
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 27 August 2021
Sec. Pulmonary Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.694029
This article is part of the Research TopicCOVID-19 Related Acute Vascular Distress Syndrome: from Physiopathology to TreatmentView all 10 articles
Angiotensin converting enzyme 2 (ACE2) seems to be a central actor in the pathophysiology of SARS-Cov-2 infection. First, it acts as the receptor for the virus and permits its attachment to cells expressing ACE2. Second, the relative deficiency of ACE2 during infection could be linked to several clinical features encountered during the disease, like ARDS and coagulation abnormalities. This study explores the strong link between ACE2 and the majority of risk factors for the severe evolution of COVID-19. It seems that all these risks factors are linked to an increased level of ACE2 and/or imbalance in ACE/ACE2.
COVID-19 is a worldwide progressing pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus type 2 (SARS-CoV-2). Present pneumonia (pneumonitis) is an acute respiratory illness associated with a new droplet-borne SARS-CoV-2, which caused the global population to be put under lockdown, with many patients clustered in hospitals. It has a wide spectrum of clinical severity, ranging from asymptomatic to fatal outcomes. The virus possesses 4 main structural proteins: spike, membrane, envelope, and nucleocapsid (1). Of special interest for our discussion is the spike protein, which attaches to human cells through the angiotensin converting enzyme 2 (ACE2) (2). Such a mechanism is common in the two SARS virusus (1, 3). Following host cell binding, with the priming by the transmembrane serine protease 2 (TMPRSS2) and other proteins, the virus and cell membrane fuse, enabling the virus to enter the cell and infect it (1). These interactions with the SARS-CoV-2, ACE2 play a crucial role in viral pathology since it is the viral receptor that provides the opportunity for the virus to invade cells expressing such enzymes. Other than this role as a viral receptor, the physiological role of ACE2 is crucial, as it reduces angiotensin 2 levels (breakdown) and so plays a role as a regulator in the renin angiotensin system balance.
ACE2 has been known for 20 years and has brought major insight to understanding of the complex renin-angiotensin system (RAS) (4, 5). ACE2 is an enzyme, a carboxypeptidase, which cleaves angiotensin 1 into angiotensin 1–9 and angiotensin-2 into angiotensin 1–7 (6). Through those reactions, ACE2 plays a crucial role as a regulator of the RAS. If they are both peptidases, ACE and ACE2 have a different active site and the two enzymes manage to counterbalance each other (5, 7). ACE2 reduces the level of angiotensin 2, thereby reducing its capacity of action as a potent vasopressor and pro-inflammatory signal (6). Furthermore, ACE2 products, mostly angiotensin 1–7, act through a specific pathway to counter angiotensin 2 and mitigate the action of the ligation between angiotensin 2 and the receptor AT1R. The two receptors are of main importance for these pathways. The AT2 receptor (AT2R), Mas receptor (MasR) and induced vasodilation have anti-fibrotic and anti-inflammatory properties (8). Another role of ACE2 is the cleavage of other bioactive peptides than angiotensin and especially bradykinin, more precisely des-Arg-Bradykinin (9, 10). Bradykinin, especially des-Arg-Bradykinin, binds bradykinin receptor B1 (BKB1R). Ligation to BKB1R induces the release of inflammatory chemokines. It has a role in vasodilatation, cellular proliferation, and fibrosis (9, 11). There is also an intricate role of bradykinin with the coagulation and the complement activation (9, 12). All the present findings emphasize the particular vascular features of COVID-19 disease. In this regard, the authors believe that Acute Vascular Distress Syndrome (acronym “AVDS”) seems to be more appropriate for COVID-19 than the usual ARDS (acute respiratory distress syndrome) acronym (13).
ACE2 is widely expressed inside organs, including, in the lungs, cardiovascular system, gut, kidneys, central nervous system, and adipose tissue. As a result of these roles, it is currently thought that ACE2 plays a major role as a cardioprotective actor. It has been linked to several situations of heart failure, hypertension, pulmonary hypertension, diabetes, and acute respiratory distress syndrome (5). ACE2 has well-described associations with better outcomes in the case of cardiac dysfunction and is linked to cardiac fibrosis and inflammation in several studies (5). Moreover, angiotensin 1–7 seems to play a protective role in diabetic cardiomyopathy and nephropathy (14, 15). In this regard, increased expression of ACE2 protects against hypertension (5). ACE2 is also strongly involved in acute pulmonary lesions and fibrosis, as a protector, by inducing an imbalance against RAS hyper activation (5, 16).
