- 1Department of Respiratory Medicine (Department of Respiratory and Critical Care Medicine), Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Critical Care Medicine, Xiangya Hospital, Central South University, Changsha, China
- 4Department of Rheumatology and Immunology, Xiangya Hospital, Central South University, Changsha, China
Granulomatosis with polyangiitis (GPA) is a subtype of anti-neutrophil cytoplasmic antibody-associated vasculitis with a wide range of clinical symptoms related to the systemic involvement of small blood vessels. The respiratory system is one of the most frequently involved, and life-threatening acute respiratory failure could occur due to diffusive alveolar hemorrhage and tracheal stenosis. When maximum mechanical ventilation is unable to maintain oxygenation, extracorporeal membrane oxygenation (ECMO) should be considered as the final respiratory supportive method, if available. Here we present a 32-year-old male patient with acute respiratory failure (ARF) related to GPA, who was rescued by winning time for accurate diagnosis and appropriate treatment. Additionally, we reviewed more than 60 GPA-related ARF cases on multiple online databases, summarized the clinical manifestations of these patients, and concluded that ECMO plays an important role in further respiratory support for ARF patients with GPA and assists in accurate and timely diagnosis and appropriate treatment, thus helping them recuperate.
Introduction
Granulomatosis with polyangiitis (GPA), formerly termed Wegener's granulomatosis, is the most common pathogenesis of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and highly relate to PR3-ANCA (1). The diversiform characteristics of GPA can refer to almost all body systems, particularly the lungs and kidneys (2). Pulmonary hemorrhagic nephritis syndrome is the most frequent cause of acute respiratory failure (ARF), and intensive care unit (ICU) supervision with advanced respiratory support and renal replacement treatment is necessary to help those patients recuperate (3, 4).
Extracorporeal membrane oxygenation (ECMO) is the final respiratory support technique for severe acute respiratory distress syndrome (ARDS) after maximum mechanical ventilation fails to provide optimal oxygen levels in the blood (5, 6). Although systemic anticoagulation is required during ECMO, with the advancement of technology, diffuse alveolar hemorrhage (DAH) has become a potential indication of ECMO as well.
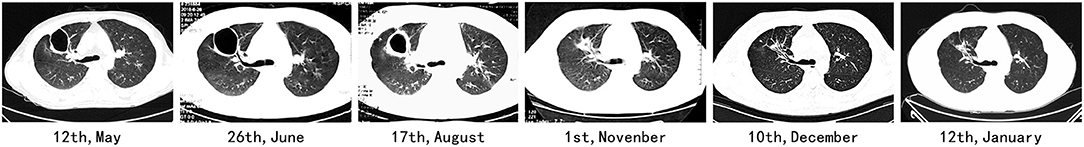
According to a literature review, only the respiratory system is involved in patients with GPA, and ARF at the onset of symptoms is rare (7). Here we report a case of GPA with ARF at the onset of the disease. The disease progressed from shortness of breath to severe respiratory failure in 3 weeks, and maximum mechanical ventilation could not maintain oxygenation. The patient eventually recovered after venovenous extracorporeal membrane oxygenation (VV-ECMO), plasmapheresis, and immunosuppressive treatment (Figure 1). Within 2 years of follow-up, the patient showed a great prognosis.
In this study, we also reviewed 60 cases of ARF caused by GPA as published in academic journals; among this series, 22 patients recovered successfully after ECMO support.
Case Report
On March 13, 2018, a 32-year-old male patient was admitted to the respiratory acute ICU (RICU) at our hospital owing to cough, expectoration, dyspnea for 3 weeks, and fever for 1 week. At 3 weeks before admission, he developed a dry cough and fever sequentially, and the fever was irregular with the highest temperature of 38.0°C, and it was difficult to get a reduction through centrifugation. In a local hospital, a computed tomography (CT) scan showed multiple shadows, and physicians prescribed demethylvancomycin, meropenem, and oseltamivir based on an empirical diagnosis of community-acquired pneumonia (CAP). However, the anti-microorganism treatment was ineffective, and the patient progressed into expectorating blood-stained sputum and dyspnea. For improved treatment, the patient was transferred to the emergency department on March 9. Before he became ill, he worked as a metal mechanical processing engineer for 8 years and had a history of metallic dust and chemical aerosol inhalation. He also had a smoking history of 1.25 pack-years.
After admission to our hospital, the patient reported aggravation of dyspnea with white mucus sputum. Physical examination showed that his body temperature was between 38.0 and 39.0°C without downtrend; the respiratory rate was 30 times/min, auscultation demonstrated only a reduction of breath sound at both sides of the lung, and no rales were revealed. There were no hemorrhagic spots or rashes on the skin. Laboratory examinations showed elevated procalcitonin, C-reactive protein, and erythrocyte sedimentation rate. On CT re-scan in this patient, multiple cavities emerged in his right lung. An empirical diagnosis of severe CAP was established. On estimation, methicillin-resistant Staphylococcus aureus or fungus was the most probable pathogen; thus, the patient was treated using linezolid, voriconazole, and levofloxacin. However, the patient continued to develop dyspnea and hyperpyrexia, indicating the probability of incorrect diagnosis. Simultaneously, the patient's blood gas analysis revealed a type I respiratory failure, with an oxygenation index of approximately 100. Although high-flow mask oxygen inhalation and non-invasive positive pressure ventilation (NIPPV) were administered sequentially, the blood oxygenation worsened, and the patient was transferred to our RICU on March 8. Considering that NIPPV did not improve oxygenation, a bedside chest film revealed consolidation in the lungs bilaterally, and the oxygenation index dropped to 60. Hence, bedside bronchoscope-guided intubation and invasive ventilation were performed. Although maximum ventilatory support was provided under sedation and analgesia, the oxygenation index did not show any improvement at 5 h later. Based on the small amount of hemorrhagic secretion in the trachea under bronchoscopy and consolidation of the gravity areas of the lower lungs by critical ultrasonic imaging, DAH was suspected, and more advanced life support systems were planned. Thus, our team performed VV-ECMO through the internal jugular and femoral veins. The ventilator was changed to synchronized intermittent mandatory ventilation sequentially, and blood gas analysis improved immediately (Table 1). Thus, the diagnosis of potential connective tissue diseases such as pulmonary vasculitis was made.
