
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 02 June 2021
Sec. Ophthalmology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.688355
This article is part of the Research TopicCorneal Disease: an UpdateView all 9 articles
Purpose: To investigate the risk of recurrent corneal erosion (RCE) in patients with atopic keratoconjunctivitis (AKC).
Methods: This national, retrospective, matched cohort study enrolled 184,166 newly-diagnosed AKC patients, selected from the Taiwan National Health Insurance Research Database and identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 372.05. The control group comprised 184,166 non-AKC patients matched by age, sex, and potential comorbidities and they were selected from the Taiwan Longitudinal Health Insurance Database, 2000. Information from patients was gathered from 1 January 2004 to 31 December 2011, and both groups were traced from the index date until December 2013. The incidence and risk of RCE (ICD-9-CM code 361.42) was compared between the groups. The adjusted hazard ratio (HR) for RCE was obtained by a Cox proportional hazard regression analysis. The Kaplan–Meier analysis was performed to calculate the cumulative incidence of RCE.
Results: In total, 564 AKC patients and 406 non-AKC controls developed RCE during the follow-up span. The incidence of RCE was 1.45 times higher in AKC patients than in controls (95% confidence interval [CI] = 1.27–1.64; P < 0.0001). After adjusting for potential confounders, including diabetes mellitus, keratoconjunctivitis sicca, corneal transplantation, ocular blunt trauma, corneal dystrophy, and band keratopathy, AKC patients were 1.36 times more likely to develop RCE than controls (adjusted HR, 1.36; 95% CI = 1.19–1.54; p < 0.05).
Conclusions: AKC Patients had an increased risk of developing RCE and should be informed of this risk.
Atopic keratoconjunctivitis (AKC), a chronic, non-infectious inflammatory ocular surface situation, is the most severe condition of allergic conjunctival disease. The disease is well-known as an ocular complication with atopic diseases. The symptoms of patients with AKC include itching, redness, tearing, pain in the eyes, and blurred vision (1, 2). Patients with AKC often present characteristics such as eyelid thickening and oedema, conjunctival congestion and thickening, tear film instability and dysfunction, and corneal scarring and neovascularization (2, 3). The pathophysiology of AKC includes eosinophil-associated inflammation, cytokine-mediated immune reactions, and immunoglobulin E-mediated mast cell degranulation (2, 4).
Recurrent corneal erosion (RCE), a relative common disorder worldwide, is characterized by recurrent detachment of the corneal epithelium from the basement membrane. The most frequent clinical presentation of RCE is sudden onset of eye pain accompanied by associated symptoms including photophobia, redness and tearing (5, 6). Corneal epithelial basement membrane dystrophies and mechanical or surgical trauma to the corneal epithelium are important risk factors for RCE (5, 7). Inflammation related to corneal surface injury results in weakening of the extra-cellular adhesion network and disruption of the basement membrane (8, 9).
Eosinophils and their toxic products may play important roles in ocular surface complications including persistent corneal epithelial defects or ulceration in patients with AKC (10, 11). Chronic eye rubbing, a common finding in AKC patients, may lead to ocular surface injury and inflammatory processes, which are well-known risk factors for RCE. Furthermore, up-regulation of matrix metalloproteinase (MMP) is found in both, patients with AKC and patients with RCE (12–14). Consequently, it is clinically relevant to survey whether AKC poses a risk for RCE.
To the best of our knowledge, no previous large-scale cohort studies have examined whether AKC poses a risk for subsequent RCE. A few studies have discussed whether AKC is a contribution factor for RCE, but these were limited to small-scale case series and case reports (10, 11). Hence, we designed a nationwide, population-based cohort study to investigate the risk of RCE in patients with AKC in Taiwan.
Since March 1, 1995, a single-payer National Health Insurance (NHI) scheme has been running, which has provided all residents with extensive medical care coverage in Taiwan. According to the data compiled after 2007, more than 98% of the total Taiwanese population, ~22.60 million individuals, were enrolled in this program.
Data used in this study were provided from the National Health Insurance Research Database (NHIRD). The Taiwan National Health Research Institute (NHRI) constructed NHIRD, which records coded information on each enrolee's demographics such as birthday, sex, residential area, as well as the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of diagnoses, prescriptions, procedures, and expenditures, irrespective of whether the patient underwent hospitalization or was under ambulatory care. The database is released by the Taiwan NHRI to the public through a formal application. The Institutional Review Board of the Chi Mei Medical Center waived the ethical approval and informed consent as no personally identifiable information was investigated in this database.
