
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 17 June 2021
Sec. Gastroenterology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.674896
Introduction: Celiac disease (CD) is a multifactorial autoimmune disorder, and studies have reported that patients with Turner syndrome (TS) are at risk for CD. This systematic review and meta-analysis aimed to quantify the weighted prevalence of CD among patients with TS and determine the weighted strength of association between TS and CD.
Methods: Studies published between January 1991 and December 2019 were retrieved from four electronic databases: PubMed, Scopus, Web of Science, and Embase. Eligible studies were identified and relevant data were extracted by two independent reviewers following specific eligibility criteria and a data extraction plan. Using the random-effects model, the pooled, overall and subgroup CD prevalence rates were determined, and sources of heterogeneity were investigated using meta-regression.
Results: Among a total of 1,116 screened citations, 36 eligible studies were included in the quantitative synthesis. Nearly two-thirds of the studies (61.1%) were from European countries. Of the 6,291 patients with TS who were tested for CD, 241 were diagnosed with CD, with a crude CD prevalence of 3.8%. The highest and lowest CD prevalence rates of 20.0 and 0.0% were reported in Sweden and Germany, respectively. The estimated overall weighted CD prevalence was 4.5% (95% confidence interval [CI], 3.3–5.9, I2, 67.4%). The weighted serology-based CD prevalence in patients with TS (3.4%, 95% CI, 1.0–6.6) was similar to the weighted biopsy-based CD prevalence (4.8%; 95% CI, 3.4–6.5). The strength of association between TS and CD was estimated in only four studies (odds ratio 18.1, 95% CI, 1.82–180; odds ratio 4.34, 95% CI, 1.48–12.75; rate ratio 14, 95% CI, 1.48–12.75; rate ratio 42.5, 95% CI, 12.4–144.8). Given the lack of uniformity in the type of reported measures of association and study design, producing a weighted effect measure to evaluate the strength of association between TS and CD was unfeasible.
Conclusion: Nearly 1 in every 22 patients with TS had CD. Regular screening for CD in patients with TS might facilitate early diagnosis and therapeutic management to prevent adverse effects of CD such as being underweight and osteoporosis.
Celiac disease (CD), also known as celiac sprue and gluten-sensitive enteropathy, is a multifactorial autoimmune disorder arising from the interaction of diverse genetic and environmental factors (1, 2). In patients with CD, the consumption of gluten-containing grains such as wheat, barley, and rye leads to an inappropriate adaptive immune response (3, 4). Although several genes have been reported to contribute to the predisposition to CD, more than 90% of patients with CD carry the HLA-DQ2 or HLA-DQ8 haplotypes (5). Gliadin consumption or repeated gastrointestinal infections in early life in genetically predisposed individuals are considered to trigger and regulate the induction of intraepithelial lymphocytes in the small intestines, leading to villous atrophy (6–8). In turn, histological changes leading to CD result in a variety of clinical manifestations. In adults, the classical clinical manifestations include chronic diarrhea, unintentional weight loss, constipation, malabsorption, and iron deficiency anemia (9). However, 50% of patients with CD present with nonclassical or atypical signs and symptoms, such as anemia, abdominal pain, osteoporosis, osteomalacia, short stature, lymphoma, liver disease, and neurological and psychological symptoms (10, 11). In pediatric patients, CD may present with unexplained growth failure, delayed puberty, chronic diarrhea, and anemia (12) and increases the risk of depression, anxiety, eating disorders, autistic spectrum disorder, and attention-deficit/hyperactivity disorder (13).
Globally, the estimated population-based prevalence of CD is approximately 1% (14). The prevalence of CD ranges from 0.8% in Europe and Oceania to 4.0% in Africa (15). The considerable increase in the prevalence of CD worldwide observed in recent decades (16, 17) has been mainly due to the increased availability of screening tests with improved sensitivity and specificity (18–20). According to current guidelines, screening for CD is not recommended for the general population but is recommended for specific patient groups who are considered at high risk for CD (21, 22), such as relatives of patients with CD as well as patients with insulin-dependent diabetes; autoimmune thyroid disease; selective IgA deficiency; and genetic disorders, including Down syndrome, Williams syndrome, and Turner syndrome (TS) (12, 23, 24).
TS is a female genetic disorder involving the X chromosome. Typical phenotypic characteristics of TS include short stature and gonadal dysgenesis (25). Female patients with TS are at high risk of developing autoimmune diseases approximately twice as high as in the general female populations (26). An increased risk of autoimmune diseases including type 1 diabetes mellitus, thyroid disease (27, 28), and CD has been reported in patients with TS (29). According to the guidelines of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition “NASPHGAN” (30), guidelines of the European Society Pediatric Gastroenterology, Hepatology and Nutrition “ESPHGAN” (31), and the guidelines and recommendation of the TS Consensus Group (32, 33), patients with TS are recommended to be screened for CD and other autoimmune disorders. On the other hand, the latest recommendation statement of the US Preventive Services Task Force (34) concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for CD in asymptomatic persons including patients who are at increased risk of developing CD such as patients with TS (34). No systematic review to date has evaluated CD in patients with TS. The aim of the present systematic review and meta-analysis was to evaluate the existing literature and provide comprehensive quantitative evidence on the prevalence of CD among patients with TS and on the strength of association between TS and CD.
This systematic review was conducted and reported following the 2009 Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (35) (Supplementary Table 1). The review followed a previously published protocol (36) that was also registered in PROSPERO (registration number, CRD42019131881). The published protocol was designed to estimate the strength of association between TS and CD. However, given the lack of sufficient and consistent quantitative effect measures, quantifying a pooled weighted measure of effect was unfeasible. Therefore, following the same protocol and search strategy, the present systematic review was slightly modified to quantify the weighted prevalence of CD among patients with TS. To adjust for the change, necessary minor amendments, including the extraction of information on the prevalence estimates, were implemented.
A comprehensive strategy was designed to search four electronic databases: PubMed, Scopus, Web of Science, and Embase. The search string was developed by an expert librarian (LÖ) and is available in the published protocol (36) and in Supplementary Box 1, which contains the results and search details for all databases. The literature search was performed in December 2019 with no restrictions on language or region. A publication year filter to encompass the period from January 1991 until the search date was applied. The year 1990 was defined as the start year for the present study based on the publication of the first modern guidelines for CD diagnosis by the European Society of Gastroenterology, Hepatology, and Nutrition in the same year (37). All records identified in the search were imported to Covidence systematic review software (38), where automatic de-duplication was performed and the references were prepared for blinded screening. A hand search of bibliographies of studies that were deemed eligible and previously published reviews was also performed.
All observational studies, abstracts, and conference papers were considered. To be deemed eligible, an observational study had to provide quantitative or quantifiable information on the prevalence of CD and/or effect measure on the association between TS and CD regardless of the age of patients with TS screened for CD. Further information on the inclusion and exclusion criteria is available in the published protocol (36).
Following the predesigned eligibility criteria, titles and abstracts of the retrieved studies were independently screened by two reviewers (GSM-AB and AH-N) to identify fully as well as potentially eligible studies; the full texts of the identified studies were retrieved and thoroughly assessed for their eligibility. Conflicts between the reviewers were discussed with a third reviewer (RH-A) and resolved by consensus.
