- 1Department of Orthopaedics, Children's Hospital of Chongqing Medical University, Chongqing, China
- 2Ministry of Education Key Laboratory of Child Development and Disorders, National Clinical Research Center for Child Health and Disorders, China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing Engineering Research Center of Stem Cell Therapy, Children's Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Neurosurgeons, Children's Hospital of Chongqing Medical University, Chongqing, China
Background: There is no uniform treatment for pathological scars, including keloids and hypertrophic scars, in clinic currently. Previously, multiple randomized controlled trials have examined the clinical efficacy of different treatments. Nonetheless, the results are inconsistent, and many treatments have not been directly compared. This makes it difficult to conclude which approach is more favorable, in terms of efficacy and safety, for the treatment of pathological scarring. This study aimed at evaluating the efficacy of different injection and topical treatment strategies for hypertrophic scar and keloid.
Methods: Relevant literature from PubMed, Medline, Embase, Scopus, the Cochrane Central Register of Controlled Trials (CCRCT), and WHO International Clinical Trials Registry Platform (WHO-ICTRP) were searched, from database inception through November 2020. Randomized clinical trials evaluating different treatment strategies of pathological scars, including triamcinolone acetonide (TAC), verapamil (VER), 5-fluorouracil (5-FU), botulinum toxin A (BTA), bleomycin (BLM), and silicone gels were included in the study.
Results: The network meta-analysis included a total of 2,009 patients from 29 studies. A network meta-analysis of injection and topical treatment strategies showed that the efficacy of TAC combined with BTA was best in the treatment of pathological scars. Combination therapies of TAC with 5-FU and TAC with BTA significantly improved the clinical efficiency. However, there was no statistically significant difference between other treatment strategies. The order of efficacy predicted by the surface under the cumulative ranking (SUCRA) curve was as follows: TAC+BTA (82.2%) > TAC+5-FU (69.8%) > BTA (67.3%) > 5-FU+silicone (59.4%) > TAC+silicone (58.3%) > 5-FU (49.8%) > BLM (42.0%) > TAC (26.7%) > VER (26.2%) > silicone (18.3%). There was no publication bias revealed based on the funnel diagram.
Conclusion: This study recommends intralesional injection of TAC-BTA and TAC-5-FU combined therapies. But for patients who cannot tolerate the side effects, the use of silicone gels in combination with TAC is recommended. However, these conclusions need to be further confirmed by more randomized controlled trials.
Introduction
Pathological scars, including hypertrophic scars and keloids, are mostly caused by the formation of a large extracellular matrix and proliferation of fibroblasts. The formation is characterized by uncontrolled deposition of collagen resulting from destruction of the balance between the catabolic and anabolic effects of collagen during wound healing (1). Patients with keloids and hypertrophic scars may present with symptoms such as pain, erythema, itching, and the persistent growth of lumps. These may lead to malformations in appearance and function as well as cause physical and psychological pain to the patient. Severe symptoms can affect the self-confidence of patients, leading to inferiority complex and seriously affecting the quality of life and mental health (2). The mechanism of pathological scar formation is not fully understood (3, 4). Some studies have suggested that the formation of scar may involve a variety of cells like fibroblast, myofibroblast, and mastocyte (5, 6), cytokines like transforming growth factor-β (TGF-β2), and tumor necrosis factor-α (7–9), extracellular matrix (deposition of collagen and glycolaminoglycan) (10), and spatial structure of tissue (excessive angiogenesis and repair of the spatial network between cells) (11). Demographic characteristics, such as race, sex, and age, as well as external factors like type of injury, also may play a significant role in scar formation (12, 13). Darker skin phenotypes, namely, Fitzpatrick's phototype classification (1975) and skin types IV and V (moderately pigmented), are prone to pathological scars after injury. In addition, women and teenagers are more prone to scars than men and adults, respectively. Elsewhere, keloids can be seen with chronic inflammatory skin diseases such as hidradenitis suppurativa and have been reported to appear spontaneously without trauma, mostly with syndromes (14–16).
There are many treatments available for keloid and hypertrophic scars, including topical therapy, drug injections, pressure therapy, laser therapy, surgery, and cryotherapy (17). Silicone gels are commonly used for topical treatment. Specifically, they are considered as first-line agents for the treatment of mild hypertrophic scars (14, 15, 18, 19). Silicone dressing can be offered to patients with predisposition to develop keloid or hypertrophic scar after future any surgical intervention. Silicone dressing is hypothesized to act by hydrating the wound, inhibiting collagen deposition, and downregulating TGF-β2 (20). Hydrated and occluded environment impairs capillary activity and the continuation of subsequent pathological regeneration signals (21). This affects fibroblast regulation and reduces synthesis of collagen (22). Hydration stabilizes mastocytes, thereby causing suppressive effects on inflammation (23). Silicone gel therapy is offered to patients as a prophylaxis and as a non-invasive treatment following excessive scarring. It is worthy to note that previous studies reported conflicting findings on silicone gels' efficacy (20). Drug injection commonly used includes triamcinolone acetonide (TAC), 5-fluorouracil (5-FU), botulinum toxin A (BTA), bleomycin (BLM), and verapamil (VER). TAC, the most used corticosteroid for the treatment of keloids and hypertrophic scars (24, 25), acts by anti-inflammatory and antimitotic mechanisms. It inhibits the growth of fibroblasts and reduces endothelial budding and synthesis of procollagen and glycosaminoglycan. Also, it enhances the degeneration of collagen and fibroblasts (26) and triggers a significant decrease in VEGF, α-1-antitrypsin, and α-2-macroglobulin levels (27–30).
The 5-FU is a pyrimidine analog that inhibits thymidine synthase, which inhibits nucleic acid synthesis and cell proliferation. In vivo and in vitro experiments have shown that it inhibits fibroblast proliferation, angiogenesis, and TGF-β-induced collagen type I expression, while increasing fibroblast apoptosis (31). BTA, isolated from Clostridium botulinum (32), is a potent neurotoxin that can block neuromuscular conduction. It can make fibroblasts stationary in G0 and G1 phases of the cell cycle (33) and non-proliferative state. This is realized by reducing the tension at the edge of the healing wound (34), which reduces the expression of TGF-β1, thereby inhibiting scar formation (35, 36). BLM, derived from Streptomyces verticillus, is cytotoxic to keratinocytes and eccrine epithelial cells (37, 38). It is an antitumor, antiviral, and antimicrobial agent that inhibits DNA, RNA, and protein synthesis. It also reduces the level of lysine oxidase, a cross-linked enzyme involved in collagen maturation (39). VER is a calcium channel blocker that has been shown to increase the synthesis of pro-collagenase in keloid, hypertrophic scars, and normal cultured fibroblasts. This leads to actin filamolymerization, cell conformational changes, and apoptosis and ultimately reduced fibrous tissue production (40, 41). It may also inhibit the frequently elevated cytokines in keloids, such as IL-6 VEGF and TGF-β1 (42).
The above treatment methods can be used as monotherapy or combination therapy. Studies have shown that each method has different degrees of efficacy (43), and each method has its corresponding side effects. However, literature regarding these methods in management of hypertrophic scars and keloids is often limited. They include small patient numbers, lack blinding and controls, and assume retrospective design and insufficient follow-up time. Also, some include both keloids and hypertrophic scars within the same treatment group. Keeping these confounding factors in mind, these studies have shown that the aforementioned methods can be efficacious in preventing the development of as well as reducing existing hypertrophic scars and keloids.
When comparing the advantages and disadvantages of three or more interventions, traditional meta-analysis can no longer provide a definite answer. Unlike the traditional meta-analysis, network meta-analysis (NMA) has the advantage that it can use direct and indirect comparison methods to rank the efficacy of different interventions and provide an overview of the optimal plan (44). There have been previous mesh meta-analyses on similar topics (45, 46), but the interventions evaluated are different from ours, and new interventions are constantly being developed for the prevention and treatment of scarring. In this study, an NMA was used to evaluate the efficacy and tolerability of TAC, 5-FU, BLM, silicone gel, BTA, and VER. Furthermore, their two-drug combination therapy, including effective rate and adverse reaction rate and recurrence rate, was explored. The findings of this study provide a clinical scientific and reliable summary that can help guide treatment decisions.
Materials and Methods
Search Strategy
This study conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (46). The existing studies from EMBASE, PubMed Medline, Scopus, the Cochrane Central Register of Controlled Trials (CCRCT), and WHO International Clinical Trials Registry Platform (WHO-ICTRP) electronic databases were searched in accordance with the Cochrane Collaboration criterion. Randomized controlled trials (RCTs) investigating the safety and efficacy of all current therapies used for the management of hypertrophic scars and keloids, from database inception through November 2020, were included in the study. Besides, the reference lists for the identified RCT were also hand-searched for possible relevant articles to avoid relevant information being missing. The search was only limited to human studies, and no language restrictions were posted on the setting. The document retrieval adopted the combination of subject word and random word. The search query was formulated based on the following keywords: “scar” or “hypertrophic scars” or “keloids” or their synonyms and “scar management” or “Triamcinolone Acetonide” or “Verapamil” or “5-fluorouracil” or “Botulinum Toxins Type A” or “Bleomycin” or “Silicone” or their synonyms.”