A total of 184,166 newly-diagnosed AKC patients with the ICD-9-CM code 372.05 were recruited for this retrospective cohort study. We collected patient information for the period from 1 January 2004 to 31 December 2011. Figure 1 shows the flowchart of our study. Initially, we included 240,493 patients with a diagnosis of AKC (ICD-9-CM code 372.05). In total, 1,253 subjects diagnosed with RCE (ICD-9-CM code 361.42) before AKC diagnosis and 205 patients with unknown sex or other missing demographic data were excluded.
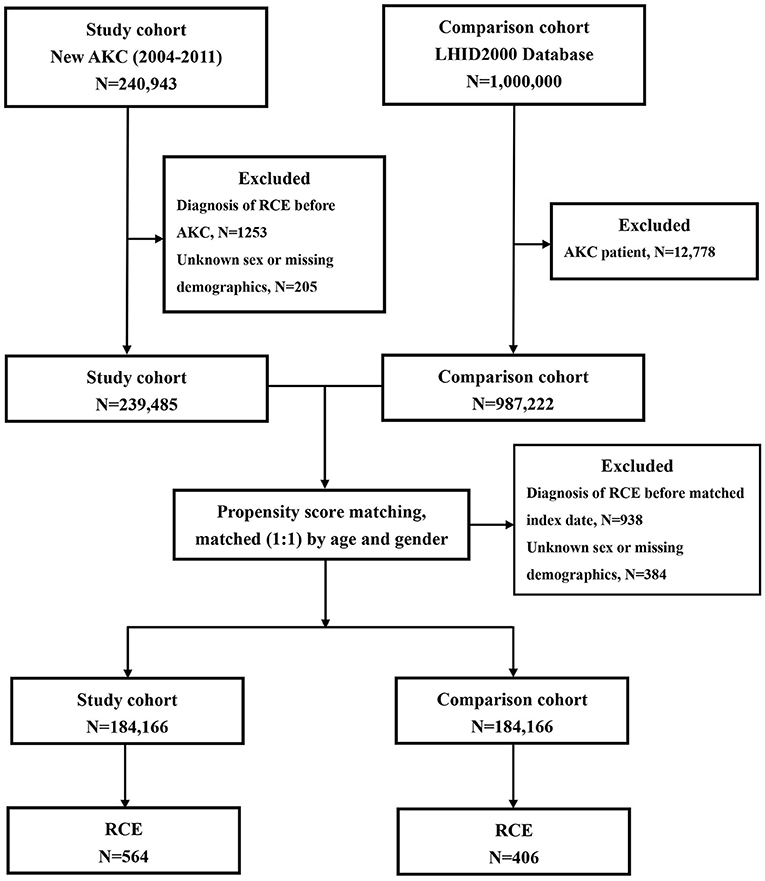
Figure 1. The flowchart for patients with atopic keratoconjunctivitis (AKC) and controls enrollment.
For each AKC patient, one non-AKC control was randomly selected from the Longitudinal Health Insurance Database 2000 (LHID 2000), a portion of the NHIRD. LHID 2000 includes the entire claims data for 1,000,000 beneficiaries from the year 2000. Initially, 12,778 subjects who had already been diagnosed with AKC, among the 1,000,000 subjects of the LHID 2000, were excluded; thereafter, additional 938 subjects diagnosed with RCE (ICD-9-CM code 361.42) before the index date and 384 patients with unknown sex or missing demographic data were excluded. The 184,166 controls without AKC were matched with the patients with AKC for age (±30 days), sex, and the index date. The index date for AKC patients was defined as the date of diagnosis of AKC; the index date for the control patients was matched with that of the AKC subjects.
To determine the incidence of RCE, we traced the participants in both groups and recorded demographic data of each participant from the index date until death or the end of 2013, whichever occurred earlier. Furthermore, data regarding risk factors, such as comorbidities, including diabetes mellitus (ICD-9-CM code 250) and keratoconjunctivitis sicca (ICD-9-CM codes 370.33 and 710.2); band keratopathy (ICD-9-CM code 371.43); corneal dystrophy (ICD-9-CM code 371.5); corneal transplantation (order codes 85212B, 85213B, 85215B, 85216B, and 85217B); and ocular blunt trauma (ICD-9-CM codes 921.1, 921.2, 921.3, 921.9, and 918.1) were assembled. Because most of the comorbidities were too rare to be assessed, we included them only if they occurred in one or more outpatient settings, or if the condition appeared in hospitalization care claims within 1 year prior to the index date.
We used SAS v. 9.4 for Windows (SAS Institute, Inc., Cary, NC, USA) for all statistical analyses. The basic demographics and comorbidities between the AKC and control groups were compared using Pearson's chi-square analysis. The RCE incidence was calculated as the total numbers of RCE patients discovered during the follow-up span, divided by the whole person-years (PY) for the respective groups according to age, sex, and chosen comorbidities. The Poisson regression analysis was performed to obtain the incidence rate ratio (IRR), to compare the risk of developing RCE between the AKC patients and non-AKC controls. The Cox proportional hazards regression was applied to determine the differences in the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of RCE. The Kaplan–Meier method was employed to construct the cumulative incidence curves and the log-rank test was applied to evaluate the differences. Statistical significance was set at p < 0.05.
