
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 14 June 2021
Sec. Nephrology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.682090
 Kam Wa Chan1†
Kam Wa Chan1† Tak Yee Chow2
Tak Yee Chow2 Kam Yan Yu1
Kam Yan Yu1 Yulong Xu3
Yulong Xu3 Nevin Lianwen Zhang4
Nevin Lianwen Zhang4 Vivian Taam Wong5
Vivian Taam Wong5 Saimei Li6*
Saimei Li6* Sydney Chi Wai Tang1*†
Sydney Chi Wai Tang1*†Background: Previous UK Biobank studies showed that symptoms and physical measurements had excellent prediction on long-term clinical outcomes in general population. Symptoms and signs could intuitively and non-invasively predict and monitor disease progression, especially for telemedicine, but related research is limited in diabetes and renal medicine.
Methods: This retrospective cohort study aimed to evaluate the predictive power of a symptom-based stratification framework and individual symptoms for diabetes. Three hundred two adult diabetic patients were consecutively sampled from outpatient clinics in Hong Kong for prospective symptom assessment. Demographics and longitudinal measures of biochemical parameters were retrospectively extracted from linked medical records. The association between estimated glomerular filtration rate (GFR) (independent variable) and biochemistry, epidemiological factors, and individual symptoms was assessed by mixed regression analyses. A symptom-based stratification framework of diabetes using symptom clusters was formulated by Delphi consensus method. Akaike information criterion (AIC) and Bayesian information criterion (BIC) were compared between statistical models with different combinations of biochemical, epidemiological, and symptom variables.
Results: In the 4.2-year follow-up period, baseline presentation of edema (−1.8 ml/min/1.73m2, 95%CI: −2.5 to −1.2, p < 0.001), epigastric bloating (−0.8 ml/min/1.73m2, 95%CI: −1.4 to −0.2, p = 0.014) and alternating dry and loose stool (−1.1 ml/min/1.73m2, 95%CI: −1.9 to −0.4, p = 0.004) were independently associated with faster annual GFR decline. Eleven symptom clusters were identified from literature, stratifying diabetes predominantly by gastrointestinal phenotypes. Using symptom clusters synchronized by Delphi consensus as the independent variable in statistical models reduced complexity and improved explanatory power when compared to using individual symptoms. Symptom-biologic-epidemiologic combined model had the lowest AIC (4,478 vs. 5,824 vs. 4,966 vs. 7,926) and BIC (4,597 vs. 5,870 vs. 5,065 vs. 8,026) compared to the symptom, symptom-epidemiologic and biologic-epidemiologic models, respectively. Patients co-presenting with a constellation of fatigue, malaise, dry mouth, and dry throat were independently associated with faster annual GFR decline (−1.1 ml/min/1.73m2, 95%CI: −1.9 to −0.2, p = 0.011).
Conclusions: Add-on symptom-based diagnosis improves the predictive power on renal function decline among diabetic patients based on key biochemical and epidemiological factors. Dynamic change of symptoms should be considered in clinical practice and research design.
The pathogenesis of diabetes is heterogeneous and there is a constant call for more personalized management (1, 2). Majority of existing studies stratified diabetes on the molecular level (1). Previous studies of UK Biobank suggested that phenomes, including symptoms and physical measures, had an excellent prediction of long-term clinical outcome in general population (3). Network medicine integrates phenome and genome to reclassify disease as the base of personalized medicine (4, 5). However, symptomatology is less explored in diabetes and kidney diseases.
Symptoms and signs are often the center of patient's care (6). Symptom-based disease stratification has been utilized in diseases with considerable heterogeneity (7–9). Patient reported outcomes and symptom-based diagnosis are increasingly used in all levels of healthcare (6) including evaluation of clinical interventions (10–12), disease diagnosis, surveillance (13), and stratification (7, 14–17). Clustering of symptom is associated with specific cluster of etiology and pathogenesis (Figure 1) (7, 15, 16, 18). Different diseases of similar symptomatology share genetic associations and protein-protein interactions (9). Assessment of phenome-genomic association could also reveal more druggable targets (19, 20).
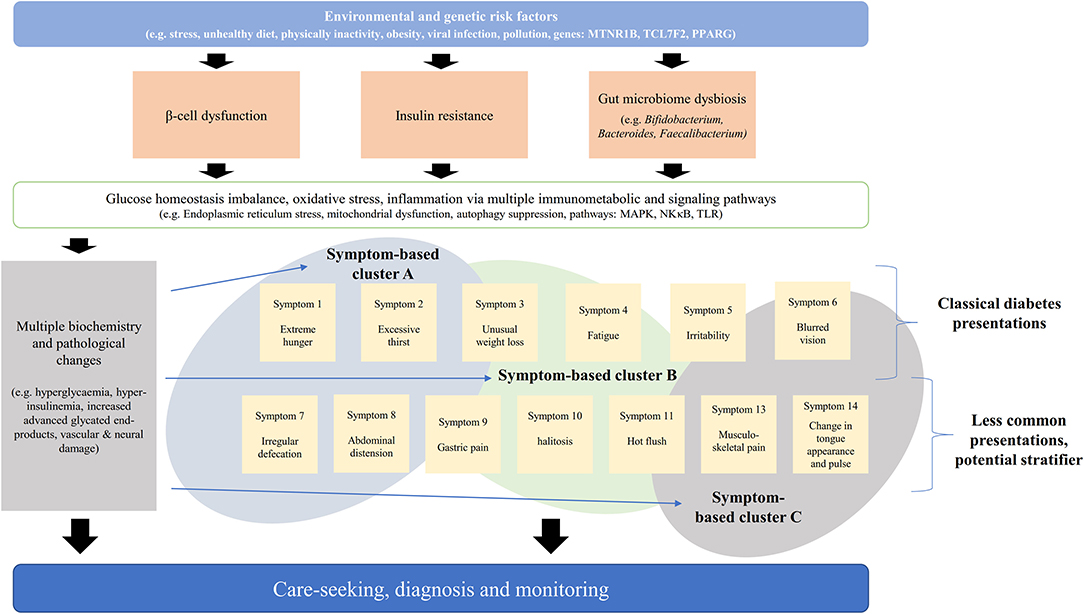
Figure 1. Schematic diagram of association between pathogenesis, biochemical parameters, and symptoms. The pathogenesis of diabetes is multifaceted and symptomatic presentation could reflect the whole-system response.
The SHIELD study showed that each of the 7 common diabetes symptoms is associated with onset of diabetes among non-diabetic population (21). Further expanding the scope of symptom analysis could identify symptoms that are specific (and therefore less commonly presented) to disease progression. However, untargeted mining of symptoms (e.g., by latent class analysis) requires an exponentially larger sample size to compensate the measurement errors arise from assessing multiple individual symptoms. Supervised and targeted analysis with qualitative inputs from clinical perspective (e.g., by Delphi consensus panel) could reduce the large number of symptoms to fewer symptom clusters for more efficient statistical modeling and clinical use. Similar dimension reduction strategy from genotype to phenotype has been used in deep learning methods to reduce sample size required to predict personalized drug response (22).
