- 1School of Health Sciences, Caritas Institute of Higher Education, Hong Kong, China
- 2Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China
- 3Department of Chemical Pathology, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China
- 4Faculty of Medicine, Macau University of Science and Technology, Macau, China
- 5Shenzhen Baoan Women's and Children's Hospital, Jinan University, Shenzhen, China
Purpose: This study was performed to investigate the effects of common polymorphisms in CYP2D6 and CYP3A5 on the plasma concentrations and antihypertensive effects of bisoprolol in hypertensive Chinese patients.
Methods: One hundred patients with essential hypertension were treated with open-label bisoprolol 2.5 mg daily for 6 weeks. Clinic blood pressure (BP) and ambulatory BP (ABP) were measured after the placebo run-in and after 6 weeks treatment. Peak plasma concentrations of bisoprolol were measured at 3 h after the first dose and 3 h after the dose after 6 weeks treatment. Trough levels were measured before the dose after 6 weeks treatment. Bisoprolol plasma concentrations were measured with a validated liquid chromatography tandem mass spectrometry method. Six common polymorphisms in CYP2D6 and the CYP3A5*3 polymorphism were genotyped by TaqMan® assay.
Results: After 6 weeks of treatment, clinic BP and heart rate were significantly reduced by 14.3 ± 10.9/8.4 ± 6.2 mmHg (P < 0.01) and 6.3 ± 7.6 BPM (P < 0.01), respectively. Similar reductions were seen in ABP values. Bisoprolol plasma concentration at 3 h after the first dose and 3 h post-dose after 6 weeks of treatment were significantly associated with baseline body weight (P < 0.001) but there was no significant effect of the CYP2D6 and CYP3A5 polymorphisms on these or the trough plasma concentrations. There was no significant association of the CYP2D6 and CYP3A5 polymorphisms or plasma bisoprolol concentrations with the clinic BP or ABP responses to bisoprolol.
Conclusion: Bisoprolol 2.5 mg daily effectively reduced BP and HR. The common polymorphisms in CYP2D6 that were examined and the CYP3A5*3 polymorphism appear to have no benefit in predicting the hemodynamic response to bisoprolol in these patients.
Introduction
Hypertension is the leading preventable cause of death globally, but there are major disparities of hypertension prevalence, awareness, treatment, and control in different countries (1). Beta-adrenoceptor antagonists or β-blockers are one of the oldest groups of drugs used to treat hypertension, although recent guidelines from the U.S. and Europe no longer recommend them as first line treatment for hypertension unless there are additional indications such as heart failure or post-myocardial infarction (2, 3). This advice is based on a meta-analysis which reported an increased risk of stroke with the use of β-blockers compared with other antihypertensive agents (4). However, in another meta-analysis which separated trials into those enrolling older patients ≥ 60 years and those enrolling younger patients <60 years, β-blockers demonstrated similar efficacy to other antihypertensive agents in younger patients but not in older patients (5). Hypertension Canada's 2020 Guidelines still recommends that β-blockers may be used as first-line monotherapy in patients younger than 60 years of age but not in patients aged ≥ 60 years (6).
The blood pressure responses to β-blockers and other antihypertensive drug classes is partly dependent on genetic variation in both pharmacodynamic and pharmacokinetic pathways (7). Several β-blockers, in particular metoprolol, are extensively metabolized by cytochrome P450 2D6 (CYP2D6) and the CYP2D6 genotype has a pronounced effect on the single dose pharmacokinetics of metoprolol which persists during long-term therapy (8). The effect of CYP2D6 genotype on the hemodynamic and clinical responses to metoprolol has not been consistent but a meta-analysis of seven studies in 2017 concluded that CYP2D6 polymorphisms significantly influenced the heart rate (HR) and diastolic blood pressure (DBP), but not the systolic blood pressure (SBP) response to metoprolol (9). A more recent meta-analysis of 15 studies found that CYP2D6 poor metabolizers (PM) had significantly greater reductions in HR, SBP and DBP compared to non-PM individuals (10).
Bisoprolol is a moderately lipophilic highly β1-selective β-blocker that is devoid of any intrinsic sympathomimetic activity (ISA), vasodilatory effects or membrane stabilizing properties. It is one of the few β-blockers approved for congestive heart failure in addition to the usual β-blocker indications of hypertension and coronary heart disease (11). Bisoprolol is eliminated with 50% renal excretion as unchanged drug and 50% via hepatic metabolism to pharmacologically inactive metabolites which are then excreted by the kidneys (11). It is reported to be metabolized by CYP3A4 and to a small extent by CYP2D6 (12, 13).
CYP3A4 is abundantly expressed in human liver and intestine, representing 30–50% and 70% of the two microsomal P450 pools, respectively (14). CYP3A5, which is expressed in intestinal enterocytes and in other extra-hepatic tissues, may contribute up to about 50% of the CYP3A pool in individuals with low CYP3A4 expression (14, 15). The CYP3A5*3 (rs776746, 6986G>A) polymorphism is a common variant occurring in all populations with an allele frequency of 0.65 in Chinese (16).
CYP2D6 is highly polymorphic with 145 variant alleles reported so far, many of these having reduced or absent function (17). CYP2D6*1, *2, *5, and *10 were the most frequent CYP2D6 alleles found in Hong Kong Chinese (18, 19). The reduced-function CYP2D6*10 allele is the most common variant in East Asians and occurs in 33–43% of these populations, including Japanese, Korean, and Chinese, but in only about 2–5% in Caucasians and African Americans (20). Conversely, the frequency of the loss of gene variant (CYP2D6*5) is similar among different ethnic groups (4–7%) (21). The CYP2D6*14B allele, which differs from the *14 allele by the absence of the C100T substitution and by the additional G1749C substitution, occurs in 2% of Chinese (22). Nozawa et al. (23) reported an association between CYP2D6 polymorphisms and plasma concentrations of metoprolol but not bisoprolol. Scoring systems have been established in an attempt to provide CYP2D6 alleles a uniform approach to quantitate the predicted functional status (24) and these have been updated recently (17). Poor metabolizers (PMs) differ from extensive metabolizers (EMs) by 5- to 15-fold if determined by rates of metabolism or by ratios of parent to metabolite concentrations (25, 26).
The influence of the CYP3A5*3 polymorphism in the overall oxidative activity of CYP3A and the possible relation of CYP3A5*3 and CYP3A4*1G polymorphisms on CYP3A activity and their potential interaction is still uncertain (27, 28). In addition, the influence of CYP2D6 genotypes on the pharmacokinetics and therapeutic responses of bisoprolol have been inconsistent (29). The present study, therefore, investigated the effect of CYP3A5 and CYP2D6 genotypes on the plasma concentrations of bisoprolol and the clinic and 24-h ambulatory blood pressure (ABP) responses in Chinese patients with mild to moderate hypertension.