After eliminating ineligible participants, 184,166 AKC patients and 184,166 control patients were enrolled. Table 1 reveals the initial demographics and comorbidities of both groups. The average age in both the groups was 31.95 (standard deviation, 19.42) years. The onset age of AKC was <12, 12–19, 20–29, 30–39, and ≥ 40 years in 35,110 (19.06%), 25,936 (14.08%), 34,834 (18.91%), 29,873 (16.22%), and 58,413 (31.72%) patients, respectively. Of the 184,166 patients with AKC, 76,361 (41.46%) were men and 107,805 (58.54%) were women. Regarding comorbidities, AKC patients demonstrated a significantly higher prevalence of previously reported risk factors for RCE, such as diabetes mellitus (p < 0.0001), keratoconjunctivitis sicca (p < 0.0001), and corneal transplantation (p = 0.0012), than the controls. There was no significant difference in prevalence of ocular blunt trauma, corneal dystrophy, and band keratopathy. The mean follow-up periods for the AKC and control group patients were 5.64 (SD, 2.28) and 5.87 (SD, 2.29) years, respectively (p < 0.0001).
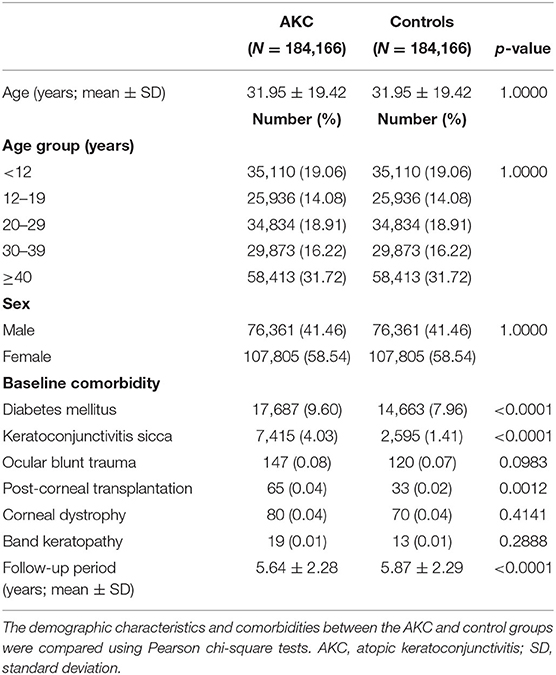
Table 1. Comparison of the demographic characteristics and comorbidities between the atopic keratoconjunctivitis (AKC) and control group.
During the follow-up span, there was a higher RCE incidence in AKC patients (5.43/10,000 PY) than in matched controls (3.76/10,000 PY), leading to a significant difference in the IRR of RCE (1.45, 95% CI = 1.27–1.64, p <0.0001; Table 2).
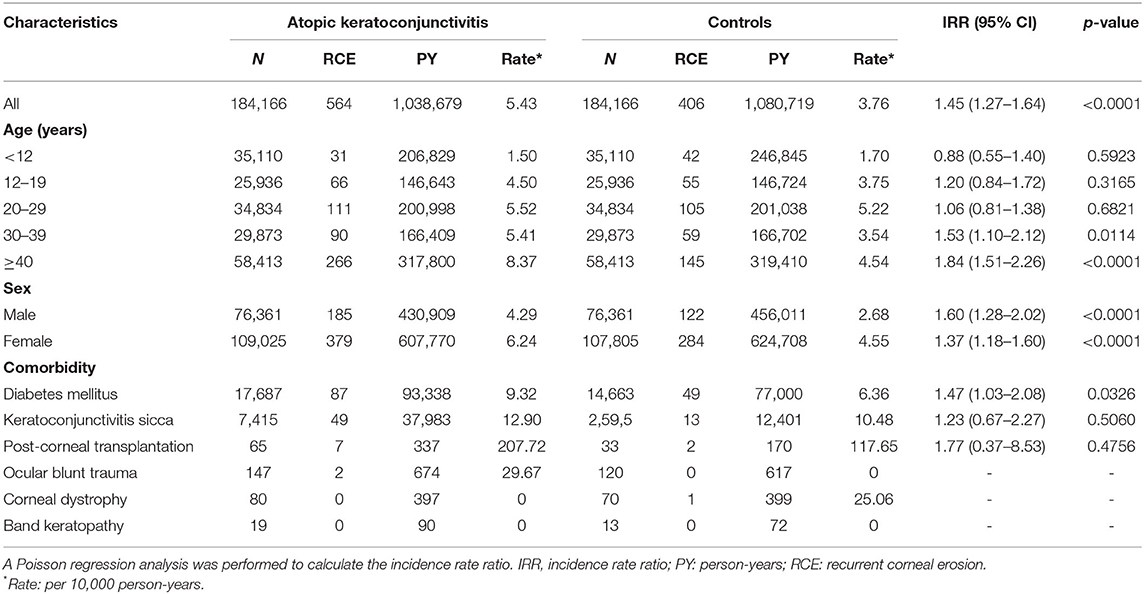
Table 2. Risk of recurrent corneal erosion (RCE) in the atopic keratoconjunctivitis and control groups.
