
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 20 May 2021
Sec. Geriatric Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.681232
This article is part of the Research Topic Impacts of Common Geriatric Syndromes and their Interaction with Chronic Diseases on Health View all 33 articles
Purpose: Sarcopenia is a major disease affecting mortality and quality of life in the elderly population. We performed a meta-analysis of studies on the community-dwelling population to investigate the prevalence of sarcopenia and its association with diabetes.
Methods: Databases were searched for studies published up to February 3, 2021, reporting the prevalence of sarcopenia in patients with and without diabetes. Data extraction and quality assessment were performed according to the Newcastle-Ottawa scale.
Results: Six articles were included in the systematic review. All the patients were Asian, aged ≥60 years (women 53.4%), and the diabetic and non-diabetic population was 1,537 and 5,485, respectively. In all six studies, the Asian Working Group for Sarcopenia criteria were used to diagnose sarcopenia. The prevalence of sarcopenia was 15.9% in diabetics and 10.8% in non-diabetics. Diabetics showed a significantly higher risk of sarcopenia than non-diabetics (pooled OR = 1.518, 95% CI = 1.110 to 2.076, Z-value = 2.611, p = 0.009).
Conclusion: Among the Asian community-dwelling geriatric population, the prevalence of sarcopenia was significantly higher in diabetics than in non-diabetics. These results suggest that strategies for the management of sarcopenia are required in Asian elderly patients, especially with diabetes.
Globally, rapid increase in the aging population has led to social and health issues associated with age-related chronic diseases. Sarcopenia is a syndrome of age-related degenerative skeletal disease characterized by a progressive and generalized reduction in muscular mass, strength, and function (1). Although controversies remain regarding definitions, assessment tools, diagnostic criteria, and treatment, sarcopenia is developing into a critical public health burden among the elderly, being related to adverse outcomes such as frailty, disability, poor quality of life, and increased mortality. Previous meta-analyses have reported the prevalence of sarcopenia in community-dwelling older adults, and the range varied from 9.9 to 40.4% depending on the definition of sarcopenia used (2, 3). Whereas, the earlier criterion was based only on muscle mass assessment (4), the recent definitions (5–7) include ethnic differences as well as muscle strength and function, which are more strongly associated with outcomes such as mortality (8).
The incidence of type 2 diabetes increases with age, and recent studies have revealed that both sarcopenia and diabetes have a bidirectional relationship (9). Age-related skeletal muscle degeneration could deteriorate insulin sensitivity and lead to the development of metabolic disorders such as diabetes, and vice versa. Diabetes-related decline in muscle mass, strength, and function leads to sarcopenia and increased disability, fracture, and mortality (10–12).
Although the decline of muscle mass and muscle strength has been compared between diabetic and non-diabetic patients in previous studies (13–16), few studies have compared diabetic and non-diabetic patients using the standardized sarcopenia diagnostic criteria reflecting qualitative changes in the skeletal muscles. To evaluate the prevalence of sarcopenia in diabetics and to compare it to that in non-diabetics, a meta-analysis of studies on the community-dwelling population using standardized criteria is required. Therefore, we systematically reviewed the literature and performed a meta-analysis to evaluate the impact of diabetes on the occurrence of sarcopenia in geriatric adults.
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. We systematically searched for relevant articles in the PubMed, Embase, Cochrane Library, SCOPUS, KoreaMed, Wangfang, and Ichushi databases for studies published from January 01, 1979 to February 03, 2021. The following keywords were used in the search: (sarcopenia OR muscle mass OR muscle strength) AND (prevalence OR epidemiology OR frequency OR incidence) AND (diabetes).
We applied the following inclusion criteria for the selection of articles: (1) the prevalence rate of sarcopenia was evaluated in the community-dwelling general population, (2) the prevalence rate of sarcopenia was investigated in diabetic and non-diabetic populations, (3) the age of included subjects was ≥60 years, and (4) loss of appendicular muscle mass and functional decline were considered for the diagnosis of sarcopenia. The exclusion criteria were as follows: (1) repeat publications and (2) lack of reporting on study outcomes.