Materials and Methods
Study Participants
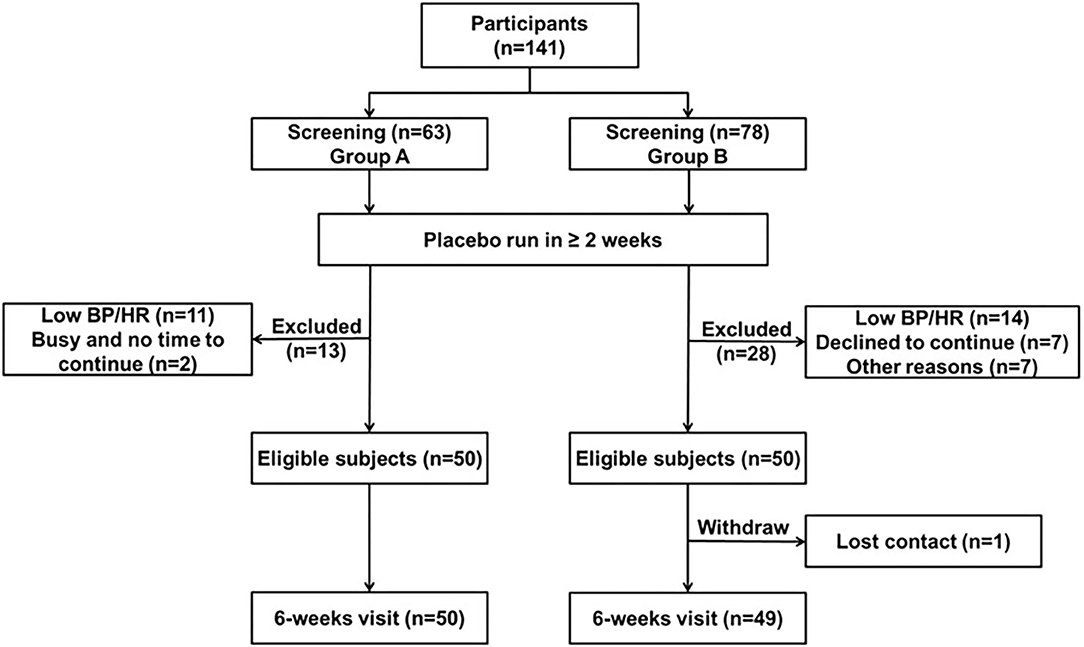
A total of 141 patients with a de novo diagnosis of primary hypertension or a previous history of primary hypertension identified from outpatient clinics in the Prince of Wales Hospital, Hong Kong were invited to participate. Sitting clinic BP levels after a placebo run-in of at least 2 weeks were required to be in the range of SBP 140–169 mmHg and/or DBP of 90–109 mmHg in otherwise healthy patients or SBP 130–169 mmHg and/or DBP of 80–109 mmHg in patients with diabetes mellitus. After informed consent was obtained, subjects were withdrawn from any previous antihypertensive therapy and given placebo once daily for at least 2 weeks. Amlodipine treatment was continued if necessary to achieve BPs in the defined range at the end of the placebo run-in. Compliance was assessed using pill counting, and any subject with compliance <80% during the placebo run-in period was excluded from the study.
Individuals with secondary hypertension, unstable angina, a history of myocardial infarction, stroke or coronary heart disease (coronary by-pass or angioplasty) in the previous 3 months before recruitment, heart failure (New York Heart Association [NYHA] II–IV), hemodynamically relevant aortic or mitral valve disease, hypertrophic obstructive cardiomyopathy, symptomatic bradycardia, second or third degree AV block, sick sinus syndrome, sinoatrial block, or HR <70 beats/min (BPM) at baseline (before starting bisoprolol treatment), primary hyperaldosteronism, renal artery stenosis, impairment of hepatic or renal function as defined by liver function values of ALT ≥ 1.5-fold the upper limit of normal or serum creatinine >150 μmol/L or upon investigator decision, and history or intolerance or with a known contraindication to β-blockers were excluded.
Study Design
This was a phase IV clinical trial registered with reference number NCT02398929 (https://clinicaltrials.gov/show/NCT02398929). Patients were enrolled into an open-label, pharmacogenetic study of bisoprolol treatment with a placebo run-in of at least 2 weeks. According to the total duration of bisoprolol treatment, the study participants were divided into two groups. In group A, 63 patients were screened and 50 were enrolled and given bisoprolol 2.5 mg once daily for 6 weeks. They had venous blood samples collected after 6 weeks of treatment before the dose for trough drug concentration assay. In group B, 78 patients were screened and 50 were enrolled and were treated with bisoprolol 2.5 mg once daily for 6 weeks and then continued treatment for a total of 24 weeks with optional titration of the dose of bisoprolol by doubling the dose after 6-week intervals up to 10 mg to achieve target BP levels. In this group, additional venous blood samples were collected 3 h after the first dose and 3 h after the dose after 6 weeks of treatment for peak plasma concentrations of bisoprolol. Clinic BP and 24-h ABP measurements were made at baseline, after the first dose of bisoprolol 2.5 mg and at the end of 6 weeks treatment with bisoprolol 2.5 mg. The patients were instructed to wear a wrist-type (BPro, HealthSTATS International, Singapore) or arm-type (A&D TM-2430, Tokyo, Japan) ABP device for 24 h and their BPs were measured at intervals automatically throughout 24 h. Some patients were fitted with both ABP devices to compare the readings. The wrist monitor showed reasonable agreement with the arm monitor in previous studies (30). Patients were encouraged to continue their usual daily activities but not to engage in vigorous physical exercise such as running, climbing, or playing sports. A daily activity record form was given to each patient.
Ethics
The study involving human participants was reviewed and approved by the Joint Clinical Research Ethics Committee of the Chinese University of Hong Kong and New Territories East Cluster (CUHK-NTEC) with reference number CRE-2011.616-T. The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki and subsequent revisions. All patients signed the Informed Consent.
Biochemical Assessments
Plasma lipid profile (total cholesterol, triglycerides, and HDL-cholesterol), glucose, renal, and liver function tests were measured on a Roche Modular Analytics system (Roche Diagnostics GmbH, Mannheim, Germany) using standard reagent kits supplied by the manufacturer of the analyzer. The analytical performance of these assays was within the manufacturer's specifications. Low-density lipoprotein cholesterol level was estimated by using the Friedewald formula (31) or directly measured when the TG level was over 4.5 mmol/L.
Glycosylated hemoglobin (HbA1c) was measured using an automated ion-exchange chromatographic method (Bio-Rad Laboratory, Hercules, CA; reference range 5.1–6.4%). The inter-assay and intra-assay coefficient of variation (CV) for HbA1c was 3.1% at values <6.5%.
Genotyping
Six common polymorphisms in CYP2D6 [*10 (100C>T, rs1065852), *4 (1934G>A, rs3892097, 1846G>A/T, rs5030865), *2 (2938C>T, rs16947, 4268G>C, rs1135840) and *5, deletion] and the CYP3A5*3 (rs776746, 6986G>A) polymorphism were selected in this study. DNA was extracted from peripheral whole blood samples by the phenol chloroform method. Genetic polymorphisms in CYP2D6 and the CYP3A5*3 polymorphism were genotyped by TaqMan® assay using the geneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, USA). The CYP3A5*3 polymorphism was determined using a previously reported polymerase chain reaction (PCR) restriction fragment length polymorphism (RFLP) method (32), while a long-PCR technology was used to detect the CYP2D6*5 variant as described previously (33). The 5 kb of the CYP2D6 gene was amplified first and then diluted 100-fold with water before performing the Taqman assay. The Taqman assays and detection were performed with ViiA7 from Life Technologies. The specific pair of primers used for PCR was as follows: Forward primer, 5′-CCA GAA GGC TTT GCA GGC TTC A-3′, and reverse primer, 5′-ACT GAG CCC TGG GAG GTA GGT A-3′. The PCR reaction conditions for CYP2D6 were: initial denaturation at 94°C for 2 min, followed by 10 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 30 s, extension at 68°C for 4 min, and followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 30 s, extension at 68°C for 4 min and 20 s, and the final extension at 68°C for 7 min.
All polymorphisms examined in this study were in Hardy–Weinberg equilibrium (χ2 test P > 0.05) and the frequencies of the minor alleles were similar to those reported in Han Chinese in HapMap. For translation of the genotypes into a qualitative measure of metabolizer group, the CYP2D6 activity score of each subject was calculated as the sum of the values assigned to each single allele (17). Subjects with an activity score of 1.25–2.25 were classified as normal metabolizers (NMs) whereas subjects with a score of 0 were classified as poor metabolizers (PMs) and subjects with a score of <1.25 was classified as intermediate metabolizers (IMs); subjects with a score > 2.25 were to be classified as ultra-rapid metabolizers (UMs) but none were identified.
Bisoprolol Assay
Plasma concentrations of bisoprolol were determined with a validated bioanalytical method. Method development and validation was performed according to the U.S. Food and Drug Administration (USFDA) guidance on Bioanalytical Method Validation (34). Briefly, liquid-liquid extraction was used to extract analyte and deuterium-labeled internal standard, bisoprolol-D7, from the biological matrix. After extraction, the target compounds were separated on a Waters ACQUITY BEH C18 UPLC column (2.1 × 50 mm, 1.7 μm), from 55% mobile phase A (0.1% formic acid in MilliQ water) to 80% mobile phase B (100% HPLC grade methanol) in 2 min, followed by 1 min washing at 95% mobile phase B and 1 min re-equilibration and detected by electrospray ionization tandem mass spectrometry using multiple reaction monitoring (MRM) in positive ion mode. Bisoprolol and the internal standard were both eluted at around 1 min and monitored by MRM transition m/z 326 > 116 and m/z 333 > 123, respectively. MRM transition 326 > 98 was used for bisoprolol as qualifier. Pooled plasma was spiked with a working solution to give 34, 1.7, and 0.34 μg/L quality control (QC) samples. An extra level of 0.1 μg/L was prepared for the lower limit of quantitation (LLOQ) validation purposes. All QC samples were aliquoted and stored at −80°C. This method covered a concentration range from 0.1 to 81.5 μg/L and total imprecision was <6% (<4% when excluding LLOQ) while inaccuracy was <13% throughout the concentration range (<4% when excluding LLOQ), which were within the acceptance criteria of Food and Drug Administration (FDA) and National Medical Products Administration (NMPA) guidelines. The imprecision and inaccuracy of LLOQ was 5.2 and −12.4%, respectively, both of which were better than the requirement by FDA and CFDA (+20%). Recovery and process efficiency of the assay at different concentrations were both on average 89%, suggesting a minimal loss of analyte during sample preparation.