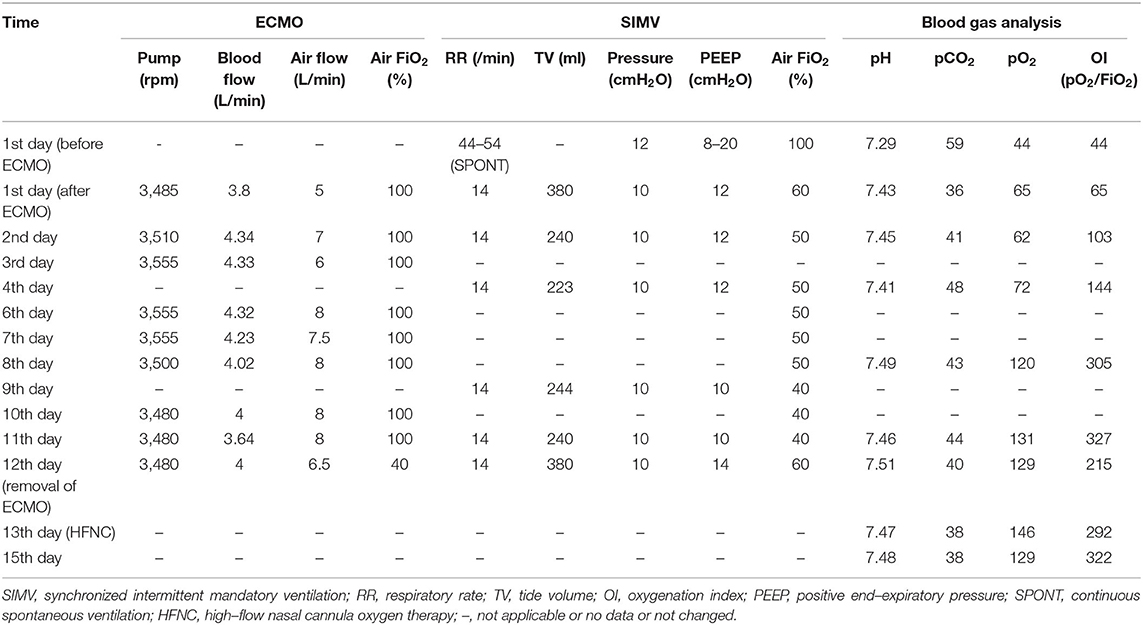
Table 1. Extracorporeal membrane oxygenation (ECMO) and SIMV parameters and results of arterial blood gas analysis.
On the day after ECMO was established, the patient's immune disorder-related test results were back, with negative anti-nucleic antibody and anti-dsDNA antibody and positive cytoplasmic anti-neutrophil cytoplasmic antibody (c-ANCA) and proteinase3 (PR3) antibodies; we confirmed the patient's diagnosis as GPA. Considering medical images, airway hemorrhagic secretion, and results of positive autoantibodies, the ARF of this patient was suspected to be due to DAH. Immunosuppressive therapy immediately replaced the antibiotics. Methylprednisolone impact therapy (March 16–18, 500 mg Qd) and maintenance therapy (March 20 and later, 40 mg Qd), cyclophosphamide (0.2 g, Qod), and plasmapheresis (March 19, 21, and 27; three times in total) were administered. Subsequently, the oxygenation and lung conditions of this patient gradually improved (Figure 1, Table 1). On the 12th day after ECMO, the latter was removed after interrupting the oxygen source of ECMO for 4 h. At 1 day later, intubation was removed after evaluation. High-flow nasal cannula oxygen therapy (HFNC) was observed to be sufficient to maintain oxygenation. On April 3, the patient was transferred to the rheumatology department and treated for another 4 days. On April 6, the patient was discharged in a good respiratory condition.
During follow-up, methylprednisolone and cyclophosphamide were maintained, and the dosages were gradually reduced, and the patient showed a stable condition. Multiple CT scans showed diminishing cavities; however, fibrosis persisted (Figure 2).
Methods
Data Collection
A literature search was performed in MEDLINE/PUBMED, Embase, and Scopus databases using the terms, “respiratory failure,” “Wegener's granulomatosis,” and “granulomatosis with polyangiitis.” The article type was limited to case reports when eligible literature was collected from Embase. Furthermore, wild retrieval was performed through Google Scholar. All conference papers and reports that were not in English were excluded, owing to a lack of medical details. By reading the abstract and full text, we collected data on patients with respiratory failure related to GPA, and the inclusion criteria were as follows: (1) diagnosed with GPA, (2) acute respiratory failure and mechanical ventilation, and (3) the patient's medical history was described in detail. We reviewed the basic information, clinical characteristics, laboratory results, radiology characteristics, ECMO application and type, treatment, and prognosis among these patients.
Statistical Analysis
We used the Wilcoxon rank-sum test to assess the statistical difference in the age between patients who were administered ECMO or not. We also used Fisher's exact test to examine differences of other clinical characteristics between the two groups. Patients who lacked specific items were excluded when performing statistical analyses on relative items. P < 0.05 was considered significant. All the analyses were performed in R software (version 3.6.3).