Patients with AKC aged ≥ 40 years showed the highest RCE incidence (8.37/10,000 PY), followed by patients aged 20–29 years (5.52/10,000 PY), 30–39 years (5.41/10,000 PY), 12–19 years (4.50/10,000 PY), and <12 years (1.50/10,000 PY). The values of the IRR were significantly higher for AKC patients than for controls in patients aged 30–39 years (1.53 [95% CI = 1.10–2.12; p = 0.0114]), and ≥ 40 years (1.84 [95% CI = 1.51–2.26; p < 0.0001]). Nevertheless, no significant difference existed in the RCE incidence between AKC patients aged <12 years, 12–19 years or 20–29 years, and their corresponding controls (Table 2). The RCE incidence was 4.29/10,000 PY for male AKC patients and 2.68/10,000 PY for male controls (IRR = 1.60; 95% CI = 1.28–2.02; p < 0.0001). A significant difference was also observed between female AKC patients and female controls (IRR = 1.37; 95% CI = 1.18–1.60; p < 0.0001; Table 2).
The RCE incidence was 9.32/10,000 PY in patients with diabetes mellitus and 6.36/10,000 PY in controls. The IRR for RCE in AKC patients with diabetes mellitus was 1.47 times higher than in controls (IRR = 1.47; 95% CI = 1.03–2.08; p = 0.0326). It is worth pointing out that the IRRs for RCE did not denote significantly greater risks in AKC patients with keratoconjunctivitis sicca, corneal transplantation, or ocular blunt trauma than in the corresponding controls (Table 2). The IRR for RCE in ocular blunt trauma, corneal dystrophy, or band keratopathy between AKC patients and the controls could not be determined, because so few patients with these conditions developed RCE in both groups (Table 2).
Table 3 displays the crude and adjusted HRs for RCE during the follow-up period. After adjusting for age, sex, and the selected comorbidities, AKC remained an independent risk of RCE (adjusted HR = 1.36; 95% CI = 1.19–1.54; p < 0.05). In both the AKC and control groups, patients aged 12–19 years (adjusted HR, 2.36; 95% CI = 1.76–3.15; p < 0.05), 20–29 years (adjusted HR, 2.93; 95% CI = 2.24–3.83; p < 0.05), 30–39 years (adjusted HR, 2.39; 95% CI = 1.80–3.17; p < 0.05), and 40–49 years (adjusted HR, 3.17; 95% CI = 2.45–4.11; p < 0.05) as well as women (adjusted HR, 1.35; 95% CI = 1.18–1.55; p < 0.05) were at a higher risk of developing RCE. Diabetes mellitus (adjusted HR, 1.32; 95% CI = 1.09–1.61; p < 0.05), keratoconjunctivitis sicca (adjusted HR, 1.85; 95% CI = 1.42–2.42; p < 0.05), and corneal transplantation (adjusted HR, 26.33; 95% CI = 13.55–51.18; p < 0.05) were significant risk factors for RCE in both groups, whereas ocular blunt trauma and corneal dystrophy were not independent risk factors for RCE. We could not appraise whether band keratopathy was a significant risk factor after adjusting for other confounding factors in the total cohort because of the lack of RCE incidence among patients with these comorbidities in both groups.
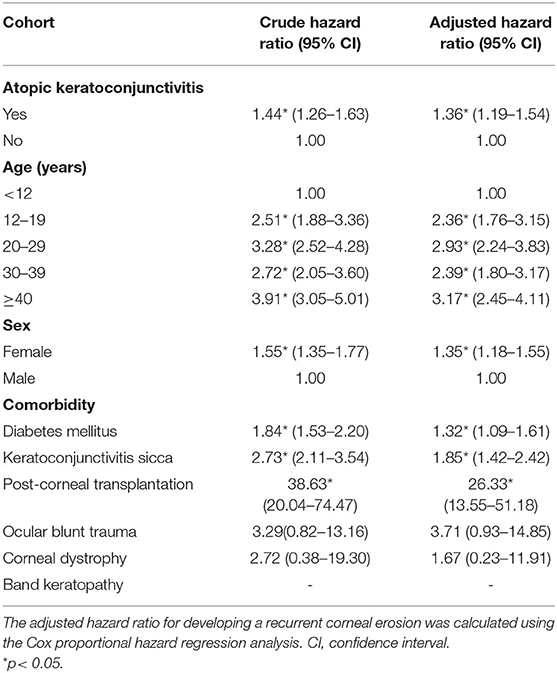
Table 3. Crude and adjusted hazard ratios for the Cox proportional hazard regression analyses and 95% confidence intervals for recurrent corneal erosion in the study cohort during the follow-up period.