Statistical Analysis
Statistical analyses were performed using IBM SPSS software (Version 26, IBM SPSS Inc., Armonk, New York, USA). Data were pooled from the two groups of subjects. The distribution of continuous data was evaluated according to the Shapiro–Wilk test. Differences in baseline characteristics, blood pressure and lipid profiles between the two studies were assessed using Student's t-test or Mann–Whitney U-test, as appropriate. χ2 test were used to test Hardy–Weinberg equilibrium and comparisons for categorical variables. Logistic regression analyses were applied to determine significantly independent predictors of BP and HR response and the pharmacogenetic analysis. Paired Student's t-test was used to compare the peak plasma levels of bisoprolol concentration 3 h post-first dose and 3 h post-dose after 6 weeks of treatment. The bisoprolol plasma concentrations were adjusted for body weight based on the univariate analysis. An independent samples t-test or an analysis of covariance (ANCOVA) followed by Tukey's multiple comparison test was used to assess the effect of the genetic polymorphisms on plasma concentrations of bisoprolol with body weight as covariate. Statistical analysis on the effect of genetic polymorphisms on the BP and HR responses to bisoprolol was performed using an independent samples t-test or a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test, as appropriate. Data are presented as mean ± standard deviation unless otherwise specified. A P < 0.05 was considered statistically significant.
Results
Study Population
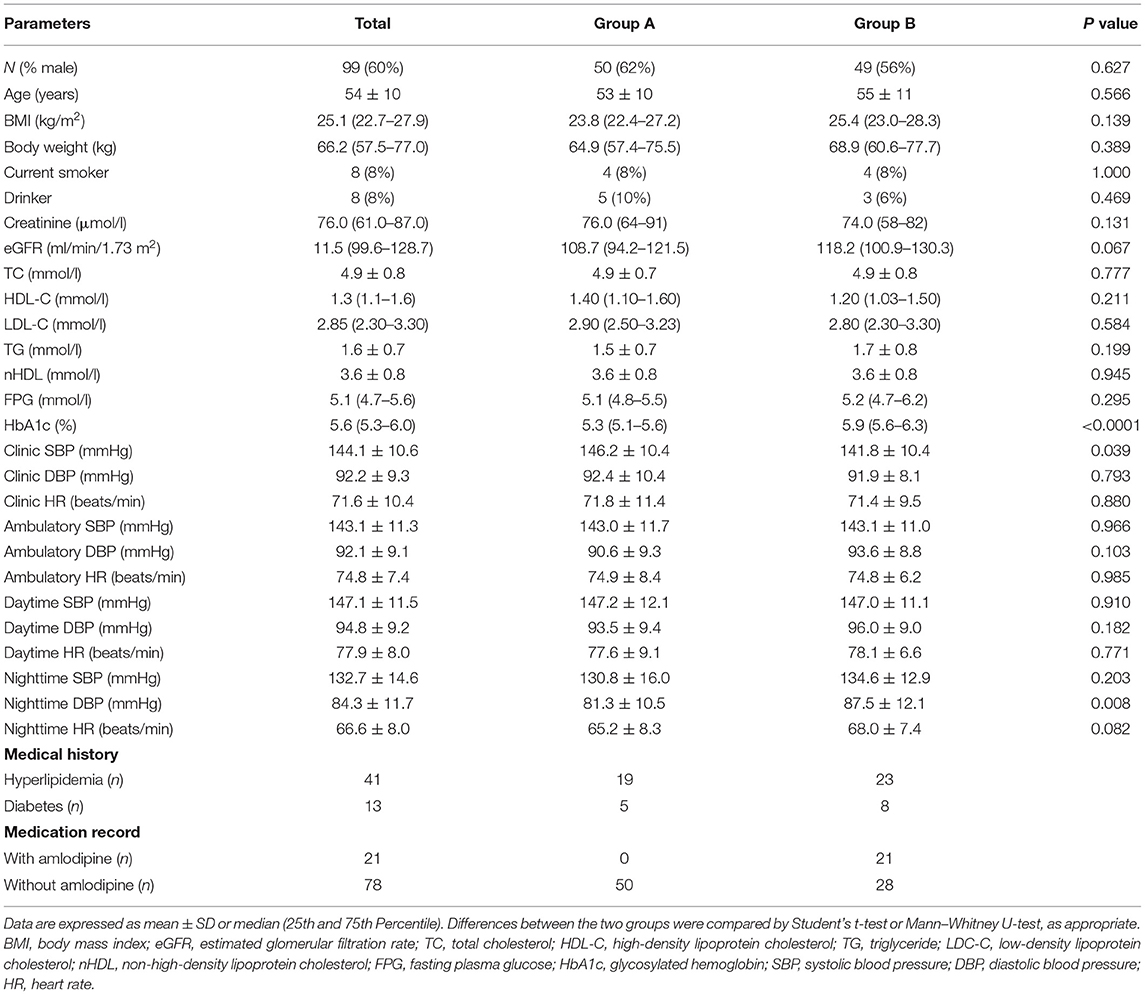
Fifty patients completed 6 weeks of bisoprolol treatment in group A and in group B, 49 patients completed the first 6 weeks treatment with bisoprolol and seven of them withdrew from the study subsequently (see Figure 1). The demographic and baseline characteristics and concomitant diseases of the study patients are shown in Table 1. All patients were of Chinese ethnicity (99 subjects), with mean (± SD) age 54 ± 10 years. The median body weight was 66.2 kg (25th−75th percentiles 57.5–77 kg) and median BMI 25.1 kg/m2 (22.7–27.9 kg/m2). The mean baseline clinic BP was 144.1 ± 10.6 mmHg/ 92.2 ± 9.3 mmHg. Patients had relatively normal lipid and glycemic profiles, as most of them were under medication control. There were no significant differences in the BPs, lipid profile, and glycemic profile between the two study groups, except the HbA1c level as there were more patients with diabetes in group B. The subjects were required to stop all the anti-hypertensive medication except amlodipine, of which 21 subjects were taking at a constant dose together with bisoprolol during the study.
Demographic data of study participants according to CYP2D6 and CYP3A5 genotypes are shown in Table 2. The observed CYP2D6 allele frequencies were 29% for *1, 11% for *2, 2% for *14B, 55% for *10, and 3% for *5. No patients carried the UM genotype in the present study. The distributions of CYP2D6 genotypes, metabolizer groups and CYP3A5 genotypes are shown in Table 2. There were two patients with a very high bisoprolol concentrations (18.5 μg/l before dose and 40.1 μg/l at 3 h post dose after 6 weeks of treatment) and they were considered as outliers possibly due to experimental error and these values were excluded from the analysis. Thus, we combined the data from 97 patients who finished 6 weeks of bisoprolol treatment in each of the two studies.
