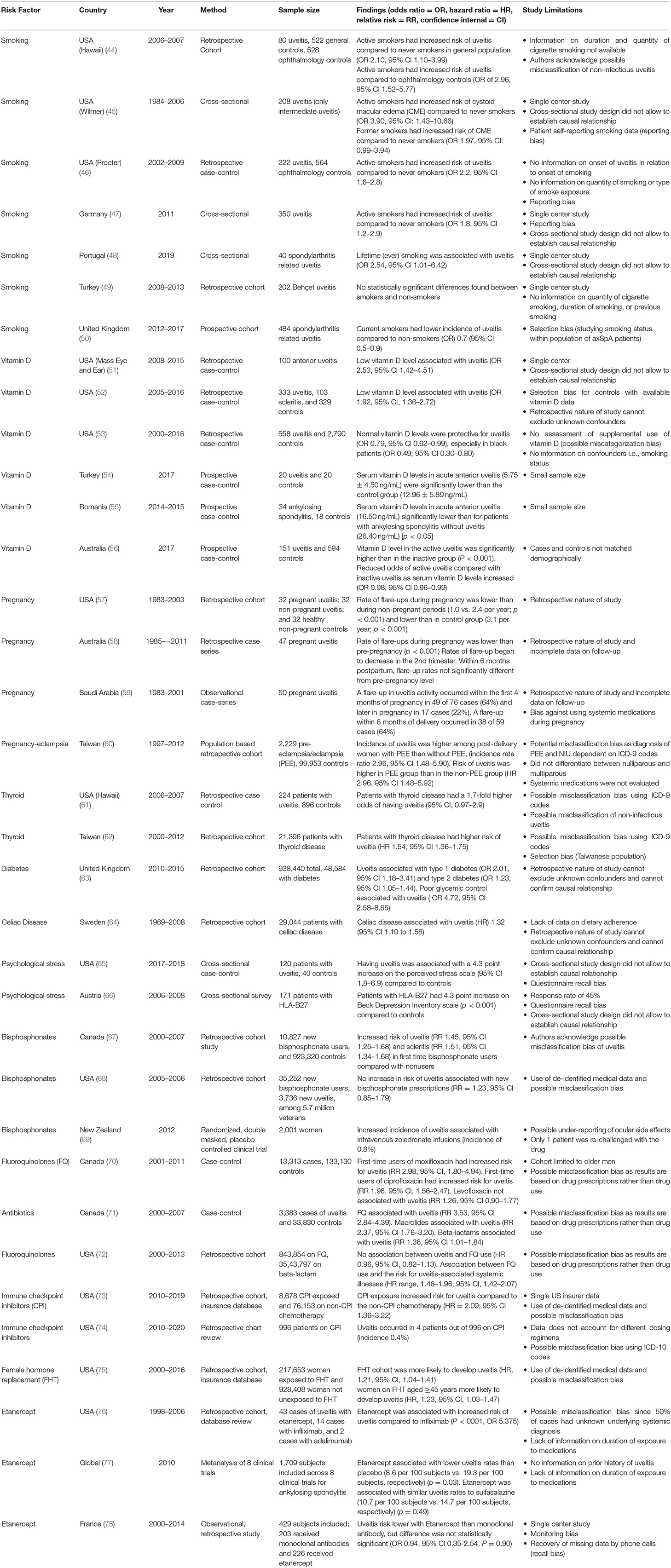
As non-infectious uveitis represents a heterogeneous group of diseases, there are many risk factors which have been associated with the development or progression of uveitis. As previously mentioned, certain demographic variables, including age, female gender, and certain races/ethnicities may be more predisposed to developing uveitis. However, there are a number of external risk factors that may contribute to development of disease. Table 3 summarizes the potential factors that influence the development of non-infectious uveitis as described over the last 50 years, including smoking, vitamin D levels, pregnancy, autoimmune disease, and certain medications. Each study included was identified according to a pre-specified protocol using the PRISMA guidelines, and limitations of each study are listed in Table 3.
Smoking
Among cross-sectional, case-control, and population-based studies conducted on the effect of smoking on uveitis, there is general agreement among studies that smoking increases the risk for development of ocular inflammation (44–48). From the Pacific Ocular Inflammation Study, smoking was associated with a 2 times greater odds of developing new onset non-infectious uveitis compared to patients who never smoked (44). In a large case-control study, smoking was found to increase the odds of having ocular inflammation in all anatomic types of uveitis, with higher odds in posterior and panuveitis compared to anterior uveitis; both current and past smokers had a 2 times higher odds of developing uveitis compared to those who had never smoked (46). A cross-sectional study of 350 non-infectious uveitis patients showed that in addition to active uveitis, smoking was also associated with younger age of uveitis activity, more frequent topical corticosteroid dosing, and a dose-dependent higher odds of macular edema and cataract (47). Smoking was associated with a 4-fold increased risk of cystoid macular edema in a dose-dependent manner in patients with intermediate uveitis; macular edema is a leading cause of vision loss in uveitis (45). It is hypothesized that smoking causes endothelial cell dysfunction with resultant increased leakage of retinal blood vessels and development of macular edema; while other factors associated with aging may also effect retinal vasculature, smoking was still associated with uveitis and macular edema when adjusting for age and other systemic diseases (45).