Relevant data were extracted from the studies that were deemed eligible. Data extraction was independently performed by two reviewers (GSM-AB and AH-N) following predefined data extraction parameters described in the published protocol (36), with minor amendments to extract data related to prevalence estimates. Discrepancies between the reviewers were discussed with a third reviewer (RH-A) and resolved by consensus. The following information was extracted from eligible studies: author names; publication year; country and city where the study was conducted; study design, setting, and period; CD diagnostic method; type of TS; number of participants tested for CD; mean or range of age of study participants at the time of CD testing; number of participants who were diagnosed with CD; number of patients with and without TS diagnosed with CD; and crude and adjusted estimates of the association between TS and CD with 95% confidence intervals (CIs), if available. The corresponding authors of the eligible articles were contacted by e-mail if the published information in the article was not sufficient.
According to our previously published protocol (36), we aimed to estimate the strength of association between TS and CD. However, due to the lack of sufficient studies reporting estimates on the strength of association between the exposure–outcome pair, we aimed to determine the burden of CD, in the form of weighted prevalence, among patients with TS.
Among the patients with TS tested for CD, the weighted CD prevalence and corresponding 95% CI was estimated using the Dersimonian–Laird random-effects model. In the meta-analysis, to estimate the weighted prevalence, variances in the prevalence measures were stabilized using the Freeman–Tukey double arcsine transformation method (39, 40). Measures of heterogeneity, Cochran's Q statistic, inconsistency I-squared (12) index, and 95% prediction interval, which estimates the 95% interval in which the true effect size in a new prevalence study will lie, were also computed and reported (41).
In addition to the overall weighted CD prevalence, the weighted CD prevalence were determined by analyzing subgroups according to TS type, sample size (<50 or ≥50 patients with TS), and CD diagnostic method (medical records, serology, biopsy, or unclear). Additionally, for each subgroup, the number of studies, number of patients with TS tested for CD, number of patients with TS diagnosed with CD, and median CD prevalence with ranges were also reported.
To determine the contribution of sample size and CD diagnostic method to the variability in CD prevalence rates across the studies, univariate and multivariate random-effects meta-regression models were performed. In the multivariate model, a p-value of ≤ 0.05 was considered indicative of statistical significance, which contributed to the heterogeneity in prevalence estimates. The number of studies in the reported subcategories was low; therefore, TS type was not used in the meta-regression analysis to preserve sufficient power.
The risk of bias (RoB) of the reviewed individual studies was evaluated using six criteria related to prevalence studies included in the National Heart, Lung, and Blood Institute risk assessment tool (42). The six quality-related criteria assessed whether the study population was clearly specified, participation rate was at least 50%, justification for the recruited sample size was provided, all the participants were selected from the same or similar populations, and the outcome measure was clearly defined, valid, reliable, and implemented consistently across all study participants. The potential answer for each of these criteria was either “yes, no, or an unclear.” For additional quality assessment, we also determined the robustness of the implemented sampling methodology (probability-based, not probability-based, or unclear sampling methodology) as the seventh criterion. Studies were considered to be of high quality if patients with TS tested for CD were selected following probability-based sampling. In the event of insufficient information on any of the quality assessment criteria, the study was categorized as unclear. The overall proportion of individual studies with potentially low RoB across the seven quality criteria was determined. The mean study quality score was also computed based on a maximum quality score of seven.
Quality assessment was independently performed by two reviewers (GSM-AB and AH-N). Any disagreements between the reviewers in the extraction phase or during quality assessment were discussed and resolved by consensus.
A contour-enhanced funnel plot was constructed to explore the effects of small studies on the pooled CD prevalence. The funnel plot was constructed by plotting each CD prevalence measure against its standard error. Asymmetry of the funnel plot was tested using Egger's test (43).
The metaprop (44) and metareg packages of Stata v15 software (45) were used for analyses.
Among a total of 1,116 citations retrieved from the four databases, 36 research articles that fulfilled the eligibility criteria were included in the quantitative meta-analysis (Figure 1). Table 1 summarizes descriptive information of the 36 research articles. These articles (29, 46–48, 50–80) were from 19 countries (Italy, Sweden, Canada, Poland, France, Iran, Germany, Denmark, The United Kingdom, Brazil, The United States of America, The Netherland, Ireland, Turkey, Czech Republic, Algeria, Tunisia, Romania, and Egypt), with the majority of the articles from Europe (47.2%) (29, 46, 47, 49–51, 53, 55, 56, 59, 61, 62, 64, 66, 67, 69, 71, 73, 76–78, 80), Canada (11.1%) (48, 60, 65, 68), and the United States of America (8.3%) (58, 60, 75). The predominantly used CD diagnostic method was biopsy in 55.6% of the research articles. These 36 research articles included 40 studies (single prevalence estimate) on CD prevalence in patients with TS. The TS type was specified in only two articles (53, 75), whereas all TS types were considered in 24 studies.
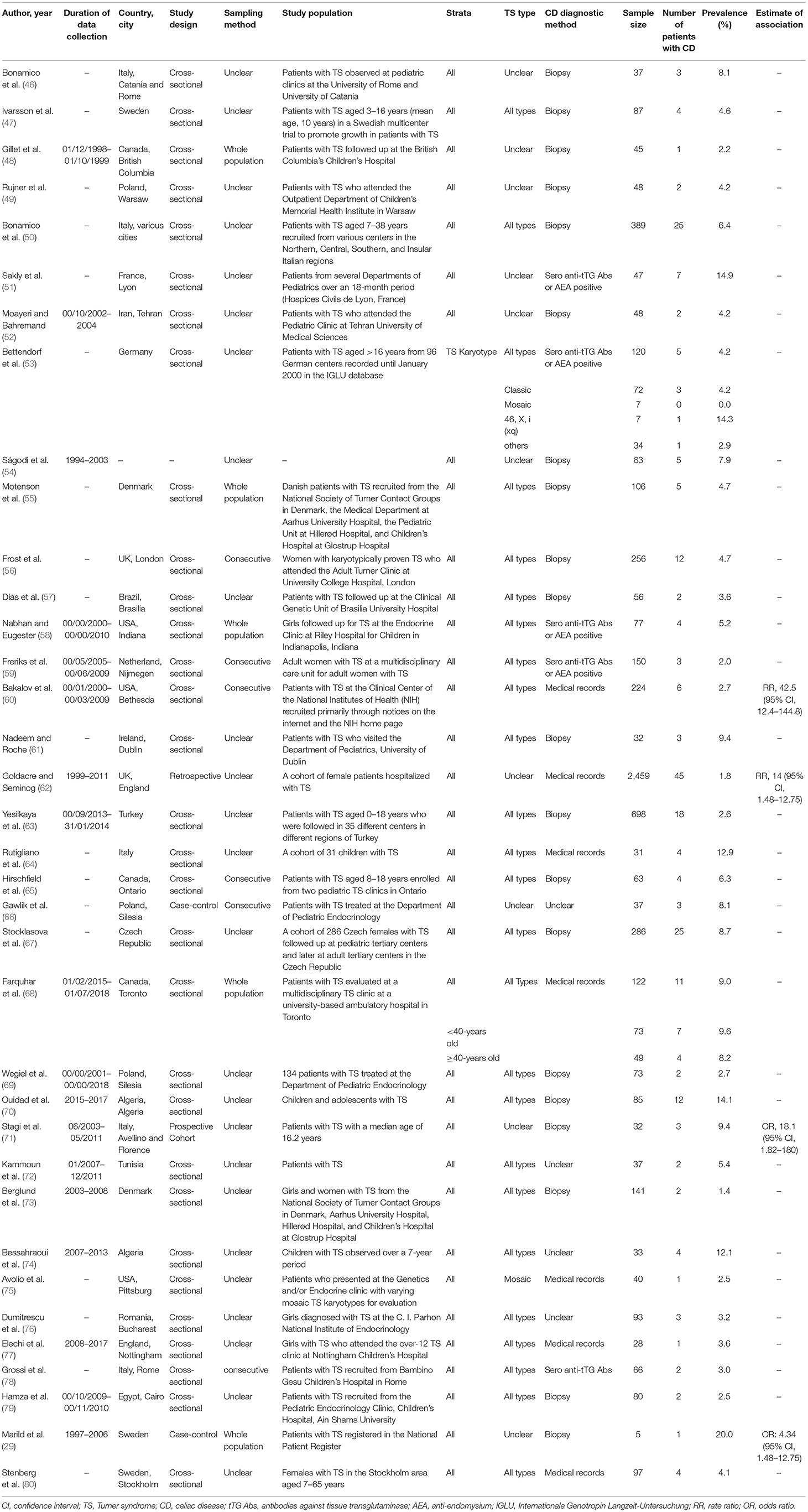
Table 1. Summary of studies reporting the prevalence of CD in patients with TS and/or association between TS and CD.