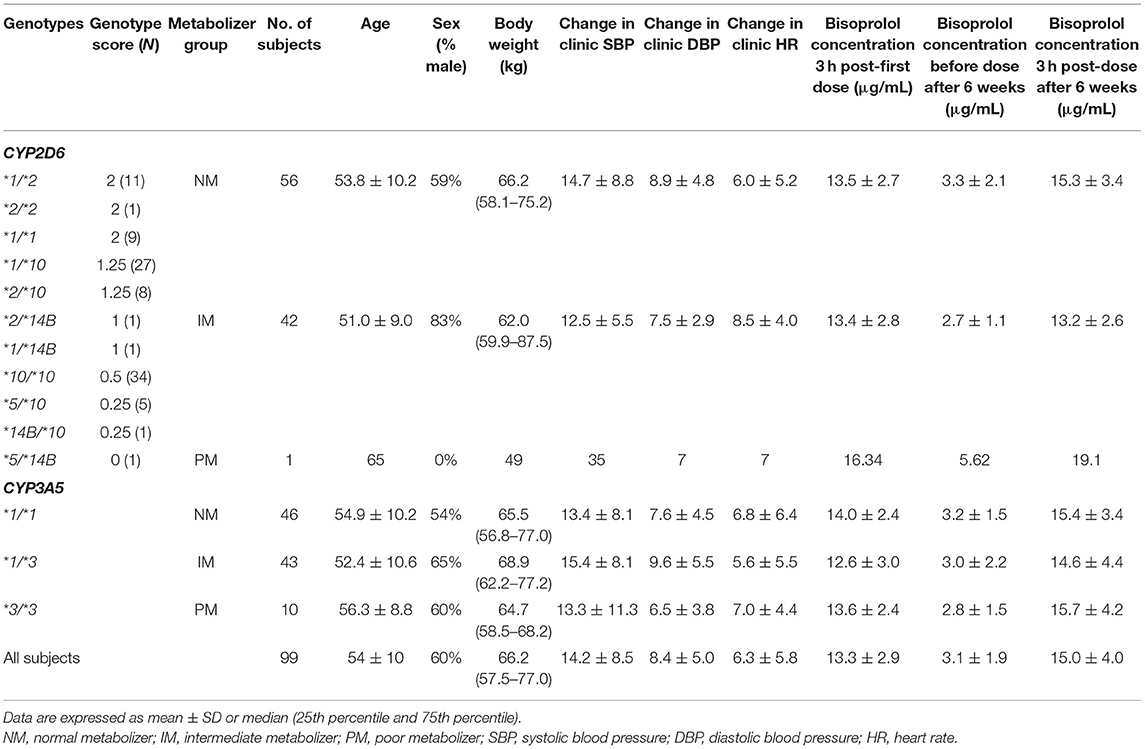
Table 2. Demographic data of study subjects (n = 99) according to CYP2D6 metabolizer and CYP3A5 genotype groups.
Pharmacogenetic Analysis of Bisoprolol Plasma Concentrations
Peak plasma concentrations of bisoprolol at 3 h after the first dose and 3 h after the dose after 6 weeks treatment were measured in 45 and 44 patients, respectively. Trough levels before the dose after 6 weeks treatment were determined in all 97 subjects. The peak levels after 6 weeks of treatment were increased by 13.6 ± 16.9% compared to the peak levels for first dose (peak level for first dose 13.2 ± 2.8, peak level at week 6 15.0 ± 4.0 μg/mL, P < 0.001). Univariate analysis showed that the bisoprolol concentration 3 h post first dose and 3 h post dose after 6 weeks of treatment was significantly related to body weight (p < 0.001) while there were no significant effects for other factors including sex, age, eGFR, concomitant treatment with amlodipine, or CYP3A5 and CYP2D6 genotype (Table 3). Only one subject had a CYP2D6 genotype score of 0 and this subject was combined with the IM group in the analysis. Multiple linear regression analyses were carried out to test the association between those candidate predictors and bisoprolol concentration (Table 3). Body weight influenced the bisoprolol concentration at 3 h post first dose (p < 0.001) and 3 h post dose (p < 0.001) after 6 weeks stable treatment, while gender and CYP2D6 metabolizer group and CYP3A5*3 genotype did not have any effect on peak bisoprolol concentrations in this study (Figures 2, 3). Moreover, a higher body weight predicted a lower peak plasma bisoprolol concentration. On the other hand, body weight and age were predictors of the trough bisoprolol concentration after 6 weeks treatment on univariate but not on multiple linear regression analysis (Table 3). There was no effect of CYP2D6 metabolizer group and CYP3A5*3 genotype on trough bisoprolol concentration (Figures 2, 3).
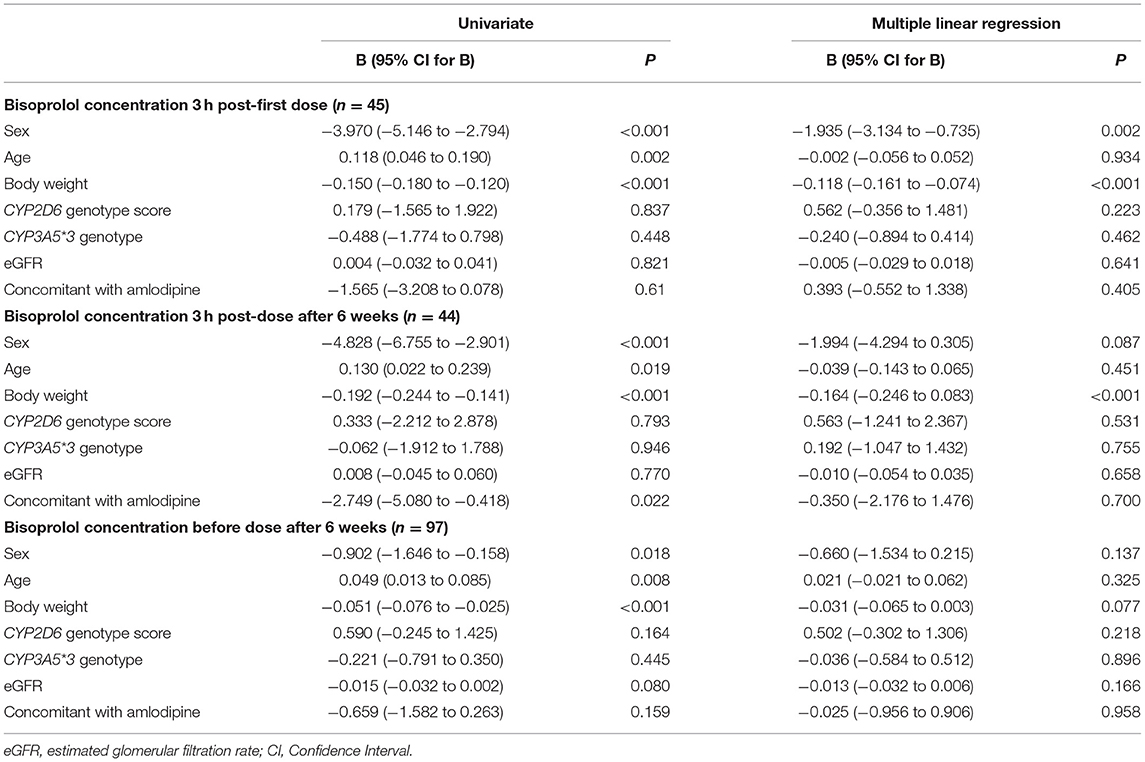
Table 3. Linear regression analysis for factors that may influence bisoprolol peak and trough plasma concentrations.
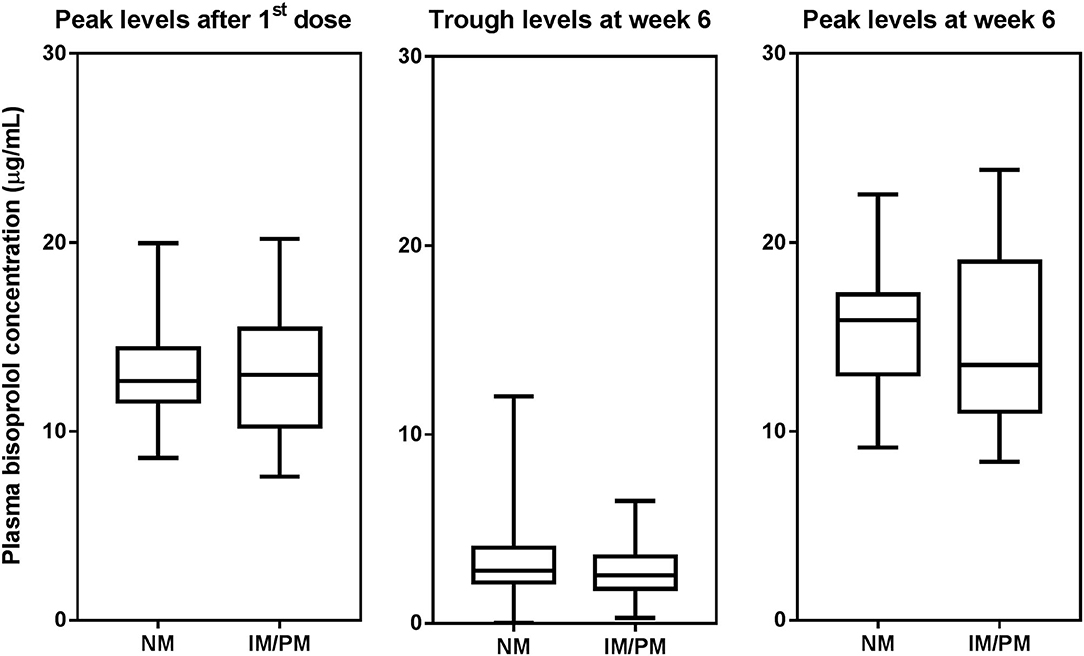
Figure 2. Box-and-whisker plot of plasma bisoprolol concentrations according to CYP2D6 metabilizer groups. The boxes represent the 25th−75th percentiles, the whiskers represent the range. There were no significant differences between metabolizer groups by independent samples t-test.