Interestingly, in a large prospective study from the United Kingdom, smoking was found to be protective against uveitis in a population of patients with spondyloarthropathies. As the authors point out, selection bias may be contributing to this finding (50). In another retrospective cohort study from Turkey, Bilgin et al. found no statistically significant association with Behçet uveitis among smokers and non-smokers. Limitations of this study include its single center patient population, and lack of information about smoking quantity, duration of smoking, and history of prior smoking (49).
Vitamin D
Vitamin D plays a role in immune regulation and vitamin D deficiency has been associated with a number of autoimmune diseases, including multiple sclerosis, inflammatory bowel disease, and rheumatoid arthritis. Low vitamin D levels have also been found in a number of studies on patients with uveitis, including patients with systemic diseases associated with uveitis (51–56).
In a small case-control study examining patients with anterior uveitis at a single institution, low vitamin D levels were seen in a significantly greater number of patients with uveitis than in control patients (51). A larger study from the same institution demonstrated that for every 1 nanogram/ml increase in vitamin D level, there was a 5% lower odds of having uveitis; this study also demonstrated association of low vitamin D levels with anterior uveitis, as well as panuveitis and scleritis (52). In a large case-control study using a national health insurer database, normal vitamin D levels were associated with a 21% lower odds of developing uveitis compared to low vitamin D levels; these results were even more pronounced in black patients with a 51% lower odds of having uveitis with normal vitamin D levels compared to low vitamin D levels (53).
A study on patients with idiopathic or HLA-B27 associated acute anterior uveitis demonstrated that these patients had significantly lower vitamin D levels compared to controls (54). In a study examining vitamin D levels and other immune markers in patients with AS, low vitamin D levels were associated with acute anterior uveitis and uveitis flares compared to AS patients without anterior uveitis (55).
One recent large case-control study demonstrated a difference in vitamin D levels among patients with active and inactive uveitis; prior studies had not differentiated between disease activity, just disease onset in relation to vitamin D levels. Patients with active uveitis had lower levels of vitamin D compared to those with inactive disease and population-based controls; increased sunlight exposure among those with vitamin D deficiency was associated with disease inactivity (56). These results provide some background for further studies into vitamin D supplementation through sunlight exposure or supplementation as a way of preventing inflammatory disease flares, although a large randomized control trial would be needed to confirm these findings.
Pregnancy
As previously discussed, non-infectious uveitis is more prevalent in female patients and can affect women of any age, including those of child-bearing potential. Because of the role of sex hormones in the development of autoimmune disease, the course of uveitis may change in pregnancy and during the post-partum period when there are significant changes in levels of estrogen and progesterone. During pregnancy, elevated levels of estrogen and progesterone are associated with a decline in Th1 type immunity in order for the immune system to tolerate the semi-allogeneic fetus (79). This decline in Th1 type immunity, with decline in cytokines such as tumor necrosis factor alpha, interleukin-12 and interferon gamma, also contributes to improvement in Th1 mediated autoimmune conditions such as multiple sclerosis and rheumatoid arthritis. Conversely, there may be potential worsening of Th2 mediated and antibody mediated conditions such as systemic lupus erythematosus. Many forms of uveitis are also thought to be Th1 mediated and several small series have indicated that the course of uveitis improves in pregnancy (57–59). In one study, uveitis activity levels were found to decline during pregnancy (second and third trimesters) compared to pre-pregnancy, with flare up rates of 0.540 and 1.188 per person year, respectively (58). The rate of uveitis flares was not significantly different from pre-pregnancy levels by 6 months post-partum. These findings supported a prior study that found that uveitis flare ups peaked in the first trimester of pregnancy and then declined in the second and third trimesters with a similar rate of flare ups pre-pregnancy and post-partum (57).
A recent population based cohort study in Taiwan examining the relationship between pre-eclampsia/eclampsia and non-infectious uveitis demonstrated a three times higher incidence rate of non-infectious uveitis in women who had pre-eclampsia/eclampsia compared to age, urbanization, and income-matched controls (60). None of these women with pre-eclampsia/eclampsia who developed non-infectious uveitis had underlying autoimmune or thyroid disease. It has been postulated that during pre-eclampsia/eclampsia, there may be a shift from Th2 back to Th1 mediated immunity, which may be why pre-eclampsia/eclampsia represent a potential risk factor for development of non-infectious uveitis (60).
Thyroid Disease
The association of thyroid disease and non-infectious uveitis has been studied (61, 62). From the Pacific Ocular Inflammation study, patients with thyroid disease had a 1.7 higher odds of developing non-infectious uveitis compared to controls without thyroid disease (61). More recently, a large cohort study from Taiwan found a 1.54 higher risk of developing non-infectious uveitis in patients with thyroid disease. Males without thyroid disease had a lower risk of developing uveitis than females (62). Both thyroid disease and uveitis have an autoimmune component, which may be why thyroid disease is a potential risk factor for non-infectious uveitis.
Diabetes
Both diabetes and uveitis can cause vision loss through various mechanisms and disruption of the blood-ocular barrier. One large cohort study in the United Kingdom examined the relationship between diabetes, glycemic control and uveitis using a large primary care database of over 1 million patients; they found that acute anterior uveitis occurred more commonly in patients with diabetes than without diabetes (63). Poor glycemic control, defined as HbA1c >11.3, and proliferative retinopathy were also strongly associated with acute uveitis. This study was limited by using diagnosis codes from a primary care database which do not specify the etiology of uveitis. It may also have ascertainment bias since patients with diabetes are more likely to have routine eye exams than healthy controls. The finding of type 1 diabetes having a stronger association with uveitis may reflect the fact that both result from immune system dysfunction and are considered autoimmune diseases.