Results
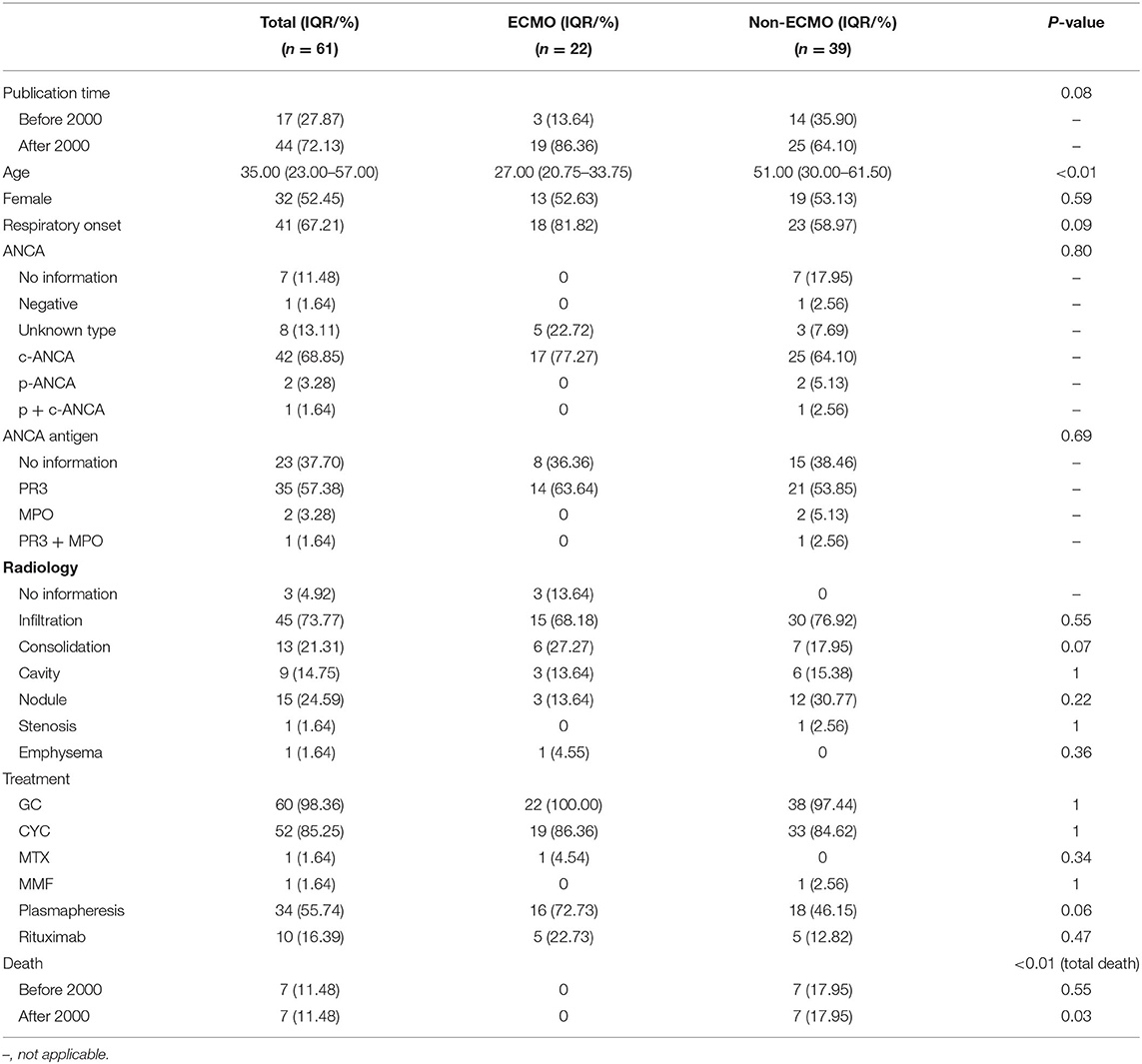
The overall schematic general information and clinical manifestations are presented in Table 2. Among the 61 cases in this review (including our case), 32 were women (32/61). The mean age was 35.0 years (interquartile range, IQR: 32.0–57.0). However, the mean age of patients who received ECMO was 27.0 years (IQR, 20.8–33.8), those who did not receive ECMO was 51.0, and their IQR was 30.0–61.5, much higher than that of patients who received ECMO treatment (Table 3).
In this series, 41 patients had respiratory symptoms at the onset of their disorders (41/61); among them, patients who received ECMO treatment had a higher proportion (18/22 vs. 23/39), but without statistical significance. Except for our patients and the patient reported by Falk et al. (58), all patients in this series presented with symptoms of extrapulmonary involvement (59/61). Regarding respiratory failure, DAH was the main reason for GPA patients developing ARF (59/61), while in the other two cases, airway stenosis-related ventilation dysfunction was considered to be the etiology. There were three cases infected with cytomegalovirus, one with pneumocystis jirovecii pneumonia, and one with mucormycosis after immunosuppressive treatment.
By examining imaging manifestations, after excluding patients without radiological information, we found that the extent of involvement was related to the severity of the respiratory failure. Patients with DAH mainly displaying infiltration (45/58) and other imaging findings such as cavities (9/58), consolidation (13/58), and nodules (15/58) were relatively rare. Comparing patients who received and did not receive ECMO (Table 3), the infiltration proportion was much higher in those who received ECMO treatment (15/18 vs. 30/39), while the number of nodules was much lower (3/19 vs. 12/39). Among the two cases of airway stenosis, stenosis was detected in the radiological image directly in one patient, while another displayed pneumothorax and emphysema, which indirectly indicated airway stenosis. However, there were no statistical differences between the two groups in terms of radiological manifestations.
Except for cases not mentioned, tested, or classified, most of the patients in the series had a positive c-ANCA (42/46), and the most frequent antigen type was PR3 (35/38). P-ANCA (2/46) and MPO (2/38) were positive in only two patients, while c/p-ANCA double-positive and PR3/MPO double-positive were found in one patient each.