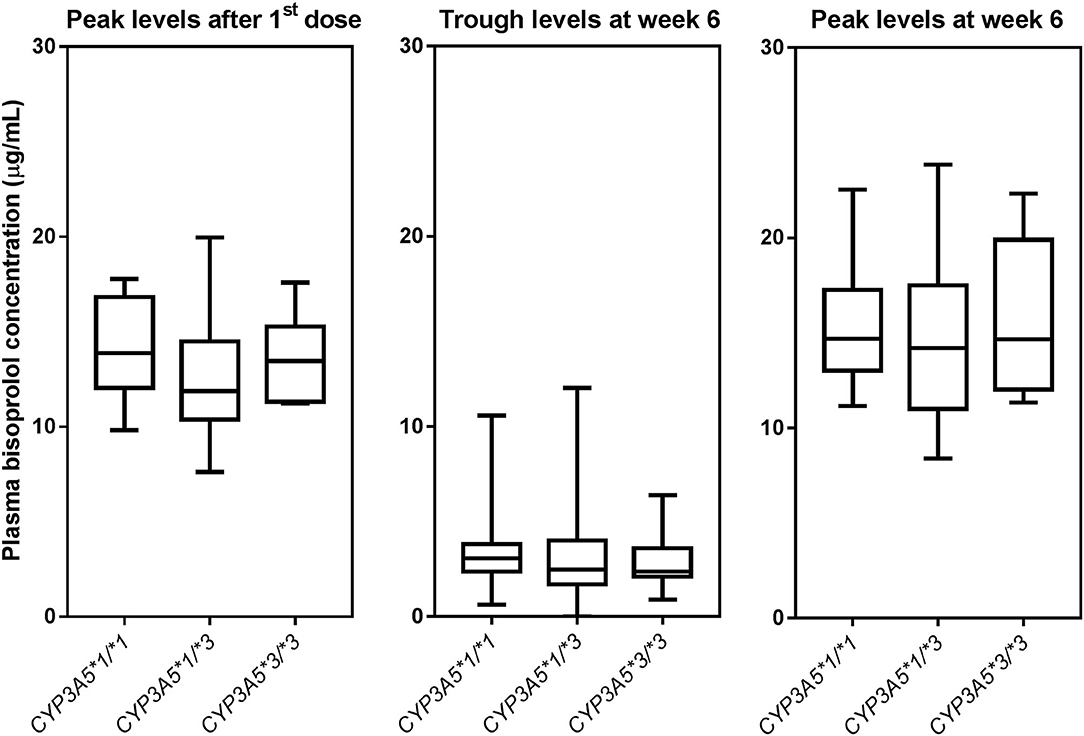
Figure 3. Box-and-whisker plot of plasma bisoprolol concentrations according to CYP3A5 genotypes. The boxes represent the 25th−75th percentiles, the whiskers represent the range. There were no significant differences between genotype groups by one-way ANOVA, with and without adjustment for body weight.
Effect of CYP2D6 and CYP3A5 Genotypes on the BP and HR Response to Bisoprolol
After 6 weeks of treatment with bisoprolol 2.5 mg daily, reductions in clinic BP and HR were 14.3 ± 10.9/8.4 ± 6.2 mmHg (P < 0.01) and 6.3 ± 7.6 BPM (P < 0.01), respectively, and there were similar reductions in the ABP and HR values (data not shown). Univariate analysis showed that BP and HR responses to bisoprolol were significantly related to baseline BP and HR (Table 4). The bisoprolol concentration before dose after 6 weeks of treatment was not related to changes in BP and HR but the peak level at week 6 was significantly associate with changes in SBP (P < 0.01) and DBP (P < 0.01) but not HR. There was no significant effect for sex, age, body weight, and concomitant treatment with amlodipine (Table 4). The subject with a CYP2D6 genotype score of 0 was combined with the IM group in the analysis and this subject actually had a small increase in BP with bisoprolol treatment. There was no significant difference in the clinical and ambulatory BP reductions among CYP2D6 metabolizer groups and CYP3A5 genotypes (Figures 4A,B). Similarly, no significant difference was observed in the clinic and ambulatory HR according to CYP2D6 metabolizer groups and CYP3A5 genotypes (Figures 5A,B).
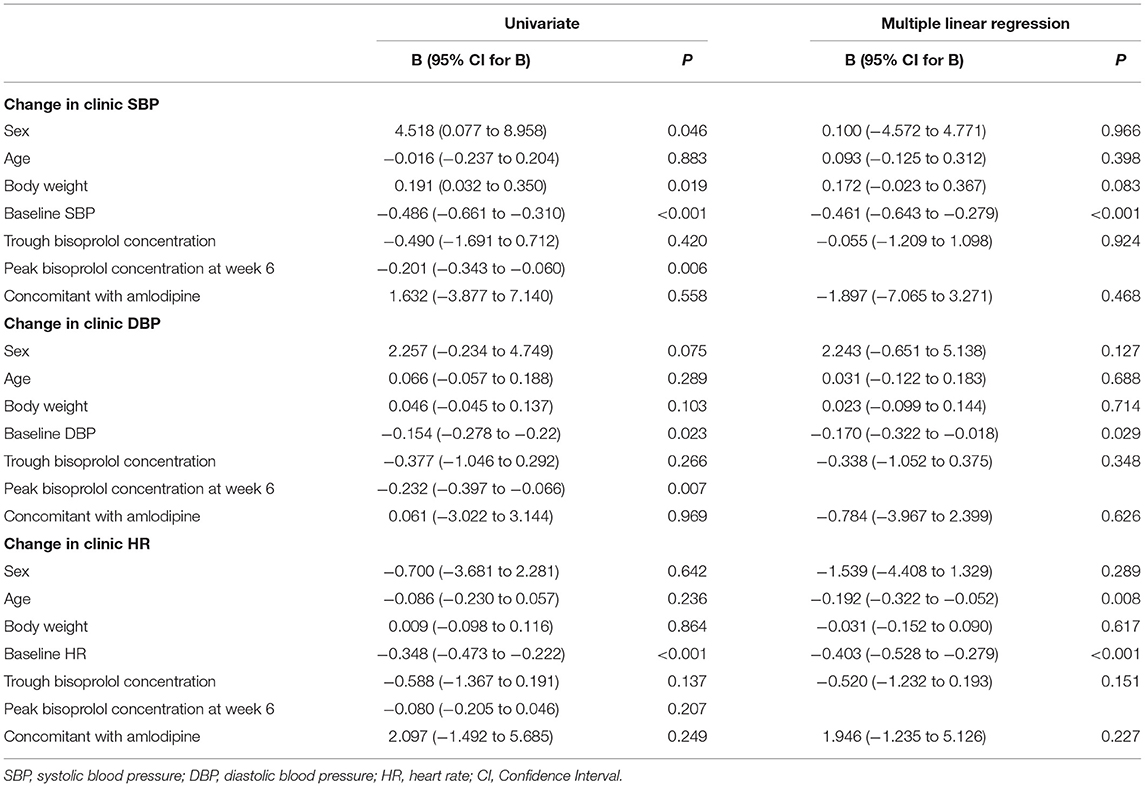
Table 4. Linear regression analysis for the factors that may influence BP and HR reductions after 6 weeks of treatment.
