- 1School of Medicine, Ningbo University, Ningbo, China
- 2Department of General Practice, Ningbo First Hospital, Ningbo, China
- 3Department of Infectious Disease, Ningbo First Hospital, Ningbo, China
- 4Department of Hematology, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, China
Disseminated tuberculosis (TB) is a rare disease and mainly occurs in immunodeficient patients. It is marked by hematogenous or lymphatic dissemination of Mycobacterium tuberculosis, causing tuberculous infection involving any organ system. Here, we report a case of disseminated TB involving lung, liver, spine, mediastinum, and prostate in an immunocompetent man. The present patient found a hepatic mass without any symptom during health examination. In the next 2 years, further examinations revealed multiple lesions in the lung, mediastinum, spine, and prostate. Imaging examinations, such as contrast-enhanced abdominal CT, F-18 FDG-PET/CT, and radionuclide bone scan, suggested the diagnosis of malignancy or metastatic tumor. Furthermore, histopathological results of the biopsies of the hepatic mass, mediastinal mass, and prostatic mass demonstrated granulomatous inflammation. Therefore, metagenomic next-generation sequencing (mNGS) was utilized to confirm the diagnosis. Mycobacterium tuberculosis complex was simultaneously detected in the spinal surgical resection specimens and bronchoalveolar lavage fluid (BALF), indicating the diagnosis of disseminated TB. mNGS is an emerging molecular diagnostic technology, and its application in disseminated TB has been rarely reported. We highlight that disseminated TB should be considered even in an immunocompetent patient, and mNGS can be performed when the diagnosis is difficult.
Introduction
Tuberculosis (TB) is a widespread infectious disease caused by Mycobacterium tuberculosis (MTB), which can involve any organ system and mostly the lungs (1, 2). Extrapulmonary infection of MTB was also reported in recent decades, including lymph glands, pleura, bones, joints, urogenital tract, and central nervous system (CNS) (3–7). Besides, disseminated TB is a rare form accounting for about 1–5% of all TB cases and defined as tuberculous infection involving two or more non-adjacent body sites via hematogenous or lymphatic spread of MTB from the primary lesion (8). The dissemination mainly occurs in patients with risk factors including HIV immunodeficiency, long-term use of immunosuppressants, poorly controlled diabetes, hematologic diseases, and alcohol abuse (9). However, it is less likely to appear in immunocompetent individuals.
Metagenomic next-generation sequencing (mNGS) is a new molecular diagnostic technology. mNGS application in TB of single organ has been reported, such as pulmonary TB, osteoarticular TB, and tuberculous meningitis, but rarely in disseminated TB until now (10–12). Here, we report a case of disseminated TB with a hepatic mass as the first manifestation. The patient failed to be diagnosed despite several biopsies of liver mass, mediastinal mass, and prostatic nodules. With the application of mNGS, the patient was finally diagnosed with disseminated TB.
Case Presentation
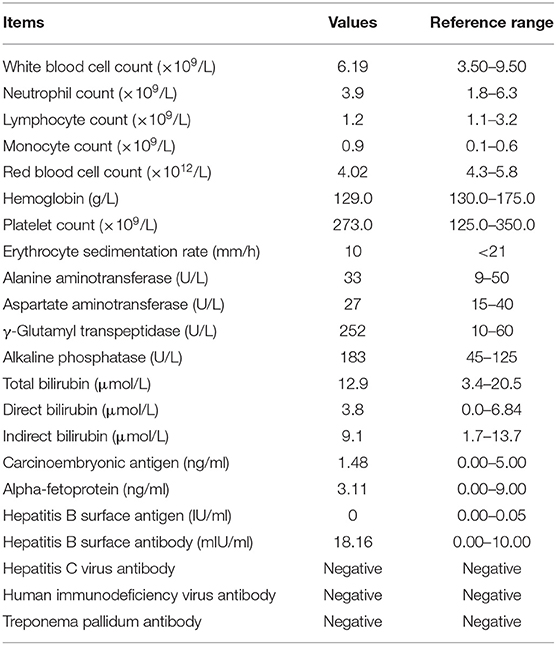
On December 14, 2017, a 51-year-old man visited our General Internal Medicine department for finding a hepatic mass without any discomfort during health examination. The entire process of diagnosis and treatment is briefly depicted in Figure 1. The patient claimed no history of hepatitis or TB and no family history of liver cancer. Physical examination was unremarkable. Detailed laboratory examination data are presented in Table 1. Contrast-enhanced abdominal computed tomography (CT) showed a hypodense lesion with mild to moderate enhancement in the right hepatic lobe near hepatic hilar, which indicated the possibility of cholangiocellular carcinoma; in addition, a soft tissue density lesion with heterogeneous enhancement in the left posterior mediastinum suggested the possibility of neurogenic tumor (Figures 1A,B).