About ECMO, 22 patients received ECMO support in this series (22/61). Among those ECMO patients, 17 received VV-ECMO (20/22), one received VA-ECMO (1/22), and one received VA-ECMO and VV-ECMO (1/22) sequentially. All patients, except the one who died before diagnosis, were administered glucocorticoids (60/61); cyclophosphamide was another important immunosuppressive drug for combinational utilization (52/61), while other immunosuppressive drugs such as methotrexate (1/61) and mycophenolate mofetil (1/61) were seldom used. Ten patients in this series received rituximab, a drug developed in recent years. Additionally, plasma exchange was also widely used in this series (34/61), and the proportion of patients receiving ECMO was 16/22, while that in non-ECMO was 18/39 (Table 3).
Finally, 14 patients died in this series due to ARF (14/61); none of them received ECMO support (Table 3), and the results showed a higher survival rate of patients in the ECMO group.
Discussion
GPA is a type of AAV; 85% of the patients with GPA show positive ANCA, mainly PR3-ANCA, and only a few demonstrate MPO-ANCAs (62). GPA is a multisystem vasculitis syndrome characterized by granulomatous lesions and necrotizing vasculitis, and all small blood vessels could be involved. The clinical symptoms of GPA vary and cover a wide range of symptoms, including fever, fatigue, weight loss, poor appetite, anorexia, arteritis, night sweats, cough, and dyspnea (63). Treatment strategies generally involve 3–6 months of remission induction plus more than 24 months of maintenance treatment using glucocorticoids in combination with immunosuppressors; sometimes, rituximab is added during the induction, and maintenance period (7).
More than 90% of patients with GPA have upper or lower respiratory tract involvement; however, the clinical manifestations can be asymptomatic to life-threatening respiratory failure. Among them, DAH and subglottic stenosis are the most common characteristics of GPA respiratory tract involvement (64, 65). Including the case, we reported that the main cause of respiratory failure was DAH in our series, and subglottic stenosis was relatively rare. It is noteworthy that patients with GPA may be associated with opportunistic infections of the lungs due to immunosuppressive therapy.
GPA-related DAH occurs in 7–45% of patients with GPA (65, 66). Fewer than 10% of cases with DAH are serious with life-threatening conditions (29); most of them are GPA patients with alveolar hemorrhage, and their mortality rate is six times that of those without alveolar hemorrhage (67). Among patients with GPA, 60% are caused by DAH (43). Any degree of DAH should be considered a serious manifestation of the disease as it can quickly progress to respiratory failure (62). The underlying pathophysiological mechanism of GPA-related DAH is linked to antibodies related to ANCA, which interact with inflammatory cells (neutrophils, monocytes, and lymphocytes) and cytokines (particularly Th1 cytokines, namely, tumor necrosis factor and interferon-γ) and then initiate and perpetuate vasculitis (68, 69). The basement membrane of capillaries is destroyed during these processes, and erythrocytes are allowed to enter the alveolar space with fluid (69). These processes damage the gas exchange of the affected lung area. However, the symptoms of DAH, including dyspnea, hypoxia, anemia, and lack of imaging findings of diffuse alveolar filling, are not specific; however, hemoptysis is relatively rare in DAH. In clinical practice, when a case with imaging findings indicates extensive infiltration but is refractory to anti-infective treatment, bronchial alveolar lavage under bronchoscopy is recommended to ascertain the occurrence of DAH (62), and immunological examination should also be conducted to obtain a definitive diagnosis that can help clinicians arrange immunosuppressive treatment earlier.
Moreover, similar to DAH, the symptoms of subglottic stenosis lack specificity, ranging from coughing and dyspnea to life-threatening stridor (65). Unlike DAH, subglottic stenosis generally does not show any positive findings on radiology, bronchoscopy is needed to confirm the diagnosis, and the severity of respiratory involvement is related to the location, length, and degree of stenosis (65). In addition, rapid progress to respiratory failure may occur among these patients soon after admission (41, 58).
Our patient has a severe case of the disease that progressed rapidly to respiratory failure within 3 days of admission, and respiratory support was upgraded from HFNC and NIPPV to intermittent mandatory ventilation (IMV) and ECMO in a short period. Additionally, the patient had no extrapulmonary manifestations, and severe infective pneumonia was the first clinical diagnosis. GPA was not diagnosed until a positive ANCA result was reported. At this time, ECMO was used for approximately 12 h. This case showed that GPA may present with unspecified clinical symptoms and radiological manifestations with rapid progress into respiratory failure, which leads to difficulties in early diagnosis and proper treatment.
Therefore, when respiratory symptoms occur in patients with GPA, etiological treatment and powerful respiratory support should be provided as soon as possible. Glucocorticoids and cyclophosphamide are the first-line drugs for impact and maintenance treatment. Rituximab and plasmapheresis should be considered when feasible (7). Respiratory support should be chosen based on oxygenation conditions, and HFNC should be upgraded early to IMV or ECMO when necessary and available (5) to further timely diagnose and treat. In this case series, all patients received ECMO as they were unable to maintain oxygenation through basic respiratory support; among them, one patient received VV-ECMO and VA-ECMO sequentially due to circulatory dysfunction related to mediastinal emphysema.