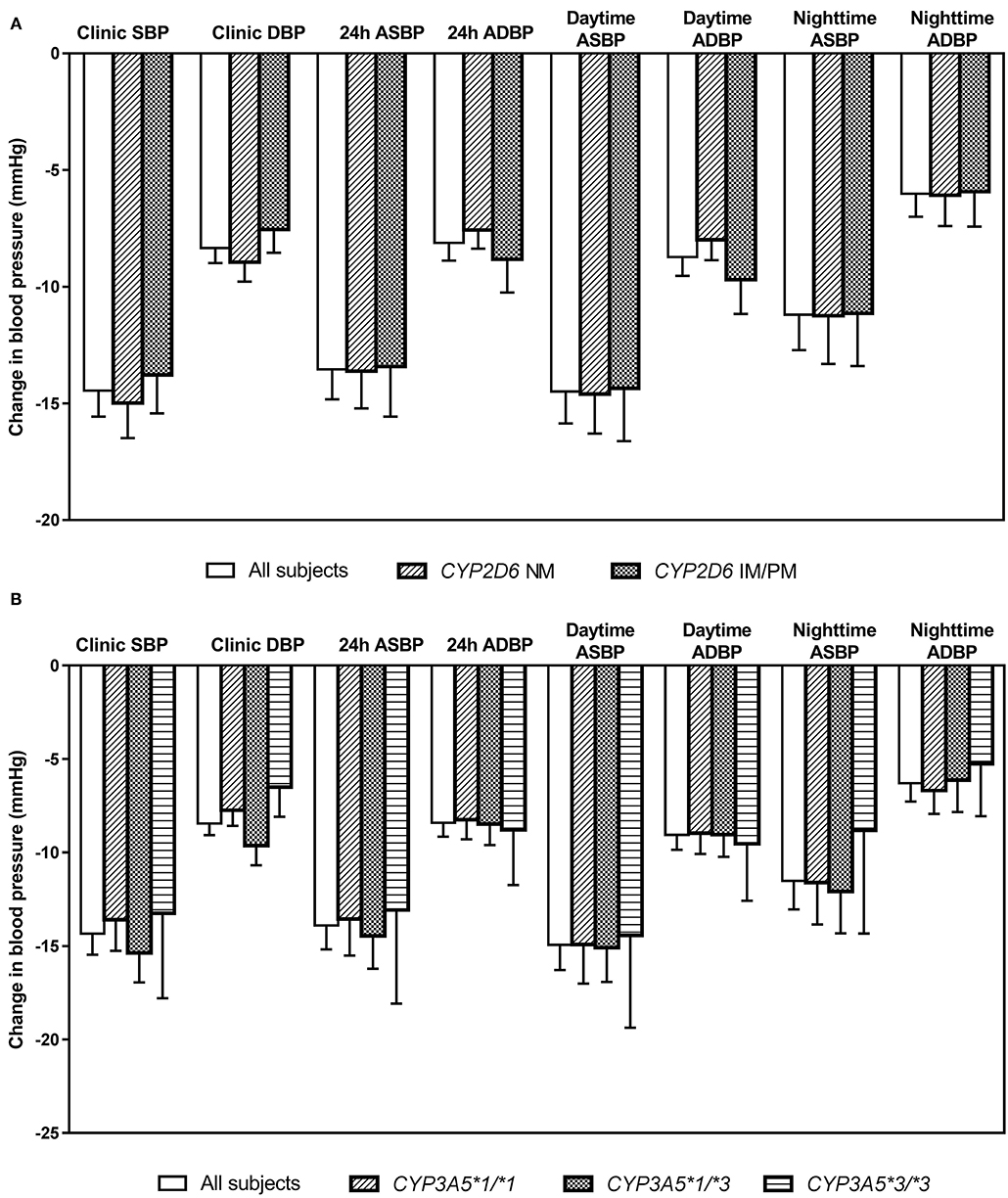
Figure 4. Changes in clinic and ambulatory blood pressure after 6 weeks treatment with bisoprolol 2.5 mg daily according to (A) CYP2D6 metabolizer groups, and (B) CYP3A5 genotypes. Data are presented as mean ± SEM. There is no significant difference between groups (independent samples t-test or one-way ANOVA, as appropriate).
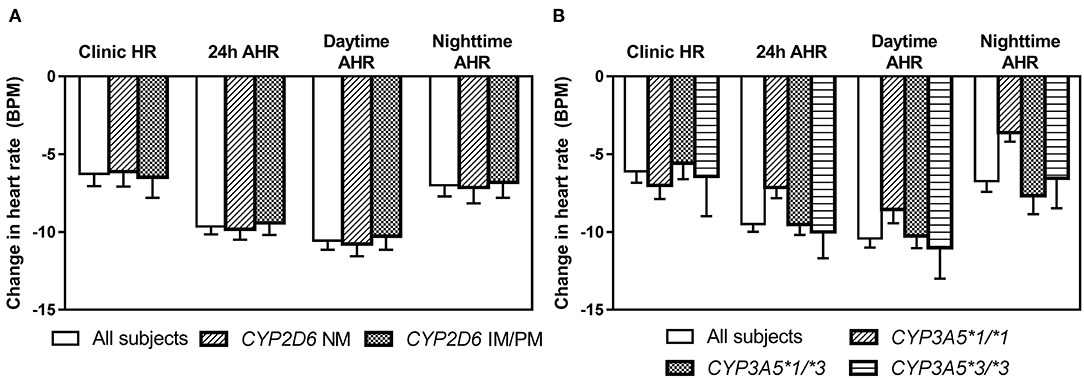
Figure 5. Changes in clinic heart rate and ambulatory heart rate (AHR) after 6 weeks treatment with bisoprolol 2.5 mg daily according to (A) CYP2D6 metabolizer groups (B) CYP3A5 genotypes. Data are presented as mean ± SEM. There is not significant difference between groups (independent samples t-test or one-way ANOVA, as appropriate).
Discussion
Bisoprolol is moderately lipophilic with a volume of distribution of about 3.5 L/kg and its plasma protein binding is ~30% (34, 35). It has been reported previously that the oral clearance of bisoprolol correlated with body weight and GFR (12). We found significant associations of bisoprolol plasma concentrations with body weight but not with eGFR, possibly because all the subjects had normal renal function. We found a mean increase in the peak bisoprolol plasma concentrations of 13.6% from the first dose to the dose at 6 weeks, comparable with the reported accumulation factor of 1.1–1.3 (34, 35).
Bisoprolol is metabolized by CYP3A4 and to a lesser extent by CYP2D6 (36), but we did not find any significant effect of the CYP2D6 metabolizer groups and CYP3A5 polymorphisms examined on the peak and trough plasma concentrations of bisoprolol. Previous studies have generally found no effect of CYP2D6 polymorphisms on bisoprolol pharmacokinetics (12, 23, 37, 38), but one study reported there were effects of CYP2D6*4 on the dose of bisoprolol used (39) and another reported effects of CYP2D6*2A on the plasma concentration and BP response (40). Overall, there is no evidence that the CYP2D6*10 polymorphism, which is common in East Asians (41) has any effect on bisoprolol pharmacokinetics, in contrast to propranolol and metoprolol pharmacokinetics, which are highly influenced by the CYP2D6*10 polymorphism (42, 43). To our knowledge there is no previous study on the effects of the CYP3A5*3 polymorphism or any polymorphism in CYP3A4 on bisoprolol pharmacokinetics and pharmacodynamics.
The reductions in BP and HR were related to the baseline values for SBP and HR but there was no association with bisoprolol plasma concentrations or the polymorphisms in CYP2D6 and CYP3A5 examined. There are conflicting reports on the effects of CYP2D6 genotype on the hemodynamic and clinical responses to metoprolol but recent meta-analyses found there was a significant effect on HR and BP responses corresponding with the marked effect on pharmacokinetics. The Dutch Pharmacogenomic Working Group recommended screening the CYP2D6 genotype when metoprolol is prescribed (44). However, no effect of the CYP2D6 genotype on the BP response to bisoprolol has been found previously (45). and genetic variants in the pharmacodynamic pathways such as the β1-adrenoceptor (ADRB1) may be more useful in predicting the response to β-blockers in general (46).