Only four research articles (29, 60, 62, 72) reported quantified or quantifiable information on the strength of association between TS and CD, with a heterogeneous study design and type of effect estimates.
The 40 studies that examined CD prevalence tested 6,291 patients with TS, yielding a crude CD prevalence of 3.8% (Table 2). The lowest CD prevalence of 0.0% was reported in a study of seven patients with mosaic TS in Germany (53), whereas the highest CD prevalence of 20.0% was reported in a study of 97 patients with TS registered in the National Patient Register in Sweden (80).
The estimated weighted CD prevalence was 4.5% (95% CI, 3.3–5.9, I2, 67.4%; Table 2 and Figure 2). The weighted CD prevalence was similar between studies that included <50 patients with TS (5.9%, 95% CI, 3.9–8.3, I2, 0.0%) and those that included ≥50 patients with TS (4.4%, 95% CI, 3.1–5.8, I2, 74.7%; Table 2). The analysis according to the CD diagnostic methods used revealed that the highest estimated weighted CD prevalence was obtained from five studies including “unclear” as a diagnostic method (6.8%, 95% CI, 3.1–5.9, I2, 26.2%), followed by an estimated weighted CD prevalence of 4.8% (95% CI, 3.4–6.5, I2, 55.3%) obtained from 20 studies using biopsy. The 95% CI of the CD prevalence according to the four CD diagnostic method categories was overlapping (Table 2).
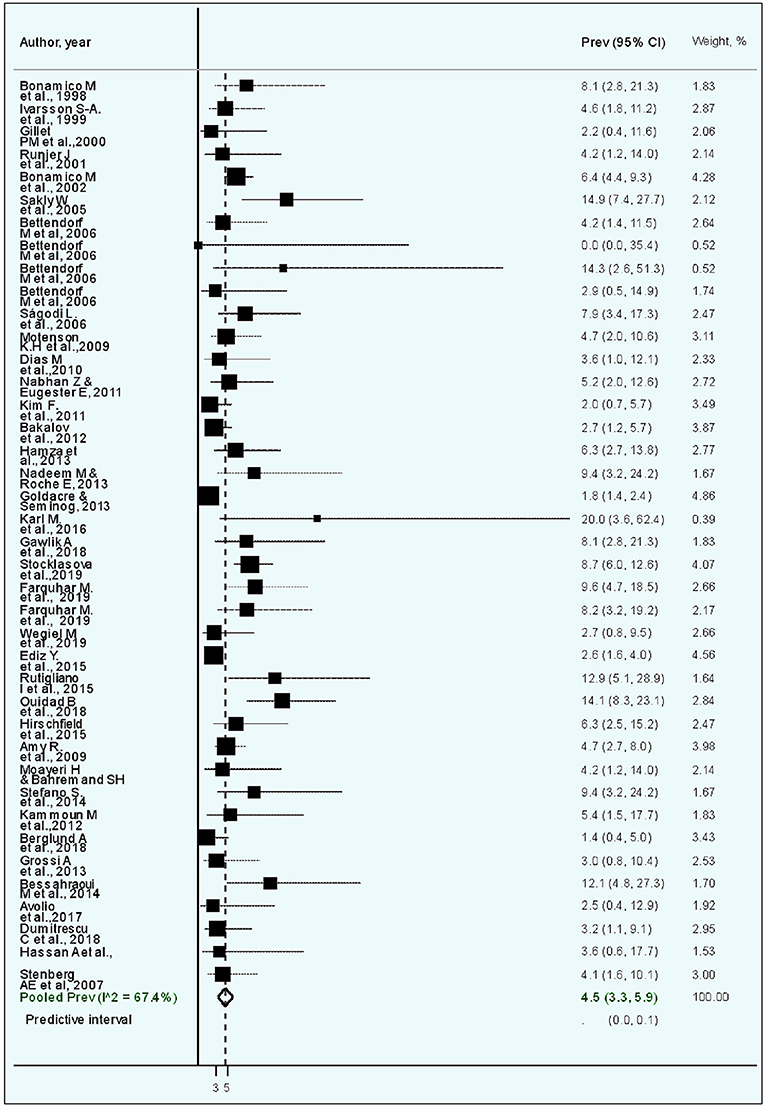
Figure 2. Forest plot of the meta-analysis of studies on celiac disease in patients with Turner syndrome. The diamond is centered on the summary prevalence estimate, and the width indicates the corresponding 95% confidence interval (CI).
In the univariate meta-regression model, only sample size exhibited a significant association with variability in CD prevalence. The CD prevalence was 40% lower in studies that included ≥50 patients with TS (odds ratio 0.60, p = 0.022) than in studies that included <50 patients with TS. The observed significance in variability remained in the meta-regression model adjusted for the CD diagnostic method (adjusted odds ratio 0.61, 95% CI, 0.39–0.97; Table 3).

Table 3. Univariate and multivariable meta-regression analyses to identify the sources of heterogeneity in studies reporting the prevalence of CD in patients with Turner syndrome based on different characteristics.
The statistical assessment (Egger's test, p < 0.001) of the funnel plot to determine the potential of publication bias due to the small-study effect suggested that there was asymmetry in the funnel plot, implicating the role of the small-study effect in CD prevalence (Figures 3A,B).