ARF is one of the indications for ECMO support. Since the first patient with ARDS was rescued using ECMO in 1972 (70), it has been increasingly used in ARF (71). Without ECMO support, our patient would not have survived. In our series, none of the patients could maintain oxygenation under mechanical ventilation before ECMO, indicating that ECMO can improve the prognosis of respiratory failure caused by GPA (59) and provide the possibility of later recovery. In other studies, such as the conventional ventilatory support vs. extracorporeal membrane oxygenation for severe acute respiratory failure in 2009, ECMO significantly improved the disability-free survival rate of patients with severe ARF (72). ECMO to rescue lung injury in severe ARDS (EOLIA) in 2008 showed that ECMO can significantly reduce the probability of 60-day treatment failure in patients with severe ARDS and increase the days of improved oxygenation, and no renal failure has occurred (73). However, whether ECMO can improve the prognosis of ARF remains controversial. There was no statistical significance in terms of prognosis between ECMO and non-ECMO users among patients with ARDS in the EOLIA study (73). Among patients with immune-related DAH, ECMO did not improve the survival rates (20 vs. 38%, p = 0.323) (74). Although these studies showed negative results, we should note that the early termination of the EOLIA study and the high proportion of patients who received remedial treatments from the traditional treatment group transferred to the ECMO group may lead to no statistically significant difference in mortality between the two groups. In studies of patients with ARDS owing to DAH (74), those who required ECMO generally had a lower oxygenation index (87 vs. 62, p = 0.017), more days of mechanical ventilation (21 vs. 9.5, p < 0.001), and higher alveolar–arterial gradient (450 vs. 586, p = 0.017), which indicates that even if these two groups of patients are included in the study under the same criteria, the oxygenation index may be much worse in patients who used ECMO, which may generate negative results.
Hence, regardless of the conclusions of future clinical studies on whether ECMO can improve the prognosis of patients with GAP-related respiratory failure, we recommend ECMO as a transitional method for those with respiratory failure caused by GPA who cannot maintain oxygenation through mechanical ventilation. Moreover, ECMO could win some time for these patients to be correctly diagnosed and treated later (74).
Conclusion
ECMO provides the final respiratory support for GPA-related critical respiratory failure and assists patients who cannot maintain oxygenation using mechanical ventilation for further definitive diagnosis and treatment. However, whether it can improve the clinical outcomes of GPA-related respiratory failure remains to be further studied.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RW and YL reviewed the lectures and wrote the manuscript. WenY, XM, WeiY, YZ, RL, and YF collected and arranged the materials. CH, QC, and PP viewed the complete manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the Natural Science Foundation of Hunan Province (2019JJ50939), the National Natural Science Foundation of China (81873406), and the National Key R&D Program of China (2018YFC1311900).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
References
1. Nasser M, Cottin V. Alveolar hemorrhage in vasculitis (Primary and Secondary). Semin Respir Crit Care Med. (2018) 39:482–93. doi: 10.1055/s-0038-1668533
2. Lamprecht P, Kerstein A, Klapa S, Schinke S, Karsten CM, Yu X, et al. Pathogenetic and clinical aspects of anti-neutrophil cytoplasmic autoantibody-associated vasculitides. Front Immunol. (2018) 9:680. doi: 10.3389/fimmu.2018.00680
3. Geri G, Terrier B, Heshmati F, Moussaoui H, Massot J, Mira JP, et al. Effect of plasma exchange in acute respiratory failure due to Anti-neutrophil cytoplasmic antibody-associated vasculitis. Critic Care. (2018) 22:328. doi: 10.1186/s13054-018-2264-x
4. Demiselle J, Auchabie J, Beloncle F, Gatault P, Grange S, Du Cheyron D, et al. Patients with ANCA-associated vasculitis admitted to the intensive care unit with acute vasculitis manifestations: a retrospective and comparative multicentric study. Ann Intensive Care. (2017) 7:39. doi: 10.1186/s13613-017-0262-9
5. Goligher EC, Ferguson ND, Brochard LJ. Clinical challenges in mechanical ventilation. Lancet. (2016) 387:1856–66. doi: 10.1016/S0140-6736(16)30176-3
6. Combes A, Pesenti A, Ranieri VM. Fifty years of research in ARDS. Is extracorporeal circulation the future of acute respiratory distress syndrome management? Am J Respir Crit Care Med. (2017) 195:1161–70. doi: 10.1164/rccm.201701-0217CP
7. Salvador F. ANCA associated vasculitis. Eur J Intern Med. (2020) 74:18–28. doi: 10.1016/j.ejim.2020.01.011
8. Leak D, Clein GP. Acute Wegener's granulomatosis. Thorax. (1967) 22:437–43. doi: 10.1136/thx.22.5.437
9. Hensley MJ, Feldman NT, Lazarus JM, Galvanek EG. Diffuse pulmonary hemorrhage and rapidly progressive renal failure. an uncommon presentation of Wegener's granulomatosis. Am J Med. (1979) 66:894–8. doi: 10.1016/0002-9343(79)91149-5