After discarding duplicate studies, two reviewers (SC and MC) independently evaluated the potentially eligible studies. The articles were screened for eligibility based on a review of the title and abstract, and disagreements were resolved through consensus. After screening, the full texts of the eligible articles were read independently by the two reviewers, and the eligibility of each article was re-assessed. Subsequently, the data, including the first author, publication date, study type, number of patients, and demographic information (age, sex, and other participant details) were extracted.
The Newcastle-Ottawa scale (NOS) for cross-sectional study was used for quality assessment (17), considering the three aspects: selection of subjects, comparability of groups, and assessment of outcome. The NOS allows four stars for selection of subjects, two for comparability of groups, and three for assessment of outcomes, a maximum of nine stars in total. Judgment of bias was expressed as “unsatisfactory studies,” “satisfactory studies,” “good studies,” or “very good studies.” The quality of each study was graded as low (0–3), moderate (4–6), or high (7–9). All divergences were resolved by consensus.
The overall quality (certainty) of evidence was assessed using the GRADE system which grades evidence as high, moderate, low, or very low quality. Randomized controlled trials are graded as high-quality evidence by default. Scores can then be downgraded on the basis of the following prespecified criteria: risk of bias (weight of studies shows important risk of bias), inconsistency (substantial unexplained interstudy heterogeneity), indirectness (present of factors that limit the generalizability of the results), imprecision (95% confidence interval [CI] for risk estimates are wide), and publication bias (evidence of small-study effects) (18).
The extracted data were analyzed statistically using Comprehensive Meta-analysis Version 2.0 (Biostat Inc., Englewood, New Jersey). For each analysis, a heterogeneity test was conducted using the I2 statistic, which helps to evaluate the extent of inconsistency among the results. If I2 values were ≥50%, the data were considered to have substantial heterogeneity, and the random-effects model was used for data analysis. In contrast, if I2 values were <50%, the pooled data were considered homogenous, and the fixed-effects model was applied for data analysis.
The pooled prevalence and 95% CI for sarcopenia were also calculated. Additionally, to analyze the difference in the risk of sarcopenia between diabetes and non-diabetes, we analyzed the odds ratio (OR), and 95% CIs from the raw prevalence data. Statistical significance was set at P < 0.05.
A total of 7,239 potentially relevant studies were selected for the preliminary search from all databases (Figure 1). We initially excluded 1,119 duplicate studies, and excluded an additional 6,016 publications after reviewing their titles and abstracts. The remaining studies were assessed through a full text review of the articles. The European Working Group on Sarcopenia (EWGS) and Asian Working Group for Sarcopenia (AWGS) were considered as the standard criteria for the diagnosis of sarcopenia (6, 7). After the systematic review, six articles were included (Table 1) (19–24). In all six articles, the AWGS criteria were used to diagnose sarcopenia (7).
The selected studies included 1,537 diabetics and 5,485 non-diabetics. All patients were aged ≥60 years, and 53.4% were women. One study used dual-energy X-ray absorptiometry (DXA) to evaluate body composition, and another used bioelectrical impedance analysis (BIA).
Three included studies [by Han et al. (19), Kang et al. (21), Nakamura et al. (23), and Wang et al. (24)] were rated nine stars (selection of subjects: fout stars; comparability of groups: two stars; and assessment of outcome: three stars). The remaining two studies [by Han et al. (20) and Keng et al. (22)] were rated eight stars (selection of subjects: four stars; comparability of groups: one star; and assessment of outcome: three stars). Therefore, the quality of all included studies assessed using NOS was considered high.
The overall quality (certainty) of evidence of all the included studies was rated as low.
To evaluate the prevalence of sarcopenia among the diabetic and non-diabetic population, the random effects model was used (diabetic: I2 = 88.472; non-diabetic: I2 = 89.158), and the total prevalence was 15.9% (95% CI = 10.6 to 23.2%) (Figure 2A) and 10.8% (95% CI = 8.4 to 13.7%) (Figure 2B), respectively.