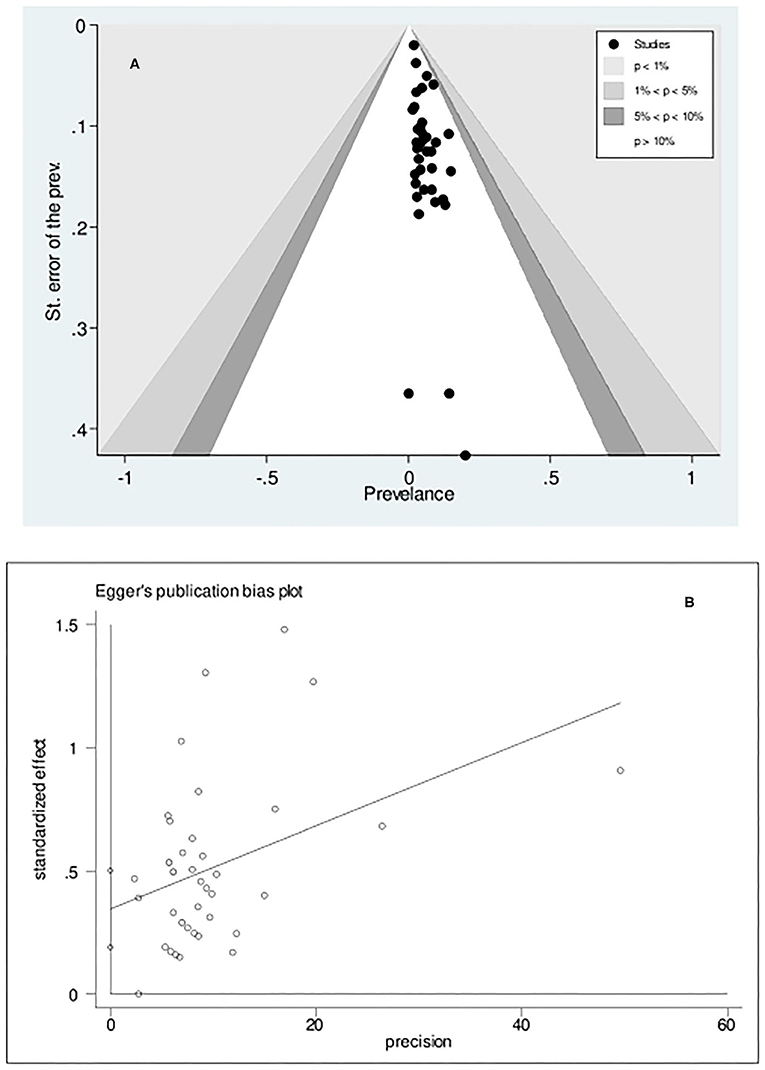
Figure 3. Contour-enhanced Funnel plot (A) and Egger's publication bias plot (B) examining small-study effects on the pooled celiac disease prevalence among patients with Turner syndrome. The estimated bias coefficient is 0.346 with a standard error of 0.077, indicating a p-value of <0.001.
Supplementary Table 2 and Supplementary Figure 1 provide the details of RoB assessment using the seven assessment criteria. Briefly, the study population was clearly specified and defined in 72.2% of the reviewed articles, the selection of the TS population from the same or similar populations was clearly mentioned in 75.0% of the articles, and the outcome of CD was clearly defined in 83.3% of the articles. The sample size justification and power calculation were not reported in 86.1% of the articles. Overall, more than half (55.6%) of the 36 articles were deemed to have low RoB based on at least five of the seven RoB assessment criteria. Of a maximum score of 7, the mean RoB score was 3.8 for the 36 reviewed articles.
The present systematic review and meta-analysis summarized the burden of CD in patients with TS by evaluating its prevalence. The systematic review included 36 research articles yielding 40 prevalence studies that included a total of 6,291 patients with TS. The meta-analysis revealed that the CD prevalence was 4.5% in patients with TS. The estimated CD burden in patients with TS in the present meta-analysis is similar to that reported by other meta-analyses of different subject cohorts with chronic conditions or genetic disorders, including patients with type 1 diabetes mellitus (5%, 95% CI, 3–7) (81), iron deficiency anemia (3.2%, 95% CI, 2.6–3.9) (82), and irritable bowel syndrome (6.13%, 95% CI, 4.11–9.05) (83). The quantified weighted CD prevalence based on serology and biopsy (3.4 and 4.8%, respectively) in patients with TS is 2.4- and 6.4-fold higher, respectively, than the recently estimated pooled global (1.4%) CD prevalence in the general population (15) and higher than the biopsy-confirmed CD in general populations in several countries including Australia (0.46%) (84), Cuba (0.5%) (85), Finland (2.1%) (86), Iran (0.3%) (87), India (1.1%) (88), United Kingdom (0.6%) (89), and Germany (0.4%) (90). To the best of our knowledge, this is the first study to report the global pooled CD prevalence in patients with TS.
Previous studies have considered patients with TS as a group at risk of developing CD (30, 37, 91), and the present study provides evidence on the susceptibility of patients with TS to the development of CD. This finding is supported by evidence presented in three individual studies that reported an increased risk of CD in patients with TS (60, 62, 71). However, there was a lack of uniformity in the reported measures of association, including relative risk, risk ratio, and odds ratio, and study design. Therefore, producing a weighted measure of association was unfeasible. Moreover, the results of our meta-analysis support the findings of a review by Lleo et al. (92), who indicated that one of the most prominent characteristics of patients with TS was increased susceptibility to autoimmune diseases. Short stature in patients with TS has been related to genetic, skeletal, and growth hormone secretion abnormalities (93). Given these genetic abnormalities, there is an accumulative evidence on the increased susceptibility of patients with TS to develop autoimmune diseases (26, 94, 95) including CD and on the association between CD and other autoimmune diseases such as thyroiditis (28). Although the exact underlying pathophysiological mechanism between TS and CD is still unclear (55), humoral and cellular immune responses (96–99) as well as genetic contribution (100, 101) such as the alteration in the expression of the X-linked FoxP3 gene (102) have been suggested.
At the light of results of more prevalent CD in patients with TS compared to general populations, a comprehensive autoimmune screening would be advised in patients with TS syndrome assessing autoantibodies that can show associated autoimmune diseases/disorders in TS-CD patients (103–105). This supported by the recently published recommendations by the TS Consensus Group (32) that has specifically addressed the diagnostic screening process and the management of several comorbidities including CD in TS in the childhood (33). Moreover, given that cutaneous stigmata can provide critical clues for early detection of TS (106) and the high prevalence of CD in patients with atopic dermatitis (107), then patients with TS presented with atopic dermatitis or with cutaneous stigmata should be prioritized for the early screening and detection of CD. Additionally, since CD patients are also susceptible to neurological manifestations (108, 109), screening for the anti-neuronal antibodies is also recommended to be assessed in the work-up of patients with TS. A study suggested that CD in patients with TS is responsible for a failure of growth hormone therapy (46), hence early screening and management of CD in patients with TS could improve treatment outcomes and controlling for other comorbidities. Evidence-based guidelines in the management of not only CD but also other autoimmune disorders in patients with TS is warranted.