As we have seen, the cardioprotective effect of ACE2 could be attributed to several mechanisms, including the degradation of angiotensin 1 and angiotensin 2, and so limits activation of AT1R, production of angiotensin 1–7 and 1–9, which have a direct cardioprotective role, and also contributing to the degradation of bradykinine and thereby limiting its pro-inflammatory effects.
There are several described risk factors for the severe evolution of COVID-19, summarized in Table 1. The earliest studies on the subject clearly showed such an association (17, 18). An important fact to underline is the strong link between ACE2 and the majority of these risk factors. It seems that all these risks factors are linked to an ACE2 increased level and/or imbalance in ACE/ACE2. A study dosing the soluble ACE2, as a surrogate marker for the level of ACE2, showed significantly increased amounts of soluble ACE2 in patients with diabetes, heart failure, older age, and male gender (19). It could be suggested that such a situation, with an increased ACE2 at baseline due to an already imbalanced RAS, may be prone to more severe SARS-CoV-2 infection (20). Hypertension was very early reported as a risk factor for fatal outcomes in COVID-19 (21, 22). Hypertension may be linked to a state of hyper activation of ACE2 to counter regulate the high blood pressure, meaning these patients have a higher number of targets for the virus to attach to. Male gender is also a risk factor for more severe COVID-19 (23). It could be linked to the potential impact of sex hormones on ACE2 expression, RAS balance, or a difference in the proportion of comorbidities (24). As an illustration, ACE2 could be found in higher concentrations in the sputum of asthmatic men or plasma of male patients with cardiac failure (25, 26). Patients of Black ethnicity are also at risk of severe COVID-19 and death from COVID-19 (27, 28). However, such risk is currently not well understood, as even if a higher proportion of patients are hospitalized or have fatal outcomes, patients of Black ethnicity seem to have a higher risk when adjusted for multiple factors (28). Patients from Black ethnicities often have comorbidities such as diabetes or hypertension, risk factors that have already been described for severe COVID-19 (28). Moreover, social disparities such as disadvantages in housing and more globally systemic structural disadvantages put such a population at higher risk. This may explain the increased risk for patients of Black ethnicities. Another reason for these comorbidity and population characteristics is that a potential risk factor for severe COVID-19 is decreased levels of ACE2 at baseline.
An interesting paper by Peters et al. on COVID-19 related gene expression in the sputum in asthmatic patients discusses these points of view (25). Patients of Black ethnicities seem to have an increased expression of ACE2 in sputum cells, along with male gender and diabetic patients. This raises the question of specific risk linked to increased ACE2 in Black people. However, the link between COVID-19, ethnicity, and prognosis remains difficult to prove, as underlined by the recent study of Colarusso et al. (9). The authors showed that if Black ethnicities were admitted to ICU they died more frequently during the first “wave”. This was not obvious during the second wave, as the authors only had an increased risk of ICU admission without an increase in mortality (29). These papers underline the already discussed risk factors of male gender and diabetes. Being overweight and obese, are also risk factors. It is common knowledge that obesity is linked to hypertension, diabetes, and heart failure, as already discussed. Moreover, obese patients may have a pro-inflammatory state, predisposing them to a higher impact of RAS imbalance (11).
An important question is the place of treatment for hypertension targeting the ACE, including ACE-inhibitors of Angiotensin receptor blockers. Such treatment is deeply linked to the RAS and has been used in a large proportion of patients with hypertension, diabetes, or obesity, as all these comorbidities are often associated. Interestingly, these medications were linked to less severe disease (and even better outcome) in pneumonia related to influenza infection and so raised the question of their role in COVID-19 infection (30–32). However, such benefits for COVID-19 patients undergoing pneumonia treatment are currently unproven and unfounded (33, 34).
In focussing on ACE2, we see that all these risk factors could be linked to the more severe features of COVID-19 disease. There are populations for which specific research needs to be done in order to investigate the impact of ACE2 and therapy aiming at restoring the RAS balance.
In case of a SARS Cov-2 infection, there is ligation of the virus to ACE2 with its spike protein. Such ligation to ACE2 caused its internalization and down-regulation following SARS-CoV-2 cellular entry (6, 11). In such a case, the decrease in ACE2 activity creates an imbalance in signaling by ACE1 and ACE2 due to deficiency in ACE2. As we discussed earlier, such decreases in ACE2 lead to an imbalance, where angiotensin 2 is the main actor, as shown in Figure 1.
