- 1Department of Pharmacy Practice, Faculty of Pharmacy, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 2Department of Pulmonology, Bahawal Victoria Hospital, Bahawalpur, Pakistan
- 3Rural Health Center, Khanewal, Pakistan
- 4Department of Pharmacy Practice, Faculty of Pharmacy and Health Sciences, University of Balochistan, Quetta, Pakistan
Background: This study involves the analysis of spectrum of microorganisms, antibiotic resistance pattern, and treatment outcomes among empyema thoracis patients. This study also analyzes the factors associated with unsuccessful treatment outcome and duration of hospital stay among the patients.
Methods: This was a descriptive, cross-sectional study carried out in the Pulmonology Ward of the Bahawal Victoria hospital, Bahawalpur, Pakistan. All patients with empyema thoracis registered at the study site during the period of 1 year were included in the study. Multivariate regression analysis was used to analyze the factors associated with duration of hospital stay and unsuccessful treatment outcome among the patients.
Results: A total 110 patients were included in the study. Most of the patients (n = 73, 66.4%) were treated with piperacillin/tazobactam alone and in combination with either one or more than one antibiotics as an empiric therapy. Culture was positive in 58 (52.7%) patients and the most commonly identified organisms included, gram-negative Pseudomonas aeruginosa (n = 20; 18.8%) and Klebsiella sp. (n = 11, 10%) followed by same proportion of E. coli. The most commonly identified bacterial isolates showed high level of resistance against antibiotics used as an empiric therapy, while these showed low level of resistance against amoxicillin, clarithromycin, ertapenem, colistin, tigecycline, fosfomycin, rifampicin, and vancomycin. In this study, 82 (74.5%) patients successfully completed the treatment, while 12 (11%) showed no clinical improvement, 5 (4.5%) lost to follow up and 11 (10%) died. In multivariate binary logistic regression analysis, none of the patient attributes were significantly associated with unsuccessful treatment outcome, while in multivariate linear regression analysis, the factors which were significantly associated with duration of hospital stay included; duration of symptoms <2 weeks prior to admission (p = 0.008, beta = −0.247) and resistance to five antibiotic classes (p = 0.02, beta = 0.280).
Conclusion: Close to 25% of the patients did not complete the treatment successfully. Most of the common bacterial isolates showed high level of resistance against the broad-spectrum antibiotics used as an empiric therapy. This is alarming. However, better sensitivity of common bacterial isolates against standardized first line treatment for empyema thoracis is promising.
Introduction
Empyema thoracis is an infectious disease which causes the accumulation of frank pus in the pleural space of lungs (1). It mostly appears as a complication of hospital and community acquired pneumonia, however, it also occurs due to other causes like thoracic injuries, chest trauma, bronchogenic carcinoma, esophageal rupture, immune-compromised states, and other postsurgical infections (1, 2). The clinical sign and symptoms of empyema include; pleuritic chest pain, cough, fever, chills, weight loss, anorexia, dyspnea, and night sweats (1, 3). The diagnosis of empyema is established by the presence of pus and fluid in the pleural space followed by microbiological assay of pleural fluid, while gene expert and acid fast bacilli (AFB) smear examination are used for the detection of Mycobacterium tuberculosis (2). The major aim of empyema treatment is to eliminate the infection and re-expansion of the lungs which is usually achieved by eradicating the bacterial growth from the pleural fluid by the use of appropriate antibiotic therapy along with drainage process (1, 2, 4, 5).
Epidemiological data about empyema thoracis is limited. However, over the last few years, the incidence rate of this disease is increasing globally, whereas, developing countries accounts for high burden of empyema thoracis (2, 6–8). Each year, in the United States (US), ~1 million patients were hospitalized with pneumonia. Among those hospitalized patients, 20–40% had para pneumonic effusion, while 5–10% of these patients progressed to empyema thoracis (2). Unfortunately, very scarce data on empyema thoracis is available from Pakistan. According to a study conducted in Pakistan, the empyema thoracis is a complication that accounts 18% of thoracic injuries (9).
The leading pathogens in community acquired empyema include, gram-negative Escherichia coli (E.coli), Klebsiella pneumoniae, and gram-positive Staphylococcus and Streptococcus species, while, in hospital acquired empyema, the most common pathogens are gram-negative Pseudomonas, Enterobacter species, and Methicillin resistant Staphylococcus aureus (MRSA) (1). All these pathogens causing empyema are the same well-known pathogens causing community and hospital acquired infection, worldwide (10–15). Moreover, Mycobacterium tuberculosis is also a common pathogen which may cause empyema (16). In case, microbiology reports are not available, the differential diagnosis can be made on patient history (17).