10. Feinstein EI, Kitt D, Collins JF, Boylen T, Koss M. Hemoptysis and acute renal failure in a young man. Am J Nephrol. (1985) 5:64–70. doi: 10.1159/000166908
11. Grupe WE, Colvin RB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 12-1986. A 15-year-old boy with hemoptysis and occult blood in the urine. N Engl J Med. (1986) 314:834–44. doi: 10.1056/NEJM198603273141307
12. Lenclud C, De Vuyst P, Dupont E, Depierreux M, Ketelbant P, Goldman M. Wegener's granulomatosis presenting as acute respiratory failure with anti-neutrophil-cytoplasm antibodies. Chest. (1989) 96:345–7. doi: 10.1378/chest.96.2.345
13. Coggins CH, Niles JL, Fienberg R. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 25-1989. A 56-year-old man with hemoptysis and microscopic hematuria. N Engl J Med. (1989) 320:1677–86. doi: 10.1056/NEJM198906223202508
14. Sanchez-Masiques J, Ettensohn DB. Alveolar hemorrhage in Wegener's granulomatosis. Am J Med Sci. (1989) 297:390–3. doi: 10.1097/00000441-198906000-00013
15. Misset B, Glotz D, Escudier B, Nochy D, Bosq J, Gilles E, et al. Wegener's granulomatosis presenting as diffuse pulmonary hemorrhage. Intensive Care Med. (1991) 17:118–20. doi: 10.1007/BF01691435
16. Yoshimura N, Matsubara O, Tamura A, Kasuga T, Mark EJ. Wegener's granulomatosis. associated with diffuse pulmonary hemorrhage. Acta Pathol Jpn. (1992) 42:657–61. doi: 10.1111/j.1440-1827.1992.tb03047.x
17. Odeh M, Best LA, Kerner H, Bassan H, Oliven A. Localized Wegener's granulomatosis relapsing as diffuse massive intra-alveolar hemorrhage. Chest. (1993) 104:955–6. doi: 10.1378/chest.104.3.955
18. Pradhan M, Meyers KE, Guttenberg M, Kaplan BS. Wegener granulomatosis–an atypical case. Pediatr Nephrol. (2000) 14:862–71. doi: 10.1007/PL00013445
19. Ullmer E, Mayr M, Binet I, Ebnother-Staub C, Dalquen P, Soler M, et al. Granulomatous Pneumocystis carinii pneumonia in Wegener's granulomatosis. Eur Respir J. (2000) 15:213–6. doi: 10.1183/09031936.00.15121300
20. Hermon MM, Golej J, Emminger W, Puig S, Szepfalusi Z, Trittenwein G. Acute hemorrhagic respiratory failure caused by Wegener's granulomatosis successfully treated by bronchoalveolar lavage with diluted surfactant. Wien Klin Wochenschr. (2003) 115:793–6. doi: 10.1007/BF03040505
21. Senf R, Jürgensen JS, Teichgräber U, Kampf D, Schindler R. Ruptured arterial aneurysm of the kidney in a patient with Wegener's granulomatosis. Nephrol Dialysis Transp. (2003) 18:2671–3. doi: 10.1093/ndt/gfg380
22. Prutkin JM, Barry P, Zaas D. Cases from the osler medical service at Johns Hopkins University. Am J Med. (2003) 115:150–3. doi: 10.1016/S0002-9343(03)00356-5
23. Griffith M, Brett S. The pulmonary physician in critical care · illustrative case 3: pulmonary vasculitis. Thorax. (2003) 58:543–6. doi: 10.1136/thorax.58.6.543
24. Steinau F, Deja M, Weber-Carstens S, Busch T, Kaisers U. Onset of acute respiratory distress syndrome following severe pulmonary hemorrhage in a patient with anti-neutrophil cytoplasmic antibody associated vasculitis. Intensive Care Med. (2003) 29:504. doi: 10.1007/s00134-003-1647-9
25. Nguyen T, Martin MK, Indrikovs AJ. Plasmapheresis for diffuse alveolar hemorrhage in a patient with Wegener's granulomatosis: case report and review of the literature. J Clin Apher. (2005) 20:230–4. doi: 10.1002/jca.20069
26. Mera A, Wada M, Miyajima M. [A case of Wegener's granulomatosis presenting as severe acute respiratory failure]. Nihon Kokyuki Gakkai Zasshi. (2007) 45:262–6.
27. Mukhopadhyay S, Hensley RG, Tazelaar HD. Cardiac involvement in Wegener granulomatosis diagnosed at autopsy. Cardiovasc Pathol. (2010) 19:312–5. doi: 10.1016/j.carpath.2009.06.011
28. Esposito S, Corona F, Defilippi A, Petaccia A, Chidini G, Dell'Era L, et al. Wegener's granulomatosis presenting with life-threatening lung hemorrhage in a 7-year-old child. Rheumatol Int. (2010) 30:1665–8. doi: 10.1007/s00296-009-1132-z
29. Berthoux E, Padilla M, Chavez L, Colombe B, Bosseray A, Massot C. Unusual evolution in Wegener's granulomatosis: recovery of pulmonary involvement while renal disease progressed to end-stage. Ren Fail. (2011) 33:1032–6. doi: 10.3109/0886022X.2011.610547
30. Mahajan V, Whig J, Kashyap A, Gupta S. Diffuse alveolar hemorrhage in Wegener's granulomatosis. Lung India. (2011) 28:52–5. doi: 10.4103/0970-2113.76302
31. Marina M, Maria A. A fatal case of treatment-related adverse effects in granulomatosis with polyangiitis (Wegener's Granulomatosis). Open J Rheumatol Autoimmune Dis. (2011) 1:5–9. doi: 10.4236/ojra.2011.12002
32. Cardenas-Garcia J, Farmakiotis D, Baldovino BP, Kim P. Wegener's granulomatosis in a middle-aged woman presenting with dyspnea, rash, hemoptysis and recurrent eye complaints: a case report. J Med Case Rep. (2012) 6:335. doi: 10.1186/1752-1947-6-335
33. Ishiguro T, Takayanagi N, Yamaguchi S, Shimizu Y, Yanagisawa T, Sugita Y, et al. Pulmonary capillaritis in Wegener's granulomatosis detected via transbronchial lung biopsy. Intern Med. (2012) 51:905–9. doi: 10.2169/internalmedicine.51.6518
34. Kaya H, Yilmaz S, Sezgi C, Abakay O, Taylan M, Sen H, et al. Two cases of extrapulmonary onset granulomatosis with polyangiitis which caused diffuse alveolar haemorrhage. Respir Med Case Rep. (2014) 13:32–6. doi: 10.1016/j.rmcr.2014.09.002