The reduction in clinic BP of 14.3 ± 10.9/8.4 ± 6.2 mmHg with bisoprolol 2.5 mg daily is greater than that reported with 5 mg daily (10.2/8.0 mmHg) in the bisoprolol prescribing information (34, 35). There was a placebo run-in but no parallel placebo group in our study so some of the BP change may be a placebo effect, although the changes in ABP were similar to the clinic BPs and ABP is less influenced by placebo effects. It is known from empirical observation that Chinese patients are more sensitive to propranolol than Caucasians and smaller doses are generally used. Zhou et al. (47) showed that Chinese men had greater sensitivity than white men to the effects of propranolol on HR and BP based on the responses in relation to plasma concentrations of the drug. The authors concluded that the increased sensitivity may have been partly related to decreased protein binding of propranolol, but considered that other factors must be involved. The clearance of propranolol was significantly greater in the Chinese subjects compared to the white group in that study, although it would be expected that Caucasians would have greater clearance of propranolol than Chinese subjects overall based on the high frequency of CYP2D6*10 IMs in Chinese causing reduced propranolol clearance (43). It is not known if Chinese subjects are more sensitive than Caucasians to other β-blockers but there are no obvious differences in the frequency of genetic variants in the pharmacodynamic pathways such as the G protein-coupled receptor kinase 4 (GRK4) variants, which may be related to reduced sensitivity to β-blockers in people of African origin (48).
This study had several important limitations. The single blood samples taken for peak levels were all taken at 3 h post dose but these will have missed the true peak levels in many patients which are reported to occur at a median of 3–4 h post dose. We only examined the polymorphisms in CYP2D6 that are common in Hong Kong Chinese patients and we did not test for rare variants or for gene duplications or tandem repeats which are relatively common in this population (19), so the CYP2D6 activity score may not be accurate. There were few PMs among these subjects and only one subject with no functional CYP2D6 alleles so we cannot be certain about the effect of total lack of CYP2D6 activity on bisoprolol pharmacokinetics. Likewise, the CYP3A5*3 polymorphism does not predict total CYP3A activity and there may be an advantage to assess the effect of CYP3A combined genotypes (49). However, the reduced function CYP3A4*22 (rs35599367) variant is usually absent in East Asians whereas the CYP3A4*1G (rs2242480) variant is common with an allele frequency of about 27% but its function is uncertain (50). We did not analyze the CYP3A4*1G variant in this study so we cannot exclude an effect of CYP3A combined genotypes on bisoprolol pharmacokinetics. Lastly, the number of subjects in the study is relatively small so we cannot exclude a small effect of these genotypes.
Conclusion
There was no significant effect of the common polymorphisms in CYP2D6 and the CYP3A5*3 polymorphism on the peak and trough plasma concentrations of bisoprolol or the BP and HR responses after 6 weeks treatment with bisoprolol 2.5 mg daily in Chinese hypertensive patients in this study. Genotyping for these variants would appear to have no benefit in predicting the hemodynamic response to bisoprolol in this population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study involving human participants were reviewed and approved by the Joint Clinical Research Ethics Committee of the Chinese University of Hong Kong and New Territories East Cluster (CUHK-NTEC). The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki and subsequent revisions. All patients signed the Informed Consent.
Author Contributions
SC and WZ analyzed the data and wrote this manuscript. BT and TC designed the research project. TC and WZ included the patients and followed this study. WZ and CH performed the experiments. BT, AK, and CH revised this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
BT has acted as consultant or speaker for Merck Serono for which he received honoraria.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The clinical trial was partially supported by Merck Pharmaceutical (HK) Limited. We would like to thank Ms. Evelyn Chau, Ms. Maybo Lin and other research staff for helping with study procedures, Professor Timothy H. Rainer and Dr. Miya Cheng for helping with patient recruitment, Dr. Richard Kam for helping with the bisoprolol assay and Ms. Emily Poon for helping with genotyping and other laboratory work.
References
1. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. (2016) 134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912
2. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. (2018) 138:e484–e594. doi: 10.1161/CIR.0000000000000596
3. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1097/HJH.0000000000001940
4. Lindholm LH, Carlberg B, Samuelsson O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. (2005) 366:1545–53. doi: 10.1016/S0140-6736(05)67573-3
5. Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ. (2006) 174:1737–42. doi: 10.1503/cmaj.060110
6. Rabi DM, McBrien KA, Sapir-Pichhadze R, Nakhla M, Ahmed SB, Dumanski SM, et al. Hypertension Canada's 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. (2020) 36:596–624. doi: 10.1016/j.cjca.2020.02.086
7. Oliveira-Paula GH, Pereira SC, Tanus-Santos JE, Lacchini R. Pharmacogenomics and hypertension: current insights. Pharmgenomics Pers Med. (2019) 12:341–59. doi: 10.2147/PGPM.S230201
8. Rau T, Heide R, Bergmann K, Wuttke H, Werner U, Feifel N, et al. Effect of the CYP2D6 genotype on metoprolol metabolism persists during long-term treatment. Pharmacogenetics. (2002) 12:465–72. doi: 10.1097/00008571-200208000-00007
9. Li S, Lin H, Sun W, Wang Y, Ding Y, Zhao H, et al. A meta-analysis of the effect of CYP2D6 polymorphism on the pharmacokinetics and pharmacodynamics of metoprolol. Int J Clin Pharmacol Ther. (2017) 55:483–92. doi: 10.5414/CP202545
10. Meloche M, Khazaka M, Kassem I, Barhdadi A, Dubé M-P, de Denus S. CYP2D6 polymorphism and its impact on the clinical response to metoprolol: a systematic review and meta-analysis. Br J Clin Pharmacol. (2020) 86:1015–33. doi: 10.1111/bcp.14247
11. McGavin JK, Keating GM. Bisoprolol: a review of its use in chronic heart failure. Drugs. (2002) 62:2677–96. doi: 10.2165/00003495-200262180-00017
12. Taguchi M, Nozawa T, Igawa A, Inoue H, Takesono C, Tahara K, et al. Pharmacokinetic variability of routinely administered bisoprolol in middle-aged and elderly Japanese patients. Biol Pharm Bull. (2005) 28:876–81. doi: 10.1248/bpb.28.876
13. Horikiri Y, Suzuki T, Mizobe M. Stereoselective metabolism of bisoprolol enantiomers in dogs and humans. Life Sci. (1998) 63:1097–108. doi: 10.1016/S0024-3205(98)00371-3
14. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. (2013) 138:103–41. doi: 10.1016/j.pharmthera.2012.12.007
15. Klein K, Zanger UM. Pharmacogenomics of cytochrome P450 3A4: recent progress toward the “missing heritability” problem. Front Genet. (2013) 4:12. doi: 10.3389/fgene.2013.00012
16. Lamba J, Hebert JM, Schuetz EG, Klein TE, Altman RB. PharmGKB summary: very important pharmacogene information for CYP3A5. Pharmacogenet Genomics. (2012) 22:555–8. doi: 10.1097/FPC.0b013e328351d47f
17. Nofziger C, Turner AJ, Sangkuhl K, Whirl-Carrillo M, Agundez JAG, Black JL, et al. PharmVar GeneFocus: CYP2D6. Clin Pharmacol Ther. (2020) 107:154–70. doi: 10.1002/cpt.1643
18. Garcia-Barcelo M, Chow LY, Chiu HFK, Wing YK, Lee DTS, Lam KL, et al. Genetic analysis of the CYP2D6 locus in a Hong Kong Chinese population. Clin Chem. (2000) 46:18–23. doi: 10.1093/clinchem/46.1.18