According to the American Association for Thoracic Surgery (AATS) consensus guidelines for the management of empyema, the diseases should be treated initially by drainage process along with antibiotics (2). The guidelines further suggest that for community acquired empyema thoracis, cephalosporins like ceftriaxone along with metronidazole, or amino-penicillin with beta lactamase inhibitors (e.g., ampicillin/sulbactam) should be preferred. While, vancomycin along with Cefepime and metronidazole or with piperacillin/tazobactam should be used for hospital acquired empyema (2). Despite these defined treatment recommendations, achievement of treatment success and reduction in mortality rates among empyema thoracis patients continues to be a problem for healthcare professionals, predominantly due to antibiotic resistance (18, 19). Unfortunately, antibiotic resistance is much higher in developing countries, for example, in India and Pakistan, due to common inappropriate use of antibiotics (20–22). Importantly, a change in the pathogen profile and pattern of susceptibility has been observed in different countries against antimicrobials, and it changes over time (23, 24). This phenomena necessitates that antibiotic resistance and sensitivity pattern should be observed over time for infectious diseases (24).
Despite of having a knowledge about increasing incidence of this disease, only few studies (25, 26) were conducted in Pakistan among empyema thoracis patients. However, the pattern of antibiotic resistance and final treatment outcomes were not addressed in depth. Moreover, patient's attributes associated with final treatment outcome and length of hospital stay are still under investigation in Pakistan. Therefore, the aim of this study was to investigate the clinical characteristics, spectrum of microorganisms, antibiotic resistance pattern, and treatment outcomes among empyema thoracis patients in Pakistan. Moreover, we also analyzed the independent factors associated with unsuccessful treatment outcome and duration of hospital stay among the patients.
Materials and Methods
Study Setting
This study was conducted in the chest disease unit (CDU) of the Bahawal Victoria hospital (BVH), Bahawalpur, Punjab, Pakistan. This tertiary care hospital has a capacity of over 1,600 beds (27). The CDU serves both indoor and outdoor patients suffering from different forms of lung diseases. The CDU has five to six consultants, 15–18 doctors, two pharmacists and has a bed capacity of 64 (27).
Study Design and Study Population
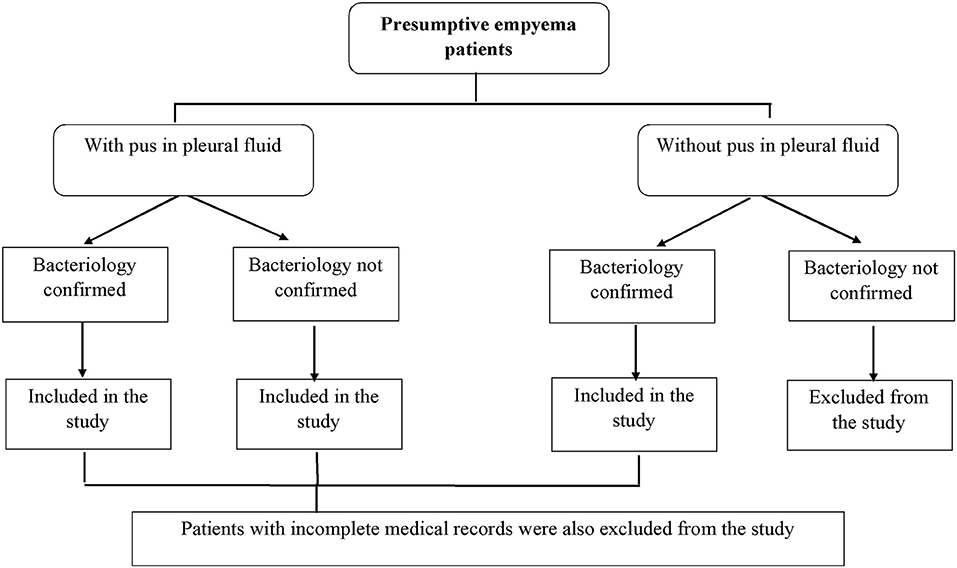
This was a descriptive cross-sectional study. A detailed information about the patients who met the inclusion criteria is presented in Figure 1.
Microbiological Examination
The BVH follows standard operating procedures for microbiological examination of culture specimens (2). For empyema patients, the specimen (pleural fluid) for culture was taken via drainage technique (thoracentesis). The specimen was drawn from freshly drained fluid to avoid the contamination and then inoculated in the aerobic and anaerobic sterile standard culture bottles (BACTEC culture bottles) for culture and staining (2, 28). The specimen was then incubated in the BACTEC960 culture bottles at 35°C for 2–3 days. Gram staining, gene expert and AFB smear examination (for M. Tuberculosis) were performed on the culture positive bottles and the culture was examined daily to check the growth of microorganisms. The identification of organism and DST was performed by subculture technique in a suitable solid media (24, 28). Standard methodology for characterization of pathogens and antibiotic concentrations in DST were used.