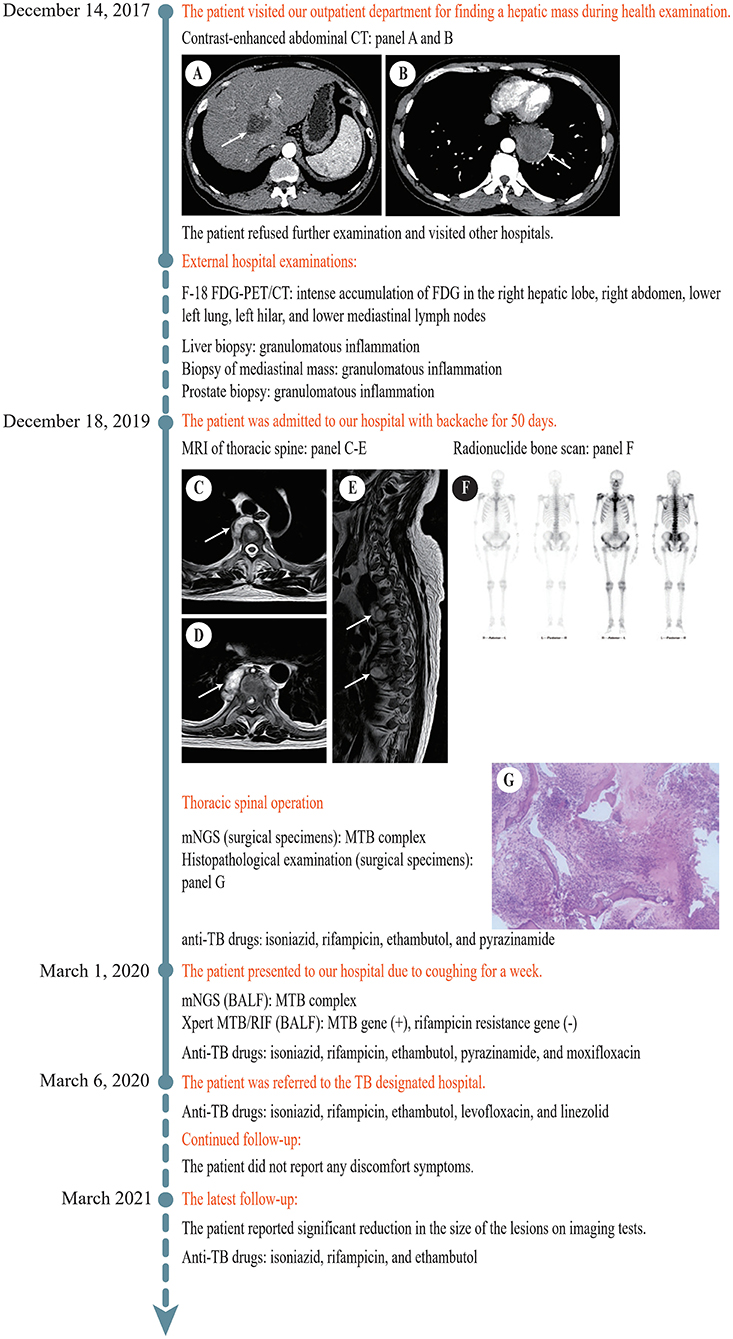
Figure 1. Timeline of the diagnosis and treatment process. (A) Contrast-enhanced abdominal CT revealed a hypodense lesion (white arrow) with mild to moderate enhancement in the right hepatic lobe near hepatic hilar. (B) Contrast-enhanced abdominal CT revealed a soft tissue density lesion (white arrow) with heterogeneous enhancement in the left posterior mediastinum. (C) MRI of thoracic spine revealed a soft tissue lesion (white arrow) at T3/T4. (D) MRI of thoracic spine showed a soft tissue lesion (white arrow) at T7/T8. (E) MRI of thoracic spine displayed multiple bone destructions (white arrows) at T3/T4 and T7/T8 with surrounding soft tissue lesions. (F) Radionuclide bone scan showed that the metabolism of vertebral bodies of T4 and T8 was abnormal. (G) Histopathological examination of the lesion at T7/T8 showed caseous necrosis with a significant number of inflammatory cells and multinucleate giant cells. CT, computed tomography; F-18 FDG-PET, 18F-fluorodeoxyglucose positron emission tomography; MRI, magnetic resonance imaging; T, thoracic vertebra; BALF, bronchoalveolar lavage fluid.