The strength of this study is the inclusion of studies from four large databases, which yielded a substantial sample size of patients with TS (n = 6,291) screened for CD. The review of the articles and the extraction of data by independent reviewers contributed to reducing the potential human error. Extracting and pooling stratified CD prevalence estimates as well as subgroup analyses according to the CD diagnostic methods also provided more stringent and potentially less biased prevalence estimates. A further strength of this study is the identification of gaps in evidence, specifically the lack of data on the burden of CD in patients with TS from several countries worldwide.
Conversely, we acknowledge some limitations that should be considered when interpreting the findings. First, there was lack of uniformity in the CD diagnostic methods among the studies, with the highest prevalence of CD reported in studies with no clear CD diagnostic method, which might have led to over- or under-estimation of CD prevalence. Second, most studies were from Western countries, which might affect the generalizability of the results at the regional, sub-regional, and global levels. Third, the publication bias assessment showed an asymmetry of the funnel plot, which might be a result of the small-study effect.
In conclusion, ~1 in 22 patients with TS had CD. Regular screening of patients with TS for CD will facilitate the early identification of asymptomatic CD, with early and better intervention ultimately leading to improvements in case management and health outcomes. Further studies are needed from countries that lack data on the burden of CD among various patient populations at risk for CD, including those with TS.
The raw data supporting the conclusions of this article will be made available by the authors upon justifiable requests.
RH-A performed data analysis and interpretation. GA-B and AA drafted the manuscript. RH-A and SA-S critically reviewed the drafted manuscript. All authors conceptualized the study objectives and design and reviewed and approved the final submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.674896/full#supplementary-material
TS, Turner syndrome; CD, celiac disease, CI, confidence interval; RoB, Risk of bias.
1. Holtmeier W, Caspary WF. Celiac disease. Orphanet J Rare Dis. (2006) 1:3. doi: 10.1186/1750-1172-1-3
2. Taylor AK, Lebwohl B, Snyder CL, Green PHR. Celiac Disease. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A, editors. GeneReviews [Internet]. Seattle, WA: University of Washington, Seattle (2008).
3. La Vieille S, Pulido OM, Abbott M, Koerner TB, Godefroy S. Celiac disease and gluten-free oats: a Canadian position based on a literature review. Can J Gastroenterol Hepatol. (2016) 2016:1870305. doi: 10.1155/2016/1870305
4. Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr. (2014) 168:272–8. doi: 10.1001/jamapediatrics.2013.3858
5. Andren Aronsson C, Kurppa K, Agardh D. Gluten in infants and celiac disease risk. Expert Rev Gastroenterol Hepatol. (2016) 10:669–70. doi: 10.1586/17474124.2016.1169922
6. Kemppainen KM, Lynch KF, Liu E, Lonnrot M, Simell V, Briese T, et al. Factors that increase risk of celiac disease autoimmunity after a gastrointestinal infection in early life. Clin Gastroenterol Hepatol. (2017) 15:694–702 e5. doi: 10.1016/j.cgh.2016.10.033
8. Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nat Rev Immunol. (2013) 13:294–302. doi: 10.1038/nri3407
9. Constantin C, Huber WD, Granditsch G, Weghofer M, Valenta R. Different profiles of wheat antigens are recognised by patients suffering from coeliac disease and IgE-mediated food allergy. Int Arch Allergy Immunol. (2005) 138:257–66. doi: 10.1159/000088727
10. Laurikka P, Nurminen S, Kivela L, Kurppa K. Extraintestinal manifestations of celiac disease: early detection for better long-term outcomes. Nutrients. (2018) 10:1015. doi: 10.3390/nu10081015
11. Ludvigsson JF, Bai JC, Biagi F, Card TR, Ciacci C, Ciclitira PJ, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. (2014) 63:1210–28. doi: 10.1136/gutjnl-2013-306578
12. Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. (2012) 54:136–60. doi: 10.1097/MPG.0b013e31821a23d0
13. Clappison E, Hadjivassiliou M, Zis P. Psychiatric manifestations of coeliac disease, a systematic review and meta-analysis. Nutrients. (2020) 12:142. doi: 10.3390/nu12010142
14. Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. (2015) 3:151–5. doi: 10.12691/ijcd-3-4-8
15. Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2018) 16:823–36.e2. doi: 10.1016/j.cgh.2017.06.037
16. Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. (2009) 137:88–93. doi: 10.1053/j.gastro.2009.03.059
17. Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. (2007) 26:1217–25. doi: 10.1111/j.1365-2036.2007.03502.x
18. van den Broeck HC, de Jong HC, Salentijn EM, Dekking L, Bosch D, Hamer RJ, et al. Presence of celiac disease epitopes in modern and old hexaploid wheat varieties: wheat breeding may have contributed to increased prevalence of celiac disease. Theor Appl Genet. (2010) 121:1527–39. doi: 10.1007/s00122-010-1408-4
19. Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol. (2013) 10:383–92. doi: 10.1038/cmi.2013.28
20. Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. (2014) 14:194. doi: 10.1186/s12876-014-0194-x
21. Mearin ML. The prevention of coeliac disease. Best Pract Res Clin Gastroenterol. (2015) 29:493–501. doi: 10.1016/j.bpg.2015.04.003
22. Aggarwal S, Lebwohl B, Green PH. Screening for celiac disease in average-risk and high-risk populations. Therap Adv Gastroenterol. (2012) 5:37–47. doi: 10.1177/1756283X11417038
23. Richey R, Howdle P, Shaw E, Stokes T, Guideline development G. Recognition and assessment of coeliac disease in children and adults: summary of NICE guidance. BMJ. (2009) 338:b1684. doi: 10.1136/bmj.b1684
24. Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. (2005) 40:1–19. doi: 10.1097/00005176-200501000-00001
25. Moore CC, Grumbach MM. Sex determination and gonadogenesis: a transcription cascade of sex chromosome and autosome genes. Semin Perinatol. (1992) 16:266–78.