Kaplan–Meier analyses revealed a higher cumulative incidence of RCE in the AKC group than in the control group, and the log-rank test findings were also significant (p < 0.0001; Figure 2).
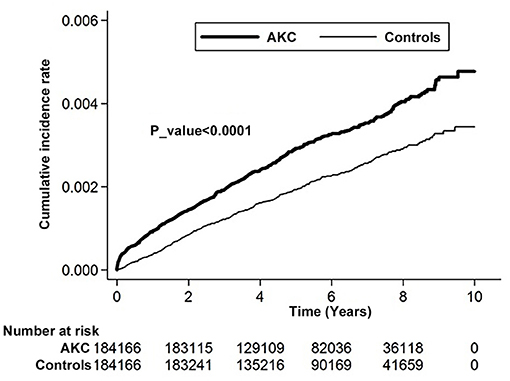
Figure 2. Cumulative incidence rate of recurrent corneal erosion in patients with atopic keratoconjunctivitis and controls during the follow-up period.
To the best of our knowledge, our study is the most large-scale population-based investigation to examine the relation between AKC and RCE. We investigated 184,166 AKC patients and 184,166 controls and discovered that the RCE incidence in AKC patients was 1.45 times higher than that in controls, and the relative risk of developing RCE in AKC patients increased 1.36 times in the whole cohort after adjustment for age, sex, diabetes mellitus, keratoconjunctivitis sicca, corneal transplantation, ocular blunt trauma, corneal dystrophy, and band keratopathy.
The association between AKC and RCE has been discussed in a few previous reports (10, 11). Messmer et al. (11) evaluated three corneal buttons of a patient with AKC having RCE or ulcerations, and detected a linear subepithelial deposition of eosinophil granular substances such as major basic protein and eosinophil cationic protein above the Bowman's membrane in all corneal buttons. They proposed that toxic eosinophil proteins may be involved in the pathophysiology of RCE in patients with AKC. Fukagawa collected tear samples from 38 eyes of 24 patients with AKC with different corneal conditions including RCE, superficial punctate keratopathy, and no additional pathology, and found significantly high concentrations of eotaxin and eosinophilic infiltration in the tears of patients with AKC with RCE (10). It is worth noting that eosinophils are known to be responsible for corneal damage through the release of toxic mediators (15). In addition, eosinophils play an pathogenic role in ocular surface disorders in AKC patients, and the clinical status of ocular surface disorders of AKC patients is associated with the tear eosinophil cationic protein levels (16–18). The high concentration of eosinophil granular substances in patients with AKC may be a possible explanation for the association between AKC and RCE.
Frequent eye rubbing is a common physiologic response to fatigue, itchy sensation, and discomfort in the eyes in AKC patients (19). Prolonged and vigorous eye rubbing may lead to an ocular surface injury in AKC patients, which is a possible risk factor for the development of RCE. Besides mechanical trauma to the ocular surface due to eye rubbing, inflammatory processes following eye rubbing have been found in patients with AKC (19). Several reports have shown that inflammatory mediators, such as interleukin (IL)-1, IL-6, and tumour necrosis factor -α, are higher in the tears of AKC patients than in those of healthy controls (20–22). The release of inflammatory cytokines, including IL-1 and IL-6, is also triggered by corneal injuries, and leads to corneal basement membrane disruption and weakening of the extra-cellular adhesion network (8, 9). Although these cytokines play an important role in wound healing by up-regulation of the integrin receptor for fibronectin or over-expression of keratinocyte growth factor, and a synergistic effect with epithelial growth factor during the wound healing process, a balance between healing and disruption has to be maintained (23–25). Candar et al. (8) reported that the levels of IL-1 and IL-6 were increased in patients with RCE when compared with healthy controls. In addition, they showed that the positive correlation between the levels of these cytokines and epithelial growth factor in healthy controls was disrupted in patients with RCE. The elevated IL-1 and IL-6 in AKC patients possibly aggravate the imbalance, which could elucidate the causal association between AKC and the later development of RCE.