In the following 2 years, he had visited several hospitals and had underwent a huge number of blood tests, CT scans, and magnetic resonance imaging (MRI) examinations. Mantoux test was positive (24 ×22 mm). The results of T-SPOT.TB tests elevated progressively from 14 to 84 pg/ml. Repeated sputum smear examinations for acid-fast bacilli (AFB) were all negative. Chest CT displayed multiple small lung nodules and a left posterior mediastinal mass. 18F-fluorodeoxyglucose positron emission tomography (F-18 FDG-PET)/CT showed intense accumulation of FDG in the right hepatic lobe, right abdomen, lower left lung, left hilar, and lower mediastinal lymph nodes, suggesting the possibility of malignancy. MRI showed multiple cystic nodules in the prostate. Histopathological examinations of specimens obtained by fine-needle aspiration biopsy from the liver mass, mediastinal mass, and prostatic nodules suggested granulomatous inflammation, and special stains including acid-fast staining, Grocott's methenamine silver (GMS), Periodic acid-Schiff (PAS), and gram were all negative. The diagnosis remained unclear.
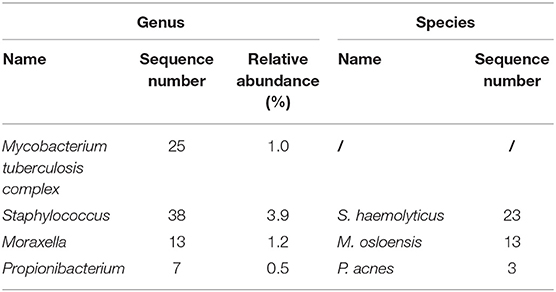
On December 18, 2019, the patient was admitted to our hospital again with backache for 50 days. MRI of thoracic spine demonstrated multiple bone destructions at T3/T4 and T7/T8 with surrounding soft tissue lesions, suggesting spinal TB with cold abscesses (Figures 1C–E). Radionuclide bone scan demonstrated that the metabolism of vertebral bodies of T4 and T8 was abnormal, and metastatic bone tumors were considered (Figure 1F). He underwent a thoracic spinal operation. Considering the possibility of TB, the surgical specimens were subjected to both mNGS and histopathological examination. The process of mNGS is provided in the Supplementary Material. The mNGS revealed 25 total reads of MTB complex (Table 2). Significantly, because of the high internal homology and relatively insufficient coverage, the species within the MTB complex was not determined (13). Meanwhile, histopathological examination showed caseous necrosis with a significant number of inflammatory cells and multinucleate giant cells (Figure 1G). Therefore, the patient was diagnosed with spinal TB and immediately treated with anti-TB drugs including isoniazid, rifampicin, ethambutol, and pyrazinamide.
On March 1, 2020, the patient presented to our hospital for the third time due to coughing for a week. His bronchoalveolar lavage fluid (BALF) was obtained by fiberoptic bronchoscopy for mNGS and Xpert MTB/RIF assay. The process of Xpert MTB/RIF is provided in the Supplementary Material. MTB complex was successfully detected by mNGS. In addition, the result of Xpert MTB/RIF was positive for MTB gene and negative for rifampicin resistance. Based on the available evidence, it was eventually considered that the patient suffered from disseminated TB with systemic multi-organ involvement, including the lung, spine, mediastinum, liver, and prostate. To enhance the treatment effect, moxifloxacin was added to the original anti-TB treatment regimen.
According to the national laws and regulations, the patient was subsequently referred to the TB designated hospital for systematic treatment because of intrapulmonary TB. Through telephone follow-up, we learned that pyrazinamide was discontinued because of hyperuricemia shortly after the referral, and then the patient received anti-TB treatment with isoniazid, rifampicin, ethambutol, levofloxacin, and linezolid for 1 year. Diammonium glycyrrhizinate, which protects the liver, was continuous in the course of anti-TB therapy. The anti-TB drug regimen was well-tolerated and achieved a remarkable effect. At the latest follow-up, the patient reported significant reduction in the size of the lesions on imaging tests, reconfirming the diagnosis of TB, and started receiving anti-TB treatment with isoniazid, rifampicin, and ethambutol. He thanked us for our contribution to the diagnosis.