Celiac Disease
As an immune-mediated disease of the small intestine associated with ingestion of gluten, celiac disease has also been associated with the development of other autoimmune diseases, including uveitis (80). In a large nationwide cohort study in Sweden, individuals with biopsy-proven celiac disease had a higher risk of development of uveitis (hazard ratio 1.32, 95% CI 1.10–1.58) compared to controls (64). However, this study did not specify the etiology of uveitis. Nonetheless, in those individuals with celiac disease and uveitis, both diseases may respond to strict adherence to a gluten-free diet. The microbiome and disruptions in the microbiome have also been hypothesized to play a role in autoimmune diseases, including celiac disease, inflammatory bowel disease, and uveitis (80).
Psychological Stress
Psychological stress can alter immune function, and chronic stress is associated with an attenuated immune response over time (81, 82). In a cross-sectional survey study from Austria, it was found that patients with HLA-B27 associated uveitis had significantly higher scores on the Beck Depression Inventory scale, a standardized survey for depression, compared to healthy controls. Almost 60 percent of patients with HLA-B27 associated uveitis in this study reported life events and psychological distress as potential triggers for uveitis flares (66). In a recent case-control study, Berlinberg et al. found that patients with non-infectious uveitis self-reported a 4.3-point increased score on a 10-item perceived stress scale compared to patients without uveitis. In multivariate analysis, female sex and history of depression were most strongly associated with an increased perceived stress score. However, there was no difference in the perceived stress score between active and controlled uveitis patients. Measures of diurnal salivary cortisol levels were not significantly different between patients with and without uveitis (65). It remains unclear if psychological stress is a risk factor of development of non-infectious uveitis, but it appears to play a role regardless of disease activity.
Medication-Induced Uveitis
Various medications have been associated with the development of uveitis. Most cases of drug-induced uveitis are single case reports or small case series rather than larger cohort or case-control studies. Some reports may also come from post-marketing surveillance of drugs, but may lack specific details on the course of uveitis.
Bisphosphonates
The bisphosphonates are the most commonly used class of medications to prevent the development of osteoporosis. Both oral and intravenous bisphosphonates have been associated with an increased risk for development of uveitis and scleritis, possibly due to the release of inflammatory cytokines such as tumor necrosis factor alpha and interleukin-6. One large retrospective cohort study from British Columbia found 1.45 increased risk for the development of uveitis in first-time oral bisphosphonate users compared to those not on bisphosphonates. However, they did not specifically identify the uveitis as non-infectious (67). Another study from a veterans population found that there was no significant difference in rates of uveitis among those prescribed bisphosphonates and the general veteran population (relative risk: 1.23, 95% CI 0.85–1.79) and that overall rates for uveitis were low (68). Another study using data from a randomized controlled trial of women receiving intravenous zoledronate compared to placebo found that the incidence of uveitis was 0.8% and occurred only in the zoledronate arm of the study (69). Unlike the other 2 studies which were performed using diagnosis codes and ran the risk of misclassification bias, the study on intravenous zoledronate by Patel et al. included only uveitis cases confirmed by an ophthalmologist (69).
Antibiotics
Certain antimicrobial medications have been associated with development of uveitis. Fluoroquinolones have been associated with bilateral acute transillumination defects, iris atrophy and pigment dispersion with anterior uveitis. In a case-control study conducted using a cohort of men from a large health claims database in British Columbia, first time users of moxifloxacin had an increased risk for uveitis (rate ratio: 2.98, 95% CI 1.80–4.94) compared to nonusers of fluoroquinolone antibiotics (70). The rate of uveitis was higher with moxifloxacin compared to other fluoroquinolones such as ciprofloxacin and levofloxacin (70). A nested case-control study done in the same population of patients in British Columbia examined the use of fluoroquinolone antibiotics as well as macrolide and beta-lactam antibiotics in new cases of uveitis compared to age-matched controls (71). An increased risk for uveitis was found among fluoroquinolone users, but also among those on macrolide and beta-lactam antibiotics which have not been associated with uveitis; the authors concluded that there was no concrete evidence of an association between fluoroquinolone use and uveitis. In fact, it is possible the uveitis was post-infectious or part of the systemic prodrome.
A large cohort study using the Optum medical claims database in the United States also compared patients taking fluoroquinolones to those prescribed a beta-lactam antibiotic since they may be prescribed for similar conditions; there was no association between uveitis and fluoroquinolone use, but there was an association between systemic illnesses that may be associated with uveitis and fluoroquinolone use (72). These authors proposed that fluoroquinolones may be more likely to be prescribed in patients with a systemic illness that may predispose individuals to develop uveitis rather than directly causing the uveitis.