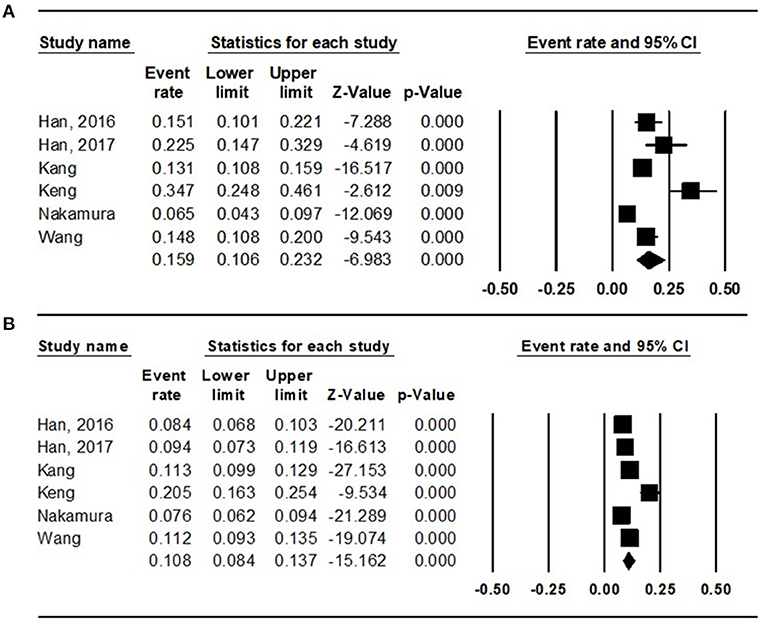
Figure 2. Results of the meta-analysis on sarcopenia prevalence among the (A) diabetes population and that among the (B) non-diabetes population.
To analyze the difference in the risk of sarcopenia between the diabetics and non-diabetics, the random effects model was used (I2 = 65.292). The diabetics showed a significantly higher risk of sarcopenia than the non-diabetics (pooled OR = 1.518, 95% CI = 1.110 to 2.076, Z-value = 2.611, p = 0.009) (Figure 3).
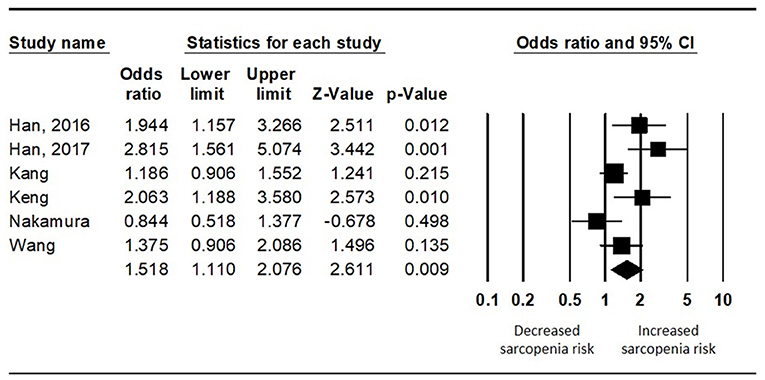
Figure 3. Results of the meta-analysis on the difference in the risk of sarcopenia between the diabetes population and the non-diabetes population.
Funnel plot analysis was performed, which showed visually a symmetrical distribution of published studies in all the analyses (prevalence of sarcopenia among the diabetics and non-diabetics and difference in the risk of sarcopenia between the diabetics and non-diabetics) (Figure 4).