Discussion
The clinical presentation of disseminated TB can vary greatly according to the involved organ system. It can present with atypical extrapulmonary symptoms as the first manifestation, often leading to a diagnostic dilemma. In our case, the patient was immunocompetent and came to our hospital due to a mass in the liver without any typical symptoms. For most clinicians, malignancy and infectious diseases are primary considerations among the broad differential diagnosis. Their identification needs evidence from additional tests.
WHO reported that nearly 30% TB cases failed to be diagnosed (14). The hepatic TB are largely non-specific and exhibit an extensive overlap with more common primary or metastatic liver carcinoma under image findings (15). Initially, this case was misdiagnosed as hepatic carcinoma according to the contrast-enhanced abdominal CT scan. Recently, F-18 FDG-PET/CT has emerged as an effective tool for the diagnosis and evaluation of malignant tumors. However, it is difficult for F-18 FDG-PET/CT to distinguish between inflammatory and malignant lesions because of the strong accumulation of F-18 FDG in both tissues (16). In our case, the F-18 FDG-PET/CT report was more favorable of malignancy vs. inflammation. Regrettably, due to the influence of the above imaging results or the lack of attention by clinicians to infectious diseases, the specimens from three biopsies were just collected for histopathological examinations, but no pathogenic detections, which led to diagnostic delay and worse clinical course.
At present, the dominating methods of diagnosing disseminated TB are still pathogen culture, AFB smear, nucleic acid amplification test (NAAT), and histopathological examination (17). MTB culture is currently considered as the gold standard of diagnosis, but low sensitivity and long culture time make it cannot meet the need of rapid clinical diagnosis (18). AFB smear, as a simple and fast tool, is unsatisfying due to lower sensitivity and specificity (19, 20). Just like the biopsies in our case, histopathological examination sometimes only shows granulomas without typical caseous necrosis, which is non-specific for the diagnosis of TB. PCR is a valuable diagnostic technique, but it always requires presupposing specific pathogens, which sometimes cannot detect rare pathogens and mixed infections (21). Additionally, Xpert MTB/RIF, a new automated molecular test, has been endorsed by WHO for the initial detection of TB and rifampicin resistance (14). Nevertheless, the sensitivity of Xpert MTB/RIF varies greatly with the type of extrapulmonary samples (22, 23). Significantly, mNGS exhibits better diagnostic performance than Xpert MTB/RIF in detecting MTB among various samples. Mutual combination can stimulate further elevation of the performance (13, 24).
mNGS, an unbiased culture-independent high-throughput sequencing technology, has shown a considerable clinical application prospect in diagnosis of infectious diseases over the past few years. In our case, mNGS finally unmasked TB after 2 years of delayed diagnosis and misdiagnosis. Notably, several prospective studies have indicated that mNGS shows an excellent diagnostic performance in suspected TB patients with 62–87.5% sensitivity for intrapulmonary samples, 47.4–60% sensitivity for extrapulmonary samples, and almost 100% specificity in various samples (13, 24, 25). In contrast to some traditional microbiological tests designed to specifically detect just one or a limited spectrum of known pathogens at a time, mNGS can simultaneously detect thousands of pathogens, including bacteria, fungi, viruses, and even parasites, without requiring any prior knowledge (26). Additionally, the turnaround time of mNGS, usually 2–3 days, is shorter than several weeks of traditional MTB culture, which might benefit clinicians in making rapid diagnosis and guiding precise antimicrobial therapy. On the other hand, mNGS can obtain all nucleotide sequence information in clinical samples, including nucleotide sequences from human, contaminant during operations, harmless parasitic microbes, and true pathogens, thus can create challenges in data processing and interpretation of results (27). MTB, as one of the intracellular bacteria, is characterized by releasing fewer nucleic acids into extracellular environment, resulting in a small number of sequences detected, which may also lead to the difficulty of detecting MTB by mNGS (28). The detection of resistance genes and the strain discrimination of the MTB complex are hard to achieve clinically because they highly depend on genome coverage, and combining with targeted PCR might be a good choice (13). The combination of mNGS and Xpert MTB/RIF shows great potential in detecting MTB, which is superior to traditional pathogenic detection methods. In our case, MTB was reconfirmed by fiberoptic bronchoscopy combining with mNGS and Xpert MTB/RIF, meeting the requirement for etiology diagnosis and proving the clinical value of the combined application. Thus, mNGS alone or in combination with Xpert MTB/RIF provides clinicians with a reliable TB diagnosis method.