Chinese medicine (CM) has a long history in symptom-based disease management as biochemical and molecular investigations were not available at the time of theory development (23–25). Previous big data studies showed that add-on symptom-based CM treatment led to reduced risk of end-stage kidney disease and mortality among chronic kidney disease (CKD) patients with diabetes (26–28). CM subclassifies diseases based on different symptom combinations on top of conventional medicine diagnosis to formulate personalized regimens (12, 17, 29). In each symptom-based subtype (SS) of diabetes, related symptoms are weighed into core and supplementary criteria based on the importance to diagnosis (30). Although CM stratification system has the potential to refine diabetes classification, there is no synchronized framework. The benefit of add-on symptom-based stratification remains undetermined for diabetes.
Symptom could serve as an intuitive and non-invasive tool to predict and monitor clinical progression, especially for telemedicine. Previous study shows that retarding renal function decline is the priority among diabetic patients with no/early CKD (25). This study aimed to investigate the association between the presence of symptoms, SSs and renal function deterioration among diabetic patients, and to establish a synchronized symptom-based framework for stratifying diabetes based on the quality of different statistical models in predicting renal function.
Retrospective cohort study on the cross-sectional and longitudinal association between renal function and risk factors (Figure 2). Delphi technique (31–33) was used as the consensus methodology to construct a symptom-based classification framework for diabetes that comprised of symptom clusters. Explanatory power and complexity of statistical models with different combinations of biochemical, epidemiological, and symptom variables were compared by information criteria used in machine learning.
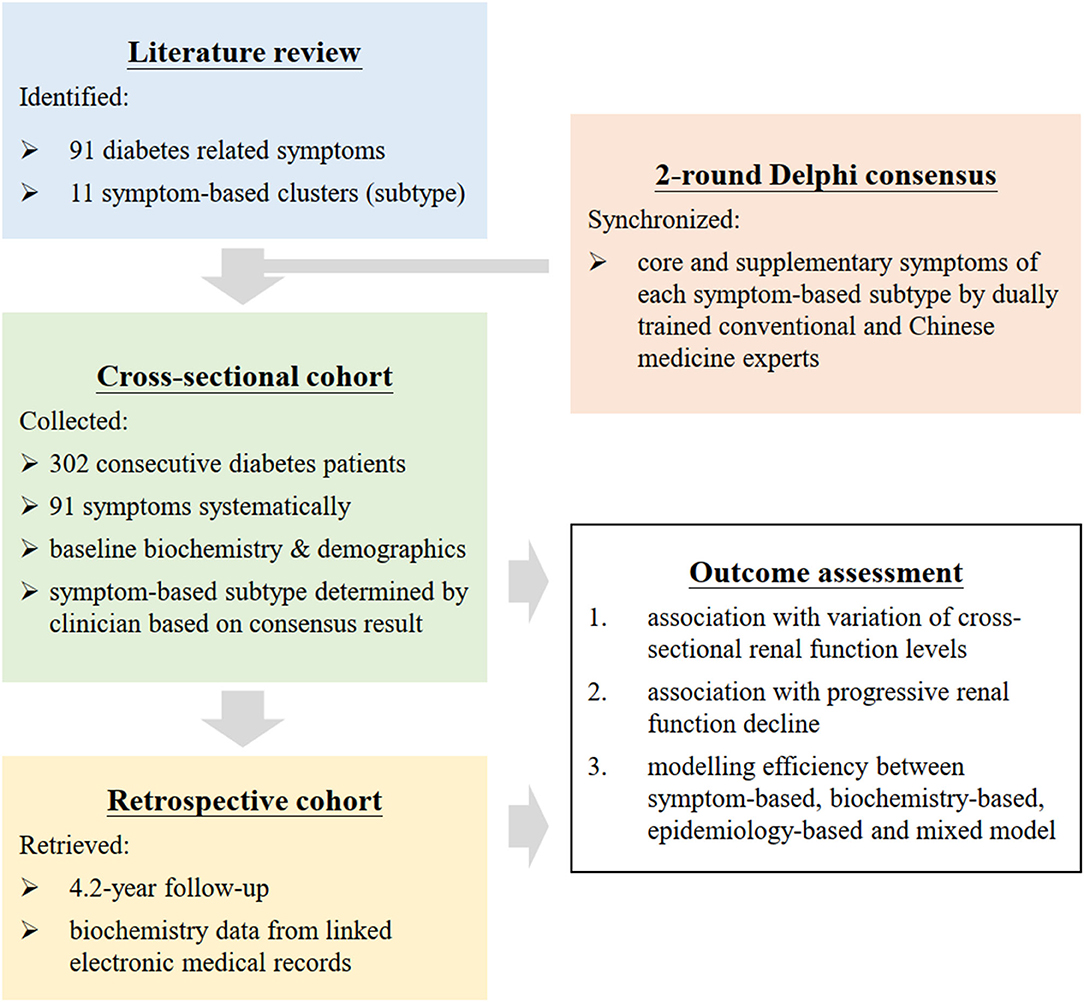
Figure 2. Flow diagram of research. Symptoms of diabetes were analyzed as individual symptom and symptom clusters. Presence of 91 symptoms was recorded with field tested questionnaire by 2 synchronized physicians. Symptom clusters was constructed by extraction from literature followed by a consensus panel. The explanatory power and complexity of different models with combinations of symptoms, biochemistry, and demographics were compared by Akaike information criterion and Bayesian information criterion used in machine learning.
Three hundred two diabetes subjects aged 18 or above on antidiabetic medication were recruited consecutively from general outpatient clinics of public hospitals in Hong Kong through a 30-min health service programme from July 2014 to December 2019. Although there is no consensus on sample size calculation for comparing model quality, modeling bias is shown to be small and independent to sampling design empirically when sample size is over 100 (34).
Ten conventional medicine and CM dually trained experts in internal medicine and classical CM theory (Supplementary Material 6) were purposively sampled to join the Delphi consensus panel to diversify the schools of theory on symptom-based diabetes diagnosis.
Ninety-one symptoms, signs and behavioral factors (Supplementary Material 5) were extracted from existing literature. The presence of symptoms were prospectively collected face-to-face by physicians based on a standardized, structured, and field-tested questionnaire through the service program (35). Presence of symptoms was quantified by the days of presence within 2 weeks of consultation. Diagnosis of the 11 SSs was based on the presence of symptoms with reference to the symptom-based diagnosis framework derived from the expert consensus described below. To ensure data consistency, only 2 trained and synchronized physicians collected the data.
Biochemistry and demographics (listed in Data Analysis) of patients were retrieved from linked electronic medical record including a comprehensive and standardized annual multidisciplinary Risk Assessment and Management Programme (36). The Programme captures patients' demographics assessed by trained nurse, and the continuous change of key biochemical parameters. All outcome assessors were blinded from the analysis plan and biochemistry data to avoid bias. Longitudinal data was retrieved from the electronic medical system until 1 December 2020 by 2 researchers (K.W.C., K.Y.Y.) independently with a standardized form.
Consensus methods are commonly used in constructing agreeable frameworks of existing practice in medicine (31). Delphi technique, which involve iterative anonymous rating, has advantage in handling dominant opinion and geographical constraint (32). The consensus panel was formed based on their academic capacity, peer reputation, geographical coverage, and school of thoughts to maximize the coverage of opinion while being specific to diabetes. A panel of 10 was recruited to maximize participation and consensus efficiency (32, 37). The consensus result was used to reduce the dimension of symptom analysis from individual symptoms to symptom clusters.