Immune Checkpoint Inhibitors
Immunotherapies are a newer treatment modality for many types of cancers. Immune checkpoint inhibitors represent one of the classes of immunotherapies that are monoclonal antibodies that block inhibitory receptors often activated by tumor cells to evade the immune response; two such targets include programmed death-1 (PD-1) and cytotoxic T lymphocyte antigen-4 (83). Because of the action of these therapies in promoting T cell responses to cancer cells, they also increase the likelihood of autoimmune adverse effects, including uveitis. Several medications, including ipilimumab, nivolumab, and pembrolizumab, have been associated with ocular adverse effects. Using the Food and Drug Administration Adverse Events Reporting system, one study conducted a disproportionality analysis to compare adverse drug reactions associated with immune checkpoint inhibitors with rates reported with all other drugs (83). Ipilimumab and nivolimumab had the strongest associations with uveitis (reporting odds ratio: 10.53, 95% CI 7.30–15.22 and 8.73, 95% CI 6.25–12.20, respectively), whereas pembrolizumab had a higher association with ocular myasthenia (83). Unlike epidemiologic studies using cohort or case-control designs, disproportionality analysis does not account or adjust for other factors such as co-morbidities that may predispose to autoimmune disease or patient demographic information.
From a recent large retrospective cohort study using insurance claims database, Xia et al. showed that patients exposed to checkpoint inhibitors had a 0.3% incidence of non-infectious uveitis, which was significantly higher compared to patients on non-checkpoint inhibitor chemotherapy (73). Similarly, Fortes at el. found a 0.4% incidence of uveitis in patients exposed to checkpoint inhibitors, although they did not specifically identify uveitis as non-infectious (74).
Hormone Replacement Therapy
As previously discussed, female sex hormones play a role in the development of autoimmune disease. In a recent study, Sobrin et al. found that patients on female hormone therapy (FHT), which includes menopausal hormonal replacement and hormone contraceptive therapy, had ~20% increased chance of developing non-infectious uveitis. This study also showed that this association was highest for anterior uveitis (HR 1.23, 95% CI, 1.05–1.45) and for women > 44 years of age (HR 1.23, 95% CI, 1.03–1.47). The trend was similar in women <44 years old, but not statistically significant. Overall, the absolute risk of non-infectious uveitis with FHT was fairly low, suggesting that it is a safe therapy for most patients (75).
Etanercept
Anti-Tumor necrosis factor alpha (Anti-TNF-alpha) agents are increasingly used in the treatment of immune-mediated disorders. Etanercept (Enbrel) is an anti- TNF-alpha agent which also has activity against TNF-beta, and is commonly used in the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Several reports have identified cases of non-infectious uveitis after initiation of etanercept therapy (76, 84). In a study by Lim et al. the authors reviewed two large databases for cases of uveitis reported in the US associated with etanercept, infliximab, and adalimumab therapy. After adjusting for underlying disease, age and sex, they found that the number of uveitis cases associated with etanercept (20 cases) exceeded that of infliximab (4 cases) and adalimumab (2 cases) (p < 0.001). In four cases of uveitis associated with etanercept, the inflammation resolved with cessation of etanercept and recurred upon re-challenging in two of the patients. The study however is limited by incomplete clinical information available in the databases (i.e., underlying disease unknown in 50% of cases). Thus, is it possible that some of the included cases of uveitis associated with anti-TNF-alpha inhibitors, are actually being treated with the drugs (85). In a meta-analysis of uveitis rates associated with etanercept for ankylosing spondylitis across 8 clinical trials, Sieper et al. found that uveitis rates were lower for etanercept than for placebo, in placebo-controlled trials. This study was limited in that prior history of uveitis was not specifically reported (77). In a recent retrospective study from France, the risk of anterior uveitis occurrence did not appear to differ in patients with spondyloarthropathies treated with etanercept and monoclonal antibodies (infliximab and adalimumab). This study was also limited by single center nature, monitoring bias, and recall bias (78).
Conclusions and Future Directions
Non-infectious uveitis is a leading cause of visual impairment and blindness worldwide. In this systematic review we summarized the prevalence and incidence of uveitis, and described the modifiable and non-modifiable risk factors specific for non-infectious uveitis in adults over the last 50 years. Understanding the epidemiology of non-infectious uveitis can help clarify the burden of disease and populations at risk, which in turn can help with resource allocation for managing chronic disease. Non-infectious uveitis can be a particularly difficult diagnosis for both patients and physicians. Patients struggle to understand why there is an immunologic process affecting their vision, and physicians often cannot find a “cause” for the immunologic process. The aim of this systematic review is to help clinicians identify potential disease contributing factors for patients with non-infectious uveitis, which is important in diagnosis and management of disease.
Several risk factors have been identified as potentially contributing to the development of uveitis, including tobacco exposure, vitamin D deficiency, and pre-eclampsia/eclampsia. Pregnancy has been found to be protective for uveitis flares. Systemic medical conditions including diabetes, thyroid disease and celiac disease have been found to be associated with an increased risk of non-infectious uveitis. Also, certain medications such as bisphosphonates, fluoroquinolones, immune checkpoint inhibitors, female hormone replacement therapy, and etanercept have been associated with increased risk of non-infectious uveitis. In the clinical setting, uveitis patients' medications should be thoroughly reviewed to identify any culprit medications that may have increased the risk of non-infectious uveitis. Counseling on smoking cessation should also be part of the discussion in the management of chronic non-infectious uveitis.