Several studies have presented that MMPs, particularly MMP-2 and MMP-9, were upregulated by inflammatory events in patients with AKC (19, 22, 26). The higher concentration of MMP in the tears of patients with AKC than in those of healthy controls has been reported by several studies (22, 26, 27). It is worth noting that MMP-2 and MMP-9 are also highly expressed in the corneal epithelium and tears of patients with RCE (13, 14). Enhanced expression of MMP in patients with RCE promotes cleavage of adhesion molecules and collagens leading to hemi-desmosome dysfunction (12, 14). These findings with regards to the upregulation of MMPs may imply a causal relationship between AKC and RCE.
We found that the incidence of RCE was higher in women with AKC (Table 2). The risk of developing RCE is 1.35 times higher in female patients than in male patients (Table 3). This finding can be possibly explained by the higher probability of development of RCE in women, demonstrated by most of the major studies (28–30).
Several studies have shown that comorbidities, such as diabetes mellitus (7, 31), keratoconjunctivitis sicca (6, 31, 32), corneal transplantation (9, 33), ocular blunt trauma (9, 33), corneal dystrophy, and band keratopathy are associated with RCE. In this cohort study, we evaluated these comorbidities in AKC patients and discovered that AKC patients with diabetes mellitus had a significantly higher IRR for RCE than the controls (Table 2), and diabetes mellitus was a significant risk factor for the development of RCE in the whole cohort (Table 3). This finding is consistent with several previous studies (6, 7, 31). Nanba et al. (7) conducted a retrospective study including 21 eyes of 21 patients with RCE and found that diabetes mellitus is an important risk factor for RCE. Our previous retrospective, nationwide, matched cohort study that included 239,854 patients with diabetes mellitus showed that patients with diabetes mellitus were 1.35 times (95% CI, 1.24–1.48) more likely to develop RCE than the total sample cohort (31). The association between diabetes mellitus and RCE may be explained by the influence of hyperglycaemia in patients with diabetes mellitus on corneal morphology, and physiology including delayed epithelial wound healing, reduced basal epithelial cell density, and decreased sub-basal nerve density in the cornea of patients with diabetes mellitus (34).
Further, we found that keratoconjunctivitis sicca is an independent risk factor for the development of RCE in the total cohort (Table 3), although keratoconjunctivitis sicca did not lead to a significantly higher RCE incidence in the AKC patients and controls (Table 2). The association between keratoconjunctivitis sicca and RCE has been reported in several papers (31, 32). Our previous study showed the relationship between keratoconjunctivitis sicca and RCE in the total cohort of 239,854 patients with diabetes mellitus and 239,854 matched control (31). Keratoconjunctivitis sicca, characterised by inflammation and a loss of homeostasis of the tear film, is a complex ocular surface disorder and a multifactorial condition accompanied by many ocular symptoms, including discomfort, visual disturbance, and foreign body sensation (35). The most important pathophysiology of keratoconjunctivitis sicca is a vicious cycle including lacrimal gland destruction, corneal sensory nerve damage, and reduced tear production (36). The association between keratoconjunctivitis sicca and RCE may be due to the common pathophysiological mechanisms.
There are several vantages in our study. First, our large-scale cohort study had great precision of risk appraisal and statistical power because of a large number of enrolees, including 184,166 AKC patients and 184,166 controls. Moreover, the potential confounding bias was reduced, by adjusted for diabetes mellitus, keratoconjunctivitis sicca, corneal transplantation, ocular blunt trauma, corneal dystrophy, and band keratopathy in our cohort study with longitudinal data of up to 10 years.
This study also had limitations. The history of AKC before January 1996 in the controls could not be verified, because the medical history of each participant in the database could only be traced back to 1996. Additionally, band keratopathy, a well-known important confounding factor, could not be evaluated truly because no band keratopathy developed RCE in the AKC and control groups. Moreover, some important confounders including the use of contact lenses, mild ocular trauma, and record of ocular refractive surgery were not assessed. Finally, the diagnosis of AKC, RCE, and other diseases depended on ICD-9-codes, so incorrect classification was possible.
In brief, this study revealed that AKC patients have a significantly higher risk of developing RCE. AKC remained an independent risk factor after adjustment for confounders in the total cohort. These results indicate that clinicians should notify AKC patients of their increased RCE risk.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The requirements for ethical approval and informed consent were waived by the Institutional Review Board of the Chi-Mei Medical Center, because no identifiable personal information was analysed using the public database.
R-LJ and Y-SC: conceptualization. R-LJ, S-FW, and Y-SC: formal analysis, methodology, and writing—original draft. J-JW: resources and software. S-HT and Y-SC: writing—review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Taiwan Bureau of National Health Insurance and Department of Health provided the National Health Insurance Research Database. The conclusions and interpretations incorporated here do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes. We thank the Center for Medical Informatics and Statistics of Kaohsiung Medical University for providing administrative and funding support.