The Delphi consensus was divided into 2 rounds. A questionnaire with Likert scale of 1–9 was designed as the medium for rating (Supplementary Material 7). Since CM has a rich history of using symptom-based diagnosis (23, 24), we sought to expand the scope of symptom analysis by extracting CM literature. Ninety-one baseline symptoms were extracted from all 11 documented SSs from key symptom-based diabetes guideline published by academy, academic society and government regulatory bodies (Supplementary Material 2) (38–41), and standardized by a national standard of medical terminology (42). The baseline questionnaire contained 300 symptoms and could be completed in 30 min in field tests.
In the first round, experts rated anonymously the importance of the symptoms in diagnosing each SS and were invited to supplement necessary symptoms. Consensus was pre-defined as two-third majority rating 1–3 (agreed as insignificant) or 7–9 (agreed as significant). Symptoms reached consensus were then removed from the questionnaire. The result in the first round was disseminated back to and confirmed by the consensus panel. Dissonant symptoms were circulated again in the second round along with the additional symptoms collected in the first round.
Symptoms reached consensus in either rounds were defined as core criteria for the diagnosis of the corresponding SS. Sensitivity analyses with different consensus definition (average score of 6.7) and handling of missing values (last-observation-carry-forward and extreme values) were conducted. Symptoms reached consensus in sensitivity analyses were defined as supplementary criteria. Results were confirmed by panel members.
Estimated GFR was calculated by the CKD-EPI equation (43). Education (no formal/primary/secondary/tertiary), type of diabetes (type 1/type 2/gestational), obesity (optimal weight/underweight/over-weight/moderately to severely obese), and smoking (non-/ex-/current smoker) and alcohol (non-/ex-/social/current drinker) history were further stratified. Univariable and backward stepwise multivariable regression models on the correlation between GFR and binary presence of individual symptoms were evaluated. Missing values were replaced by multiple imputation with 20 sets of data using age as auxiliary variable for the cross-sectional analysis.
Annual rate of GFR change over the follow-up period (in weeks) with repeated measures was analyzed by mixed models with unstructured covariance and adjusted for (1) baseline levels of hemoglobin A1c (HbA1c), systolic blood pressure, low-density lipoprotein, log-transformed urine albumin-to-creatinine ratio (UACR), and GFR, (2) baseline presence of symptoms or SSs, (3) gender, type of diabetes, smoking history, alcohol consumption, and obesity, (4) interaction between baseline GFR and visit, (5) and interaction between presence of symptoms or SSs and visit. The included biochemical parameters and epidemiological factors are known predictors of renal function decline. Baseline presence of symptoms was used as the explanatory variable over continuous change of symptoms as the analyses were focused on prediction instead of continuous association. The slope of change was analyzed with a linear growth model with sensitivity analysis of using repeated measures of biochemical parameters and blood pressures as covariates instead of baseline values, as previous studies showed that the majority of GFR decline is linear (44).
Five multivariable regression models were built using GFR as the dependent variable to assess the power of explaining the variance in GFR, namely (1) Individual Symptom-based Model, (2) Traditional CM Model (with SSs), (3) Traditional CM-Epidemiology Model (with SSs and epidemiological variables), (4) Conventional Medicine Model (with biochemical and epidemiological variables), and (5) Integrative Chinese and Conventional Medicine Model (with SSs, biochemical and epidemiological variables) (Table 1). Both cross-sectional regression models and longitudinal mixed models were evaluated.
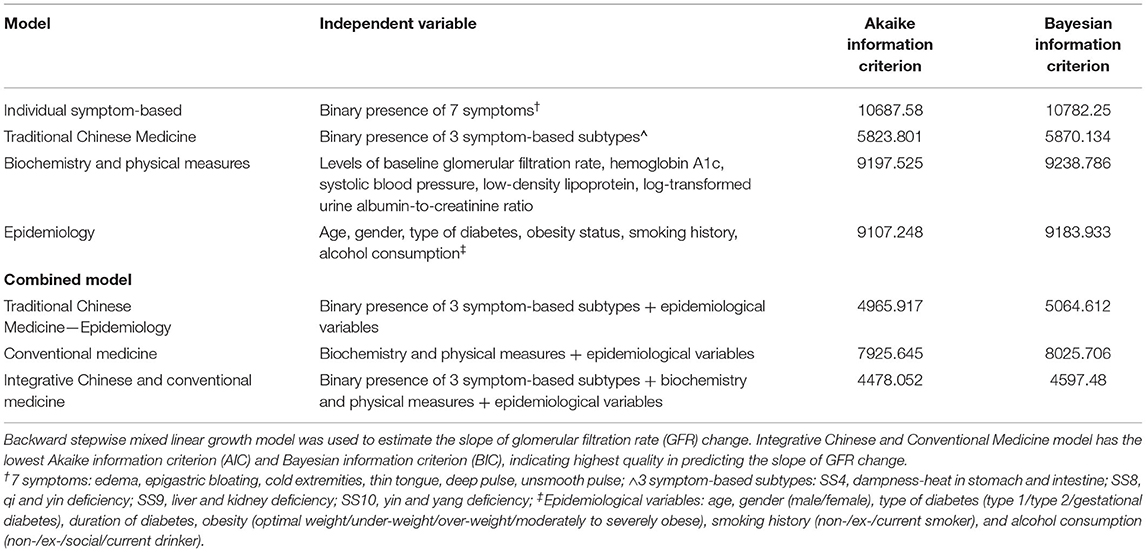
Table 1. Performance of regression models on longitudinal measurements of glomerular filtration rate.
Quality of the statistical models was assessed by Akaike information criterion (AIC) and Bayesian information criterion (BIC). AIC and BIC assessed the quality of statistical models in predicting GFR with different discount on model complexity, and are commonly used as model selection criteria in machine learning (45). Lower AIC and BIC refers to model that explain the variance better with less complexity, and is regarded as a better model. GFR was selected as the dependent variable for the models as diabetic kidney disease (DKD) is the most prevalent and costly complication of diabetes with limited known progression risk factors (44). Prevention of renal function decline has been suggested as the top priority among diabetic patients in our previous focus group interview series (25). The efficiency of diagnostic models in predicting rate of renal function has clinical significance (46). STATA 15.1 was used for analysis.
Majority of the 302 patients had type 2 diabetes, were non-smoker, centrally obese, non-insulin user and complicated with dyslipidemia, stage 2 CKD, and microalbuminuria (3.0 ± 1.1 mg/mmol) (Table 2). The mean age, GFR and HbA1c were 59.7 ± 0.7 years, 77.3 ± 1.5 ml/min/1.73m2 and 7.7 ± 0.1%, respectively. Most presented symptoms and signs were susceptible to infections (80.8%), nocturnal polyuria (67.4%), forgetfulness (65.6%), past frequent intake of high fat diet (59.6%), malar flush (58.3%), malaise (56.3%) knee buckling (54.6%), lumbago (54.0%), dry skin (52.3%), and fatigue (49.7%) (Supplementary Material 6). Eight patients' record were irretrievable due to missing personal identifier. Median follow-up period was 220 weeks (4.2 years) [IQR: 212–234]. Median change of GFR was −2.3 ml/min/1.73m2 [IQR: −8.7 to 1.4] over the period. 286 (94.7%) and 280 (92.7%) patients had annual GFR measurements until the 3rd and 4th year of follow-up.