Inclusion and Exclusion Criteria of Studies
Inclusion Criteria
(1) RCT, no language limitation; (2) any patient with hypertrophic scar or keloid, regardless of age, regardless of whether the diagnosis of the scars was clinical or histopathological; (3) TAC or VER or 5-FU or BTA or BLM or silicone, whether it is the monotherapy of the above therapies or the combination of the two therapies; and (4) outcome indicators: must include effective rate, including but not limited to adverse reaction rate and recurrence rate. The effective rate of each therapeutic strategy was calculated using the following formula: [n (effective events)/n (total events)]. Exclusion criteria: (1) case control studies, case reports, abstracts from conference proceedings, and non-human studies, among others; (2) research on special populations, such as pregnant women with immunodeficiency; (3) study on non-above five interventions; (4) lack of relevant outcome data; and (5) <10 people per group.
Data Extraction and Quality Assessment
Two reviewers (YS and LYJ) extracted data independently using a predefined data extraction form. After the duplicates were eliminated, qualified studies were preliminarily screened, full text was downloaded by browsing the titles and abstracts, and finally, the included studies were determined. Disagreements were resolved by discussion or consensus with a third reviewer (LC). The data extracted included the first author; country, study characteristics (i.e., year and duration); participant characteristics (i.e., mean age, proportion of male and sample size); of the experimental and control group treatments; and measured outcomes. For studies with insufficient information, the reviewers contacted the primary authors, when possible, to acquire and verify the data.
Statistical Analysis
Data for the included literature were analyzed using R software and Stata software. The heterogeneity was evaluated using the I2 test, with I2 < 50% scoring for low heterogeneity, while I2 >50% scoring for significant heterogeneity. The risk ratio (RR) was used as an effect statistical index for the effective rate; adverse reaction rate, recurrence rate, and its 95% confidence interval (CI) were calculated. Four Markov chains were used for the initial value setting model. The number of iterations for the first update was set as 50,000; and the number of iterations for the further update was set as 100,000. The first 50,000 annealing times were used to eliminate the influence of the initial value, and the sampling started from 50,001. The random-effects model was used within Bayesian NMA. A random-effects model with small deviance information criterion (DIC) was selected in this study. The smaller the DIC value, the better the model fitting effect (47). Convergence of iteration was evaluated using the Brooks–Gelman–Rubin method. In the case of a closed loop, the consistency between direct and indirect comparisons was judged based on node analysis. A p-value < 0.05 scored positive for inconsistency; thus, the inconsistency model was used for analysis. Local inconsistency was tested using the node-split Model, and a p-value >0.05 indicated that the heterogeneity of the included studies was small, so the consistency model was used for analysis. The potential scale reduction factor (PSRF) reflects convergence. When PSRF is close to 1 or equal to 1, it indicates that good convergence efficiency has been achieved, and the reliability of the results obtained using consistency model analysis is high (48). A scatter plot was drawn according to surface under the cumulative ranking of the efficacy and tolerance of each therapeutic measure. The bias risk of the included literature was determined using Reviewer Manager (RevMan) 5.3 software. Publication bias was evaluated with a funnel plot (49). A p-value < 0.05 was regarded as statistically significant.
Results
Literature Search and Characteristics of Included Studies
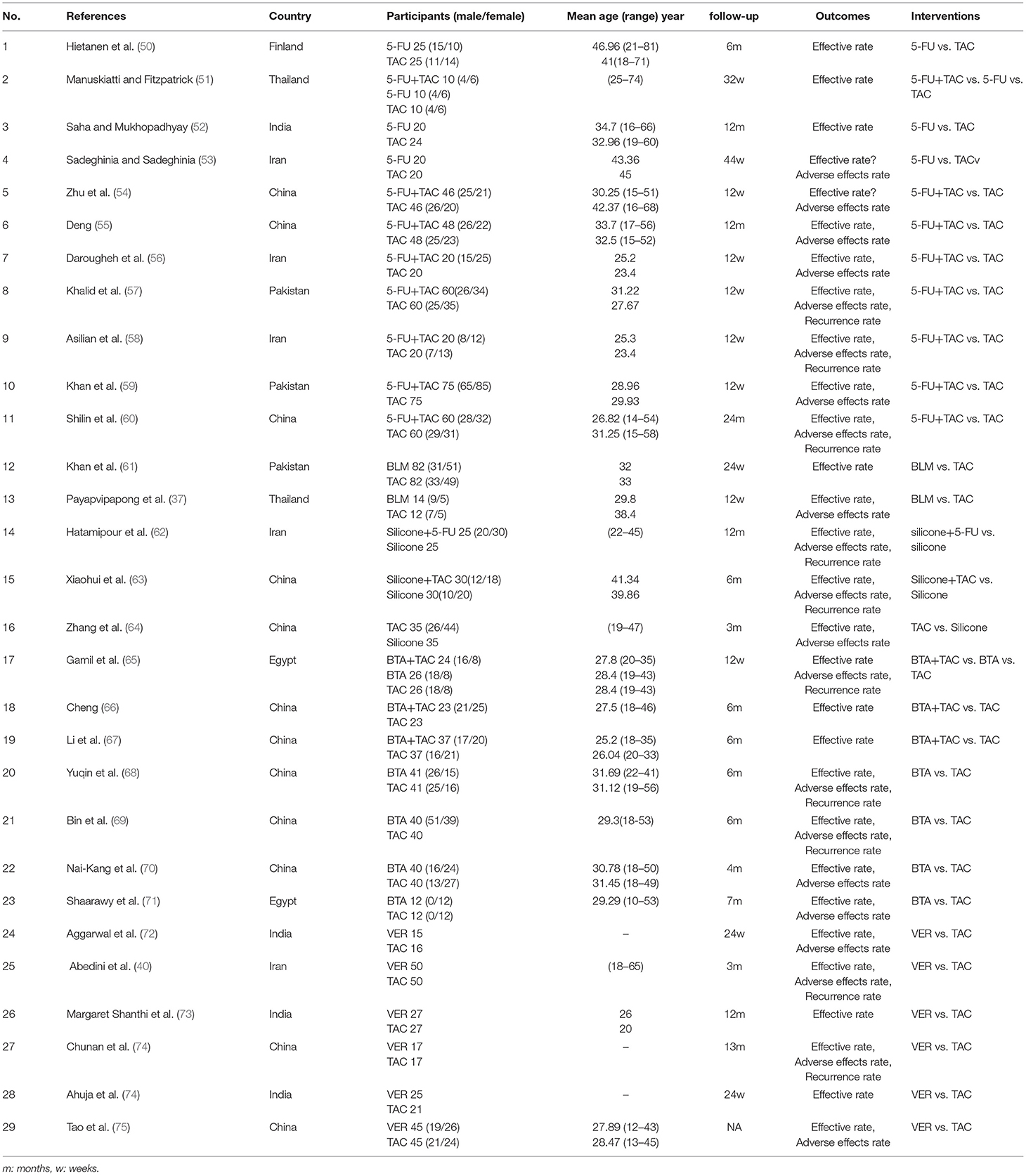
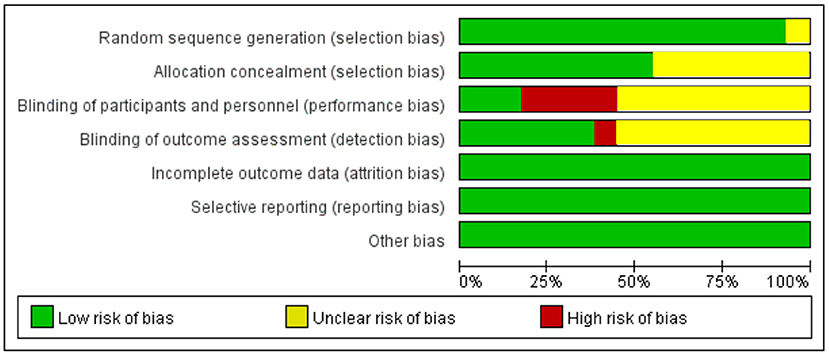
A detailed overview of the selection process is shown in Figure 1. A total of 29 studies, including 2,009 patients, were enrolled in the meta-analysis. The characteristics and methodology assessment of individual studies included in the meta-analysis are described in Table 1. The risk of bias assessment is shown in Figure 2.