Diagnosis and Treatment of Patients
In this study setting, presumptive patients of empyema were diagnosed through clinical presentation of patients (i.e., pleuritic chest pain, cough, fever, chills, weight loss, anorexia, dyspnea, and night sweats), pleural ultrasound (US), conventional chest x-ray (CXR), computed tomography (CT-scan) and pleural fluid analysis (1, 2). Immediately after clinical diagnosis patients were started on empiric therapy. Once pleural fluid analysis (i.e., microbiological analysis) reports became available, the empiric therapy was replaced with appropriate modified regimen. In case, patient's culture was negative (only for those who had pus in pleural fluid), the patient was still considered as clinically diagnosed case and asked to complete the empyema treatment (29). Pleural fluid drainage was done on the basis of clinician's suggestion for the improvement of patient's condition (2).
Outcomes Variables
Outcome variables included; spectrum of microorganisms, antibiotic resistance pattern and treatment outcomes.
Treatment outcomes were broadly divided into following categories; treatment completed with clinical improvement, no clinical improvement, loss to follow up and death.
Treatment Completed With Clinical Improvement
The patients with empyema thoracis who completed treatment duration of 2–6 weeks with clinical improvement. Such patients were classified as successfully treated patients (2, 6).
No Clinical Improvement
The patients with empyema thoracis who did not improve at any stage of treatment.
Loss to Follow-Up
The patients with empyema thoracis who left the hospital on their own before treatment completion (27).
Death
Patients died during the treatment.
Unsuccessful Outcome
No clinical improvement, loss to follow up due to any reason and death during treatment were broadly classified as unsuccessful treatment outcome.
Data Collection
The study cohort included all those confirmed cases of empyema thoracis who were registered at the study site from October, 2019 to September, 2020. The data collection form was designed through literature review (5, 6, 30). It consisted of information on respondent's sociodemographic and clinical characteristics, antibiotic sensitivity and resistance pattern, treatment regimen, duration of symptoms, duration of hospital stay, comorbidities, and final treatment outcomes. Microbiological reports and medical records of the patients were used to obtain patient data.
Data Analysis
Data analysis was performed by using Statistical Package for Social Sciences (IBM SPSS statistics for windows version 20.0, Armonk, NY: IBM Corp) (27). Continuous variables were presented as mean and standard deviation (SD), whereas counts (n) and proportions (%) were used to present categorical variables (27). Multivariate binary logistic regression analysis was used to identify the factors associated with unsuccessful treatment outcomes and multivariate linear regression analysis was used to identify the factors associated with duration of hospital stay. The variable with a p < 0.05 in univariate analysis were entered into multivariate analysis (27).