In conclusion, we emphasize that disseminated TB should also be considered in an immunocompetent patient even when imaging results favor malignant lesions based on the experience from this case. It is essential for clinicians to perform pathogenic examinations in time when suspicious specimens are encountered, and mNGS can be used as a valuable complementary means for TB diagnosis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patient provided his written informed consent to participate in this study.
Author Contributions
YY and NY analyzed and interpreted the clinical data. YY, NY, and JZ drafted the manuscript. GQ and JC revised the manuscript. All authors have read and approved the final manuscript.
Funding
This research was supported by Key Program of Natural Science Foundation of Ningbo under Grant No. 202003N4019.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.687984/full#supplementary-material
References
1. Click ES, Moonan PK, Winston CA, Cowan LS, Oeltmann JE. Relationship between Mycobacterium tuberculosis phylogenetic lineage and clinical site of tuberculosis. Clin Infect Dis. (2012) 54:211–9. doi: 10.1093/cid/cir788
2. Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. Deutsches Aerzteblatt Online. (2019) 116:729–35. doi: 10.3238/arztebl.2019.0729
3. Fujikawa T, Senoo A. Recurrent erythema nodosum as a warning of tuberculous lymphadenitis. Mayo Clin Proc. (2019) 94:174–5. doi: 10.1016/j.mayocp.2018.11.018
4. Nishizawa S, Tobino K. Asymptomatic tuberculous pleurisy mimicking mesothelioma. Am J Respir Crit Care Med. (2020) 201:1149. doi: 10.1164/rccm.201906-1223IM
5. Mahjoub S, Lahmar AA, Zaraa M, Belhaj G, Abdelkafi M, Mbarek M. Extensive lytic tuberculosis of the humeral head. Joint Bone Spine. (2018) 85:767. doi: 10.1016/j.jbspin.2018.04.005
6. Ran P, Liang X, Zhang Y, Sun P, Dong A, FDG. PET/CT in a case of bilateral tuberculous epididymo-orchitis. Clin Nucl Med. (2019) 44:757–60. doi: 10.1097/RLU.0000000000002606
7. Machida A, Amano E, Otsu S, Akagawa S. Corticosteroid-dependent tuberculous meningitis: a case report. J Neurol Sci. (2019) 396:232–4. doi: 10.1016/j.jns.2018.11.021
8. Suarez I, Maria Funger S, Jung N, Lehmann C, Reimer RP, Mehrkens D, et al. Severe disseminated tuberculosis in HIV-negative refugees. Lancet Infect Dis. (2019) 19:e352–9. doi: 10.1016/S1473-3099(19)30162-8
9. Crump JA, Reller LB. Two decades of disseminated tuberculosis at a university medical center: the expanding role of mycobacterial blood culture. Clin Infect Dis. (2003) 37:1037–43. doi: 10.1086/378273
10. Shi CL, Han P, Tang PJ, Chen MM, Ye ZJ, Wu MY, et al. Clinical metagenomic sequencing for diagnosis of pulmonary tuberculosis. J Infect. (2020) 81:567–74. doi: 10.1016/j.jinf.2020.08.004
11. Zhao M, Tang K, Liu F, Zhou W, Fan J, Yan G, et al. Metagenomic next-generation sequencing improves diagnosis of osteoarticular infections from abscess specimens: a multicenter retrospective study. Front Microbiol. (2020) 11:2034. doi: 10.3389/fmicb.2020.02034
12. Yan L, Sun W, Lu Z, Fan L. Metagenomic Next-Generation Sequencing (mNGS) in cerebrospinal fluid for rapid diagnosis of Tuberculosis meningitis in HIV-negative population. Int J Infect Dis. (2020) 96:270–5. doi: 10.1016/j.ijid.2020.04.048
13. Zhou X, Wu H, Ruan Q, Jiang N, Chen X, Shen Y, et al. Clinical evaluation of diagnosis efficacy of active Mycobacterium tuberculosis complex infection via metagenomic next-generation sequencing of direct clinical samples. Front Cell Infect Microbiol. (2019) 9:351. doi: 10.3389/fcimb.2019.00351
14. WHO. Global Tuberculosis Report 2020. WHO (2020). Available online at: https://www.who.int/publications-detail-redirect/9789240013131 (accessed October 15, 2020).