The association between GFR and individual symptoms are summarized in Table 3. The frequency of nocturnal polyuria, defined as the reported average frequency of urination during sleep, demonstrated a strong inverse dose-dependent response with GFR (Supplementary Material 3) in the univariable analysis. In the multivariable symptom-based regression model, frequent urination (−9.1 ml/min/1.73m2, 95%CI: −15.7 to −2.5, p = 0.007), nocturnal polyuria (−12.1 ml/min/1.73m2, 95%CI: −17.7 to −6.5, p < 0.001), darkened complexion (−9.7 ml/min/1.73m2, 95%CI: −17.6 to −1.8, p = 0.017), dark lips (−10.7 ml/min/1.73m2, 95%CI: −16.5 to −4.8, p < 0.001) and dull purple color on lower limb (−13.5 ml/min/1.73m2, 95%CI: −21.8 to −5.1, p = 0.002) are associated with reduced renal function after adjusting the presence of other symptoms. Further adjustment with biochemical parameters and demographics showed that edema (−7.1 ml/min/1.73m2, 95%CI: −12.1 to −2.2, p = 0.005) and crenated tongue (−4.2 ml/min/1.73m2, 95%CI: −8.2 to −0.3, p = 0.037) are independently associated with reduced GFR.
The 11 SSs extracted from literature described diabetes patients that co-present with: (1) SS1: lower gastrointestinal symptoms and obesity; (2) SS2: lower gastrointestinal symptoms and malaise; (3) SS3: upper gastrointestinal symptoms that related to emotion; (4) SS4: both upper and lower gastrointestinal symptoms; (5) SS5: upper gastrointestinal symptoms and fatigue/malaise; (6) SS6: upper gastrointestinal symptoms and cold extremities; (7) SS7: general dry and hot sensation; (8) SS8: dry sensation and fatigue/malaise; (9) SS9: waist and knee musculoskeletal and otorhinolaryngological symptoms; (10) SS10: general weakness, waist and knee musculoskeletal symptoms, nocturia/frothy urine, and hyposexuality; and (11) SS11: signs of poor circulation (Supplementary Material 1).
Invitation was sent to 11 experts. One refused due to work engagement and 10 experts joined the consensus panel (Supplementary Material 6). After 2 rounds of consensus, 90 symptoms reached consensus in total for the 11 SSs (Table 4). The inter-rater Cohen's Kappa between consensus definition of two-third majority and average score of 6.7 was 0.90 overall (0.89 and 0.90 for the first and second round of consensus, respectively). Sensitivity analyses showed that 39 symptoms reached consensus with consensus definition of 6.7 average score or after imputation of missing values. They were listed as supplementary criteria that add likelihood for the classification of the corresponding SS (Table 4).
The AIC and BIC of the 5 models are presented in Table 1. The Integrative Chinese and Conventional Medicine Model had the lowest AIC (4,478 vs. 5,824 vs. 4,966 vs. 7,926) and BIC (4,597 vs. 5,870 vs. 5,065 vs. 8,026) compared to the Traditional CM, Traditional CM-Epidemiology, and Conventional Medicine models, respectively. Using SS as the explanatory variable in statistical models resulted in better model quality with lower AIC and BIC when compared to the models using individual symptoms.
From the multivariable regression model of Integrative Chinese and Conventional Medicine, the presence of SS8 (fatigue, malaise, dry mouth, dry throat, weak pulse) is associated −1.1 ml/min/1.73m2, 95%CI: −1.9 to −0.2, p = 0.011 more annual GFR drop after adjusting key biochemical parameters and demographics. In the symptom-based multivariable model using individual symptoms instead of SSs, edema (−1.8 ml/min/1.73m2, 95%CI: −2.5 to −1.2, p < 0.001), epigastric bloating (−0.8 ml/min/1.73m2, 95%CI: −1.4 to −0.2, p = 0.014), alternating dry and loose stool (−1.1 ml/min/1.73m2, 95%CI: −1.9 to −0.4, p = 0.004), thin tongue (−3.0 ml/min/1.73m2, 95%CI: −4.5 to −1.4, p < 0.001), deep pulse (−1.5 ml/min/1.73m2, 95%CI: −2.2 to −0.9, p < 0.001), and unsmooth pulse (−1.2 ml/min/1.73m2, 95%CI: −2.0 to −0.5, p = 0.002) were significantly and independently associated with faster GFR decline (Figure 3, Table 5). Result is robust in sensitivity analyses using repeated measures of biochemical parameters and blood pressures as covariates for adjustment.