Ethical Approval
Pharmacy research and ethics committee (PREC) at the Islamia university of Bahawalpur (Reference no: 109-2020-/PREC) approved the design and conduct of the study.
Results
Description of the Patients
At the time of data collection, 140 presumptive cases of empyema thoracis were reported at the study site, while, 110 cases of empyema thoracis were finely included in the study. Thirty patients with no pus in the pleural fluid (with no bacterial growth) (n = 20) and the patients with incomplete medical records (n = 10) were excluded from the study.
Sociodemographic and Clinical Characteristics of the Patients
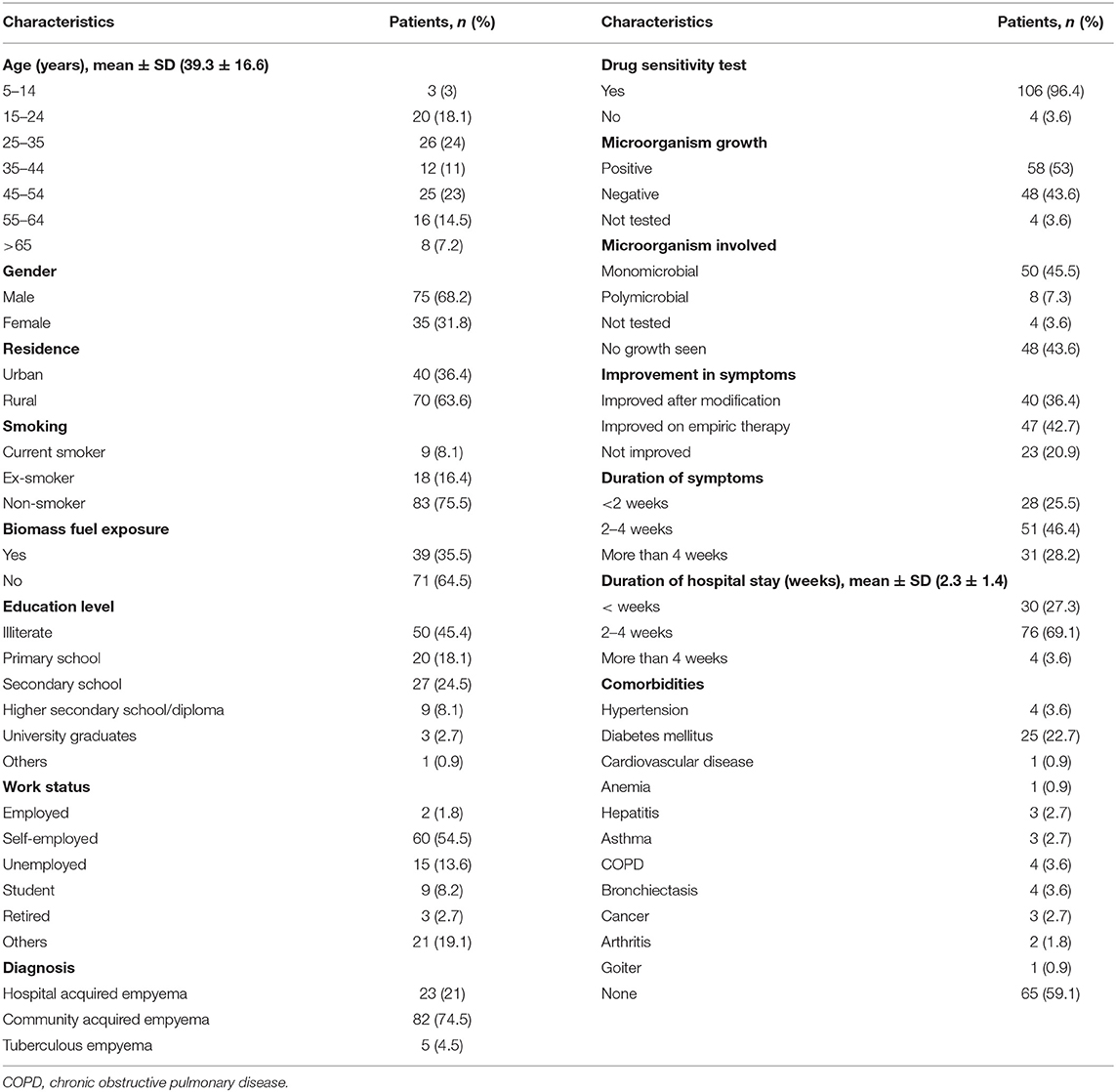
Out of the total 110 patients, 75 (68.2%) were male, 70 (63.6%) were resident of rural areas, and 50 (45.4%) were illiterate. The mean age of the patients was 39.3 (SD = 16.6) years. Out of the total, 71 (64.5%) patients were not exposed to biomass fuel. The community acquired empyema was diagnosed in 82 (74.5%) patients. A total of 58 (52.7%) patients were culture positive, whereas, 48 (43.6%) were culture negative. Close to half of the patients (46.4%) had symptom duration of 2–4 weeks. The mean duration of hospital stay was 2.3 (SD = 1.4) weeks (Table 1).
Empiric Therapy Started Among the Patients
At the beginning of therapy, 73 (66.4%) patients were treated with piperacillin+ tazobactam; alone in 14 (12.7%) cases and in combination with moxifloxacin 26 (23.6%) followed by linezolid 9 (8.1%) and ceftriaxone 6 (5.4%). Remaining 37 (34%) patients were treated with other antibiotics used alone or in combination which included; moxifloxacin (n = 56; 50.9%), ceftriaxone (n = 37; 33.6%), linezolid (n = 17; 15.5%), vancomycin (n = 10; 9.1%), ciprofloxacin (n = 8; 7.3%), clarithromycin (n = 5; 4.5%), imipenem (n = 5; 4.5%) and levofloxacin (n = 1; 0.9%).
Organisms Isolated From the Specimen
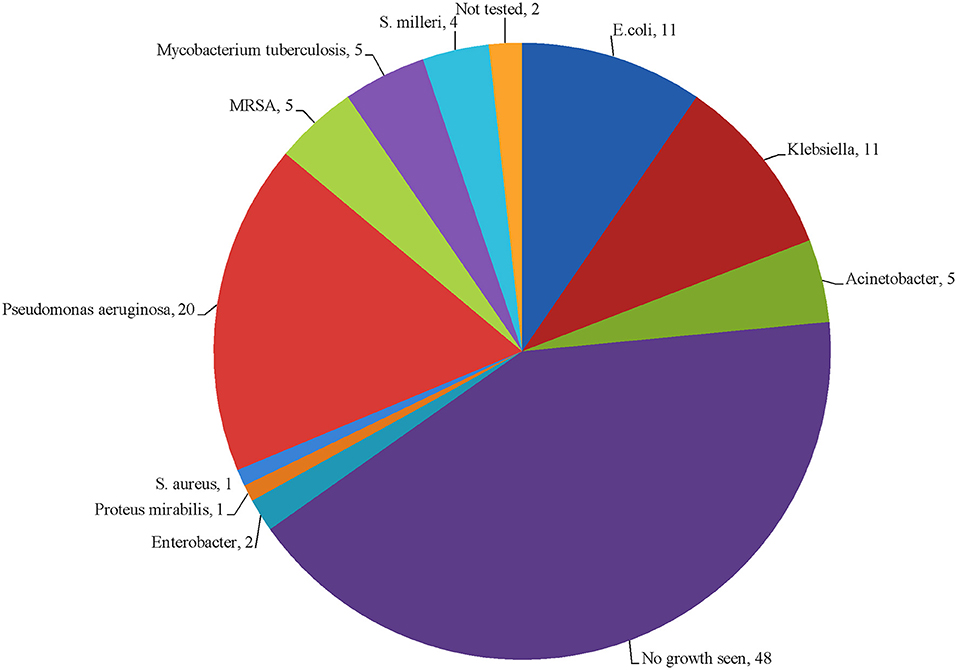
Culture yielded a total of 65 bacterial isolates from 58 (52.7%) culture positive specimens. Out of these 58 specimens, 50 (86.2%) were mono-microbial and 8 (13.7%) were poly-microbial. In 48 (43.6%) specimens, there was no growth of microorganism, while 4 (3.6%) specimens were not tested for culture growth. The isolated organisms were categorized as gram-negative (n = 50, 43.4%), gram-positive (n = 10, 9%), and Mycobacterium tuberculosis (n = 5, 4.3%). Among them, Pseudomonas aeruginosa was dominant 20 (18.8%), while E. coli and Klebsiella sp. shared same proportions (n = 11, 10%). The type of bacterial species identified in this study is presented in Figure 2.