Future studies in uveitis will include more large-scale, multi-center studies that focus on risk factor and lifestyle modifications in the prevention and management of non-infectious uveitis. Analysis of big data, including the Intelligent Research in Sight (IRIS) registry developed through the American Academy of Ophthalmology as the nation's first comprehensive eye disease clinical registry, may allow for innovative ways to perform epidemiologic studies and outcomes-based studies on patients with uveitis. Understanding populations at risk and modifiable factors that may be involved in the development of uveitis can help in disease prevention, monitoring and control to decrease overall morbidity from this potentially blinding condition.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
KJ and A-ML-C contributed to the study design, literature review, data analysis, and writing of the manuscript. A-ML-C received funding for support of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants: P30 EY001792, NIH/NEI K12 EY021475, UIC CCTS 2019-01, unrestricted departmental funding from Research to Prevent Blindness.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. (1996) 80:332–6. doi: 10.1136/bjo.80.4.332
2. Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. (2004) 88:1159–62. doi: 10.1136/bjo.2003.037226
3. Rosenbaum JT, Bodaghi B, Couto C, Zierhut M, Acharya N, Pavesio C, et al. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: a review. Semin Arthritis Rheum. (2019) 49:438–45. doi: 10.1016/j.semarthrit.2019.06.004
4. Rothova A, Buitenhuis HJ, Meenken C, Brinkman CJ, Linssen A, Alberts C, et al. Uveitis and systemic disease. Br J Ophthalmol. (1992) 76:137–41. doi: 10.1136/bjo.76.3.137
5. Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. (2018) 26:2–16. doi: 10.1080/09273948.2016.1196713
6. Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. (2004) 111:491–500; discussion 500. doi: 10.1016/j.ophtha.2003.06.014
7. Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, et al. Prevalence of noninfectious uveitis in the united states: a claims-based analysis. JAMA Ophthalmol. (2016) 134:1237–45. doi: 10.1001/jamaophthalmol.2016.3229
8. Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, Papo T, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine. (2001) 80:263–70. doi: 10.1097/00005792-200107000-00005
9. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
10. Hsu Y-R, Huang JC-C, Tao Y, Kaburaki T, Lee CS, Lin T-C, et al. Noninfectious uveitis in the Asia-Pacific region. Eye. (2019) 33:66–77. doi: 10.1038/s41433-018-0223-z
11. García-Aparicio Á, García de Yébenes MJ, Otón T, Muñoz-Fernández S. Prevalence and incidence of uveitis: a systematic review and meta-analysis. Ophthalmic Epidemiol. (2021) 1–8. doi: 10.1080/09286586.2021.1882506
12. Acharya NR, Tham VM, Esterberg E, Borkar DS, Parker J V, Vinoya AC, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. (2013) 131:1405–12. doi: 10.1001/jamaophthalmol.2013.4237
13. Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol. (2008) 146:890–6.e8. doi: 10.1016/j.ajo.2008.09.014
14. Rim TH, Kim SS, Ham D-I, Yu S-Y, Chung EJ, Lee SC. Incidence and prevalence of uveitis in South Korea: a nationwide cohort study. Br J Ophthalmol. (2018) 102:79–83. doi: 10.1136/bjophthalmol-2016-309829
15. Hwang D-K, Chou Y-J, Pu C-Y, Chou P. Epidemiology of uveitis among the Chinese population in Taiwan: a population-based study. Ophthalmology. (2012) 119:2371–76. doi: 10.1016/j.ophtha.2012.05.026
16. Rathinam SR, Krishnadas R, Ramakrishnan R, Thulasiraj RD, Tielsch JM, Katz J, et al. Population-based prevalence of uveitis in Southern India. Br J Ophthalmol. (2011) 95:463–7. doi: 10.1136/bjo.2010.182311
17. Dandona L, Dandona R, John RK, McCarty CA, Rao GN. Population based assessment of uveitis in an urban population in southern India. Br J Ophthalmol. (2000) 84:706–9. doi: 10.1136/bjo.84.7.706
18. Hart CT, Zhu EY, Crock C, Rogers SL, Lim LL. Epidemiology of uveitis in urban Australia. Clin Experiment Ophthalmol. (2019) 47:733–40. doi: 10.1111/ceo.13517
19. Hermann L, Fernando F-R, Luís F. Epidemiology of Uveitis in a tertiary care centre in Portugal. Semin Ophthalmol. (2021) 1–7. doi: 10.1080/08820538.2021.1885721
20. García-Aparicio A, Alonso Martín L, López Lancho R, Quirós Zamorano R, Del Olmo Perez L, Sánchez Fernández S, et al. Epidemiology of Uveitis in a Spanish region: prevalence and etiology. Ophthalmic Epidemiol. (2020) 28:227–36. doi: 10.1080/09286586.2020.1815802
21. Bro T, Tallstedt L. Epidemiology of uveitis in a region of southern Sweden. Acta Ophthalmol. (2020) 98:32–5. doi: 10.1111/aos.14130
22. Keorochana N. Pattern and outcome of uveitis in a tertiary military hospital in Thailand. Ocul Immunol Inflamm. (2020) 28:424–32. doi: 10.1080/09273948.2019.1589527
23. Yang P, Zhong Z, Du L, Li F, Chen Z, Zhu Y, et al. Prevalence and clinical features of systemic diseases in Chinese patients with uveitis. Br J Ophthalmol. (2021) 105:75–82. doi: 10.1136/bjophthalmol-2020-315960
24. Jabs DA, Nussenblatt RB, Rosenbaum JT, Atmaca LS, Becker MD, Brezin AP, et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. (2005) 140:509–16. doi: 10.1016/j.ajo.2005.03.057
25. Jones NP. The Manchester Uveitis Clinic: the first 3000 patients–epidemiology and casemix. Ocul Immunol Inflamm. (2015) 23:118–26. doi: 10.3109/09273948.2013.855799
26. Garcia GE, Aucoin J, Gladstone G. Extended wear rigid gas permeable lenses used for correction of aphakia. CLAO J. (1990) 16:195–9.