35. Powers B, Uppalapati A, Gogineni S, Jamkhana ZA. Rituximab-a drug with many facets and cures: a treatment for acute refractory hypoxemic respiratory failure secondary to severe granulomatosis with polyangiitis. Case Rep Crit Care. (2013) 2013:123134. doi: 10.1155/2013/123134
36. Haupt ME, Pires-Ervoes J, Brannen ML, Klein-Gitelman MS, Prestridge AL, Nevin MA. Successful use of plasmapheresis for granulomatosis with polyangiitis presenting as diffuse alveolar hemorrhage. Pediatr Pulmonol. (2013) 48:614–6. doi: 10.1002/ppul.22666
37. Pinto B, Dhir V, Singh PK, Gowda KK, Sharma A. Granulomatosis with polyangiitis and severe respiratory involvement. J Emerg Med. (2014) 47:e79–81. doi: 10.1016/j.jemermed.2014.01.037
38. Moreno-González G, Corral-Ansa L, Sabater-Riera J, Solanich-Moreno X, Mañez-Mendiluce R. Pulmonary thromboembolism and diffuse alveolar hemorrhage in granulomatosis with polyangiitis vasculitis. Respiratory Care. (2014) 59:e206–e9. doi: 10.4187/respcare.03162
39. Hilal T. Fatal cytomegalovirus disease after combination therapy with corticosteroids and rituximab for granulomatosis with polyangiitis. Case Rep Rheumatol. (2015) 2015:538137. doi: 10.1155/2015/538137
40. Fukui S, Iwamoto N, Tsuji S, Umeda M, Nishino A, Nakashima Y, et al. Diffuse alveolar hemorrhage emerging one week after starting high-dose corticosteroid therapy for granulomatosis with polyangiitis (GPA) with systemic lupus erythematosus (SLE). Intern Med. (2015) 54:2681–6. doi: 10.2169/internalmedicine.54.5299
41. Tajarernmuang P, Limsukon A, Liwsrisakun C, Wannasopha Y. Severe bilateral bronchial stenosis with acute respiratory failure from granulomatosis with polyangiitis. Respirol Case Rep. (2016) 4:e00189. doi: 10.1002/rcr2.189
42. Ning S, Zhang X, Xu C, Dang X, Cheng H, Zhu K, et al. Methylprednisolone and plasmapheresis are effective for life-threatening diffuse alveolar hemorrhage and gastrointestinal hemorrhage in granulomatosis with polyangiitis: a case report and literature review. Medicine. (2018) 97:e0592. doi: 10.1097/MD.0000000000010592
43. Sattar Y, Susheela AT, Ullah W, Usman N, Zafrullah F. Use of plasmapheresis and immunosuppressants to treat diffuse alveolar hemorrhage in a patient with granulomatosis with polyangiitis. Medicina. (2019) 55:378. doi: 10.3390/medicina55070378
44. Hartmann A, Nordal KP, Svennevig J, Noddeland H, Pedersen T, Skarbovik AJ, et al. Successful use of artificial lung (ECMO) and kidney in the treatment of a 20-year-old female with Wegener's syndrome. Nephrol Dial Transplant. (1994) 9:316–9.
45. Loscar M, Hummel T, Haller M, Briegel J, Wiebecke B, Samtleben W, et al. [ARDS and Wegener granulomatosis]. Anaesthesist. (1997) 46:969–73. doi: 10.1007/s001010050494
46. Matsumoto T, Ueki K, Tamura S, Ideura H, Tsukada Y, Maezawa A, et al. Extracorporeal membrane oxygenation for the management of respiratory failure due to ANCA-associated vasculitis. Scand J Rheumatol. (2000) 29:195–7. doi: 10.1080/030097400750002111
47. Rosengarten A, Elmore P, Epstein J. Long distance road transport of a patient with Wegener's Granulomatosis and respiratory failure using extracorporeal membrane oxygenation. Emerg Med. (2002) 14:181–7. doi: 10.1046/j.1442-2026.2002.00315.x
48. Hernandez ME, Lovrekovic G, Schears G, Helfaer M, Friedman D, Stafford P, et al. Acute onset of Wegener's granulomatosis and diffuse alveolar hemorrhage treated successfully by extracorporeal membrane oxygenation. Pediatric Criti Care Med. (2002) 3:63–6. doi: 10.1097/00130478-200201000-00014
49. Ahmed SH, Aziz T, Cochran J, Highland K. Use of extracorporeal membrane oxygenation in a patient with diffuse alveolar hemorrhage. Chest. (2004) 126:305–9. doi: 10.1378/chest.126.1.305
50. Gay SE, Ankney N, Cochran JB, Highland KB. Critical care challenges in the adult ECMO patient. Dimens Crit Care Nurs. (2005) 24:157–62; quiz 63–4. doi: 10.1097/00003465-200507000-00001
51. Balasubramanian SK, Tiruvoipati R, Chatterjee S, Sosnowski A, Firmin RK. Extracorporeal membrane oxygenation with lepirudin anticoagulation for Wegener's granulomatosis with heparin-induced thrombocytopenia. Asaio J. (2005) 51:477–9. doi: 10.1097/01.mat.0000169123.21946.31
52. Joseph M, Charles AG. Early extracorporeal life support as rescue for Wegener granulomatosis with diffuse alveolar hemorrhage and acute respiratory distress syndrome: a case report and literature review. Pediatr Emerg Care. (2011) 27:1163–6. doi: 10.1097/PEC.0b013e31823b01a2
53. Barnes SL, Naughton M, Douglass J, Murphy D. Extracorporeal membrane oxygenation with plasma exchange in a patient with alveolar haemorrhage secondary to Wegener's granulomatosis. Intern Med J. (2012) 42:341–2. doi: 10.1111/j.1445-5994.2012.02720.x
54. Hohenforst-Schmidt W, Petermann A, Visouli A, Zarogoulidis P, Darwiche K, Kougioumtzi I, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther. (2013) 7:627–33. doi: 10.2147/DDDT.S47156
55. Yusuff H, Malagon I, Robson K, Parmar J, Hamilton P, Falter F. Extracorporeal membrane oxygenation for Life-threatening ANCA-positive pulmonary capillaritis. a review of UK experience. Heart Lung Vessel. (2015) 7:159–67.