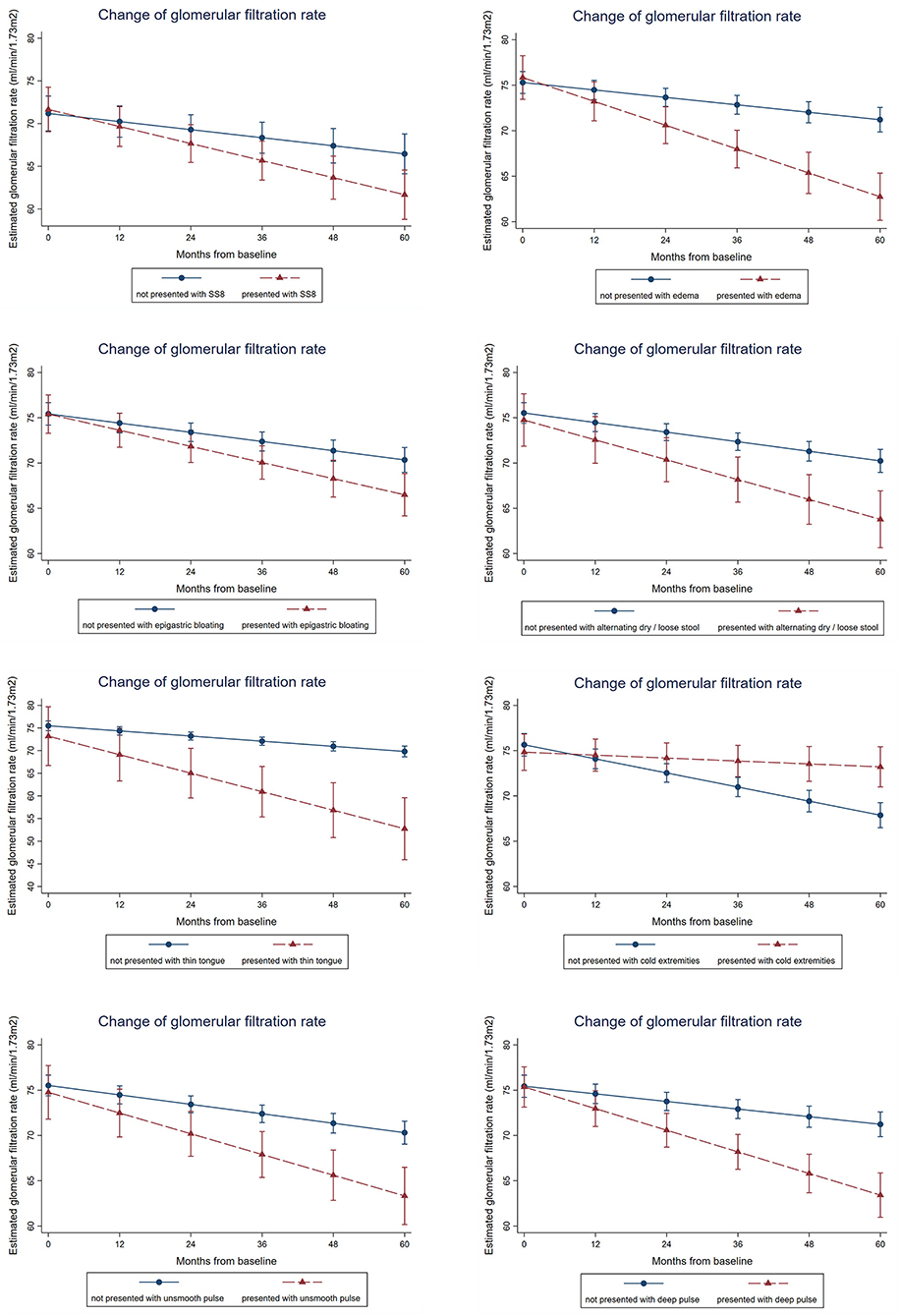
Figure 3. Association between presence of symptoms, symptom clusters and renal function decline over time. Mixed linear growth model on longitudinal repeated measures was used to estimate the slope of GFR change. After adjusting age, gender, type of diabetes, smoking history, alcohol consumption, obesity status, the baseline hemoglobin A1c, systolic blood pressure, low-density lipoprotein, and log-transformed urine albumin-to-creatinine ratio, patients presented with symptom-based subtype 8 (SS8) is associated with 1.1 ml/min/1.73m2 more annual GFR drop, whereas patients presented with edema, epigastric bloating, alternating dry and loose stool, thin tongue, deep pulse, or unsmooth pulse is associated with 1.8, 0.8, 1.1, 3.0, 1.5, or 1.2 ml/min/1.73m2 more annual GFR drop. Result is robust in sensitivity analyses using repeated measures of biochemical parameters and blood pressures as covariates for adjustment.
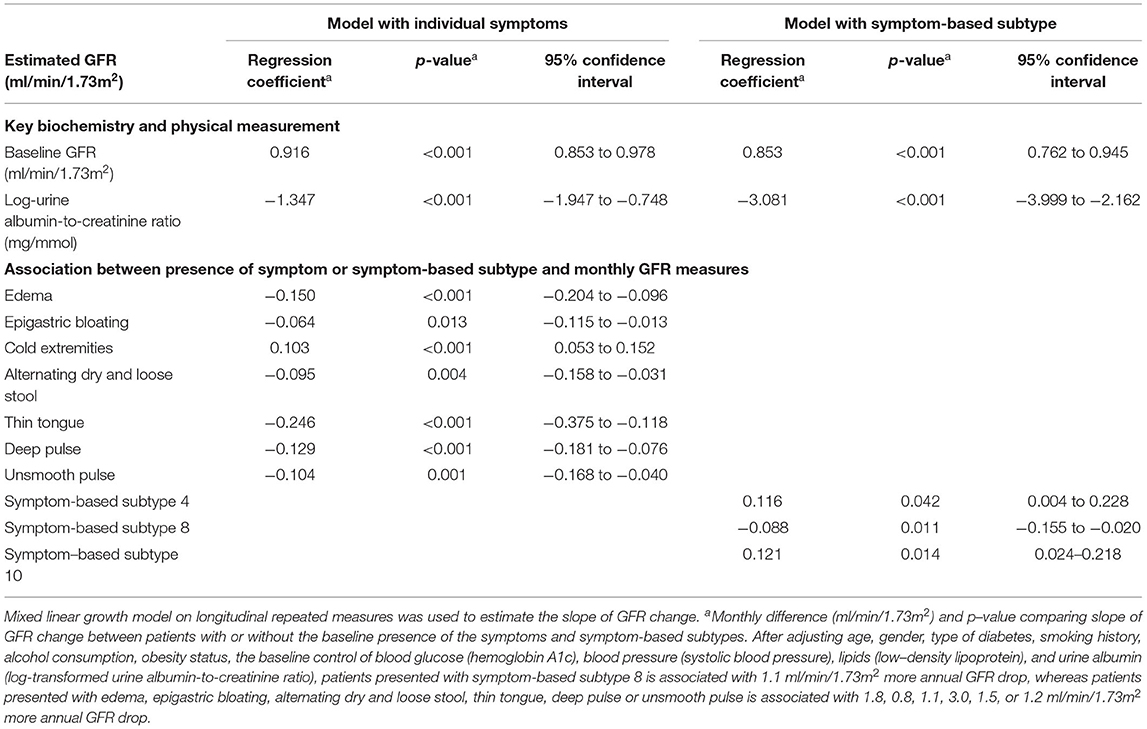
Table 5. Longitudinal association between slope of glomerular filtration rate change, epidemiological factors, symptoms, and biochemical parameters by follow-up repeated measures.
This is the first attempt to compare modeling efficiency of renal function in diabetes with different combinations of biochemical parameters, demographics, and symptoms. We synchronized the symptom-based stratification framework through expert consensus and identified individual symptoms which better predicts longitudinal renal function decline. The demographics of our cohort was comparable to that from general outpatient clinics in Hong Kong and other studies globally with considerable generalizability (47). Patients had a moderate level of HbA1c and the symptoms presented were likely residual to glycemic control which require attention.
Symptoms developed from clustering of pathophysiology (9, 13, 20) and have been used in disease monitoring, or as a treatment response predictor (7, 14). Distinctive clustering of frequent urination, excessive thirst, extreme hunger and unusual weight loss has characterized diabetes since 500 B.C.. Increased fatigue, irritability and blurry vision were supplemented since then. These symptoms have also been demonstrated to correlate with diabetes onset among non-diabetic population in the SHIELD study (21).
Independent associations have been established between symptoms and CKD. For instance, dermatological changes (e.g., pruritus, xerosis) were shown to correlate with uricemia (48). DKD was also defined as a composite of clinical presentations (edema, heart failure), epidemiology (long standing diabetes, hypertension), histopathology (nodular glomerulosclerosis, thickened basement membranes), and biochemistry (increased urine albumin) by Paul Kimmelstiel in 1936 (49). Symptoms that associate with the renal progression could further stratify patients for personalized management.
Currently, there is no standardized symptom-based stratification framework for diabetes (50). We identified discrepancies in the listing of commonly presented SSs and the related symptoms across different guidelines. Our newly synchronized framework is more concise. For instance, the defining symptoms of SS1 were simplified from 31 possible symptoms to 9 key symptoms (Supplementary Material 1). This facilitates the comparison of utility between individual symptoms and symptom clusters.
Infection susceptibility, forgetfulness, lumbago, knee buckling, and malar flush were commonly presented in our cohort. Classical presentation of frequent thirst and excessive hunger was less frequent, likely because the patients were already on hypoglycaemic agents that alleviated these symptoms.