27. McCannel CA, Holland GN, Helm CJ, Cornell PJ, Winston JV, Rimmer TG. Causes of uveitis in the general practice of ophthalmology. UCLA Community-Based Uveitis Study Group. Am J Ophthalmol. (1996) 121:35–46. doi: 10.1016/S0002-9394(14)70532-X
28. Reddy AK, Patnaik JL, Miller DC, Lynch AM, Palestine AG, Pantcheva MB. Risk factors associated with persistent anterior uveitis after cataract surgery. Am J Ophthalmol. (2019) 206:82–6. doi: 10.1016/j.ajo.2019.02.016
29. Hasler P, Zouali M. Immune receptor signaling, aging, and autoimmunity. Cell Immunol. (2005) 233:102–8. doi: 10.1016/j.cellimm.2005.04.012
30. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. (2018) 9:2279. doi: 10.3389/fimmu.2018.02279
31. Maldini C, Druce K, Basu N, LaValley MP, Mahr A. Exploring the variability in Behçet's disease prevalence: a meta-analytical approach. Rheumatology. (2018) 57:185–195. doi: 10.1093/rheumatology/kew486
32. Gao F, Zhao C, Cheng G, Pei M, Liu X, Wang M, et al. Clinical patterns of uveitis in a tertiary center in north China. Ocul Immunol Inflamm. (2017) 25:S1–7. doi: 10.3109/09273948.2016.1158279
33. Reddy AK, John FT, Justin GA, Dahr SS. Vogt-Koyanagi-Harada disease in a Native American population in Oklahoma. Int Ophthalmol. (2021) 41:915–22. doi: 10.1007/s10792-020-01647-3
34. Reveille JD, Hirsch R, Dillon CF, Carroll MD, Weisman MH. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum. (2012) 64:1407–11. doi: 10.1002/art.33503
35. Gran JT, Mellby AS, Husby G. The prevalence of HLA-B27 in Northern Norway. Scand J Rheumatol. (1984) 13:173–6. doi: 10.3109/03009748409100382
36. Hukuda S, Minami M, Saito T, Mitsui H, Matsui N, Komatsubara Y, et al. Spondyloarthropathies in Japan: nationwide questionnaire survey performed by the Japan Ankylosing Spondylitis Society. J Rheumatol. (2001) 28:554–9.
37. Chang JH, McCluskey PJ, Wakefield D. Acute anterior uveitis and HLA-B27. Surv Ophthalmol. (2005) 50:364–88. doi: 10.1016/j.survophthal.2005.04.003
38. Leirisalo-Repo M, Hannu T, Mattila L. Microbial factors in spondyloarthropathies: insights from population studies. Curr Opin Rheumatol. (2003) 15:408–12. doi: 10.1097/00002281-200307000-00006
39. Oh B-L, Lee JS, Lee EY, Lee HY, Yu HG. Recurrent anterior uveitis and subsequent incidence of ankylosing spondylitis: a nationwide cohort study from 2002 to 2013. Arthritis Res Ther. (2018) 20:22. doi: 10.1186/s13075-018-1522-2
40. Rothova A, Alberts C, Glasius E, Kijlstra A, Buitenhuis HJ, Breebaart AC. Risk factors for ocular sarcoidosis. Doc Ophthalmol. (1989) 72:287–96. doi: 10.1007/BF00153496
41. Ma SP, Rogers SL, Hall AJ, Hodgson L, Brennan J, Stawell RJ, et al. Sarcoidosis-related uveitis: clinical presentation, disease course, and rates of systemic disease progression after uveitis diagnosis. Am J Ophthalmol. (2019) 198:30–6. doi: 10.1016/j.ajo.2018.09.013
42. Sota J, Rigante D, Lopalco G, Emmi G, Gentileschi S, Gaggiano C, et al. Clinical profile and evolution of patients with juvenile-onset Behçet's syndrome over a 25-year period: insights from the AIDA network. Intern Emerg Med. (2021) doi: 10.1007/s11739-021-02725-9
43. Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol. (2004) 138:373–80. doi: 10.1016/j.ajo.2004.03.022
44. Yuen BG, Tham VM, Browne EN, Weinrib R, Borkar DS, Parker J V, et al. Association between smoking and uveitis: results from the Pacific Ocular Inflammation Study. Ophthalmology. (2015) 122:1257–61. doi: 10.1016/j.ophtha.2015.02.034
45. Thorne JE, Daniel E, Jabs DA, Kedhar SR, Peters GB, Dunn JP. Smoking as a risk factor for cystoid macular edema complicating intermediate uveitis. Am J Ophthalmol. (2008) 145:841–6. doi: 10.1016/j.ajo.2007.12.032
46. Lin P, Loh AR, Margolis TP, Acharya NR. Cigarette smoking as a risk factor for uveitis. Ophthalmology. (2010) 117:585–90. doi: 10.1016/j.ophtha.2009.08.011
47. Roesel M, Ruttig A, Schumacher C, Heinz C, Heiligenhaus A. Smoking complicates the course of non-infectious uveitis. Graefe's Arch Clin Exp Ophthalmol. (2011) 249:903–7. doi: 10.1007/s00417-010-1597-1
48. Costa E, Almeida D, Cerqueira M, Costa JR, Ribeiro AR, Sousa-Neves J. Smoking as associated factor for spondyloarthritis related uveitis: results from a single centre cross-sectional study. Acta Reumatol Port. (2020) 45:265–9. doi: 10.1136/annrheumdis-2020-eular.3802