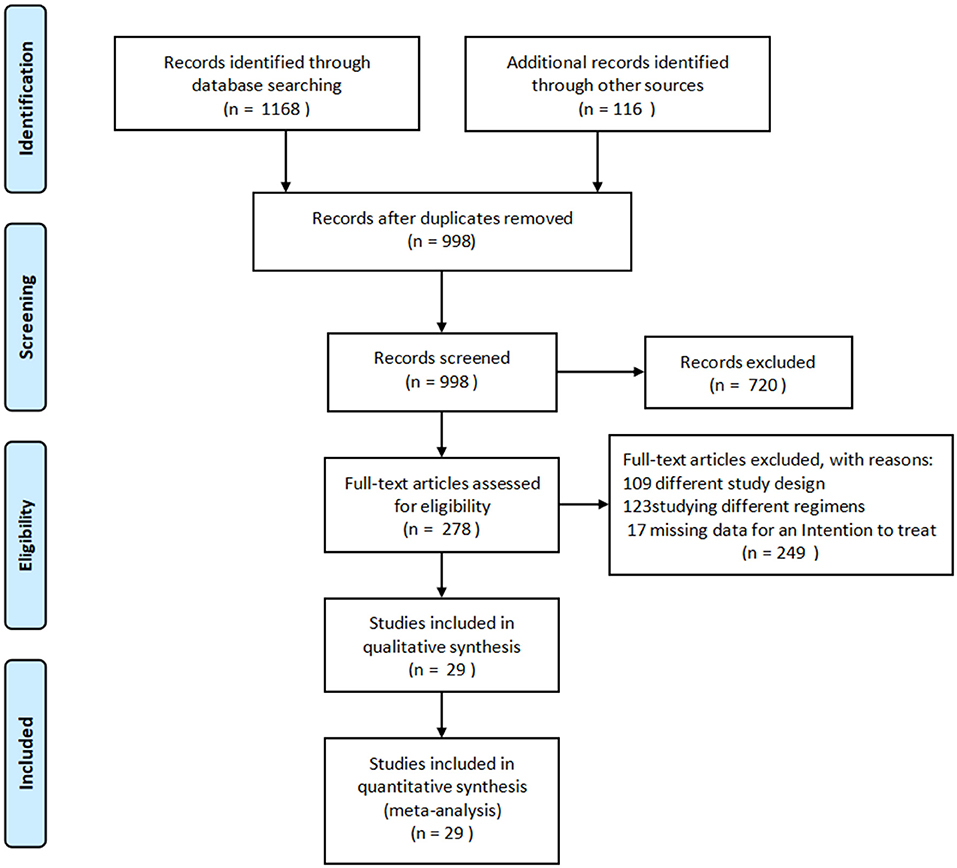
Figure 1. PRISMA flow diagram of the study selection process. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Effective Rate
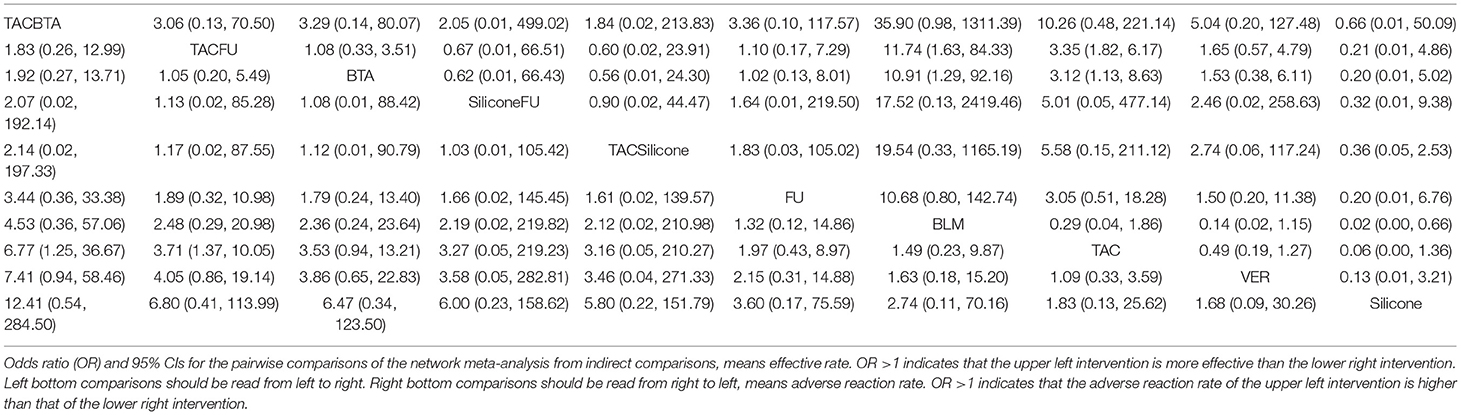
Effective rates were reported in 29 studies (37, 40, 50–76), including six monotherapy measures and four combination measures. The former comprised A, TAC; B, 5-FU; C, BLM; D, silicone gels; E, BTA; and F, VER. The latter comprised A+B, TAC combined with 5-FU; D+A, silicone gels combined with TAC; D+B, silicone gels combined with 5-FU; and A+E, TAC combined with BTA. The network diagram of effective rate is shown in Figure 3. The 10 treatment measures formed 45 pair comparisons. In terms of treatment effectiveness, there were 11 direct comparisons among 29 studies, and the rest were indirect comparisons.
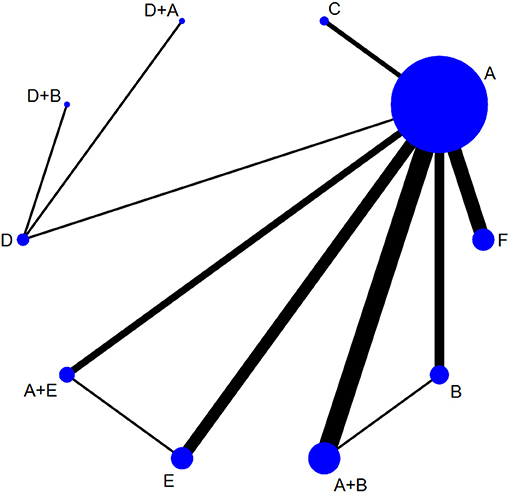
Figure 3. Network diagram of effective rate. A, TAC; B, 5-FU; C, BLM; D, silicone; E, BTA; F, VER; A+B, TAC+5-FU; D+A, silicone+TAC; D+B, silicone+5-FU; A+E, TAC+BTA. TAC, triamcinolone acetonide; 5-FU, 5-fluorouracil; BLM, bleomycin; VER, verapamil.
Detection of Inconsistency
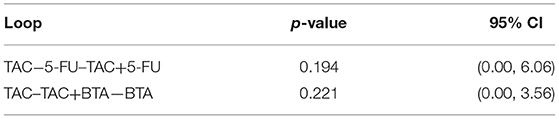
Eleven direct comparisons constituted two triangular closed loops. The Z-test results showed that the lower limits of 95% CI were 0. This implies that each closed loop was consistent, as shown in Table 2. The results of the node-splitting model showed consistency in the direct and indirect comparison results all at p > 0.05 (Table 3).
Results of Network Meta-Analysis and Publication Bias
Results of NMA showed that compared with TAC, only TAC combined with BTA and TAC combined with 5-FU could improve efficacy rate. The difference in the efficacy rate among these therapies was statistical significant at p < 0.05. Interestingly, there was no statistically significant difference among other interventions at p < 0.05 (Figure 4; Table 4). The funnel diagram showed good symmetry, indicating no obvious publication bias. This is illustrated in Figure 5.
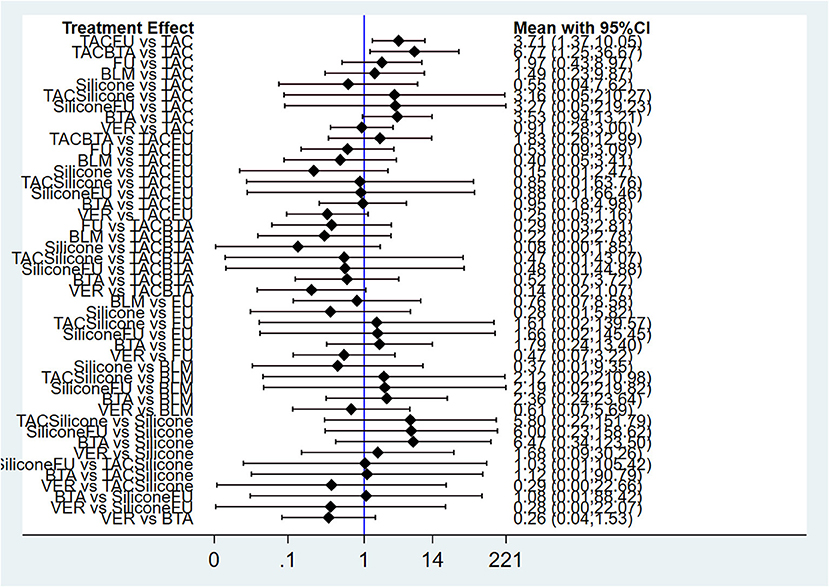
Figure 4. Forest plot of efficacy rate for pairwise treatment comparison. FU, 5-FU; TACFU, TAC+5-FU; TACBTA, TAC+BTA; TACSilicone, TAC+silicone; SiliconeFU, silicone+5-FU. TAC, triamcinolone acetonide; 5-FU, 5-fluorouracil.