AKC, atopic keratoconjunctivitis; CI, confidence interval; HR, hazard ratio; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IL, interleukin; IRR, incidence rate ratio; LHID 2000, Longitudinal Health Insurance Database 2000; MMP, matrix metalloproteinase; NHIRD, National Health Insurance Research Database; NHRI, National Health Research Institute; PY, person-years; RCE, recurrent corneal erosion; SD, standard deviation.
1. Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. (2010) 10:478–85. doi: 10.1097/ACI.0b013e32833e16e4
2. Chen JJ, Applebaum DS, Sun GS, Pflugfelder SC. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. (2014) 70:569–75. doi: 10.1016/j.jaad.2013.10.036
3. Power WJ, Tugal-Tutkun I, Foster CS. Long-term follow-up of patients with atopic keratoconjunctivitis. Ophthalmology. (1998) 105:637–42. doi: 10.1016/S0161-6420(98)94017-9
4. Bonini S. Atopic keratoconjunctivitis. Allergy. (2004) 59(Suppl. 78):71–3. doi: 10.1111/j.1398-9995.2004.00570.x
5. Das S, Seitz B. Recurrent corneal erosion syndrome. Surv Ophthalmol. (2008) 53:3–15. doi: 10.1016/j.survophthal.2007.10.011
6. Ramamurthi S, Rahman MQ, Dutton GN, Ramaesh K. Pathogenesis, clinical features and management of recurrent corneal erosions. Eye (Lond). (2006) 20:635–44. doi: 10.1038/sj.eye.6702005
7. Nanba H, Mimura T, Mizuno Y, Matsumoto K, Hamano S, Ubukata S, et al. Clinical course and risk factors of recurrent corneal erosion: observational study. Medicine. (2019) 98:e14964. doi: 10.1097/MD.0000000000014964
8. Candar T, Asena L, Alkayid H, Altinors DD. Galectin-3, il-1a, il-6, and egf levels in corneal epithelium of patients with recurrent corneal erosion syndrome. Cornea. (2020) 39:1354–8. doi: 10.1097/ICO.0000000000002422
9. Miller DD, Hasan SA, Simmons NL, Stewart MW. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. (2019) 13:325–35. doi: 10.2147/OPTH.S157430
10. Fukagawa K, Nakajima T, Tsubota K, Shimmura S, Saito H, Hirai K. Presence of eotaxin in tears of patients with atopic keratoconjunctivitis with severe corneal damage. J Allergy Clin Immunol. (1999) 103:1220–1. doi: 10.1016/S0091-6749(99)70206-X
11. Messmer EM, May CA, Stefani FH, Welge-Luessen U, Kampik A. Toxic eosinophil granule protein deposition in corneal ulcerations and scars associated with atopic keratoconjunctivitis. Am J Ophthalmol. (2002) 134:816–21. doi: 10.1016/S0002-9394(02)01726-9
12. Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. (2013) 54:6390–400. doi: 10.1167/iovs.13-12547
13. Sakimoto T, Shoji J, Yamada A, Sawa M. Upregulation of matrix metalloproteinase in tear fluid of patients with recurrent corneal erosion. J J Ophthalmol. (2007) 51:343–6. doi: 10.1007/s10384-007-0455-0
14. Garrana RM, Zieske JD, Assouline M, Gipson IK. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest Ophthalmol Vis Sci. (1999) 40:1266–70.
15. Trocme SD, Hallberg CK, Gill KS, Gleich GJ, Tyring SK, Brysk MM. Effects of eosinophil granule proteins on human corneal epithelial cell viability and morphology. Invest Ophthalmol Vis Sci. (1997) 38:593–9.