19. Chan W, Li MS, Sundaram SK, Tomlinson B, Cheung PY, Tzang CH. CYP2D6 allele frequencies, copy number variants, and tandems in the population of Hong Kong. J Clin Lab Anal. (2019) 33:e22634. doi: 10.1002/jcla.22634
20. Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics. (2002) 3:229–43. doi: 10.1517/14622416.3.2.229
21. Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. (2004) 14:1–18. doi: 10.1097/00008571-200401000-00001
22. Ji L, Pan S, Marti-Jaun J, Hanseler E, Rentsch K, Hersberger M. Single-step assays to analyze CYP2D6 gene polymorphisms in Asians: allele frequencies and a novel *14B allele in mainland Chinese. Clin Chem. (2002) 48:983–8. doi: 10.1093/clinchem/48.7.983
23. Nozawa T, Taguchi M, Tahara K, Hashimoto Y, Igarashi N, Nonomura M, et al. Influence of CYP2D6 genotype on metoprolol plasma concentration and beta-adrenergic inhibition during long-term treatment: a comparison with bisoprolol. J Cardiovasc Pharmacol. (2005) 46:713–20. doi: 10.1097/01.fjc.0000184117.76188.68
24. Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther. (2012) 91:321–6. doi: 10.1038/clpt.2011.287
25. Rau T, Wuttke H, Michels LM, Werner U, Bergmann K, Kreft M, et al. Impact of the CYP2D6 genotype on the clinical effects of metoprolol: a prospective longitudinal study. Clin Pharmacol Ther. (2009) 85:269–72. doi: 10.1038/clpt.2008.218
26. Lefebvre J, Poirier L, Poirier P, Turgeon J, Lacourciere Y. The influence of CYP2D6 phenotype on the clinical response of nebivolol in patients with essential hypertension. Br J Clin Pharmacol. (2007) 63:575–82. doi: 10.1111/j.1365-2125.2006.02796.x
27. Chan SW, Xiao Y, Hu M, Yin OQ, Chu TT, Fok BS, et al. Associations of the CYP3A5*3 and CYP3A4*1G polymorphisms with the pharmacokinetics of oral midazolam and the urinary 6beta-hydroxycortisol/cortisol ratio as markers of CYP3A activity in healthy male Chinese. J Clin Pharm Ther. (2016) 41:552–8. doi: 10.1111/jcpt.12433
28. Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, et al. Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther. (2007) 82:410–26. doi: 10.1038/sj.clpt.6100237
29. Chan SW, Hu M, Tomlinson B. The pharmacogenetics of beta-adrenergic receptor antagonists in the treatment of hypertension and heart failure. Expert Opin Drug Metab Toxicol. (2012) 8:767–90. doi: 10.1517/17425255.2012.685157
30. Komori T, Eguchi K, Hoshide S, Williams B, Kario K. Comparison of wrist-type and arm-type 24-h blood pressure monitoring devices for ambulatory use. Blood Press Monit. (2013) 18:57–62. doi: 10.1097/MBP.0b013e32835d124f
31. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. (1972) 18:499–502. doi: 10.1093/clinchem/18.6.499
32. Wang Y, Wang C, Li J, Wang X, Zhu G, Chen X, et al. Effect of genetic polymorphisms of CYP3A5 and MDR1 on cyclosporine concentration during the early stage after renal transplantation in Chinese patients co-treated with diltiazem. Eur J Clin Pharmacol. (2009) 65:239–47. doi: 10.1007/s00228-008-0577-4
33. Stamer UM, Bayerer B, Wolf S, Hoeft A, Stuber F. Rapid and reliable method for cytochrome P450 2D6 genotyping. Clin Chem. (2002) 48:1412–7. doi: 10.1093/clinchem/48.9.1412
34. U.S. Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. (2018). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed July 22, 2021).
35. U.S. Food and Drug Administration. Duramed Pharmaceuticals, Inc. Zebeta (Bisoprolol Fumarate) Tablet Prescribing Information. (2007). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/019982s014lbl.pdf (accessed May, 2007).
36. Horikiri Y, Suzuki T, Mizobe M. Pharmacokinetics and metabolism of bisoprolol enantiomers in humans. J Pharm Sci. (1998) 87:289–94. doi: 10.1021/js970316d
37. Deroubaix X, Lins RL, Lens S, Demblon C, Jeanbaptiste B, Poelaert D, et al. Comparative bioavailability of a metoprolol controlled release formulation and a bisoprolol normal release tablet after single oral dose administration in healthy volunteers. Int J Clin Pharmacol Ther. (1996) 34:61–70.
38. Brytkova Ia V, Ignat'ev IV, Kazakov RE, Sokova EA, Striuk RI. Efficacy and safety of bisoprolol in pregnant women with chronic arterial hypertension do not depend on genetic CYP2D6 polymorphism. Kardiologiia. (2009) 49:50–5.
39. Klein L, O'Connor CM, Gattis WA, Zampino M, de Luca L, Vitarelli A, et al. Pharmacologic therapy for patients with chronic heart failure and reduced systolic function: review of trials and practical considerations. Am J Cardiol. (2003) 91:18F–40F. doi: 10.1016/S0002-9149(02)03336-2
40. Mohammed Alkreathy H, Mohammed Eid Alsayyid K, Alaama JY, Al Ghalayini K, Karim S, Esmat A, et al. Bisoprolol responses (PK/PD) in hypertensive patients: a cytochrome P450 (CYP) 2D6 targeted polymorphism study. Saudi J Biol Sci. (2020) 27:2727–32. doi: 10.1016/j.sjbs.2020.06.022
41. Gaedigk A. Complexities of CYP2D6 gene analysis and interpretation. Int Rev Psychiatry. (2013) 25:534–53. doi: 10.3109/09540261.2013.825581
42. Huang J, Chuang SK, Cheng CL, Lai ML. Pharmacokinetics of metoprolol enantiomers in Chinese subjects of major CYP2D6 genotypes. Clin Pharmacol Ther. (1999) 65:402–7. doi: 10.1016/S0009-9236(99)70134-7
43. Lai ML, Wang SL, Lai MD, Lin ET, Tse M, Huang JD. Propranolol disposition in Chinese subjects of different CYP2D6 genotypes. Clin Pharmacol Ther. (1995) 58:264–8. doi: 10.1016/0009-9236(95)90242-2
44. Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, et al. Pharmacogenetics: from bench to byte–an update of guidelines. Clin Pharmacol Ther. (2011) 89:662–73. doi: 10.1038/clpt.2011.34
45. Hiltunen TP, Donner KM, Sarin AP, Saarela J, Ripatti S, Chapman AB, et al. Pharmacogenomics of hypertension: a genome-wide, placebo-controlled cross-over study, using four classes of antihypertensive drugs. J Am Heart Assoc. (2015) 4:e001521. doi: 10.1161/JAHA.114.001521
46. Cunningham PN, Chapman AB. The future of pharmacogenetics in the treatment of hypertension. Pharmacogenomics. (2019) 20:129–32. doi: 10.2217/pgs-2018-0191
47. Zhou HH, Koshakji RP, Silberstein DJ, Wilkinson GR, Wood AJ. Altered sensitivity to and clearance of propranolol in men of Chinese descent as compared with American whites. N Engl J Med. (1989) 320:565–70. doi: 10.1056/NEJM198903023200905
48. Bhatnagar V, O'Connor DT, Brophy VH, Schork NJ, Richard E, Salem RM, et al. G-protein-coupled receptor kinase 4 polymorphisms and blood pressure response to metoprolol among African Americans: sex-specificity and interactions. Am J Hypertens. (2009) 22:332–8. doi: 10.1038/ajh.2008.341
49. Sanchez Spitman AB, Moes D, Gelderblom H, Dezentje VO, Swen JJ, Guchelaar HJ. Effect of CYP3A4*22, CYP3A5*3, and CYP3A combined genotypes on tamoxifen metabolism. Eur J Clin Pharmacol. (2017) 73:1589–98. doi: 10.1007/s00228-017-2323-2
Keywords: bisoprolol, blood pressure, CYP2D6, CYP3A5, pharmacokinetics, polymorphisms
Citation: Chan SW, Chu TTW, Ho CS, Kong APS, Tomlinson B and Zeng W (2021) Influence of CYP2D6 and CYP3A5 Polymorphisms on the Pharmacokinetics and Pharmacodynamics of Bisoprolol in Hypertensive Chinese Patients. Front. Med. 8:683498. doi: 10.3389/fmed.2021.683498
Received: 21 March 2021; Accepted: 16 August 2021;
Published: 09 September 2021.
Edited by:
Alice Chen, National Cancer Institute (NCI), United StatesReviewed by:
Dora Koller, Yale University, United StatesPablo Zubiaur, Princess University Hospital, Spain
Copyright © 2021 Chan, Chu, Ho, Kong, Tomlinson and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Zeng, end3c3ByaW5nQDEyNi5jb20=; Brian Tomlinson, YnRvbWxpbnNvbkBtdXN0LmVkdS5tbw==
 Sze Wa Chan
Sze Wa Chan Tanya T. W. Chu2
Tanya T. W. Chu2 Alice P. S. Kong
Alice P. S. Kong Brian Tomlinson
Brian Tomlinson Weiwei Zeng
Weiwei Zeng