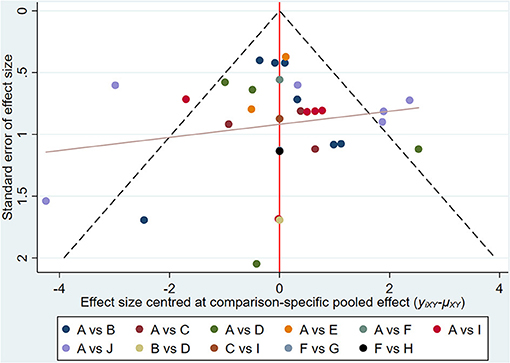
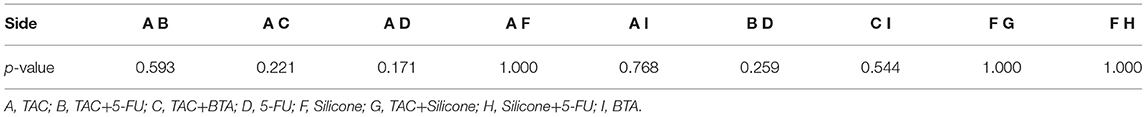
Figure 5. Funnel diagram for effective rate. A, TAC; B, TAC+5-FU; C, TAC+BTA; D, 5-FU; E, BLM; F, silicone; G, TAC+silicone; H, silicone+5-FU; I, BTA; J, VER. TAC, triamcinolone acetonide; 5-FU, 5-fluorouracil; BLM, bleomycin; VER, verapamil.
Ranking Results
Results of the convergence analysis showed that PSRF was equal to 1, indicating that the model had good convergence. Based on the Bayesian theory of Markov chain Monte Carlo (MCMC) method, random-effects model for NMA results showed the following: TAC+BTA > TAC+5-FU > BTA > 5-FU+silicone > TAC+silicone > 5-FU > BLM > TAC > VER > silicone (Figure 6) (The dose and interval for the combination of TCA with BTA was given in Supplementary Table 1 to help readers understand the regimens easily, as this is per our study the most effective).
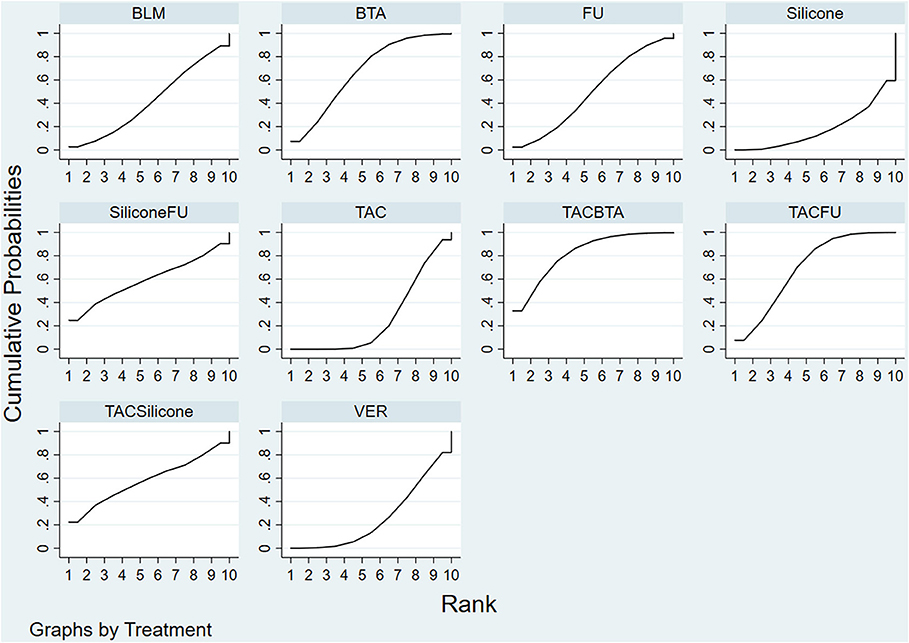
Figure 6. SUCRA efficacy rate ranking curve. FU, 5-FU; SiliconeFU, silicone+5-FU; TACBTA, TAC+BTA; TACFU, TAC+5-FU; TACSilicone, TAC+silicone. SUCRA, surface under the cumulative ranking; TAC, triamcinolone acetonide; 5-FU, 5-fluorouracil.
Adverse Effect Rate
Adverse effect rates were reported in 21 studies (37, 40, 53–60, 62–65, 68–73, 75, 76), with a total of 10 measures. The former comprised A, TAC; B, 5-FU; C, BLM; D, Silicone gels; E, BTA, and F, VER. The latter comprised A+B, TAC combined with 5-FU; D+A, Silicone gels combined with TAC; D+B, Silicone gels combined with 5-FU; and A+E, TAC combined with BTA. The 10 treatment measures formed 45 different pair comparisons. In terms of treatment effectiveness, there are 11 direct comparisons among 21 studies; the rest were indirect comparisons. See Supplementary Figure 1.
Detection of Inconsistency
Eleven direct comparisons constituted one triangular closed loop. The Z-test results showed that the lower limits of 95% CI were 0. This implies that each closed loop was consistent, as shown in Supplementary Table 2. The results of the node-splitting model showed consistency in the direct and indirect comparison results, all at p > 0.05 (Supplementary Table 3).
Results of Network Meta-Analysis and Publication Bias
Results of NMA showed that compared with TAC, only TAC combined with 5-FU and BTA could decrease adverse effect rate. In addition, compared with BLM, BTA, silicone gel, and TAC combined with 5-FU could decrease adverse effect rate. The difference in the efficacy rate among these therapies was statistical significant at p < 0.05. Interestingly, there was no statistically significant difference among other interventions at p < 0.05 (Figure 7; Table 4). The funnel diagram showed good symmetry, indicating no obvious publication bias. This is illustrated in Supplementary Figure 2.
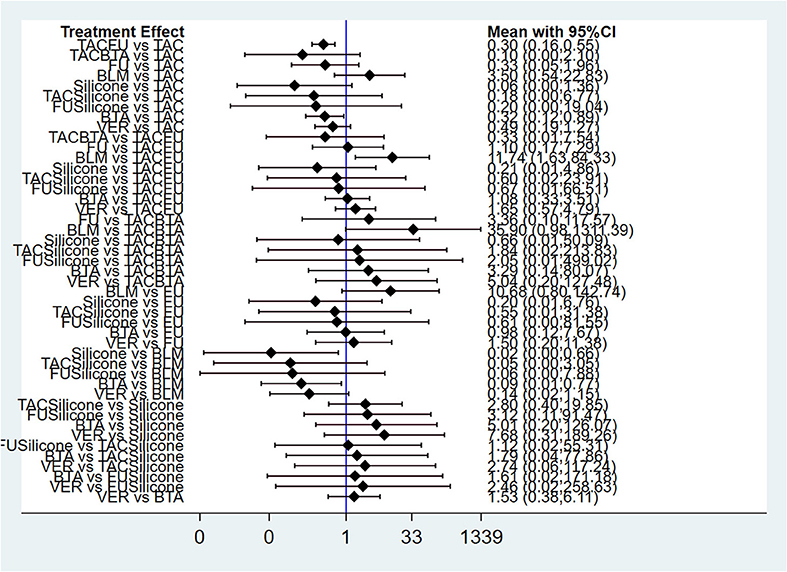
Figure 7. Forest plot of adverse effect rate for pairwise treatment comparison. FU, 5-FU; TACFU, TAC+5-FU; TACBTA, TAC+BTA; TACSilicone, TAC+silicone; SiliconeFU, silicone+5-FU. TAC, triamcinolone acetonide; 5-FU, 5-fluorouracil.
Ranking Results
Results of the convergence analysis showed that PSRF was equal to 1, indicating that the model had good convergence. Based on the Bayesian theory of MCMC method, use random-effects model for NMA results showed the following: silicone (83.1%) > TAC+BTA (73.4%) > TAC+silicone (59.4%) > TAC+5-FU (57.4%) > silicone+5-FU (56.5%) > BTA (54.0%) > 5-FU (52.8%) > VER (40.2%) > TAC (18.2%) > BLM (4.9%) (Figure 8).