From the regression analysis without adjustment of biochemical and epidemiological factors, a strong inverse dose-dependent relationship between GFR and nocturnal polyuria was observed, indicating a quantifiable assessment on nocturnal polyuria in clinical practice. Frequent urination, nocturnal polyuria, darkened complexion, dark lips, and dull purple color on lower limb are associated with lower GFR, which is commonly observed in practice.
After adjusting biochemical parameters and demographics including age, gender, HbA1c, and UACR, a set of different symptoms (edema, crenated tongue) were associated with poorer renal function independently. This is due to the collinearity between symptoms and biochemical parameters (e.g., frequent urination and UACR).
Using symptoms from the unadjusted symptom-based model can estimate renal function in resources limited setting or in mass population screening without requiring laboratory investigations. Symptoms from the adjusted model, meanwhile, can increase the explanatory power on top of known risk factors and indicated pathophysiology independent to the control of glucose, blood pressure, lipid, and albuminuria.
Model quality assessment showed that using symptom clusters provides better quality in modeling renal function when compared to using individual symptoms. This is due to the natural collinearity of symptoms in which the local dependence between symptoms can be better analyzed by grouping symptoms with shared pathophysiology and predictive power on renal function (51).
From the 4-year follow-up cohort, symptom clusters provide additional explanatory power on top of biochemical and epidemiological factors in stratifying the decline of renal function. Early diabetes presented with SS8, which included fatigue, malaise, dry mouth and dry throat, were associated with 1.1 ml/min/1.73m2 faster renal function decline annually.
The presence of epigastric bloating, and alternating dry and loose stool are associated with faster GFR decline after adjusting blood glucose, blood pressure, lipid and urine albumin. From the 11 SSs that documented in CM literature, diabetes was stratified into three main groups (Figure 1): (1) SS1–SS6: with gastrointestinal presentation, (2) SS7, SS8: with classical diabetes presentations, and (3) SS9–SS11: with neurological and vascular presentations. The symptom-based stratification of diabetes depends heavily (6 of 11 SSs) on gastrointestinal presentations.
The symptom analysis and expert consensus converged to suggest an important role of gastrointestinal involvement in the renal progression of diabetes. Previous studies showed that gut microbiome is involved in the inflammatory and immune response in metabolic diseases, CKD and related phenotypical changes (52, 53). We previously demonstrated in vivo that toll-like receptor signaling pathway regulate DKD progression (54–56). Activation of the pathway via Paneth cells in small intestine due to microbiota dysbiosis can be a possible mechanism warrants further study (57). Endotoxin (e.g., lipopolysaccharide) translocation to systemic circulation due to disruption of gut barrier can also accelerate CKD progression (53).
Symptom-based research offers a new perspective in assessing correlations in pathogenesis among diseases, accelerating the discovery of secondary application of existing drugs (29). In this study, edema is a strongly independent cross-sectional and longitudinal predictor of reduced renal function. Further multi-level omics analysis stratifying the presence of edema with matching GFR and other confounders could reveal novel mechanisms in renal function decline among early diabetic patients.
Key challenge in symptom-based research is the standardization of subjective symptoms (9). Although the unharmonized definition of symptoms is likely to introduce random measurement error instead of systematic bias, and is unlikely to increase type I error in assessing model quality, a large sample size is needed to demonstrate statistical significance. In this study, thin tongue, deep pulse, and unsmooth pulse were strongly associated with faster renal function decline. Although these symptoms have long been suggested as signs of reduced kidney function and poor circulation in CM theory, standardized, and digitalized measurement is lacking until the past decade (58–60). Recent advancement in phenotype-oriented network in multi-omics data provided a platform to link up phenotype, genotype, omics, and drug targets for the integration of clinical presentation, genetics, and epigenetics (5, 9, 50, 61, 62). These advancements enhance the clinical translation and generalizability of symptom-based diagnosis research to the other parts of the world. Nevertheless, the description of pulse would require substantial work on standardization before being widely utilized.
Our cohort was conveniently sampled from public general outpatient clinics. Although the sampling is not random, selection bias was minimized by consecutive sampling. Majority of the diabetes patients in Hong Kong have follow-ups in public general outpatient clinics. The demographics of our cohort matched the territory-wide demographics (47). Besides, there is no standardized questionnaire for the assessment of symptoms internationally. We developed a data entry form for this purpose with field tests, and only recorded the presence of symptoms to minimize the error due to physicians' judgment. Error in symptom assessment is likely random error instead of systematic bias as the assessors were blinded from data analysis plan and biochemistry data. The measurement error is unlikely to increase type I error in assessing model quality. Furthermore, only a small group of experts with outstanding and diversified academic and clinical track record and was purposively sampled to form the consensus panel for better participation and coordination, which common for Delphi technique (32). Further large-scale untargeted mining by latent class analysis can identify the natural clustering of symptoms. In the analysis, the prescription was not analyzed as the control of blood glucose, pressure, lipid, and urine albumin were reflected by the change of HbA1c, systolic blood pressure, low-density lipoprotein, and UACR. Lastly, this study was designed to compare the quality of model performance and was not powered to evaluate the longitudinal predictiveness of each SS. The SSs that did not reach statistical significance are subject to type II error. Further large-scale correlation study is needed to precisely assess the clinical and statistical significance of each SS.
Our findings showed that symptoms, in particular gastrointestinal symptoms, are strongly and independently associated with renal function decline among diabetic patients. Add-on symptom-based stratification improved the classification of diabetes in predicting glomerular filtration rate decline. Our study proved the concept and clinical value of using symptoms and symptom clusters to predict disease progression either alone or in combination with key biochemical and epidemiological factors. Further prediction algorithms of mixed molecular-symptom diagnosis are warranted. Dynamic change of symptoms should be considered in clinical practice and research design.
Previous UK Biobank studies showed that phenomes, including symptoms and physical measurements, had excellent prediction on long-term clinical outcomes in general population. Symptom-based research in diabetes and renal medicine is limited when compared to other disciplines.
• In this 4-year cohort, edema, and gastrointestinal symptoms were independently associated with faster renal function decline among diabetic patients after adjusting epidemiological factors and control of blood glucose, blood pressure, lipid, and albuminuria.
• Using symptom clusters instead of individual symptoms as the independent variable in statistical models reduced computational dimension and improved model quality (increased explanatory power and less complexity).
• Clustering analysis revealed that patients presenting with a constellation of fatigue, malaise, dry mouth and dry throat were independently associated with faster renal function decline.
Add-on symptom-based diagnosis improves the predictive power on renal function decline among diabetic patients in addition to key biochemistry and epidemiological factors. Further prediction algorithms of mixed molecular-symptom diagnosis are warranted. Dynamic change of symptoms should be considered in clinical practice and research design.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KC, SL, and ST conceived the study. KC, SL, and VW coordinated the Delphi interview. KC collected the data. KC, TC, and VW coordinated the cross-sectional study. TC collected the symptom data. KY retrieved longitudinal follow-up biochemistry and epidemiology data. KC, YX, and NZ performed the analysis. KC and ST drafted the manuscript. All authors involved in the interpretation of data and manuscript revision.