49. Bilgin AB, Turkoglu EB, Ilhan HD, Unal M, Apaydin KC. Is smoking a risk factor in ocular Behçet disease? Ocul Immunol Inflamm. (2015) 23:283–6. doi: 10.3109/09273948.2014.909047
50. Zhao S, Jones GT, Macfarlane GJ, Hughes DM, Dean LE, Moots RJ, et al. Associations between smoking and extra-axial manifestations and disease severity in axial spondyloarthritis: results from the BSR Biologics Register for Ankylosing Spondylitis (BSRBR-AS). Rheumatology. (2019) 58:811–9. doi: 10.1093/rheumatology/key371
51. Grotting LA, Davoudi S, Palenzuela D, Papaliodis GN, Sobrin L. Association of low vitamin d levels with noninfectious anterior uveitis. JAMA Ophthalmol. (2017) 135:150–3. doi: 10.1001/jamaophthalmol.2016.4888
52. Llop SM, Davoudi S, Stanwyck LK, Sathe S, Tom L, Ahmadi T, et al. Association of low vitamin D levels with noninfectious uveitis and scleritis. Ocul Immunol Inflamm. (2019) 27:602–9. doi: 10.1080/09273948.2018.1434208
53. Sobrin L, Stanwyck LK, Pan W, Hubbard RA, Kempen JH, VanderBeek BL. Association of hypovitaminosis D with increased risk of uveitis in a large health care claims database. JAMA Ophthalmol. (2018) 136:548–52. doi: 10.1001/jamaophthalmol.2018.0642
54. Dadaci Z, Cetinkaya S, Oncel Acir N, Oncel M, Borazan M. Serum vitamin D levels in patients with acute anterior uveitis. Ocul Immunol Inflamm. (2017) 25:492–6. doi: 10.3109/09273948.2016.1139735
55. Mitulescu TC, Stavaru C, Voinea LM, Banica LM, Matache C, Predeteanu D. The role of vitamin D in immuno-inflammatory responses in ankylosing spondylitis patients with and without Acute Anterior Uveitis. J Med Life. (2016) 9:26–33.
56. Chiu ZK, Lim LL, Rogers SL, Hall AJ. Patterns of vitamin D levels and exposures in active and inactive noninfectious uveitis patients. Ophthalmology. (2020) 127:230–7. doi: 10.1016/j.ophtha.2019.06.030
57. Kump LI, Cervantes-Castañeda RA, Androudi SN, Foster CS, Christen WG. Patterns of exacerbations of chronic non-infectious uveitis in pregnancy and puerperium. Ocul Immunol Inflamm. (2006) 14:99–104. doi: 10.1080/09273940500557027
58. Chiam NPY, Hall AJH, Stawell RJ, Busija L, Lim LLP. The course of uveitis in pregnancy and postpartum. Br J Ophthalmol. (2013) 97:1284–8. doi: 10.1136/bjophthalmol-2013-303358
59. Rabiah PK, Vitale AT. Noninfectious uveitis and pregnancy. Am J Ophthalmol. (2003) 136:91–8. doi: 10.1016/S0002-9394(03)00110-7
60. Chen W-D, Yang Y-H, Lee C-Y, Lai C-H, Liu C-Y, Lai L-J. Pre-eclampsia/eclampsia as a risk factor of noninfectious uveitis among postdelivery women. Am J Ophthalmol. (2019) 198:166–73. doi: 10.1016/j.ajo.2018.10.009
61. Borkar DS, Homayounfar G, Tham VM, Ray KJ, Vinoya AC, Uchida A, et al. Association between thyroid disease and uveitis: results from the pacific ocular inflammation study. JAMA Ophthalmol. (2017) 135:594–9. doi: 10.1001/jamaophthalmol.2017.1009
62. Lin C-J, Tien P-T, Chang C-HMD, Hsia N-Y, Yang Y-C, Lai C-T, et al. Relationship between uveitis and thyroid disease: a 13-year nationwide population-based cohort study in Taiwan. Ocul Immunol Inflamm. (2020) 1–7. doi: 10.1080/09273948.2020.1762899
63. Ansari AS, de Lusignan S, Hinton W, Munro N, Taylor S, McGovern A. Glycemic control is an important modifiable risk factor for uveitis in patients with diabetes: a retrospective cohort study establishing clinical risk and ophthalmic disease burden. J Diabetes Complicat. (2018) 32:602–8. doi: 10.1016/j.jdiacomp.2018.03.008
64. Mollazadegan K, Kugelberg M, Tallstedt L, Ludvigsson JF. Increased risk of uveitis in coeliac disease: a nationwide cohort study. Br J Ophthalmol. (2012) 96:857–61. doi: 10.1136/bjophthalmol-2011-301051
65. Berlinberg EJ, Gonzales JA, Doan T, Acharya NR. Association between noninfectious uveitis and psychological stress. JAMA Ophthalmol. (2019) 137:199–205. doi: 10.1001/jamaophthalmol.2018.5893
66. Maca SM, Schiesser AW, Sobala A, Gruber K, Pakesch G, Prause C, et al. Distress, depression and coping in HLA-B27-associated anterior uveitis with focus on gender differences. Br J Ophthalmol. (2011) 95:699–704. doi: 10.1136/bjo.2009.174839