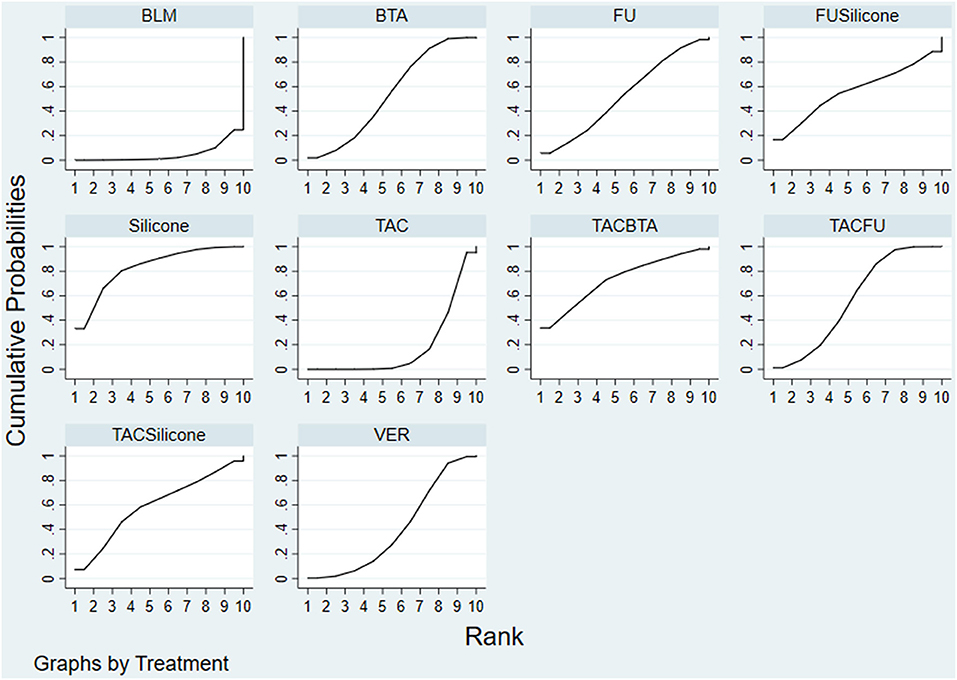
Figure 8. SUCRA adverse effect rate ranking curve. FU, 5-FU; SiliconeFU, silicone+5-FU; TACBTA, TAC+BTA; TACFU, TAC+5-FU; TACSilicone, TAC+silicone. SUCRA, surface under the cumulative ranking; TAC, triamcinolone acetonide; 5-FU, 5-fluorouracil.
Recurrence Rate
Recurrence rates were reported in 11 studies (37, 40, 53–60, 62–65, 68–73, 75, 76), with a total of eight measures. The former comprised A, TAC; D, silicone gels; E, BTA; and F, VER. The latter comprised A+B, TAC combined with 5-FU; D+A, silicone gels combined with TAC; D+B, silicone gels combined with 5-FU; and A+E, TAC combined with BTA. The eight treatment measures formed 28 different pair comparisons. In terms of recurrence rate, there are eight direct comparisons among 11 studies; the rest were indirect comparisons (Supplementary Figure 3).
Detection of Inconsistency
Eight direct comparisons constituted one triangular closed loop. The Z-test results showed that the lower limits of 95% CI were 0. This implies that each closed loop was consistent, as shown in Supplementary Table 4. The results of the node-splitting model showed consistency in the direct and indirect comparison results, all at p > 0.05 (Supplementary Table 5).
Results of Network Meta-Analysis and Publication Bias
Results of NMA showed that compared with silicone, only TAC, VER, BTA, and BTA combined with 5-FU significantly could decrease recurrence rate. The difference in the efficacy rate among these therapies was statistical significant at p < 0.05. Interestingly, there was no statistically significant difference among other interventions at p < 0.05 (Supplementary Figure 5). The funnel diagram showed good symmetry, indicating no obvious publication bias. This is illustrated in Supplementary Figure 4.
Ranking Results
Results of the convergence analysis showed that PSRF was equal to 1, indicating that the model had good convergence. Based on the Bayesian theory of MCMC method, random-effects model for NMA results showed the following: VER (78%) > VER (72.7%) > BTA (70.4%) > TAC+5-FU (58.2%) > silicone+5-FU (45%) > TAC (42.4%) > silicone+TAC (29%) > silicone (4.3%) (Supplementary Figure 6).
Efficacy and Tolerability
From the comprehensive data, TAC combined with BTA was highly effective and well-tolerated. Furthermore, the combination therapy was superior to TAC or BTA monotherapy in terms of efficacy and tolerability. The combined therapies for BTA or TAC with 5-FU, silicone gel with TAC, and silicone gel with 5-FU revealed a better efficacy and fewer side effects. These therapies were superior to TAC, 5-FU, or silicone gel monotherapies in terms of efficacy and tolerability. Silicone gels had the best tolerance but poor therapeutic response. On the other hand, BLM had poor tolerance (Figure 9).
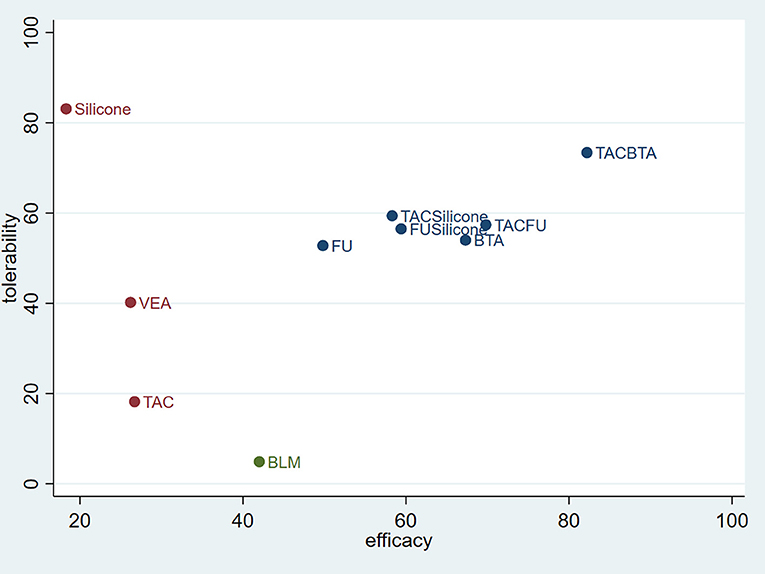
Figure 9. Coordinate figure for efficacy and tolerability. FU, 5-FU; SiliconeFU, silicone+5-FU; TACBTA, TAC+BTA; TACFU, TAC+5-FU; TACSilicone, TAC+silicone. TAC, triamcinolone acetonide; 5-FU, 5-fluorouracil.
Discussion
At present, pathological scars are still an unsolved problem worldwide. The readily available drugs and methods for treating pathological scars are limited mainly due to incomplete understanding of the mechanism of scar formation. Comparative analysis on the methods for treating pathological scars like hypertrophic scar and keloid is critical for clinical management (43). Traditional meta-analysis does not compare and analyze the effects of three or more treatments. Therefore, in the present study, a network meta-analysis was performed to evaluate the efficacy and safety of injection with TAC, 5-FU, BTA, VER, BLM, and commonly used topical drugs in the treatment of hypertrophic scar and keloid.
The results of NMA showed that the combined therapies were better than the two therapeutic modalities separately. Good efficacy and tolerability were revealed when TAC was combined with BTA, followed by TAC combined with 5-FU. These findings imply that TAC-BTA and TAC-5-FU therapies are more effective and safer in the treatment of keloid and hypertrophic scars. Silicone gels may be considered good for patients who cannot tolerate side effects. A previous meta-analysis by Ren et al. concluded that TAC+5-FU was safer and more effective in the treatment of pathological scars than TAC. Also, the effect of combined medication was significantly better than that of 5-FU (77). In recent years, a meta-analysis also found that TAC combined with 5-FU in the treatment of keloid and hypertrophic scars was more effective and safer than the monotherapy of TAC and 5-FU (78). The authors further reported that TAC combined with 5-FU was more effective in the treatment of keloid and hypertrophic scars than was intralesional injection TAC. The combined therapy showed significant improvements in scar height, erythema, and Observer and Patient Scar Assessment Scale. Most studies support that combined therapy is safer and more acceptable to patients, with fewer complications and a lower recurrence rate than intralesional TAC. In the present study, the effective concentration of TAC was 10 to 40 mg/ml for monotherapy, but how many units of BTA and TAC were used in combination therapy was an underexamined area. However, on administering a combined therapy of 5-FU and TAC, the concentration of TAC was far lower than the effective concentration. Therefore, we hypothesized that TAC might play an anti-inflammatory role in the combination therapy and offset most of the side effects of 5-FU, but further research is needed to verify this.