This project was made possible in part through the support of Fu Tak Iam Foundation, Health and Medical Research Fund (Ref: 12133341, 14151731) and Hong Kong Society of Nephrology Research Grant. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the contribution of the experts participated in the consensus panel for their expert opinion, Ms. Wong Wai Yan Margaret for the symptom data collection of the validation cohort, clinicians for facilitating the health service programme, and Mr. Zheng Ho for the coordination of patient and clinicians.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.682090/full#supplementary-material
AIC, Akaike information criterion; BIC, Bayesian information criterion; CI, confidence interval; CKD, chronic kidney disease; CM, Chinese medicine; DKD, diabetic kidney disease; GFR, glomerular filtration rate; HbA1c, hemoglobin A1c; SS, symptom-based subtype; UACR, urine albumin-to-creatinine ratio.
1. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. (2017) 66:241–55. doi: 10.2337/db16-0806
2. Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. (2018) 6:361–9. doi: 10.1016/S2213-8587(18)30051-2
3. Ganna A, Ingelsson E. 5 year mortality predictors in 498,103 UK Biobank participants: a prospective population-based study. Lancet. (2015) 386:533–40. doi: 10.1016/S0140-6736(15)60175-1
4. Barabási A-L, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. (2011) 12:56–68. doi: 10.1038/nrg2918
5. Cheng F, Desai RJ, Handy DE, Wang R, Schneeweiss S, Barabási A-L, et al. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat Commun. (2018) 9:2691. doi: 10.1038/s41467-018-05116-5
6. Nelson EC, Eftimovska E, Lind C, Hager A, Wasson JH, Lindblad S. Patient reported outcome measures in practice. BMJ. (2015) 350:g7818. doi: 10.1136/bmj.g7818
7. Sood R, Law GR, Ford AC. Diagnosis of IBS: symptoms, symptom-based criteria, biomarkers or 'psychomarkers'? Nat Rev Gastroenterol Hepatol. (2014) 11:683–91. doi: 10.1038/nrgastro.2014.127
8. Idborg H. Symptom-based stratification of autoimmune diseases. Lancet Rheumatol. (2019) 1:e76–7. doi: 10.1016/S2665-9913(19)30047-5
9. Zhou X, Menche J, Barabasi AL, Sharma A. Human symptoms-disease network. Nat Commun. (2014) 5:4212. doi: 10.1038/ncomms5212
10. Calvert MJ, O'Connor DJ, Basch EM. Harnessing the patient voice in real-world evidence: the essential role of patient-reported outcomes. Nat Rev Drug discov. (2019) 18:731–2. doi: 10.1038/d41573-019-00088-7
11. Evans JM, Glazer A, Lum R, Heale E, MacKinnon M, Blake PG, et al. Implementing a patient-reported outcome measure for hemodialysis patients in routine clinical care. Clin J Am Soc Nephrol. (2020) 15:1299. doi: 10.2215/CJN.01840220
12. Chan KW, Kwong ASK, Tsui PN, Cheung SCY, Chan GCW, Choi WF, et al. Efficacy, safety and response predictors of adjuvant astragalus for diabetic kidney disease (READY): study protocol of an add-on, assessor-blind, parallel, pragmatic randomised controlled trial. BMJ Open. (2021) 11:e042686. doi: 10.1136/bmjopen-2020-042686
13. Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. (2020) 26:1037–40. doi: 10.1038/s41591-020-0916-2
14. Tarn JR, Howard-Tripp N, Lendrem DW, Mariette X, Saraux A, Devauchelle-Pensec V, et al. Symptom-based stratification of patients with primary Sjogren's syndrome: multi-dimensional characterisation of international observational cohorts and reanalyses of randomised clinical trials. Lancet Rheumat. (2019) 1:e85–94. doi: 10.1016/S2665-9913(19)30042-6
15. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. (2019) 394:1949–64. doi: 10.1016/S0140-6736(19)32563-2
16. Wagner R, Heni M, Tabák AG, Machann J, Schick F, Randrianarisoa E, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med. (2021) 27:49–57. doi: 10.1101/2020.10.12.20210062
17. Chan KW, Wong Taam V, Tang SC. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-western medicine for the management of 2019. novel coronavirus disease. Am J Chin Med. (2020) 48:737–62. doi: 10.1142/S0192415X20500378
18. Agusti A, Celli B, Faner R. What does endotyping mean for treatment in chronic obstructive pulmonary disease? Lancet. (2017) 390:980–7. doi: 10.1016/S0140-6736(17)32136-0
19. Pividori M, Rajagopal PS, Barbeira A, Liang Y, Melia O, Bastarache L, et al. PhenomeXcan: mapping the genome to the phenome through the transcriptome. Sci Adv. (2020) 6:eaba2083. doi: 10.1126/sciadv.aba2083
20. Bush WS, Oetjens MT, Crawford DC. Unravelling the human genome-phenome relationship using phenome-wide association studies. Nat Rev Genet. (2016) 17:129–45. doi: 10.1038/nrg.2015.36
21. Clark NG, Fox KM, Grandy S. Symptoms of diabetes and their association with the risk and presence of diabetes: findings from the Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD). Diabetes Care. (2007) 30:2868–73. doi: 10.2337/dc07-0816
22. Rampášek L, Hidru D, Smirnov P, Haibe-Kains B, Goldenberg A. Dr.VAE: improving drug response prediction via modeling of drug perturbation effects. Bioinformatics. (2019) 35:3743–51. doi: 10.1093/bioinformatics/btz158
23. Tong XL, Dong L, Chen L, Zhen Z. Treatment of diabetes using traditional Chinese medicine: past, present and future. Am J Chin Med. (2012) 40:877–86. doi: 10.1142/S0192415X12500656
24. Ferreira AS, Lopes AJ. Chinese medicine pattern differentiation and its implications for clinical practice. Chin J Integr Med. (2011) 17:818–23. doi: 10.1007/s11655-011-0892-y
25. Chan KW, Lee PW, Leung CPS, Chan GCW, Yiu WH, Cheung HM, et al. Patients' and clinicians' expectations on integrative medicine Services for Diabetes: a focus group study. BMC Complement Med Ther. (2020) 20:205. doi: 10.1186/s12906-020-02994-5
26. Lin MY, Chiu YW, Chang JS, Lin HL, Lee CT, Chiu GF, et al. Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int. (2015) 5:226. doi: 10.1038/ki.2015.226
27. Huang KC, Su YC, Sun MF, Huang ST. Chinese herbal medicine improves the long-term survival rate of patients with chronic kidney disease in taiwan: a nationwide retrospective population-based cohort study. Front Pharmacol. (2018) 9:1117. doi: 10.3389/fphar.2018.01117
28. Hsieh CF, Huang SL, Chen CL, Chen WT, Chang HC, Yang CC. Non-aristolochic acid prescribed Chinese herbal medicines and the risk of mortality in patients with chronic kidney disease: results from a population-based follow-up study. BMJ Open. (2014) 4:e004033. doi: 10.1136/bmjopen-2013-004033
29. Shu Z, Chang K, Zhou Y, Peng C, Li X, Cai W, et al. Add-On semi-individualized Chinese medicine for Coronavirus Disease (2019). (ACCORD): a retrospective cohort study of hospital registries. Am J Chin Med. (2021) 49:543–75. doi: 10.1142/S0192415X21500257
30. Guo J, Chen H, Song J, Wang J, Zhao L, Tong X. Syndrome differentiation of diabetes by the traditional Chinese medicine according to evidence-based medicine and expert consensus opinion. Evid Based Complement Alternat Med. (2014) 2014:492193. doi: 10.1155/2014/492193
31. Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. (1995) 311:376–80. doi: 10.1136/bmj.311.7001.376
32. Waggoner J, Carline JD, Durning SJ. Is there a consensus on consensus methodology? Descriptions and recommendations for future consensus research. Acad Med. (2016) 91:663–8. doi: 10.1097/ACM.0000000000001092
33. Graham B, Regehr G, Wright JG. Delphi as a method to establish consensus for diagnostic criteria. J Clin Epidemiol. (2003) 56:1150–6. doi: 10.1016/S0895-4356(03)00211-7
34. Voncken L, Albers CJ, Timmerman ME. Model selection in continuous test norming with GAMLSS. Assessment. (2019) 26:1329–46. doi: 10.1177/1073191117715113
35. Zhang QM, Liu BY, Wang YY. Chinese Medicine Symptomatology Research. Beijing: Chinese Medicine Ancient Literature Press (2013).