26. De Sanctis V, Khater D. Autoimmune diseases in Turner syndrome: an overview. Acta Biomed. (2019) 90:341–4. doi: 10.23750/abm.v90i3.8737
27. Witkowska-Sedek E, Borowiec A, Kucharska A, Chacewicz K, Ruminska M, Demkow U, et al. Thyroid autoimmunity in girls with turner syndrome. Adv Exp Med Biol. (2017) 1022:71–6. doi: 10.1007/5584_2017_42
28. Volta U, Ravaglia G, Granito A, Forti P, Maioli F, Petrolini N, et al. Coeliac disease in patients with autoimmune thyroiditis. Digestion. (2001) 64:61–5. doi: 10.1159/000048840
29. Marild K, Stordal K, Hagman A, Ludvigsson JF. Turner syndrome and celiac disease: a case-control study. Pediatrics. (2016) 137:e20152232. doi: 10.1542/peds.2015-2232
30. Hill ID, Fasano A, Guandalini S, Hoffenberg E, Levy J, Reilly N, et al. NASPGHAN clinical report on the diagnosis and treatment of gluten-related disorders. J Pediatr Gastroenterol Nutr. (2016) 63:156–65. doi: 10.1097/MPG.0000000000001216
31. Husby S, Koletzko S, Korponay-Szabo I, Kurppa K, Mearin ML, Ribes-Koninckx C, et al. European Society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. (2020) 70:141–56. doi: 10.1097/MPG.0000000000002497
32. Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. (2017) 177:G1–G70. doi: 10.1530/EJE-17-0430
33. Shankar RK, Backeljauw PF. Current best practice in the management of Turner syndrome. Ther Adv Endocrinol Metab. (2018) 9:33–40. doi: 10.1177/2042018817746291
34. Force USPST, Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, et al. Screening for celiac disease: US Preventive Services Task Force Recommendation Statement. JAMA. (2017) 317:1252–7. doi: 10.1001/jama.2017.1462
35. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
36. Al-Bluwi GSM, Alnababteh AH, Al-Shamsi S, Al-Rifai RH. Strength of the association between Turner syndrome and coeliac disease: protocol for a systematic review and meta-analysis. BMJ Open. (2020) 10:e037478. doi: 10.1136/bmjopen-2020-037478
37. Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of paediatric gastroenterology and nutrition. Arch Dis Child. (1990) 65:909–11. doi: 10.1136/adc.65.8.909
38. Veritas Health Innovation. Covidence Systematic Review Software. (2021). Available online at: https://www.covidence.org
39. Tukey MFFaJW. Transformations related to the angular and the square root. Ann Math Stat. (1950) 21:607–6011. doi: 10.1214/aoms/1177729756
40. Miller JJ. The inverse of the Freeman - Tukey Double Arcsine Transformation. Am Stat. (1978) 32:138. doi: 10.2307/2682942
41. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. New York, NY: John Wiley & Sons, Ltd. (2009).
42. National Institutes of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. (2014). Available online at: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (accessed January, 2021).
43. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. (2001) 54:1046–55. doi: 10.1016/S0895-4356(01)00377-8
44. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. (2014) 72:39. doi: 10.1186/2049-3258-72-39
46. Bonamico M, Bottaro G, Pasquino AM, Caruso-Nicoletti M, Mariani P, Gemme G, et al. Celiac disease and Turner syndrome. J Pediatr Gastroenterol Nutr. (1998) 26:496–9. doi: 10.1097/00005176-199805000-00002
47. Ivarsson SA, Carlsson A, Bredberg A, Alm J, Aronsson S, Gustafsson J, et al. Prevalence of coeliac disease in Turner syndrome. Acta Paediatr. (1999) 88:933–6. doi: 10.1111/j.1651-2227.1999.tb00184.x
48. Gillett PM, Gillett HR, Israel DM, Metzger DL, Stewart L, Chanoine JP, et al. Increased prevalence of celiac disease in girls with Turner syndrome detected using antibodies to endomysium and tissue transglutaminase. Can J Gastroenterol. (2000) 14:915–8. doi: 10.1155/2000/172914
49. Rujner J, Wisniewski A, Gregorek H, Wozniewicz B, Mlynarski W, Witas HW. Coeliac disease and HLA-DQ 2 (DQA1* 0501 and DQB1* 0201) in patients with Turner syndrome. J Pediatr Gastroenterol Nutr. (2001) 32:114–5. doi: 10.1097/00005176-200101000-00033
50. Bonamico M, Pasquino AM, Mariani P, Danesi HM, Culasso F, Mazzanti L, et al. Prevalence and clinical picture of celiac disease in Turner syndrome. J Clin Endocrinol Metab. (2002) 87:5495–8. doi: 10.1210/jc.2002-020855
51. Sakly W, Bienvenu F, Peretti N, Lachaux A, Morel S, Bouvier R, et al. IgA anti-transglutaminase antibodies as a tool for screening atypical forms of coeliac disease in a French at-risk paediatric population. Eur J Gastroenterol Hepatol. (2005) 17:235–9. doi: 10.1097/00042737-200502000-00016
52. Bahremand HMSH. Prevalence of celiac disease in patients with turner's syndrome. Acta Med Iran. (2005) 43:287–90.
53. Bettendorf M, Doerr HG, Hauffa BP, Lindberg A, Mehls O, Partsch CJ, et al. Prevalence of autoantibodies associated with thyroid and celiac disease in Ullrich-Turner syndrome in relation to adult height after growth hormone treatment. J Pediatr Endocrinol Metab. (2006) 19:149–54. doi: 10.1515/JPEM.2006.19.2.149
54. Sagodi L, Solyom E, Tamasi K, Minik K. [Prevalence of celiac disease in Turner syndrome]. Orv Hetil. (2006) 147:1185–8.
55. Mortensen KH, Cleemann L, Hjerrild BE, Nexo E, Locht H, Jeppesen EM, et al. Increased prevalence of autoimmunity in Turner syndrome–influence of age. Clin Exp Immunol. (2009) 156:205–10. doi: 10.1111/j.1365-2249.2009.03895.x
56. Frost AR, Band MM, Conway GS. Serological screening for coeliac disease in adults with Turner's syndrome: prevalence and clinical significance of endomysium antibody positivity. Eur J Endocrinol. (2009) 160:675–9. doi: 10.1530/EJE-08-0846
57. Dias Mdo C, Castro LC, Gandolfi L, Almeida RC, Cordoba MS, Pratesi R. Screening for celiac disease among patients with Turner syndrome in Brasilia, DF, midwest region of Brazil. Arq Gastroenterol. (2010) 47:246–9. doi: 10.1590/S0004-28032010000300007
58. Nabhan ZM, Eugster EA. Medical care of girls with Turner Syndrome: where are we lacking? Endocr Pract. (2011) 17:747–52. doi: 10.4158/EP11059.OR
59. Freriks K, Timmermans J, Beerendonk CC, Verhaak CM, Netea-Maier RT, Otten BJ, et al. Standardized multidisciplinary evaluation yields significant previously undiagnosed morbidity in adult women with Turner syndrome. J Clin Endocrinol Metab. (2011) 96:E1517–26. doi: 10.1210/jc.2011-0346