15. Kakkar C, Polnaya AM, Koteshwara P, Smiti S, Rajagopal KV, Arora A. Hepatic tuberculosis: a multimodality imaging review. Insights Imaging. (2015) 6:647–58. doi: 10.1007/s13244-015-0440-y
16. Li YJ, Zhang Y, Gao S, Bai RJ. Systemic disseminated tuberculosis mimicking malignancy on F-18 FDG PET-CT. Clin Nucl Med. (2008) 33:49–51. doi: 10.1097/RLU.0b013e31815c5004
17. Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American thoracic society/infectious diseases society of America/centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. (2017) 64:111–5. doi: 10.1093/cid/ciw778
18. Tyrrell FC, Budnick GE, Elliott T, Gillim-Ross L, Hildred MV, Mahlmeister P, et al. Probability of negative mycobacterium tuberculosis complex cultures based on time to detection of positive cultures: a multicenter evaluation of commercial-broth-based culture systems. J Clin Microbiol. (2012) 50:3275–82. doi: 10.1128/JCM.01225-12
19. Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. (2006) 6:570–81. doi: 10.1016/S1473-3099(06)70578-3
20. Ninet B, Rohner P, Metral C, Auckenthaler R. Assessment of use of the COBAS AMPLICOR system with BACTEC 12B cultures for rapid detection of frequently identified mycobacteria. J Clin Microbiol. (1999) 37:782–4. doi: 10.1128/JCM.37.3.782-784.1999
21. Huang Z, Zhang C, Hu D, Shi K, Li W, Zhang C, et al. Diagnosis of osteoarticular tuberculosis via metagenomic next-generation sequencing: a case report. Exp Ther Med. (2019) 18:1184–8. doi: 10.3892/etm.2019.7655
22. Tadesse M, Abebe G, Bekele A, Bezabih M, Yilma D, Apers L, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a diagnostic evaluation study. Clin Microbiol Infect. (2019) 25:1000–5. doi: 10.1016/j.cmi.2018.12.018
23. Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. (2014) 44:435–46. doi: 10.1183/09031936.00007814
24. Chen P, Sun W, He Y. Comparison of metagenomic next-generation sequencing technology, culture and GeneXpert MTB/RIF assay in the diagnosis of tuberculosis. J Thorac Dis. (2020) 12:4014–24. doi: 10.21037/jtd-20-1232
25. Sun W, Lu Z, Yan L. Clinical efficacy of metagenomic next-generation sequencing for rapid detection of Mycobacterium tuberculosis in smear-negative extrapulmonary specimens in a high tuberculosis burden area. Int J Infect Dis. (2021) 103:91–6. doi: 10.1016/j.ijid.2020.11.165
26. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. (2018) 66:778–88. doi: 10.1093/cid/cix881
27. Zhu YM, Ai JW, Xu B, Cui P, Cheng Q, Wu H, et al. Rapid and precise diagnosis of disseminated tmarneffei infection assisted by high-throughput sequencing of multifarious specimens in a HIV-negative patient: a case report. BMC Infect Dis. (2018) 18:379. doi: 10.1186/s12879-018-3276-5
Keywords: disseminated tuberculosis, metagenomic next-generation sequencing, immunocompetence, extrapulmonary tuberculosis, diagnosis
Citation: Ye Y, Yang N, Zhou J, Qian G and Chu J (2021) Case Report: Metagenomic Next-Generation Sequencing in Diagnosis of Disseminated Tuberculosis of an Immunocompetent Patient. Front. Med. 8:687984. doi: 10.3389/fmed.2021.687984
Received: 30 March 2021; Accepted: 14 June 2021;
Published: 12 July 2021.
Edited by:
Yanfei Chen, Zhejiang University, ChinaReviewed by:
Eirini Christaki, University of Cyprus, CyprusReza Yazdani, Tehran University of Medical Sciences, Iran
Copyright © 2021 Ye, Yang, Zhou, Qian and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinguo Chu, Y2h1amdAMTI2LmNvbQ==; Guoqing Qian, YmlsbC5xaWFuQG91dGxvb2suY29t
 Yuanting Ye1,2
Yuanting Ye1,2 Naibin Yang
Naibin Yang Guoqing Qian
Guoqing Qian Jinguo Chu
Jinguo Chu