67. Etminan M, Forooghian F, Maberley D. Inflammatory ocular adverse events with the use of oral bisphosphonates: a retrospective cohort study. C Can Med Assoc J. (2012) 184:E431–4. doi: 10.1503/cmaj.111752
68. French DD, Margo CE. Postmarketing surveillance rates of uveitis and scleritis with bisphosphonates among a national veteran cohort. Retina. (2008) 28:889–93. doi: 10.1097/IAE.0b013e31816576ef
69. Patel D V, Horne A, House M, Reid IR, McGhee CNJ. The incidence of acute anterior uveitis after intravenous zoledronate. Ophthalmology. (2013) 120:773–6. doi: 10.1016/j.ophtha.2012.10.028
70. Eadie B, Etminan M, Mikelberg FS. Risk for uveitis with oral moxifloxacin: a comparative safety study. JAMA Ophthalmol. (2015) 133:81–4. doi: 10.1001/jamaophthalmol.2014.3598
71. Forooghian F, Maberley D, Albiani DA, Kirker AW, Merkur AB, Etminan M. Uveitis risk following oral fluoroquinolone therapy: a nested case-control Study. Ocul Immunol Inflamm. (2013) 21:390–3. doi: 10.3109/09273948.2013.808351
72. Sandhu HS, Brucker AJ, Ma L, VanderBeek BL. Oral Fluoroquinolones and the risk of uveitis. JAMA Ophthalmol. (2016) 134:38–43. doi: 10.1001/jamaophthalmol.2015.4092
73. Xia T, Brucker AJ, McGeehan B, VanderBeek BL. Risk of non-infectious uveitis or myasthenia gravis in patients on checkpoint inhibitors in a large healthcare claims database. Br J Ophthalmol. (2020) 1–4. doi: 10.1136/bjophthalmol-2020-317060
74. Fortes BH, Liou H, Dalvin LA. Ophthalmic adverse effects of immune checkpoint inhibitors: the Mayo Clinic experience. Br J Ophthalmol. (2020) 105:1263–71. doi: 10.1136/bjophthalmol-2020-316970
75. Sobrin L, Yu Y, Susarla G, Chan W, Xia T, Kempen JH, et al. Risk of noninfectious uveitis with female hormonal therapy in a large healthcare claims database. Ophthalmology. (2020) 127:1558–66. doi: 10.1016/j.ophtha.2020.04.034
76. Suzuki J, Goto H. Uveitis associated with sarcoidosis exacerbated by etanercept therapy. Jpn J Ophthalmol. (2009) 53:439–40. doi: 10.1007/s10384-009-0691-6
77. Sieper J, Koenig A, Baumgartner S, Wishneski C, Foehl J, Vlahos B, et al. Analysis of uveitis rates across all etanercept ankylosing spondylitis clinical trials. Ann Rheum Dis. (2010) 69:226–9. doi: 10.1136/ard.2008.103192
78. Khoury G, Morel J, Combe B, Lukas C. Occurrence of anterior uveitis in patients with spondyloarthritis treated with tumor necrosis factor inhibitors: comparing the soluble receptor to monoclonal antibodies in a large observational cohort. Arthritis Res Ther. (2020) 22:94. doi: 10.1186/s13075-020-02187-y
79. Wilder RL. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci. (1998) 840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x
80. Ghanchi FD, Rembacken BJ. Inflammatory bowel disease and the eye. Surv Ophthalmol. (2003) 48:663–76. doi: 10.1016/j.survophthal.2003.08.004
81. Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity. The role of stress hormones and leukocyte trafficking. Ann N Y Acad Sci. (2000) 917:876–93. doi: 10.1111/j.1749-6632.2000.tb05454.x
82. Morey JN, Boggero IA, Scott AB, Segerstrom SC. Current directions in stress and human immune function. Curr Opin Psychol. (2015) 5:13–17. doi: 10.1016/j.copsyc.2015.03.007
83. Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. (2019) 31:319–22. doi: 10.1016/j.joco.2019.05.002
84. Reddy AR, Backhouse OC. Does etanercept induce uveitis? Br J Ophthalmol. (2003) 87:925. doi: 10.1136/bjo.87.7.925
Keywords: non-infectious uveitis, epidemiology, risk factors, systematic review, incidence, prevalence
Citation: Joltikov KA and Lobo-Chan A-M (2021) Epidemiology and Risk Factors in Non-infectious Uveitis: A Systematic Review. Front. Med. 8:695904. doi: 10.3389/fmed.2021.695904
Received: 15 April 2021; Accepted: 17 August 2021;
Published: 10 September 2021.
Edited by:
Alessandra Soriano, Case Western Reserve University, United StatesReviewed by:
Carla Gaggiano, University of Siena, ItalyFrancesco Maria D'Alterio, Imperial College Healthcare NHS Trust, United Kingdom
Copyright © 2021 Joltikov and Lobo-Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ann-Marie Lobo-Chan, YWxvYm8yJiN4MDAwNDA7dWljLmVkdQ==
 Katherine A. Joltikov
Katherine A. Joltikov Ann-Marie Lobo-Chan
Ann-Marie Lobo-Chan