60. Bakalov VK, Gutin L, Cheng CM, Zhou J, Sheth P, Shah K, et al. Autoimmune disorders in women with turner syndrome and women with karyotypically normal primary ovarian insufficiency. J Autoimmun. (2012) 38:315–21. doi: 10.1016/j.jaut.2012.01.015
61. Nadeem M, Roche EF. Coeliac disease in Turner syndrome. Arch Dis Child. (2013) 98:649–50. doi: 10.1136/archdischild-2013-304126
62. Goldacre MJ, Seminog OO. Turner syndrome and autoimmune diseases: record-linkage study. Arch Dis Child. (2014) 99:71–3. doi: 10.1136/archdischild-2013-304617
63. Yesilkaya E, Bereket A, Darendeliler F, Bas F, Poyrazoglu S, Kucukemre Aydin B, et al. Turner syndrome and associated problems in Turkish children: a multicenter study. J Clin Res Pediatr Endocrinol. (2015) 7:27–36. doi: 10.4274/jcrpe.1771
64. Rutigliano M, D'Altilia M., Pastore L., Romaniello A., Pacilio S., Cringoli, et al. Spectrum of autoimmunity in celiac disease. Digest Liver Dis. (2015) 47:e268–e9. doi: 10.1016/j.dld.2015.07.135
65. Hirschfield GM, McAssey K, Milkiewicz P, Heathcote EJ, Hamilton J. Prospective evaluation of liver biochemistry in children with turner syndrome: high prevalence of abnormalities associated with celiac disease and insulin resistance. Hepatology. (2008) 48:1038A. Available online at: https://experts.mcmaster.ca/display/publication606533
66. Gawlik AM, Berdej-Szczot E, Blat D, Klekotka R, Gawlik T, Blaszczyk E, et al. immunological profile and predisposition to autoimmunity in girls with Turner Syndrome. Front Endocrinol (Lausanne). (2018) 9:307. doi: 10.3389/fendo.2018.00307
67. Stoklasova J, Zapletalova J, Frysak Z, Hana V, Cap J, Pavlikova M, et al. An isolated Xp deletion is linked to autoimmune diseases in Turner syndrome. J Pediatr Endocrinol Metab. (2019) 32:479–88. doi: 10.1515/jpem-2019-0067
68. Farquhar M, Jacobson M, Braun C, Wolfman W, Kelly C, Allen LM, et al. Medical and gynecological comorbidities in adult women with Turner syndrome: our multidisciplinary clinic experience. Climacteric. (2020) 23:32–7. doi: 10.1080/13697137.2019.1627315
69. Wegiel M, Antosz A, Gieburowska J, Szeliga K, Hankus M, Grzybowska-Chlebowczyk U, et al. Autoimmunity predisposition in girls with Turner Syndrome. Front Endocrinol (Lausanne). (2019) 10:511. doi: 10.3389/fendo.2019.00511
70. Ouidad B, Mourad S, Samia S, Safiaa MZ. Coeliac disease in Turner Syndrome more frequent than expected. Eur Soc Paediatr Endocrinol. (2018) 89:3–223. Available online at: https://abstracts.eurospe.org/hrp/0089/hrp0089p3-p223 (accessed January, 2021).
71. Stagi S, Lapi E, D'Avanzo MG, Perferi G, Romano S, Giglio S, et al. Coeliac disease and risk for other autoimmune diseases in patients with Williams-Beuren syndrome. BMC Med Genet. (2014) 15:61. doi: 10.1186/1471-2350-15-61
72. Kammoun M, Mougou S, Brahem R, Ghali N, Bel Haj Hmida I, Dimassi S, et al. TURNER SYNDROME: A CLINICO-CYTOGENETIC STUDY OF 37 CHILDREN. Arch Dis Childhood. (2012) 97(Suppl. 2):A211. doi: 10.1136/archdischild-2012-302724.0732
73. Berglund A, Cleemann L, Oftedal BE, Holm K, Husebye ES, Gravholt CH. 21-hydroxylase autoantibodies are more prevalent in Turner syndrome but without an association to the autoimmune polyendocrine syndrome type I. Clin Exp Immunol. (2019) 195:364–8. doi: 10.1111/cei.13231
74. Bessahraoui M, Naceur M, Niar S, Zennaki A, Arbi F, Ousaleh M, et al. Descriptive analyses of Turner Syndrome. Horm Res Paediatr. (2014) 82:3–872. Available online at: https://abstracts.eurospe.org/hrp/0082/hrp0082p3-d3-872 (accessed January, 2021).
75. Avolio M, Witchel SF, Yatsenko SA. The genotypic and phenotypic diversity of mosaic turner syndrome (TS). Horm Res Paediatr. (2017) 88(Suppl. 1):1–628. Available online at: https://regroup-production.s3.amazonaws.com/documents/ReviewReference/169353558/The%20genotypic%20and%20phenotypic%20diversity%20of%20mosaic%20turner%20syndrome%20%28TS%29.pdf?AWSAccessKeyId=AKIAJBZQODCMKJA4H7DA&Expires=1622979680&Signature=IYDO1K0ybYNagUJmdwniJHro%2B28%3D (accessed January, 2021).
76. Dumitrescua C, Gherlan I, Radomir L, Vintila M, Brehar A, Caragheorgheopol A, et al. Turner syndrome and autoimmune thyroid disease: pecularities of evolution in 93 Turner syndrome patients. In: 57th Annual European Society for Paediatric Endocrinology. Athens (2018).
77. Elechi HA, James Law, Jacqui Alexander, Loiuse Denvir, Tabitha Randell, Sachdev P. Turners syndrome - clinical presentation, genetics, investigation and management: a 10-year review. Eur Soc Paediatr Endocrinol. (2019) 92:3–155. doi: 10.1530/endoabs.58.P014
78. Grossi A, Crino A, Luciano R, Lombardo A, Cappa M, Fierabracci A. Endocrine autoimmunity in Turner syndrome. Ital J Pediatr. (2013) 39:79. doi: 10.1186/1824-7288-39-79
79. Hamza RT, Raof NA, Abdallah KO. Prevalence of multiple forms of autoimmunity in Egyptian patients with Turner syndrome: relation to karyotype. J Pediatr Endocrinol Metab. (2013) 26:545–50. doi: 10.1515/jpem-2012-0265
80. Stenberg AE, Sylven L, Hedstrand H, Kampe O, Hultcrantz M. Absence of autoantibodies connected to autoimmune polyendocrine syndrome type I and II and Addison's disease in girls and women with Turner syndrome. J Negat Results Biomed. (2007) 6:10. doi: 10.1186/1477-5751-6-10
81. Gheshlagh RG, Rezaei H, Goli M, Ausili D, Dalvand S, Ghafouri H, et al. Prevalence of celiac disease in Iranian patients with type 1 diabetes: a systematic review and meta-analysis. Indian J Gastroenterol. (2020) 39:419–25. doi: 10.1007/s12664-020-01046-7
82. Mahadev S, Laszkowska M, Sundstrom J, Bjorkholm M, Lebwohl B, Green PHR, et al. Prevalence of celiac disease in patients with iron deficiency anemia-a systematic review with meta-analysis. Gastroenterology. (2018) 155:374–82.e1. doi: 10.1053/j.gastro.2018.04.016
83. Azami M, Badfar G, Abangah G, Mahmoudi L. Celiac disease in Iranian irritable bowel syndrome patients; a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. (2019) 12:85–97.