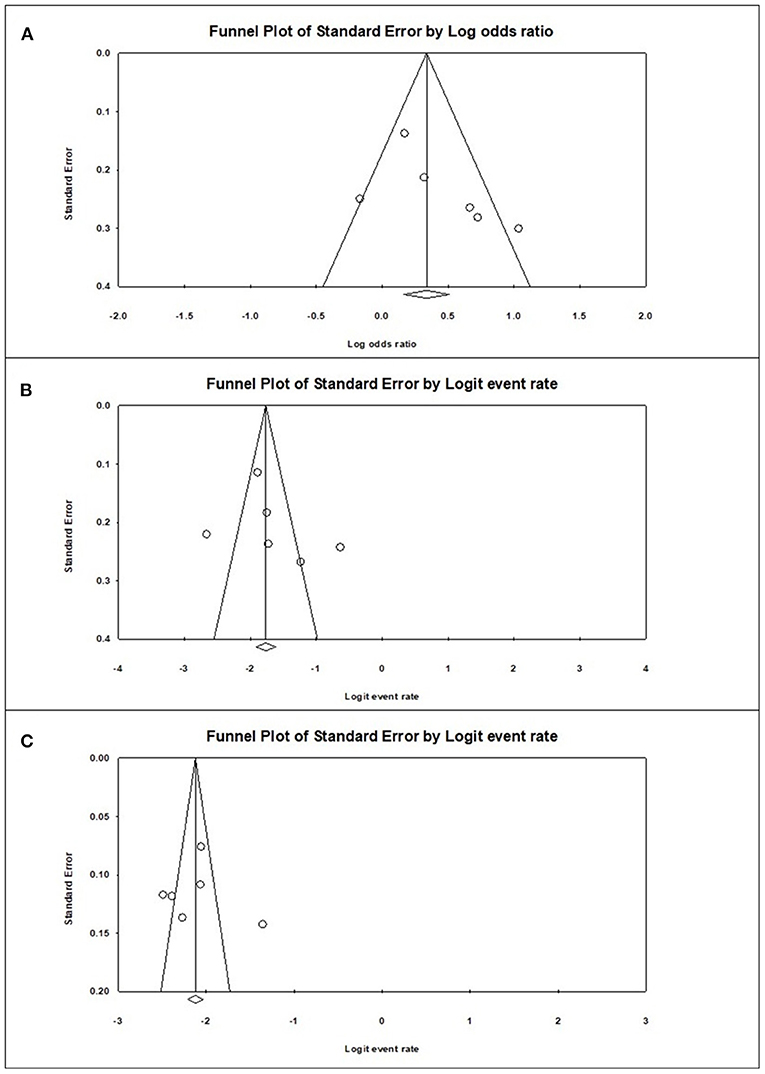
Figure 4. Graphic funnel plots of the included studies (A), diabetes population vs. non-diabetic population; (B), prevalence of sarcopenia among the diabetes population; (C), prevalence of sarcopenia among the non-diabetic population.
In this meta-analysis, we analyzed studies that evaluated the prevalence of sarcopenia in diabetes and analyzed all the following parameters: muscle strength, quality, and function in the community-dwelling general population aged ≥ 60 years. During the systemic review, we found that most studies assessed either muscle strength or quality or did not target the general population. A total of six studies, which fulfilled all inclusion criteria were finally enrolled. The prevalence of sarcopenia was significantly higher in diabetics (15.9%) than non-diabetics (10.8%).
After the concept of “sarcopenia” was introduced by Baumgartner et al. (4), various definitions and diagnostic criteria have been introduced. The earlier diagnostic criteria were simply to assess skeletal muscle mass (25, 26), but recently, indicators to evaluate muscle function and quality are known to be considered an important portion of sarcopenia (5–7). Therefore, both components should be evaluated, and the diagnostic criteria reflecting them are widely accepted. Previous meta-analysis results included a number of studies using inconsistent criteria that demonstrated a wide range of the prevalence (2, 3). Since it is well-known that not only quantitative but also a qualitative assessment of body composition reflects the clinical outcomes such as frailty (21), fall risk (27), and mortality (28), we only included studies evaluated by EWGS or AWGS criteria recognized as the standard criteria (6, 7). Therefore, our findings have an advantage including the clinical studies conducted with more relevant criteria that are more closely related to actual clinical outcomes than previous studies. In addition, the risk of selection bias was less affected because only included the studies target the general population, not patients admitted to hospitals or patients receiving treatment for other diseases.