Antibiotic Sensitivity and Resistance Pattern Against Bacterial Isolates
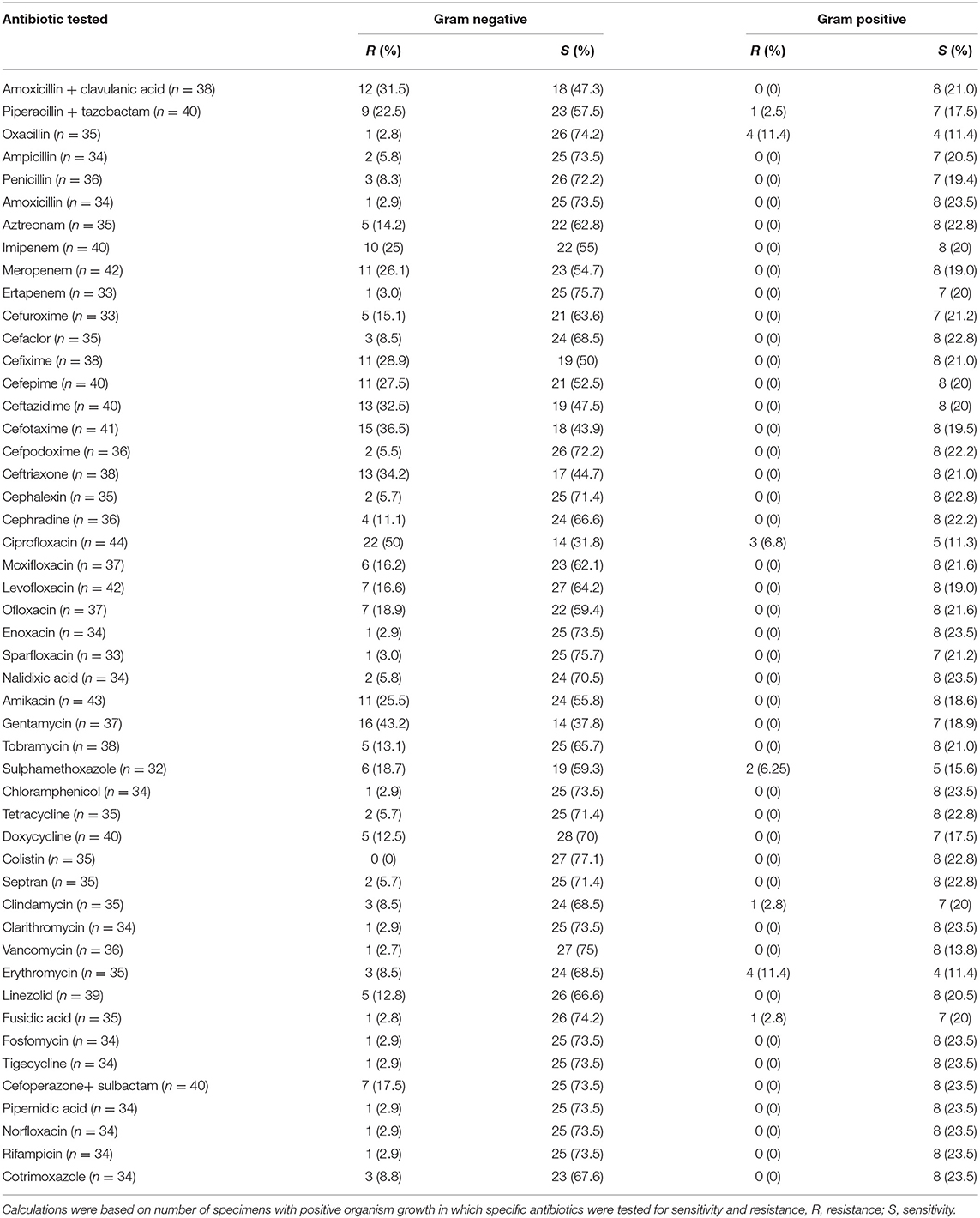
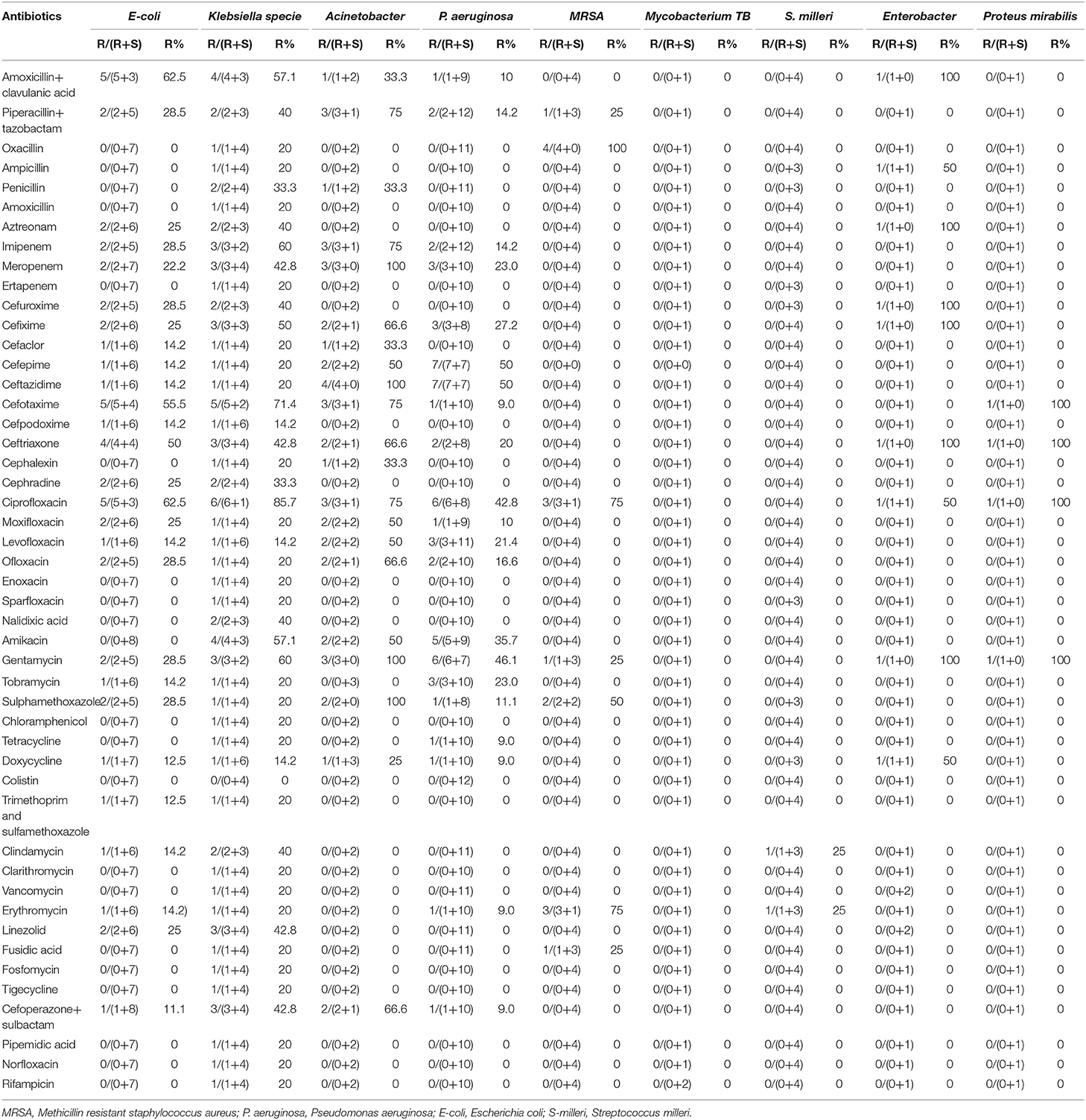
Out of the total 65 isolates, piperacillin/tazobactam was tested in 40 isolates (gram-negative = 32, gram-positive = 8). Out of these, nine (22.5%) gram-negative and one (2.5%) gram-positive bacterial isolates were resistant to aforementioned antibiotic. Moxifloxacin was tested in 37 isolates (gram-negative = 29, gram-positive = 8). Out of these, six (16.2%) gram-negative isolates were resistant to moxifloxacin. Similarly, ceftriaxone was tested in 38 isolates (gram-negative = 30, gram-positive = 8), from which 13 (34.2%) gram-negative isolates were resistant to above mentioned antibiotic. Amoxicillin/clavulanic acid, clarithromycin, colistin, vancomycin, ertapenem, and tigecycline showed better sensitivity against bacterial isolates (Table 2). A detailed information about the number of drugs resistant to each bacterial isolate is mentioned in Supplementary Table 1.