BTA inhibits muscle fiber contraction, reduces the tension at the wound healing edge (33), and enhances retention of fibroblasts in the GO and G1 phases of the cell cycle (34). Meanwhile, decreased expression of TGF-β1 may directly regulate fibroblast activity by changing apoptosis migration and fibrosis, thereby reducing scar formation (35, 36). Similar to our findings, a meta-analysis that included high-quality studies showed that intralesional injection of BTA was more effective in treating pathological scars than TAC or placebo (79). Also, it significantly reduced post-injection pain (79). A recent meta-analysis evaluating the efficacy and safety of BLM, TAC, 5-FU, TAC combined with 5-FU, and TAC combined with cryotherapy found that BLM significantly improved the treatment of pathological scars than TAC or 5-FU combined with cryotherapy (80). There was no significant difference between the BLM group and the TAC combined with 5-Fu group. In addition, their results showed that BLM reduced the recurrence rate when compared with 5-FU-TAC combined therapy. These results suggest that BLM is a more effective treatment option for keloid or hypertrophic scars than TAC and 5-FU or their combined therapies. However, the adverse reaction rate of BLM was higher (80). Interestingly, these findings are not in line with the current study's findings. This may be because the recurrence rate after BLM treatment was not reported in our included RCTs. Also, this study included controlled clinical trials, and the outcome indicators for our evaluation of the efficacy were different.
In the present study, it was revealed that BTA, glucocorticoid combined with 5-FU, VER, and 5-FU showed no statistically significant difference in the therapeutic effect. The efficacy of glucocorticoid combined with 5-FU was better than that of glucocorticoid and VER. The difference was, however, not statistically significant compared with 5-FU. Elsewhere, an NMA involving 23 studies with a total of 1,513 patients found that BTA combined with glucocorticoids had the best effect on pathological scar (45). Unlike the current study, which only included TAC, their study included four injectable drugs, including TAC and Diprospan. Another NMA sought to evaluate the efficacy of TAC in the treatment of keloids compared with placebo, pulsed dye laser (PDL), 5-FU, silicone, VER, TAC+5-FU, and TAC+5-FU+PDL (46). The authors suggested that VER is preferable to other treatments. Different from our study, their study only compared the treatment of keloid, did not include hypertrophic scar, and only included 10 RCTs.
Limitations
However, there are also limitations in our study. First of all, the included trials were followed up for a short time, ranging from 12 weeks to 24 months. The duration of the follow-up for 23 subjects was <32 weeks. Previous studies have found that 50–94% of pathological scars recurred after 1 year of treatment (81, 82). Nonetheless, long-term follow-up can be difficult because most patients lose interest in follow-up after achieving satisfactory treatment results. Secondly, hypertrophic scar and keloid were reported simultaneously in several of the studies we included (38, 40, 74, 83). Although they are both fibroproliferative diseases, they should not be confused with each other (84, 85), which may be an important cause of heterogeneity. Thirdly, the dose of each treatment and the interval between each treatment varied. Fourthly, all the studies were conducted in selected countries and thus cannot be used to generalize conclusions of the study. Scarring is a worldwide disease, and it is valuable to know how different regions and different races respond to the same treatment. Fifthly, none of the included studies classified patients according to Fitzpatrick's skin type. It is currently believed that Fitzpatrick's skin type is an important factor affecting the occurrence and development of pathological scars. Therefore, this study does not reveal whether there is a difference in the therapeutic effect of different treatment measures for different Fitzpatrick's skin types. Sixthly, although no publication bias was found according to the funnel plot, potential bias risks still exist, such as the lack of covert random allocation methods and financial support from the pharmaceutical industry, which may lead to overestimation of efficacy. Seventhly, our study did not assess the efficacy of physical or surgical modalities such as cryotherapy, lasers, and excision. Eighthly, the diagnosis of the scars in some included studies was clinical only and not confirmed by histopathology. Finally, it is important to note that adding botulinum toxin that is not covered by public insurance may have had a limit to its use.
Conclusion
Based on the Bayesian theorem, the consistency model was selected to conduct an NMA to evaluate the relative efficacy and safety of TAC, 5-FU, BLM, BTA, and VER in treatment of pathological scars. According to the main data synthesis, we concluded that more combination therapies should be recommended for the treatment of pathological scars, especially the TAC-5-FU and TAC-BTA combined therapies. These therapies have the advantage of good curative effects and fewer side effects. But for patients who cannot tolerate the side effects, we recommend the use of silicone gels in combination with TAC. However, there are still shortcomings in this NMA, and its conclusions need to be further confirmed by well-designed and rigorous RCTs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SY designed the study, processed the data, conducted statistical analyses, interpreted data, drafted article, approved the final version, and is the guarantor. CL contributed to study design, interpreted data, drafted article, and approved the final version. YL contributed to interpretation of data, drafted article, and approved the final version. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.691628/full#supplementary-material
References
1. Ud-Din S, Bayat A. Non-animal models of wound healing in cutaneous repair: in silico, in vitro, ex vivo, and in vivo models of wounds and scars in human skin. Wound Repair Regen. (2017) 25:164–76. doi: 10.1111/wrr.12513
2. Chiang RS, Borovikova AA, King K, Banyard DA, Lalezari S, Toranto JD, et al. Current concepts related to hypertrophic scarring in burn injuries. Wound Repair Regen. (2016) 24:466–77. doi: 10.1111/wrr.12432
3. Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. (2017) 43(Suppl. 1):S3–18. doi: 10.1097/DSS.0000000000000819
4. Zhu Z, Ding J, Tredget EE. The molecular basis of hypertrophic scars. Burns Trauma. (2016) 4:2. doi: 10.1186/s41038-015-0026-4
5. Hinz B. The role of myofibroblasts in wound healing. Curr Res Transl Med. (2016) 64:171–7. doi: 10.1016/j.retram.2016.09.003
6. Braga TT, Agudelo JS, Camara NO. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol. (2015) 6:602. doi: 10.3389/fimmu.2015.00602
7. van den Broek LJ, van der Veer WM, de Jong EH, Gibbs S, Niessen FB. Suppressed inflammatory gene expression during human hypertrophic scar compared to normotrophic scar formation. Exp Dermatol. (2015) 24:623–29. doi: 10.1111/exd.12739
8. Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: the paradigm of skin fibrosis - pathomechanisms and treatment. Matrix Biol. (2016) 51:37–46. doi: 10.1016/j.matbio.2016.01.013
9. Lian N, Li T. Growth factor pathways in hypertrophic scars: molecular pathogenesis and therapeutic implications. Biomed Pharmacother. (2016) 84:42–50. doi: 10.1016/j.biopha.2016.09.010
10. Rousselle P, Montmasson M, Garnier C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. (2019) 75–76:12–26. doi: 10.1016/j.matbio.2018.01.002
11. Kuehlmann B, Stern-Buchbinder Z, Wan DC, Friedstat JS, Gurtner GC. Beneath the surface: a review of laser remodeling of hypertrophic scars and burns. Adv Wound Care. (2019) 8:168–76. doi: 10.1089/wound.2018.0857
12. Ghazawi FM, Zargham R, Ghazawi FM, Zargham R, Gilardino MS, Sasseville D, et al. Insights into the pathophysiology of hypertrophic scars and keloids: how do they differ?. Adv Skin Wound Care. (2018) 31:582–95. doi: 10.1097/01.ASW.0000527576.27489.0f
13. He Y, Deng Z, Alghamdi M, Lu L, Fear MW, He L. From genetics to epigenetics: new insights into keloid scarring. Cell Prolif. (2017) 50:e12326. doi: 10.1111/cpr.12326
14. Jfri A, O'Brien E, Alavi A, Goldberg SR. Association of hidradenitis suppurativa and keloid formation: a therapeutic challenge. JAAD Case Rep. (2019) 5:675–8. doi: 10.1016/j.jdcr.2019.06.001
15. Jfri A, Alajmi A. Spontaneous keloids: a literature review. Dermatology. (2018) 234:127–30. doi: 10.1159/000491924
16. Jfri A, Rajeh N, Karkashan E. A case of multiple spontaneous keloid scars. Case Rep Dermatol. (2015) 7:156–60. doi: 10.1159/000437249
17. Gowitt GT, Hanzlick RL. Atypical autoerotic deaths. Am J Forensic Med Pathol. (1992) 13:115–9. doi: 10.1097/00000433-199206000-00007
18. Gold MH, McGuire M, Mustoe TA, Pusic A, Sachdev M, Waibel J, et al. Updated international clinical recommendations on scar management: part 2–algorithms for scar prevention and treatment. Dermatol Surg. (2014) 40:825–31. doi: 10.1111/dsu.0000000000000050
19. Meaume S, Le Pillouer-Prost A, Richert B, Roseeuw D, Vadoud J. Management of scars: updated practical guidelines and use of silicones. Eur J Dermatol. (2014) 24:435–43. doi: 10.1684/ejd.2014.2356