16. Leonardi A, Borghesan F, Faggian D, Secchi A, Plebani M. Eosinophil cationic protein in tears of normal subjects and patients affected by vernal keratoconjunctivitis. Allergy. (1995) 50:610–3. doi: 10.1111/j.1398-9995.1995.tb01209.x
17. Montan PG, van Hage-Hamsten M. Eosinophil cationic protein in tears in allergic conjunctivitis. Br J Ophthalmol. (1996) 80:556–60. doi: 10.1136/bjo.80.6.556
18. Tsubota K, Takamura E, Hasegawa T, Kobayashi T. Detection by brush cytology of mast cells and eosinophils in allergic and vernal conjunctivitis. Cornea. (1991) 10:525–31. doi: 10.1097/00003226-199111000-00011
19. Ben-Eli H, Erdinest N, Solomon A. Pathogenesis and complications of chronic eye rubbing in ocular allergy. Curr Opin Allergy Clin Immunol. (2019) 19:526–34. doi: 10.1097/ACI.0000000000000571
20. Pahuja N, Kumar NR, Shroff R, Shetty R, Nuijts RM, Ghosh A, et al. Differential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest Ophthalmol Vis Sci. (2016) 57:5372–82. doi: 10.1167/iovs.16-19677
21. Shoji J, Kawaguchi A, Gotoh A, Inada N, Sawa M. Concentration of soluble interleukin-6 receptors in tears of allergic conjunctival disease patients. Japn J Ophthalmol. (2007) 51:332–7. doi: 10.1007/s10384-007-0461-2
22. Balasubramanian SA, Pye DC, Willcox MD. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom. (2013) 96:214–8. doi: 10.1111/cxo.12038
23. Nakamura M, Nishida T. Differential effects of epidermal growth factor and interleukin 6 on corneal epithelial cells and vascular endothelial cells. Cornea. (1999) 18:452–8. doi: 10.1097/00003226-199907000-00011
24. Nishida T, Nakamura M, Mishima H, Otori T, Hikida M. Interleukin 6 facilitates corneal epithelial wound closure in vivo. Arch Ophthalmol. (1992) 110:1292–4. doi: 10.1001/archopht.1992.01080210110036
25. Boisjoly HM, Laplante C, Bernatchez SF, Salesse C, Giasson M, Joly MC. Effects of egf, il-1 and their combination on in vitro corneal epithelial wound closure and cell chemotaxis. Exp Eye Res. (1993) 57:293–300. doi: 10.1006/exer.1993.1127
26. Maatta M, Kari O, Tervahartiala T, Wahlgren J, Peltonen S, Kari M, et al. Elevated expression and activation of matrix metalloproteinase 8 in tear fluid in atopic blepharoconjunctivitis. Cornea. (2008) 27:297–301. doi: 10.1097/ICO.0b013e31815c18d6
27. Leonardi A, Brun P, Abatangelo G, Plebani M, Secchi AG. Tear levels and activity of matrix metalloproteinase (mmp)-1 and mmp-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. (2003) 44:3052–8. doi: 10.1167/iovs.02-0766
29. Reidy JJ, Paulus MP, Gona S. Recurrent erosions of the cornea: epidemiology and treatment. Cornea. (2000) 19:767–71. doi: 10.1097/00003226-200011000-00001
30. Hope-Ross MW, Chell PB, Kervick GN, McDonnell PJ. Recurrent corneal erosion: clinical features. Eye (Lond). (1994) 8:373–7. doi: 10.1038/eye.1994.89
31. Jan RL, Tai MC, Ho CH, Chu CC, Wang JJ, Tseng SH, et al. Risk of recurrent corneal erosion in patients with diabetes mellitus in taiwan: a population-based cohort study. BMJ Open. (2020) 10:e035933. doi: 10.1136/bmjopen-2019-035933
32. Kang EY, Chen HT, Hsueh YJ, Chen HC, Tan HY, Hsiao CH, et al. Corneal sensitivity and tear function in recurrent corneal erosion syndrome. Invest OphthalmolVis Sci. (2020) 61:21. doi: 10.1167/iovs.61.3.21
33. Krysik K, Wroblewska-Czajka E, Lyssek-Boron A, Wylegala EA, Dobrowolski D. Total penetrating keratoplasty: indications, therapeutic approach, and long-term follow-up. J Ophthalmol. (2018) 2018:9580292. doi: 10.1155/2018/9580292
34. Chang PY, Carrel H, Huang JS, Wang IJ, Hou YC, Chen WL, et al. Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. Am J Ophthalmol. (2006) 142:488–90. doi: 10.1016/j.ajo.2006.04.033
35. Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. Tfos dews ii report executive summary. Ocul Surf. (2017) 15:802–12. doi: 10.1016/j.jtos.2017.08.003
Keywords: atopic keratoconjunctivitis, recurrent corneal erosion, Taiwan Longitudinal Health Insurance Database, cohort study, hazard ratio
Citation: Jan R-L, Weng S-F, Wang J-J, Tseng S-H and Chang Y-S (2021) Association Between Atopic Keratoconjunctivitis and the Risk of Recurrent Corneal Erosion. Front. Med. 8:688355. doi: 10.3389/fmed.2021.688355
Received: 30 March 2021; Accepted: 12 May 2021;
Published: 02 June 2021.
Edited by:
Pedram Hamrah, Tufts Medical Center, United StatesReviewed by:
Christopher Rapuano, Wills Eye Hospital, United StatesCopyright © 2021 Jan, Weng, Wang, Tseng and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuh-Shin Chang, eXVoc2hpbmNoYW5nQHlhaG9vLmNvbS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.