Percentage Resistance of Organism Isolates Against Antibiotic
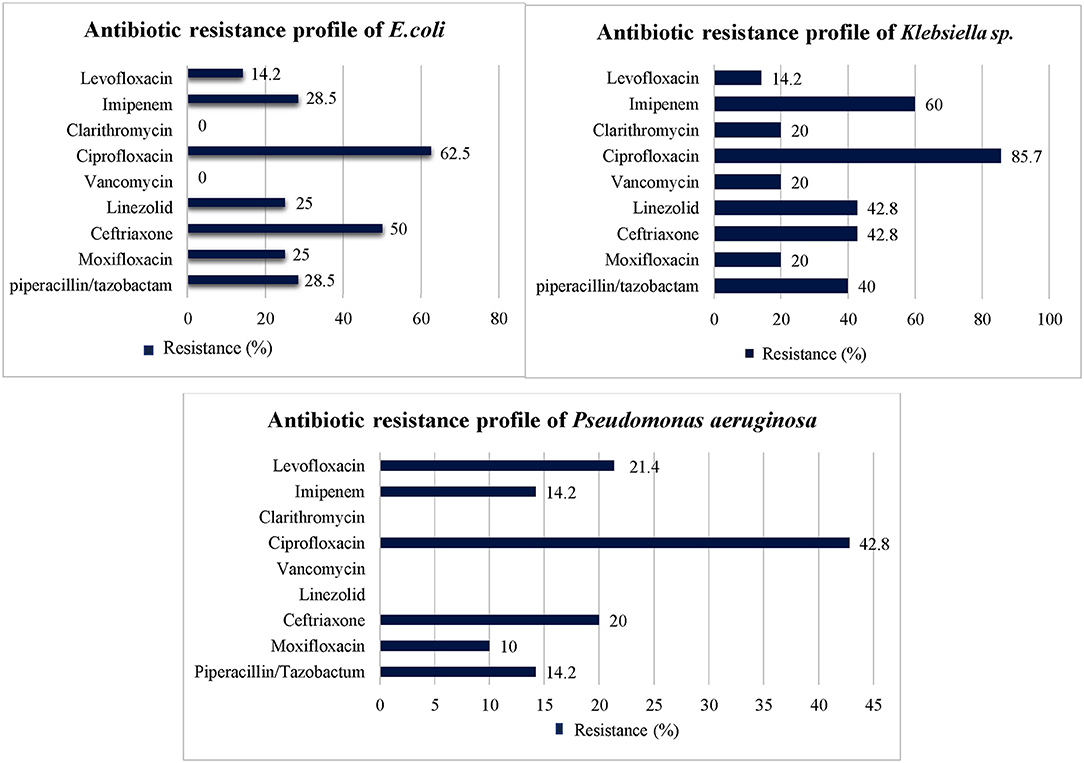
Among most common gram-negative bacterial isolates (i.e., E. coli, Klebsiella sp., and Acinetobacter species) identified in this study, highest level of resistance was seen against third generation cephalosporins (i.e., ceftriaxone, cefotaxime), piperacillin/tazobactam, imipenem, and second, third and fourth generation fluoroquinolones. However, amoxicillin/clavulanic acid, ertapenem, clarithromycin, colistin, vancomycin, fosfomycin, and tigecycline showed low level of resistance against most common gram-negative isolates (Table 3). Drug resistance index of E. coli was 0.094 and 0.86 during first and second 6 months, respectively. In case of Klebsiella sp., drug resistance index of 0.16 and 0.7 was observed during first and second 6 months, respectively. Drug resistance index of Pseudomonas aeruginosa was 0.065 and 0.23 during first and second 6 months, respectively. Details about drug resistance index of E. coli, Klebsiella sp., and Pseudomonas aeruginosa are provided in Supplementary Table 2.
The antibiotics resistance profile of the three most prevalent bacterial species against the commonly used antibiotics at the start of the treatment is presented in Figure 3.
Modification in Antibiotic Therapy Among the Patients
Among the total 110 patients, antibiotic therapy was modified in 52 (47.3%) patients based on either clinician's judgement and/or culture and sensitivity results. Out of 52 patients, initial treatment was replaced with imipenem in 20 (38.4%) patients, piperacillin/tazobactam in 13 (25%) patients and linezolid in 12 (23%) patients (Table 4).
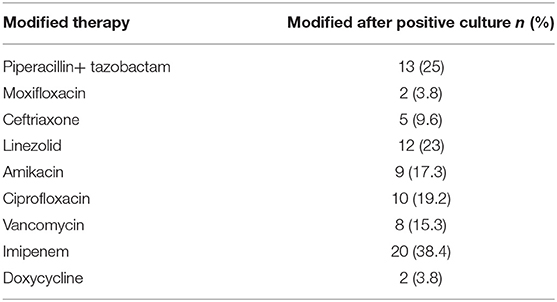
Table 4. Modification in antibiotic therapy based on clinician's suggestion and culture and sensitivity results (N = 110).