20. Atala A, Lanza R, Mikos T, Nerem R, editors. Principles of Regenerative Medicine. Academic Press (2018).
21. Kim JS, Hong JP, Choi JW, Seo DK, Lee ES, Lee HS. The efficacy of a silicone sheet in postoperative scar management. Adv Skin Wound Care. (2016) 29:414–20. doi: 10.1097/01.ASW.0000488665.03896.3d
22. Bleasdale B, Finnegan S, Murray K, Kelly S, Percival SL. The use of silicone adhesives for scar reduction. Adv Wound Care. (2015) 4:422–30. doi: 10.1089/wound.2015.0625
23. Shi X, Cantu-Crouch D, Sharma V, Pruitt J, Yao G, Fukazawa K, et al. Surface characterization of a silicone hydrogel contact lens having bioinspired 2-methacryloyloxyethyl phosphorylcholine polymer layer in hydrated state. Colloids Surf B Biointerfaces. (2020) 199:111539. doi: 10.1016/j.colsurfb.2020.111539
24. Del Toro D, Dedhia R, Tollefson TT. Advances in scar management: prevention and management of hypertrophic scars and keloids. Curr Opin Otolaryngol Head Neck Surg. (2016) 24:322–9. doi: 10.1097/MOO.0000000000000268
25. Morelli Coppola M, Salzillo R, Segreto F, Persichetti P. Triamcinolone acetonide intralesional injection for the treatment of keloid scars: patient selection and perspectives. Clin Cosmet Investig Dermatol. (2018) 11:387–96. doi: 10.2147/CCID.S133672
26. Hochman B, Locali RF, Matsuoka PK, Ferreira LM. Intralesional triamcinolone acetonide for keloid treatment: a systematic review. Aesthetic Plast Surg. (2008) 32:705–9. doi: 10.1007/s00266-008-9152-8
27. Diegelmann RF, Bryant CP, Cohen IK. Tissue alpha-globulins in keloid formation. Plast Reconstr Surg. (1977) 59:418–23. doi: 10.1097/00006534-197703000-00018
28. Huang Y, Wang Y, Wang X, Lin L, Wang P, Sun J, et al. The effects of the transforming growth factor-β1 (TGF-β1) signaling pathway on cell proliferation and cell migration are mediated by ubiquitin specific protease 4 (USP4) in hypertrophic scar tissue and primary fibroblast cultures. Med Sci Monit. (2020) 26:e920736. doi: 10.12659/MSM.920736
29. Wang JP, Yu HM, Chiang ER, Wang JY, Chou PH, Hung SC. Corticosteroid inhibits differentiation of palmar fibromatosis-derived stem cells (FSCs) through downregulation of transforming growth factor-β1 (TGF-β1). PLoS ONE. (2018) 13:e0198326. doi: 10.1371/journal.pone.0198326
30. Wu WS, Wang FS, Yang KD, Huang CC, Kuo YR. Dexamethasone induction of keloid regression through effective suppression of VEGF expression and keloid fibroblast proliferation. J Invest Dermatol. (2006) 126:1264–71. doi: 10.1038/sj.jid.5700274
31. Lee HJ, Jang YJ. Recent understandings of biology, prophylaxis and treatment strategies for hypertrophic scars and keloids. Int J Mol Sci. (2018) 19:711. doi: 10.3390/ijms19030711
32. Muñoz Lora VRM, Del Bel Cury AA, Jabbari B, Lacković Z. Botulinum toxin type a in dental medicine. J Dent Res. (2019) 98:1450–7. doi: 10.1177/0022034519875053
33. Kim SH, Lee SJ, Lee JW, Jeong HS, Suh IS. Clinical trial to evaluate the efficacy of botulinum toxin type A injection for reducing scars in patients with forehead laceration: a double-blinded, randomized controlled study. Medicine. (2019) 98:e16952. doi: 10.1097/MD.0000000000016952
34. Zhibo X, Miaobo Z. Botulinum toxin type a affects cell cycle distribution of fibroblasts derived from hypertrophic scar. J Plast Reconstr Aesthet Surg. (2008) 61:1128–29. doi: 10.1016/j.bjps.2008.05.003
35. Zhang X, Lan D, Ning S, Jia H, Yu S. Botulinum toxin type a prevents the phenotypic transformation of fibroblasts induced by TGF-β1 via the PTEN/PI3K/Akt signaling pathway. Int J Mol Med. (2019) 44:661–71. doi: 10.3892/ijmm.2019.4226
36. Schlessinger J, Gilbert E, Cohen JL, Kaufman J. New uses of abobotulinumtoxina in aesthetics. Aesthet Surg J. (2017) 37(Suppl. 1):S45–58. doi: 10.1093/asj/sjx005
37. Payapvipapong K, Niumpradit N, Piriyanand C, Buranaphalin S, Nakakes A. The treatment of keloids and hypertrophic scars with intralesional bleomycin in skin of color. J Cosmet Dermatol. (2015) 14:83–90. doi: 10.1111/jocd.12132
38. Bik L, Sangers T, Greveling K, Prens E, Haedersdal M, van Doorn M. Efficacy and tolerability of intralesional bleomycin in dermatology: a systematic review. J Am Acad Dermatol. (2020) 83:888-903. doi: 10.1016/j.jaad.2020.02.018
39. Huang M, Cai G, Baugh LM, Liu Z, Smith A, Watson M, et al. Systemic sclerosis dermal fibroblasts induce cutaneous fibrosis through lysyl oxidase-like 4: new evidence from three-dimensional skin-like tissues. Arthritis Rheumatol. (2020) 72:791–801. doi: 10.1002/art.41163
40. Abedini R, Sasani P, Mahmoudi HR, Nasimi M, Teymourpour A, Shadlou Z. Comparison of intralesional verapamil versus intralesional corticosteroids in treatment of keloids and hypertrophic scars: a randomized controlled trial. Burns. (2018) 44:1482–8. doi: 10.1016/j.burns.2018.05.005
41. Wang R, Mao Y, Zhang Z, Li Z, Chen J, Cen Y. Role of verapamil in preventing and treating hypertrophic scars and keloids. Int Wound J. (2016) 13:461–8. doi: 10.1111/iwj.12455
42. Sabry HH, Abdel Rahman SH, Hussein MS, Sanad RR, Abd El Azez TA. The efficacy of combining fractional carbon dioxide laser with verapamil hydrochloride or 5-fluorouracil in the treatment of hypertrophic scars and keloids: a clinical and immunohistochemical study. Dermatol Surg. (2019) 45:536–46. doi: 10.1097/DSS.0000000000001726
43. Cheraghi N, Cognetta A Jr, Goldberg D. Radiation therapy for the adjunctive treatment of surgically excised keloids: a review. J Clin Aesthet Dermatol. (2017) 10:12–5.
44. Watt J, Tricco AC, Straus S, Veroniki AA, Naglie G, Drucker AM. Research techniques made simple: network meta-analysis. J Invest Dermatol. (2019) 139:4–12.e1. doi: 10.1016/j.jid.2018.10.028
45. Sun P, Lu X, Zhang H, Hu Z. The efficacy of drug injection in the treatment of pathological scar: a network meta-analysis. Aesthetic Plast Surg. (2021) 45:791–805. doi: 10.1007/s00266-019-01570-8
46. Hutton B, Catalá-López F, Moher D. La extensión de la declaración PRISMA para revisiones sistemáticas que incorporan metaanálisis en red: PRISMA-NMA [The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA]. Med Clin. (2016) 147:262–6. doi: 10.1016/j.medcli.2016.02.025
47. Pooley CM, Marion G. Bayesian model evidence as a practical alternative to deviance information criterion. R Soc Open Sci. (2018) 5:171519. doi: 10.1098/rsos.171519
48. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graphical Stat. (1998) 7:434–55. doi: 10.1080/10618600.1998.10474787
49. Lin L. Graphical augmentations to sample-size-based funnel plot in meta-analysis. Res Synth Meth. (2019) 10:376–88. doi: 10.1002/jrsm.1340
50. Hietanen KE, Järvinen TA, Huhtala H, Tolonen TT, Kuokkanen HO, Kaartinen IS. Treatment of keloid scars with intralesional triamcinolone and 5-fluorouracil injections - a randomized controlled trial. J Plast Reconstr Aesthet Surg. (2019) 72:4–11. doi: 10.1016/j.bjps.2018.05.052
51. Manuskiatti W, Fitzpatrick RE. Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol. (2002) 138:1149–55. doi: 10.1001/archderm.138.9.1149
52. Saha AK, Mukhopadhyay M. A comparative clinical study on role of 5-flurouracil versus triamcinolone in the treatment of keloids. Indian J Surg. (2012) 74:326–9. doi: 10.1007/s12262-011-0399-y
53. Sadeghinia A, Sadeghinia S. Comparison of the efficacy of intralesional triamcinolone acetonide and 5-fluorouracil tattooing for the treatment of keloids. Dermatol Surg. (2012) 38:104–9. doi: 10.1111/j.1524-4725.2011.02137.x
54. Zhu XM, Huang YC, Fan RH. Fluorouracil combined with triamcinolone acetonide in the treatment of 46 cases of keloid [J]. Shanxi Med J. (2016) 09:1241–2. doi: 10.3969/j.issn.1000-7377.2016.09.068
55. Deng F. Clinical effect of local injection of triamcinolone acetonide and local injection of triamcinolone acetonide combined with fluorouracil in the treatment of keloid [J]. Mod Diag Treat. (2015) 18:4167–8.