56. Rawal G, Kumar R, Yadav S. ECMO rescue therapy in diffuse alveolar haemorrhage: a case report with review of literature. J Clin Diagn Res. (2016) 10:Od10–1. doi: 10.7860/JCDR/2016/20649.7969
57. Vanoli J, Riva M, Vergnano B, D'Andrea G, L'Imperio V, Pozzi MR, et al. Granulomatosis with polyangiitis presenting with diffuse alveolar hemorrhage requiring extracorporeal membrane oxygenation with rapid multiorgan relapse: A case report. Medicine. (2017) 96:e6024. doi: 10.1097/MD.0000000000006024
58. Falk L, Broman LM. Extracorporeal membrane oxygenation rescue in adolescent with bronchiolitis obliterans-organizing pneumonia like Wegener's granulomatosis. Clin Case Rep. (2017) 5:29–34. doi: 10.1002/ccr3.752
59. Delvino P, Monti S, Balduzzi S, Belliato M, Montecucco C, Caporali R. The role of extra-corporeal membrane oxygenation (ECMO) in the treatment of diffuse alveolar haemorrhage secondary to ANCA-associated vasculitis: report of two cases and review of the literature. Rheumatol Int. (2019) 39:367–75. doi: 10.1007/s00296-018-4116-z
60. Goel MK, Chauhan M, Kumar A, Wadwa P, Maitra G, Talegaonkkar M, et al. A case of refractory hypoxemic respiratory failure due to antineutrophil cytoplasmic antibodies-associated diffuse alveolar hemorrhage rescued by extracorporeal membrane oxygenation. Indian J Crit Care Med. (2020) 24:879–81. doi: 10.5005/jp-journals-10071-23585
61. Yin K, March RJ, Hoopes CW, Balk RA, Raman J, Lateef OB, et al. Extracorporeal membrane oxygenation in the management of granulomatosis with polyangiitis. J Cardiac Surg. (2021) 36:743–7. doi: 10.1111/jocs.15252
62. Thompson GE, Specks U. Update on the management of respiratory manifestations of the antineutrophil cytoplasmic antibodies-associated vasculitides. Clin Chest Med. (2019) 40:573–82. doi: 10.1016/j.ccm.2019.05.012
63. Almouhawis HA, Leao JC, Fedele S, Porter SR. Wegener's granulomatosis: a review of clinical features and an update in diagnosis and treatment. J Oral Pathol Med. (2013) 42:507–16. doi: 10.1111/jop.12030
64. Wick MR. Pulmonary disorders that are potentially associated with anti- neutrophilic cytoplasmic antibodies: a brief review. Semin Diagn Pathol. (2018) 35:304–14. doi: 10.1053/j.semdp.2018.08.005
65. Thickett DR, Richter AG, Nathani N, Perkins GD, Harper L. Pulmonary manifestations of anti-neutrophil cytoplasmic antibody (ANCA)-positive vasculitis. Rheumatology. (2006) 45:261–8. doi: 10.1093/rheumatology/kei217
66. Quartuccio L, Bond M, Isola M, Monti S, Felicetti M, Furini F, et al. Alveolar haemorrhage in ANCA-associated vasculitis: long-term outcome and mortality predictors. J Autoimmun. (2020) 108:102397. doi: 10.1016/j.jaut.2019.102397
67. Caetano J, Fernandes das Neves M, Oliveira S, Delgado Alves J. Refractory Wegener's granulomatosis presenting with alveolar haemorrhage, treated with rituximab. BMJ Case Rep. (2014) 2014:bcr2014208510. doi: 10.1136/bcr-2014-208510
68. Scapa JV, Fishbein GA, Wallace WD, Fishbein MC. Diffuse alveolar hemorrhage and pulmonary vasculitides: histopathologic findings. Semin Respir Crit Care Med. (2018) 39:425–33. doi: 10.1055/s-0038-1669412
69. Martinez-Martinez MU, Oostdam DAH, Abud-Mendoza C. Diffuse alveolar hemorrhage in autoimmune diseases. Curr Rheumatol Rep. (2017) 19:27. doi: 10.1007/s11926-017-0651-y
70. Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). use of the Bramson membrane lung. N Engl J Med. (1972) 286:629–34. doi: 10.1056/NEJM197203232861204
71. Parekh M, Abrams D, Brodie D, Yip NH. Extracorporeal membrane oxygenation for ARDS: optimization of lung protective ventilation. Respir Care. (2018) 63:1180–8. doi: 10.4187/respcare.06262
72. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. (2009) 374:1351–63. doi: 10.1016/S0140-6736(09)61069-2
73. Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. (2018) 378:1965–75. doi: 10.1056/NEJMoa1800385
Keywords: diffuse alveolar hemorrhage, ANCA-associated vasculitis, extracorporeal membrane oxygenation, acute respiratory failure, granulomatosis with polyangiitis
Citation: Wan R, Yang W, Ma X, Yang W, Pan P, Hu C, Chen Q, Zhou Y, Lu R, Fang Y and Li Y (2021) ECMO Rescues Patients With Acute Respiratory Failure Related to GPA. Front. Med. 8:671396. doi: 10.3389/fmed.2021.671396
Received: 23 February 2021; Accepted: 22 April 2021;
Published: 28 May 2021.
Edited by:
Huahao Shen, Zhejiang University, ChinaReviewed by:
Ying Liang, Peking University Third Hospital, ChinaAlvise Berti, Santa Chiara Hospital, Italy
Copyright © 2021 Wan, Yang, Ma, Yang, Pan, Hu, Chen, Zhou, Lu, Fang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Li, bGVlcm91bmRAY3N1LmVkdS5jbg==
 Rongjun Wan
Rongjun Wan Wenzhe Yang1
Wenzhe Yang1 Pinhua Pan
Pinhua Pan Qiong Chen
Qiong Chen Yuanyuan Li
Yuanyuan Li