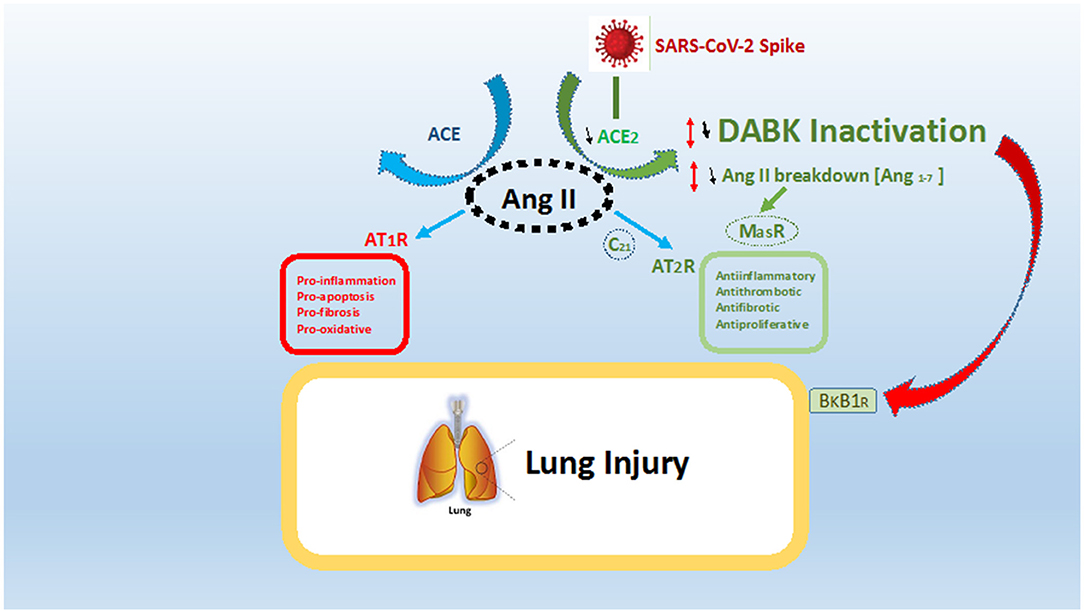
Figure 1. ACE/ACE2 imbalance and effect in case of SARS-CoV-2 infection. SARS-CoV-2 spike protein ligates angiotensin conversion enzyme 2 (ACE2) and leads to a relative deficiency in ACE2. Such deficiency leads to both an imbalance in the ACE/ACE2 system and an over activation of the ACE pathway with over production of angiotensin 2 (Ang II) ligating the receptor AT1R. The present fact leads to a global pro-inflammatory, pro-fibrotic, and pro-oxidative state (in red). ACE2, through degradation of Ang II to angiotensin 1–7, leads to both a decrease in Ang II levels and stimulation of anti-inflammatory and anti-fibrotic pathways via AT2R and MasR (in green). Moreover, ACE2 inactivate des-Arg-Bradykinin (DABK), which in the case of ligation to receptor Bradykinine-B1-receptor (BkB1R) leads to inflammation and vascular permeability (red arrow). In this regard, the final result of ACE2 deficiency is both the promotion of lung inflammation and a decrease in lung anti-inflammatory effects, inducing SARS-CoV-2 acute respiratory syndrome.
A higher level of angiotensin 2 is linked to the pro-inflammatory and pro-fibrotic situation after ligation to AT1R. Moreover, a severe decrease in ACE2 has a double effect: first, there is a decrease of Angiotensin 1-7, lowering activation of the MasR or AT2R, which impedes anti-inflammatory and anti-proliferation pathways (4, 5, 7). Second, there is an increase of D-Arg bradykinin, with inflammatory and vasoactive properties through BKB1R (9, 11). This finally leads the RAS equilibrium to imbalanced conditions characterized by pro-inflammatory, pro-apoptosis, and pro-oxidative states. Moreover, the deregulation of RAS, especially in patients who already are in a state of ACE/ACE2 imbalance, could lead to more severe COVD-19 (5).
If we look specifically at the lung, ACE2 deficiency is known to be linked to acute lung injury. It has a role in limiting the angiotensin 2 hypoxic vasoconstriction but also pro-fibrotic and inflammatory effect, both meet in case of severe SARS-CoV-2 acute respiratory distress syndrome (1, 7, 11). Diminished levels of ACE2 and an imbalance in the ACE/ACE2 system could be a major factor in the outcome of COVID-19, as previously noted in laboratory experimentation on acute lung injury. The effect of imbalance could also explain the significant impact of severe SARS-CoV-2 infection on several systems, especially cardiovascular, including systemic endothelitis, renal, and coagulation (1, 7, 11, 35, 36).