56. Darougheh A, Asilian A, Shariati F. Intralesional triamcinolone alone or in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. Clin Exp Dermatol. (2009) 34:219–23. doi: 10.1111/j.1365-2230.2007.02631.x
57. Khalid FA, Mehrose MY, Saleem M, Yousaf MA, Mujahid AM, Rehman SU, et al. Comparison of efficacy and safety of intralesional triamcinolone and combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: randomised control trial. Burns. (2019) 45:69–75. doi: 10.1016/j.burns.2018.08.011
58. Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-Fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. (2006) 32:907–15. doi: 10.1111/j.1524-4725.2006.32195.x
59. Khan MA, Bashir MM, Khan FA. Intralesional triamcinolone alone and in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. J Pak Med Assoc. (2014) 64:1003–7.
60. Shiling Q, Dingheng Z, Xian A, Taoyuan H, Renliang H. Clinical observation of triamcinolone acetonide combined with 5-fluorouracil in the treatment of keloid [J]. Chin J Clin N Med. (2018) 04:342–4. doi: 10.3969/j.issn.1674-3806.2018.04.08
61. Khan HA, Sahibzada MN, Paracha MM. Comparison of the efficacy of intralesional bleomycin versus intralesional triamcinolone acetonide in the treatment of keloids. Dermatol Ther. (2019) 32:e13036. doi: 10.1111/dth.13036
62. Hatamipour E, Mehrabi S, Hatamipour M, Ghafarian Shirazi HR. Effects of combined intralesional 5-Fluorouracil and topical silicone in prevention of keloids: a double blind randomized clinical trial study. Acta Med Iran. (2011) 49:127–30.
63. Xiaohui L, Baoxia C, Chengdong W, Yongzhi S, Xiaoli L, Xuefeng S. Quantitative analysis of silicone dressing combined with intramural injection of triamcinolone acetonide in the peri-scar period of head and neck surgery [J]. Hebei Med J. (2017) 22:34 58–60. doi: 10.3969/j.issn.1002-7386.2017.22.026
64. Zhang JF, Lou SW. Clinical control study of triamcinolone acetonide and silicon gel in the treatment of hypertrophic scar [J]. Sci Educ Wenhui. (2013) 05:87–88. doi: 10.3969/j.issn.1672-7894.2013.15.052
65. Gamil HD, Khattab FM, El Fawal MM, Eldeeb SE. Comparison of intralesional triamcinolone acetonide, botulinum toxin type A, and their combination for the treatment of keloid lesions. J Dermatolog Treat. (2020) 31:535–44. doi: 10.1080/09546634.2019.1628171
66. Cheng P. Clinical observation of botulinum toxin type A combined with triamcinolone acetonide in the treatment of hyperplastic scar [J]. J Taishan Med Univ. (2015) 04:390–2. doi: 10.3969/j.issn.1004-7115.2015.04.010
67. Li ZZ, Li L. Effect of botulinum toxin type A combined with triamcinolone acetonide in the treatment of hypertrophic scar [J]. Chin J Commun Phys. (2016) 26:74+76. doi: 10.3969/j.issn.1007-614x.2016.26.44
68. Yuqin Z, Lidan C, Cuihua S. Clinical observation of local injection of type A botulinum toxin and triamcinolone acetonide in the treatment of hypertrophic scar [J]. Clin Pract Int Trad Chin West Med. (2020) 11:135–6. doi: 10.13638/j.issn.1671-4040.2020.11.069
69. Bin L, Jing L, Huiqing H. Efficacy and safety analysis of type a botulinum toxin in the treatment of scar hyperplasia [J]. Chin J Dermatol Venerol Int Trad West Med. (2017) 01:25–27. doi: 10.3969/j.issn.1672-0709.2017.01.006
70. Nai-Kang Y, Qi S. Comparison of efficacy and safety of botulinum toxin type A and triamcinolone acetonide in the treatment of keloid [J]. Chin Aesth Med. (2020) 05:66–9.
71. Shaarawy E, Hegazy RA, Abdel Hay RM. Intralesional botulinum toxin type A equally effective and better tolerated than intralesional steroid in the treatment of keloids: a randomized controlled trial. J Cosmet Dermatol. (2015) 14:161–6. doi: 10.1111/jocd.12134
72. Aggarwal A, Ravikumar BC, Vinay KN, Raghukumar S, Yashovardhana DP. A comparative study of various modalities in the treatment of keloids. Int J Dermatol. (2018) 57:1192–200. doi: 10.1111/ijd.14069
73. Margaret Shanthi FX, Ernest K, Dhanraj P. Comparison of intralesional verapamil with intralesional triamcinolone in the treatment of hypertrophic scars and keloids. Indian J Dermatol Venereol Leprol. (2008) 74:343–8. doi: 10.4103/0378-6323.42899
74. Ahuja RB, Chatterjee P. Comparative efficacy of intralesional verapamil hydrochloride and triamcinolone acetonide in hypertrophic scars and keloids. Burns. (2014) 40:583–8. doi: 10.1016/j.burns.2013.09.029
75. Tao D, Tao D, Huile P. Effect observation of verapamil injection in the treatment of skin keloid [J]. J Rare Pediatr Dis. (2020) 04:54–6. doi: 10.3969/j.issn.1009-3257.2020.04.021
76. Chunan Q, Min L, Dongyue L, Xiaoyu W, Yue L, Shengjian T. Comparison of efficacy between verapamil and triamcinolone acetonide injection in the treatment of keloid [J]. Chin J Aesth Plast Surg. (2018) 06:366–8. doi: 10.3969/j.issn.1673-7040.2018.06.015
77. Ren Y, Zhou X, Wei Z, Lin W, Fan B, Feng S. Efficacy and safety of triamcinolone acetonide alone and in combination with 5-fluorouracil for treating hypertrophic scars and keloids: a systematic review and meta-analysis. Int Wound J. (2017) 14:480–7. doi: 10.1111/iwj.12629
78. Jiang ZY, Liao XC, Liu MZ, Fu ZH, Min DH, Yu XT, et al. Efficacy and safety of intralesional triamcinolone versus combination of triamcinolone with 5-fluorouracil in the treatment of keloids and hypertrophic scars: a systematic review and meta-analysis. Aesthetic Plast Surg. (2020) 44:1859–68. doi: 10.1007/s00266-020-01721-2
79. Bi M, Sun P, Li D, Dong Z, Chen Z. Intralesional injection of botulinum toxin type a compared with intralesional injection of corticosteroid for the treatment of hypertrophic scar and keloid: a systematic review and meta-analysis. Med Sci Monit. (2019) 25:2950–8. doi: 10.12659/MSM.916305
80. Kim WI, Kim S, Cho SW, Cho MK. The efficacy of bleomycin for treating keloid and hypertrophic scar: a systematic review and meta-analysis. J Cosmet Dermatol. (2020) 19:3357–66. doi: 10.1111/jocd.13390
81. Carvajal CC, Ibarra CM, Arbulo DL, Russo MN, Solé CP. Postoperative radiotherapy in the management of keloids. Ecancermedicalscience. (2016) 10:690. doi: 10.3332/ecancer.2016.690
82. Lee SY, Park J. Postoperative electron beam radiotherapy for keloids: treatment outcome and factors associated with occurrence and recurrence. Ann Dermatol. (2015) 27:53–8. doi: 10.5021/ad.2015.27.1.53
83. Cocci A, Di Maida F, Cito G, Verrienti P, Laruccia N, Campi R, et al. Comparison of intralesional hyaluronic acid vs. Verapamil for the treatment of acute phase peyronie's disease: a prospective, open-label non-randomized clinical study. World J Mens Health. (2021) 39:352–7. doi: 10.5534/wjmh.190108
84. Lee JY, Yang CC, Chao SC, Wong TW. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. (2004) 26:379–84. doi: 10.1097/00000372-200410000-00006
Keywords: network meta-analysis, efficacy, safety, drugs, hypertrophic scar, keloid
Citation: Yang S, Luo YJ and Luo C (2021) Network Meta-Analysis of Different Clinical Commonly Used Drugs for the Treatment of Hypertrophic Scar and Keloid. Front. Med. 8:691628. doi: 10.3389/fmed.2021.691628
Received: 03 June 2021; Accepted: 29 July 2021;
Published: 09 September 2021.
Edited by:
Andreas Recke, University of Lübeck, GermanyReviewed by:
Irina Khamaganova, Pirogov Russian National Research Medical University, RussiaAbdulhadi Hazzaa Jfri, McGill University, Canada
Copyright © 2021 Yang, Luo and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Luo, YXJlbmFzYWxsQDE2My5jb20=
 Sha Yang1,2
Sha Yang1,2 Cong Luo
Cong Luo