The evil synergistic effects between sarcopenia and metabolic disease (such as diabetes) are already established from large population studies which lead to critical outcomes such as cardiovascular disease or mortality. In a study on 6,021 (2,593 men, 3,429 women) individuals from the general population in South Korean aged 30–93 years, a low muscle mass increased the risk of metabolically unhealthy status (≥2 components of metabolic syndrome or the presence of hypertension, diabetes, or cardiovascular disease) in non-obese men by 1.88 folds (29). Of 347,130 individuals from the general population (158,959 men, 188,171 women; mean age, 55.9) in the United Kingdom, 4% were diabetic, and diabetics with low handgrip strength had a 4.05 times higher risk for cardiovascular disease mortality compared to the high handgrip strength group (30). Of 610 (diabetics, 306, non-diabetics, 304) patients who were hospitalized at a single center in Brazil, the risk of mortality after discharge was significantly higher in those with diabetes and sarcopenia (OR = 1.78) (28). Insulin resistance is known to be the one of major factors involving the development of sarcopenia (31, 32). In patients with diabetes, age-related anabolic resistance worsens, fat mass increases, and lean mass decreases more rapidly than in non-diabetics (33). Insulin plays a role in inhibiting protein catabolism in muscles, and insulin resistance induces protein dysregulation (31). Insulin resistance causes dysregulation of the potential mediators of glucose and protein metabolism in the skeletal muscles, which are as follows: those regulated by the hypothalamic-pituitary axis [glucocorticoids, insulin-like growth factor 1 (IGF-1), and androgen], as well as Akt/mammalian target of rapamycin signaling, AMP-activated protein kinase, myostatin, urocortins, and vitamin D (34). In addition, advanced glycation end-products accumulate in the skeletal muscles in chronic conditions, resulting in muscle mass reduction and sarcopenia (35). Tumor necrosis factor-α (TNF-α) and TNF-like weak inducer of apoptosis increase the risk of sarcopenia, while IGF-1, insulin, and adiponectin reduce the risk of sarcopenia (36). In elderly diabetes patients, the incidence of sarcopenic obesity (high body fat and low body mass) (37) should be evaluated and should be prevented by nutrition intake and exercise giving anabolic stimulus to skeletal muscle (38).
Among diabetic patients, there are differences in the risk of sarcopenia depending on the duration of diabetes and glucose variability. In a Japanese study on diabetic individuals aged ≥60 years (108 men and 105 women), the prevalence of sarcopenia was 19.2%. The risk of sarcopenia increased with increase in the duration of diabetes in women (OR = 1.43) (39). In another Japanese study on 746 diabetic patients aged 38–96 years, the prevalence of sarcopenia was 7%. The risk of sarcopenia was 7.2 times higher in the patients with glycated hemoglobin (HbA1c) ≥8% than that in patients with HbA1c <6.5% when blood glucose control was poor (40). In another study on Japanese diabetics aged 65–87 years (69 men, 33 women), the prevalence of sarcopenia was found to be 11.6%. Low muscle mass, low grip strength, and slow walking speed were associated with larger glucose fluctuations. Glucose fluctuation was reported to increase the risk of sarcopenia (OR = 1.045) (41). Our study assessed the association of diabetes and sarcopenia from the community-dwelling population but have some limitations regarding the effects of glycemic control status, disease duration, level of exercise, malnutrition, and hypoglycemic medications on sarcopenia in peoples with diabetes. Further large epidemiologic studies are warranted in the future. Interestingly, recent anti-diabetic therapeutics might be candidate for prevention of sarcopenia: the use of metformin, glucagon-like peptide-1(GLP-1) receptor agonists, and sodium-glucose co-transporter-2 (SGLT2) inhibitors, and metabolic surgery has been suggested as a potential strategy to reduce fat mass (33). Further prospective studies for the impact of glucose control and body composition modification by appropriate diabetes treatment strategy on sarcopenia prevention are needed.
A limitation of the current meta-analysis is that a relatively small number of relevant studies have been published that used standardized diagnostic criteria for sarcopenia in the community-dwelling general population. Despite this limitation, this systematic review retrieved comparatively large data on the difference in prevalence of sarcopenia among the diabetic and non-diabetic Asian populations.
In conclusion, this meta-analysis revealed that sarcopenia was more prevalent in the diabetic population than in the non-diabetic population. The presence of diabetes significantly affects the occurrence of sarcopenia. To reduce sarcopenia-related complications, proper nutrition intake and resistance exercises are essential. Especially in elderly individuals with diabetes, strategies to reduce glucose variability and maintain healthy body composition while managing cognitive dysfunction and accompanying cardiovascular risks are warranted.
The data analyzed in this study is subject to the following licenses/restrictions: this is a meta-analysis so the original dataset of reviewed studies is unavailable. Requests to access these datasets should be directed to Seung Min Chung, c21jaHVuZ0B5bnUuYWMua3I=.
SMC, JSM, and MCC: conception or design and drafting the work or revising. SMC and MCC: acquisition, analysis, or interpretation of data. All authors: final approval of the manuscript.