84. Chin MW, Mallon DF, Cullen DJ, Olynyk JK, Mollison LC, Pearce CB. Screening for coeliac disease using anti-tissue transglutaminase antibody assays, and prevalence of the disease in an Australian community. Med J Aust. (2009) 190:429–32. doi: 10.5694/j.1326-5377.2009.tb02491.x
85. Galvan JA, Lemos G, Fernandez de Cossio ME, Ruenes C, Martinez Y, Tejeda Y, et al. Silent celiac disease in a cohort of healthy adults. Autoimmunity. (2009) 42:705–8. doi: 10.3109/08916930903214009
86. Vilppula A, Collin P, Maki M, Valve R, Luostarinen M, Krekela I, et al. Undetected coeliac disease in the elderly: a biopsy-proven population-based study. Dig Liver Dis. (2008) 40:809–13. doi: 10.1016/j.dld.2008.03.013
87. Akbari MR, Mohammadkhani A, Fakheri H, Javad Zahedi M, Shahbazkhani B, Nouraie M, et al. Screening of the adult population in Iran for coeliac disease: comparison of the tissue-transglutaminase antibody and anti-endomysial antibody tests. Eur J Gastroenterol Hepatol. (2006) 18:1181–6. doi: 10.1097/01.meg.0000224477.51428.32
88. Makharia GK, Verma AK, Amarchand R, Bhatnagar S, Das P, Goswami A, et al. Prevalence of celiac disease in the northern part of India: a community based study. J Gastroenterol Hepatol. (2011) 26:894–900. doi: 10.1111/j.1440-1746.2010.06606.x
89. El-Hadi S, Tuthill D, Lewis E, Adisesh A, Moody M, Fifield R, et al. Unrecognised coeliac disease is common in healthcare students. Arch Dis Childhood. (2004) 89:842. doi: 10.1136/adc.2003.041459
90. Kratzer W, Kibele M, Akinli A, Porzner M, Boehm BO, Koenig W, et al. Prevalence of celiac disease in Germany: a prospective follow-up study. World J Gastroenterol. (2013) 19:2612–20. doi: 10.3748/wjg.v19.i17.2612
91. Downey L, Houten R, Murch S, Longson D. Recognition, assessment, and management of coeliac disease: summary of updated NICE guidance. BMJ. (2015) 351:h4513. doi: 10.1136/bmj.h4513
92. Lleo A, Moroni L, Caliari L, Invernizzi P. Autoimmunity and Turner's syndrome. Autoimmun Rev. (2012) 11:A538–A43. doi: 10.1016/j.autrev.2011.11.015
93. Cianfarani S, Vaccaro F, Pasquino AM, Marchione SA, Passeri F, Spadoni GL, et al. Reduced growth hormone secretion in Turner syndrome: is body weight a key factor? Horm Res. (1994) 41:27–32. doi: 10.1159/000183873
94. Mohamed SOO, Elkhidir IHE, Abuzied AIH, Noureddin A, Ibrahim GAA, Mahmoud AAA. Prevalence of autoimmune thyroid diseases among the Turner Syndrome patients: meta-analysis of cross sectional studies. BMC Res Notes. (2018) 11:842. doi: 10.1186/s13104-018-3950-0
95. Jørgensen KT, Rostgaard K, Bache I, Biggar RJ, Nielsen NM, Tommerup N, et al. Autoimmune diseases in women with Turner's syndrome. Arthritis Rheum. (2010) 62:658–66. doi: 10.1002/art.27270
96. Gupta S, Chiplunkar S, Gupta A, Gollapudi S. Increased spontaneous, tumor necrosis factor receptor- and CD95 (Fas)-mediated apoptosis in cord blood T-cell subsets from Turner's syndrome. Genes Immun. (2003) 4:239–43. doi: 10.1038/sj.gene.6363945
97. Robson SC, Potter PC. Common variable immunodeficiency in association with Turner's syndrome. J Clin Lab Immunol. (1990) 32:143–6.
98. al-Attas RA, Rahi AH, Ahmed el FE. Common variable immunodeficiency with CD4+ T lymphocytopenia and overproduction of soluble IL-2 receptor associated with Turner's syndrome and dorsal kyphoscoliosis. J Clin Pathol. (1997) 50:876–9. doi: 10.1136/jcp.50.10.876
99. Hochberg Z, Aviram M, Rubin D, Pollack S. Decreased sensitivity to insulin-like growth factor I in Turner's syndrome: a study of monocytes and T lymphocytes. Eur J Clin Invest. (1997) 27:543–7. doi: 10.1046/j.1365-2362.1997.1640702.x
100. El-Mansoury M, Bryman I, Berntorp K, Hanson C, Wilhelmsen L, Landin-Wilhelmsen K. Hypothyroidism is common in turner syndrome: results of a five-year follow-up. J Clin Endocrinol Metab. (2005) 90:2131–5. doi: 10.1210/jc.2004-1262
101. Elsheikh M, Wass JA, Conway GS. Autoimmune thyroid syndrome in women with Turner's syndrome–the association with karyotype. Clin Endocrinol (Oxf). (2001) 55:223–6. doi: 10.1046/j.1365-2265.2001.01296.x
102. Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. (2007) 3:619–26. doi: 10.1038/ncprheum0624
103. Volta U, De Giorgio R, Petrolini N, Stangbellini V, Barbara G, Granito A, et al. Clinical findings and anti-neuronal antibodies in coeliac disease with neurological disorders. Scand J Gastroenterol. (2002) 37:1276–81. doi: 10.1080/003655202761020542
104. Volta U, Rodrigo L, Granito A, Petrolini N, Muratori P, Muratori L, et al. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol. (2002) 97:2609–13. doi: 10.1111/j.1572-0241.2002.06031.x
105. Volta U, De Giorgio R, Granito A, Stanghellini V, Barbara G, Avoni P, et al. Anti-ganglioside antibodies in coeliac disease with neurological disorders. Dig Liver Dis. (2006) 38:183–7. doi: 10.1016/j.dld.2005.11.013
106. Lowenstein EJ, Kim KH, Glick SA. Turner's syndrome in dermatology. J Am Acad Dermatol. (2004) 50:767–76. doi: 10.1016/j.jaad.2003.07.031
107. Zauli D, Grassi A, Granito A, Foderaro S, De Franceschi L, Ballardini G, et al. Prevalence of silent coeliac disease in atopics. Dig Liver Dis. (2000) 32:775–9. doi: 10.1016/S1590-8658(00)80354-0
108. Cervio E, Volta U, Verri M, Boschi F, Pastoris O, Granito A, et al. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology. (2007) 133:195–206. doi: 10.1053/j.gastro.2007.04.070
Keywords: celiac disease, Turner syndrome, systematic review, weighted prevalence, meta-analysis
Citation: Al-Bluwi GSM, AlNababteh AH, Östlundh L, Al-Shamsi S and Al-Rifai RH (2021) Prevalence of Celiac Disease in Patients With Turner Syndrome: Systematic Review and Meta-Analysis. Front. Med. 8:674896. doi: 10.3389/fmed.2021.674896
Received: 02 March 2021; Accepted: 26 May 2021;
Published: 17 June 2021.
Edited by:
Alessandro Granito, University of Bologna, ItalyReviewed by:
Zsolt Szakács, University of Pécs, HungaryCopyright © 2021 Al-Bluwi, AlNababteh, Östlundh, Al-Shamsi and Al-Rifai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rami H. Al-Rifai, cnJpZmFpQHVhZXUuYWMuYWU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.