In other pulmonary infections, ACE2 and angiotensin II were also studied and potentially linked to disease severity. In particular, influenza infection, in which a link between ACE2 deficiency and lung injury severity has been observed (37, 38). Moreover, increased levels of Angiotensin-II in a patient with severe influenza infection or coxsackie virus, emphasized the key role of ACE2 in other viral lung infections leading to ARDS (39). The role of the RAS system in potential lung cytokine storm and fibrosis could explain such an association between ACE2, the RAS system, and viral ARDS (39, 40).
To conclude, ACE2 seems to be a central actor in the pathophysiology of SARS-Cov-2 infection. First, it acts as the receptor for the virus and permits its attachment to cells expressing ACE2. Second, the relative deficiency of ACE2 during infection could be linked to several clinical features encountered during the disease, such as ARDS, vascular inflammation, and coagulation abnormalities (41, 42). Further research is needed to better understand the role of ACE2 in virus pathophysiology and ACE2 as a potential therapeutic target. In this regard, a soluble form of ACE2 may both slow viral entry into cells by competitively binding with SARS-CoV-2 and protect the lung from injury through its unique enzymatic function (2).
KB contributed to the conception and design of the review and Figure 1. VC design and first draft. RG designed Table 1. All authors contributed to manuscript revision, and read and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology. (2020) 35:288–301. doi: 10.1152/physiol.00019.2020
2. Zhang H, Penninger JM Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. (2020) 46:586–90. doi: 10.1007/s00134-020-05985-9
3. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. (2003) 426:450–4. doi: 10.1038/nature02145
4. Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. (2000) 87:E1–9. doi: 10.1161/01.RES.87.5.e1
5. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. (2020) 126:1456–74. doi: 10.1161/CIRCRESAHA.120.317015
6. Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, et al. The emerging role of ACE2 in physiology and disease. J Pathol. (2007) 212:1–11. doi: 10.1002/path.2162
7. Sriram K, Insel PA. A hypothesis for pathobiology and treatment of COVID-19: the centrality of ACE1/ACE2 imbalance. Br J Pharmacol. (2020) 177:4825–44. doi: 10.1111/bph.15082
8. Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. (2005) 289:H2281–2290. doi: 10.1152/ajpheart.00618.2005
9. Colarusso C, Terlizzi M, Pinto A, Sorrentino R. A lesson from a saboteur: High-MW kininogen impact in coronavirus-induced disease 2019. Br J Pharmacol. (2020) 177:4866–72. doi: 10.1111/bph.15154
10. Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. (2002) 277:14838–43. doi: 10.1074/jbc.M200581200
11. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. (2020) 251:228–48. doi: 10.1002/path.5471
12. Coccheri S. COVID-19: The crucial role of blood coagulation and fibrinolysis. Intern Emerg Med. (2020) 15:1369–73. doi: 10.1007/s11739-020-02443-8
13. Mahjoub Y, Rodenstein DO, Jounieaux V. Severe Covid-19 disease: rather AVDS than ARDS? Crit Care. (2020) 24:327. doi: 10.1186/s13054-020-02972-w
14. Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, et al. Angiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol. (2014) 306:F812–821. doi: 10.1152/ajprenal.00655.2013
15. Patel VB, Basu R, Oudit GY. ACE2/Ang 1-7 axis: A critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte. (2016) 5:306–11. doi: 10.1080/21623945.2015.1131881
16. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. (2005) 436:112–6. doi: 10.1038/nature03712
17. Edler C, Schroder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Correction to: Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. (2020) 134:1977. doi: 10.1007/s00414-020-02336-7
18. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. (2020) 323:2052–9. doi: 10.1001/jama.2020.6775
19. Wallentin L, Lindback J, Eriksson N, Hijazi Z, Eikelboom JW, Ezekowitz MD, et al. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur Heart J. (2020) 41:4037–46. doi: 10.1093/eurheartj/ehaa697
20. Sama IE, Voors AA, van Veldhuisen DJ. New data on soluble ACE2 in patients with atrial fibrillation reveal potential value for treatment of patients with COVID-19 and cardiovascular disease. Eur Heart J. (2020) 41:4047–9. doi: 10.1093/eurheartj/ehaa761
21. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934–43. doi: 10.1001/jamainternmed.2020.0994
22. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
23. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. (2020) 8:e20. doi: 10.1016/S2213-2600(20)30117-X
24. Young MJ, Clyne CD, Chapman KE. Endocrine aspects of ACE2 regulation: RAAS, steroid hormones and SARS-CoV-2. J Endocrinol. (2020) 247:R45–62. doi: 10.1530/JOE-20-0260
25. Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19-related Genes in Sputum Cells in Asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. (2020) 202:83–90. doi: 10.1164/rccm.202003-0821OC
26. Sama IE, Voors AA. Circulating plasma angiotensin-converting enzyme 2 concentration is elevated in patients with kidney disease and diabetes. Eur Heart J. (2020) 41:3099. doi: 10.1093/eurheartj/ehaa527
27. Carethers JM. Insights into Disparities Observed with COVID-19. J Intern Med. (2020) 289:463–73. doi: 10.1111/joim.13199
28. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. (2020) 382:2534–43. doi: 10.1056/NEJMsa2011686
29. Mathur R, Rentsch CT, Morton CE, Hulme WJ, Schultze A, MacKenna B, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. (2021) 397:1711–24. doi: 10.1016/S0140-6736(21)00634-6
30. Christiansen CF, Heide-Jorgensen U, Rasmussen TB, Bodilsen J, Sogaard OS, Maeng M, et al. Renin-angiotensin system blockers and adverse outcomes of influenza and pneumonia: a danish cohort study. J Am Heart Assoc. (2020) 9:e017297. doi: 10.1161/JAHA.120.017297
31. Davis TME, Davis WA. Influence of renin-angiotensin system inhibitors on lower-respiratory tract infections in type 2 diabetes: the fremantle diabetes study phase II. Diabetes Care. (2020) 43:2113–20. doi: 10.2337/dc20-0895
32. Jeffery MM, Cummins NW, Dempsey TM, Limper AH, Shah ND, Bellolio F. Association of outpatient ACE inhibitors and angiotensin receptor blockers and outcomes of acute respiratory illness: a retrospective cohort study. BMJ Open. (2021) 11:e044010. doi: 10.1136/bmjopen-2020-044010
33. Dai XC, An ZY, Wang ZY, Wang ZZ, Wang YR. Associations Between the Use of Renin-Angiotensin System Inhibitors and the Risks of Severe COVID-19 and Mortality in COVID-19 Patients With Hypertension: A Meta-Analysis of Observational Studies. Front Cardiovasc Med. (2021) 8:609857. doi: 10.3389/fcvm.2021.609857
34. Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, et al. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann Intern Med. (2020) 173:195–203. doi: 10.7326/M20-1515
35. Nagele MP, Haubner B, Tanner FC, Ruschitzka F, Flammer AJ. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis. (2020) 314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014
36. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
37. Imai Y, Kuba K, Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. (2008) 93:543–8. doi: 10.1113/expphysiol.2007.040048
38. Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. (2014) 4:7027. doi: 10.1038/srep07027
39. Gao YL, Du Y, Zhang C, Cheng C, Yang HY, Jin YF, et al. Role of renin-angiotensin system in acute lung injury caused by viral infection. Infect Drug Resist. (2020) 13:3715–25. doi: 10.2147/IDR.S265718
40. Mascolo A, Scavone C, Rafaniello C, De Angelis A, Urbanek K, di Mauro G, et al. The Role of Renin-Angiotensin-Aldosterone System in the Heart and Lung: Focus on COVID-19. Front Pharmacol. (2021) 12:667254. doi: 10.3389/fphar.2021.667254
41. Bendjelid K. Should we use angiotensin II infusion in COVID-19-associated vasoplegic shock? Crit Care. (2020) 24:407. doi: 10.1186/s13054-020-03144-6
Keywords: pathophysiology, COVID-19 pandemic, angiotensin-converting enzyme, SARS – CoV – 2, bradykinin - analogs and derivatives, lung injury
Citation: Cousin VL, Giraud R and Bendjelid K (2021) Pathophysiology of COVID-19: Everywhere You Look You Will See ACE2! Front. Med. 8:694029. doi: 10.3389/fmed.2021.694029
Received: 12 April 2021; Accepted: 02 June 2021;
Published: 27 August 2021.
Edited by:
Yazine MAHJOUB, University Hospital Center (CHU) of Amiens, FranceReviewed by:
Arnaud Friggeri, Hospices Civils de Lyon, FranceCopyright © 2021 Cousin, Giraud and Bendjelid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karim Bendjelid, a2FyaW0uYmVuZGplbGlkQGhjdWdlLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.