This study was supported by Yeungnam University Hospital Research Grant (2019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
2. Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. (2019) 48:48–56. doi: 10.1093/ageing/afy106
3. Cruz-Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. (2014) 43:748–59. doi: 10.1093/ageing/afu115
4. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. (1998) 147:755–63. doi: 10.1093/oxfordjournals.aje.a009520
5. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
6. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
7. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
8. Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. (2006) 61:72–7. doi: 10.1093/gerona/61.1.72
9. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. (2019) 12:1057–72. doi: 10.2147/DMSO.S186600
10. Sinclair AJ, Abdelhafiz AH, Rodriguez-Manas L. Frailty and sarcopenia—newly emerging and high impact complications of diabetes. J Diabetes Complications. (2017) 31:1465–73. doi: 10.1016/j.jdiacomp.2017.05.003
11. Lee JH, Yoon JS, Lee HW, Won KC, Moon JS, Chung SM, et al. Risk factors affecting amputation in diabetic foot. Yeungnam Univ J Med. (2020) 37:314–20. doi: 10.12701/yujm.2020.00129
12. Kang SH, Do JY, Kim JC. The relationship between disability and clinical outcomes in maintenance dialysis patients. Yeungnam Univ J Med. (2020) 38:127–35. doi: 10.12701/yujm.2020.00346
13. Chen L, Nelson DR, Zhao Y, Cui Z, Johnston JA. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States. BMC Geriatr. (2013) 13:74. doi: 10.1186/1471-2318-13-74
14. Lee N, Choi CJ. Smoking and diabetes as predictive factors of accelerated loss of muscle mass in middle-aged and older women: a six-year retrospective cohort study. J Womens Health. (2019) 28:1391–8. doi: 10.1089/jwh.2018.7527
15. Kim KS, Park KS, Kim MJ, Kim SK, Cho YW, Park SW. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr Gerontol Int. (2014) 14(Suppl 1):115–21. doi: 10.1111/ggi.12189
16. Kim CR, Jeon YJ, Jeong T. Risk factors associated with low handgrip strength in the older Korean population. PLoS ONE. (2019) 14:e0214612. doi: 10.1371/journal.pone.0214612
17. Institute OHR. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed February 07, 2021).
18. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
19. Han P, Kang L, Guo Q, Wang J, Zhang W, Shen S, et al. Prevalence and factors associated with sarcopenia in suburb-dwelling older chinese using the Asian working group for sarcopenia definition. J Gerontol A Biol Sci Med Sci. (2016) 71:529–35. doi: 10.1093/gerona/glv108
20. Han P, Yu H, Ma Y, Kang L, Fu L, Jia L, et al. The increased risk of sarcopenia in patients with cardiovascular risk factors in Suburb-Dwelling older Chinese using the AWGS definition. Sci Rep. (2017) 7:9592. doi: 10.1038/s41598-017-08488-8
21. Kang S, Oh TJ, Cho BL, Park YS, Roh E, Kim HJ, et al. Sex differences in sarcopenia and frailty among community-dwelling Korean older adults with diabetes: the Korean frailty and aging cohort study. J Diabetes Investig. (2021) 12:155–64. doi: 10.1111/jdi.13348
22. Keng BMH, Gao F, Teo LLY, Lim WS, Tan RS, Ruan W, et al. Associations between skeletal muscle and myocardium in aging: a syndrome of “cardio-sarcopenia”? J Am Geriatr Soc. (2019) 67:2568–73. doi: 10.1111/jgs.16132
23. Nakamura K, Yoshida D, Honda T, Hata J, Shibata M, Hirakawa Y, et al. Prevalence and mortality of sarcopenia in a community-dwelling older Japanese population: the hisayama study. J Epidemiol. (2020) 31:320–7. doi: 10.2188/jea.JE20190289
24. Wang T, Feng X, Zhou J, Gong H, Xia S, Wei Q, et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep. (2016) 6:38937. doi: 10.1038/srep38937
25. Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. (1996) 271(6 Pt 1):E941–51. doi: 10.1152/ajpendo.1996.271.6.E941
26. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. (2002) 50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x
27. Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Hayashida I, Kusabiraki T, et al. Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European Working Group on Sarcopenia in older people. Arch Gerontol Geriatr. (2014) 59:295–9. doi: 10.1016/j.archger.2014.04.016
28. Beretta MV, Dantas Filho FF, Freiberg RE, Feldman JV, Nery C, Rodrigues TC. Sarcopenia and Type 2 diabetes mellitus as predictors of 2-year mortality after hospital discharge in a cohort of hospitalized older adults. Diabetes Res Clin Pract. (2020). 159:107969. doi: 10.1016/j.diabres.2019.107969
29. Hwang YC, Cho IJ, Jeong IK, Ahn KJ, Chung HY. Differential association between sarcopenia and metabolic phenotype in Korean young and older adults with and without obesity. Obesity. (2017) 25:244–51. doi: 10.1002/oby.21694
30. Celis-Morales CA, Petermann F, Hui L, Lyall DM, Iliodromiti S, McLaren J, et al. Associations between diabetes and both cardiovascular disease and all-cause mortality are modified by grip strength: evidence from uk biobank, a prospective population-based cohort study. Diabetes Care. (2017) 40:1710–8. doi: 10.2337/dc17-0921
31. Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. (2016) 229:R67–81. doi: 10.1530/JOE-15-0533
32. Park S, Park SY. Can antioxidants be effective therapeutics for type 2 diabetes? Yeungnam Univ J Med. (2020) 38:83–94. doi: 10.12701/yujm.2020.00563
33. Al-Sofiani ME, Ganji SS, Kalyani RR. Body composition changes in diabetes and aging. J Diabetes Complications. (2019) 33:451–9. doi: 10.1016/j.jdiacomp.2019.03.007
34. Clegg A, Hassan-Smith Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. (2018) 6:743–52. doi: 10.1016/S2213-8587(18)30110-4
35. Mori H, Kuroda A, Ishizu M, Ohishi M, Takashi Y, Otsuka Y, et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J Diabetes Investig. (2019) 10:1332–40. doi: 10.1111/jdi.13014
36. Li CW, Yu K, Shyh-Chang N, Li GX, Jiang LJ, Yu SL, et al. Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle. (2019) 10:586–600. doi: 10.1002/jcsm.12417
37. Fukuoka Y, Narita T, Fujita H, Morii T, Sato T, Sassa MH, et al. Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig. (2019) 10:322–30. doi: 10.1111/jdi.12908
38. Makanae Y, Fujita S. Role of exercise and nutrition in the prevention of sarcopenia. J Nutr Sci Vitaminol. (2015) 61(Suppl):S125–7. doi: 10.3177/jnsv.61.S125
39. Nishimura A, Harashima SI, Hosoda K, Arai H, Inagaki N. Sex-related differences in frailty factors in older persons with type 2 diabetes: a cross-sectional study. Ther Adv Endocrinol Metab. (2019) 10:2042018819833304. doi: 10.1177/2042018819833304
40. Sugimoto K, Tabara Y, Ikegami H, Takata Y, Kamide K, Ikezoe T, et al. Hyperglycemia in non-obese patients with type 2 diabetes is associated with low muscle mass: the multicenter study for clarifying evidence for sarcopenia in patients with diabetes mellitus. J Diabetes Investig. (2019) 10:1471–9. doi: 10.1111/jdi.13070
Keywords: diabetes mellitus, type 2, geriatrics, sarcopenia, prevalence, meta-analysis
Citation: Chung SM, Moon JS and Chang MC (2021) Prevalence of Sarcopenia and Its Association With Diabetes: A Meta-Analysis of Community-Dwelling Asian Population. Front. Med. 8:681232. doi: 10.3389/fmed.2021.681232
Received: 16 March 2021; Accepted: 27 April 2021;
Published: 20 May 2021.
Edited by:
Ming Yang, Sichuan University, ChinaReviewed by:
Asli Tufan, Marmara University, TurkeyCopyright © 2021 Chung, Moon and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Sung Moon, bWpzNzkxMkB5dS5hYy5rcg==; orcid.org/0000-0003-1569-3068; Min Cheol Chang, d2hlZWw2MzNAeW51LmFjLmty; orcid.org/0000-0002-7629-7213
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.