36. Wan EYF, Fung CSC, Jiao FF, Yu EYT, Chin WY, Fong DYT, et al. Five-year effectiveness of the multidisciplinary Risk Assessment and Management Programme-Diabetes Mellitus (RAMP-DM) on diabetes-related complications and health service uses-a population-based and propensity-matched cohort study. Diabetes Care. (2018) 41:49–59. doi: 10.2337/dc17-0426
37. Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. (2014) 67:401–9. doi: 10.1016/j.jclinepi.2013.12.002
38. China Association of Chinese Medicine. Guideline for traditional Chinese medicine diabetes prevention and treatment. Chinese medicine modern distance education of China. (2011) 9:148–51.
39. National Administration of Traditional Chinese Medicine. Type 2 Diabetes Clinical Practice Guideline. Beijing: China Press of Traditional Chinese Medicine (2012).
40. State Food and Drug Administration. Guiding Principle of Clinical Research on New Drugs of Chinese Medicine (Trial). Beijing: China Medical Science Press (2002).
41. China Academy of Chinese Medical Sciences. Evidence-based Guidelines of Clinical Practice in Chinese Medicine Internal Medicine. Type 2 Diabetes Mellitus Chinese Medicine Clinical Practice Guideline. Beijing: China Press of Traditional Chinese Medicine (2012). p. 95–118.
42. General Administration of Quality Supervision Inspection and Quarantine of the People's Republic of China. Clinic Terminology of Traditional Chinese Medical Diagnosis and Treatment – Syndromes. Beijing: China Zhijian Publishing House (1997).
43. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
44. Jiang G, Luk AOY, Tam CHT, Xie F, Carstensen B, Lau ESH, et al. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with Type 2 diabetes. Kidney Int. (2019) 95:178–87. doi: 10.1016/j.kint.2018.08.026
45. Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods. (2012) 17:228–43. doi: 10.1037/a0027127
46. Grams ME, Sang Y, Ballew SH, Matsushita K, Astor BC, Carrero JJ, et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol. (2019) 30:1746–55 doi: 10.1681/ASN.2019010008
47. Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia. (2018) 61:1249–60. doi: 10.1007/s00125-018-4557-7
48. Galperin TA, Cronin AJ, Leslie KS. Cutaneous manifestations of ESRD. Clin J Am Soc Nephrol. (2014) 9:201–18. doi: 10.2215/CJN.05900513
49. Kimmelstiel P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. Am J Pathol. (1936) 12:83–98.
50. Jiang M, Lu C, Zhang C, Yang J, Tan Y, Lu A, et al. Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol. (2012) 140:634–42. doi: 10.1016/j.jep.2012.01.033
51. Chen P, Zhang NL, Liu T, Poon LKM, Chen Z, Khawar F. Latent tree models for hierarchical topic detection. Artifi Intellig. (2017) 250:105–24. doi: 10.1016/j.artint.2017.06.004
52. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375:2369–79. doi: 10.1056/NEJMra1600266
53. Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. (2014) 25:657–70. doi: 10.1681/ASN.2013080905
54. Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. (2012) 23:86–102. doi: 10.1681/ASN.2010111210
55. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. (2020) 16:206–22. doi: 10.1038/s41581-019-0234-4
56. Burrows MP, Volchkov P, Kobayashi KS, Chervonsky AV. Microbiota regulates type 1 diabetes through Toll-like receptors. Proc Natl Acad Sci USA. (2015) 112:9973–7. doi: 10.1073/pnas.1508740112
57. Knauf F, Brewer JR, Flavell RA. Immunity, microbiota and kidney disease. Nat Rev Nephrol. (2019) 15:263–74. doi: 10.1038/s41581-019-0118-7
58. Wang ZC, Zhang SP, Yuen PC, Chan KW, Chan YY, Cheung CH, et al. Intra-rater and inter-rater reliability of tongue coating diagnosis in traditional Chinese medicine using smartphones: quasi-delphi study. JMIR mHealth uHealth. (2020) 8:e16018. doi: 10.2196/16018
59. Chung YF, Hu CS, Yeh CC, Luo CH. How to standardize the pulse-taking method of traditional Chinese medicine pulse diagnosis. Comput Biol Med. (2013) 43:342–9. doi: 10.1016/j.compbiomed.2012.12.010
60. Wong W, Lam CL, Su YC, Lin SJ, Ziea ET, Wong VT, et al. Measuring body constitution: validation of the Body Constitution Questionnaire (BCQ) in Hong Kong. Complement Thera Med. (2014) 22:670–82. doi: 10.1016/j.ctim.2014.05.009
61. Yoo S, Nam H, Lee D. Phenotype-oriented network analysis for discovering pharmacological effects of natural compounds. Sci Rep. (2018) 8:11667. doi: 10.1038/s41598-018-30138-w
Keywords: diabetes, diabetic kidney disease, renal medicine, epidemiology, integrative medicine, symptom, diagnosis, traditional chinese medicine
Citation: Chan KW, Chow TY, Yu KY, Xu Y, Zhang NL, Wong VT, Li S and Tang SCW (2021) SYmptom-Based STratification of DiabEtes Mellitus by Renal Function Decline (SYSTEM): A Retrospective Cohort Study and Modeling Assessment. Front. Med. 8:682090. doi: 10.3389/fmed.2021.682090
Received: 17 March 2021; Accepted: 17 May 2021;
Published: 14 June 2021.
Edited by:
Min Chen, Peking University First Hospital, ChinaReviewed by:
Xuezhou Zhou, Beijing Jiaotong University, ChinaCopyright © 2021 Chan, Chow, Yu, Xu, Zhang, Wong, Li and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saimei Li, bGlzYWltZWkyMDA0QDE2My5jb20=; Sydney Chi Wai Tang, c2N3dGFuZ0Boa3UuaGs=
†ORCID: Kam Wa Chan orcid.org/0000-0002-3175-1574
Sydney Chi Wai Tang orcid.org/0000-0002-6862-1941
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.