Treatment Outcomes Among the Patients
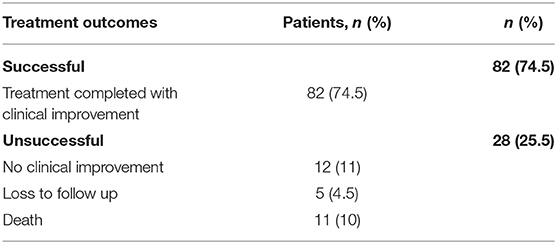
In the total 110 patients, 82 (74.5%) completed their treatment with clinical improvement, 12 (10.9%) showed no clinical improvement at any stage of treatment, five (4.5%) were lost to follow up and 11 (10%) died before treatment completion (Table 5).
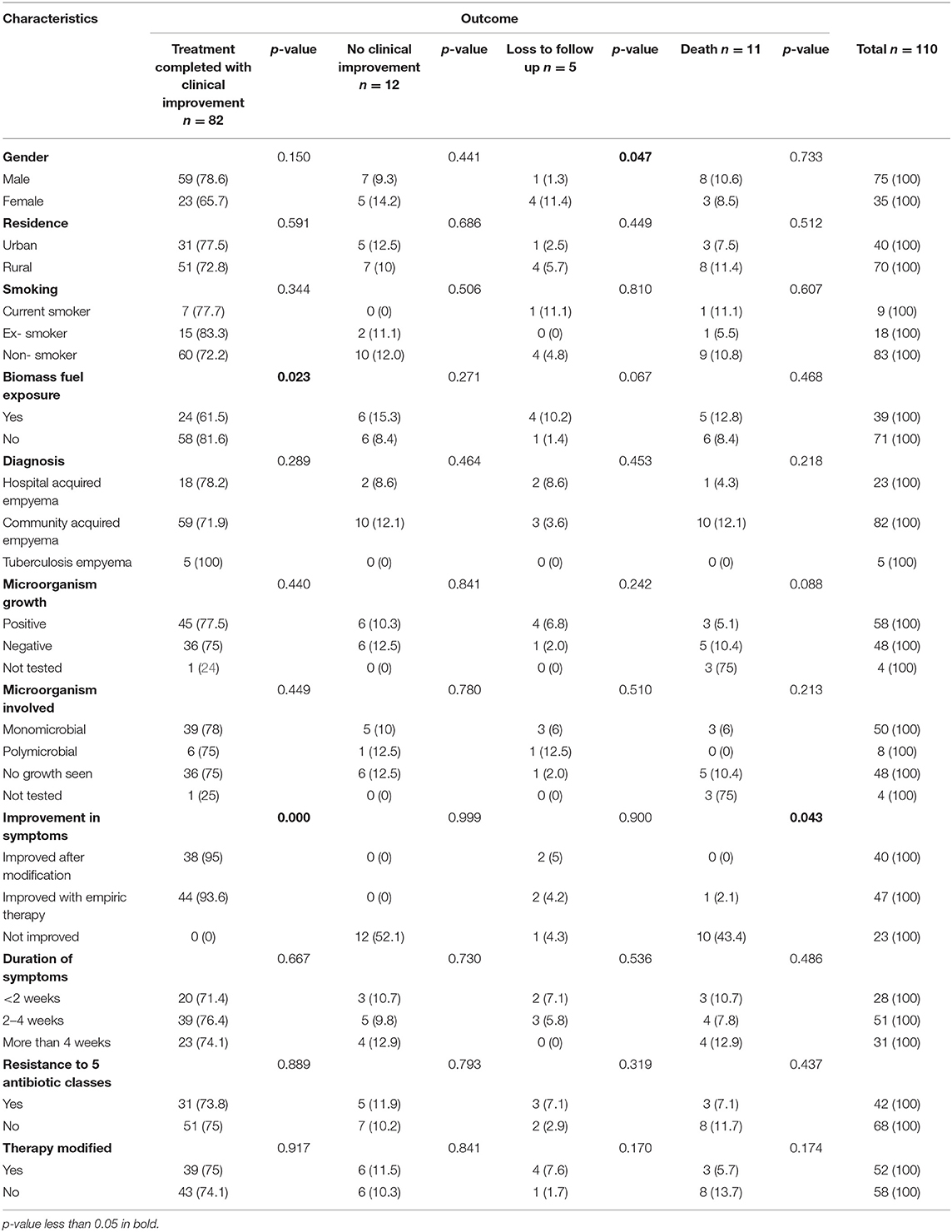
When treatment outcomes with respect to patient's characteristics, a statistically significant difference was found in male (i.e., p < 0.05) with respect to loss to follow up. Likewise, statistically significant difference was found in biomass (i.e., p < 0.05) with regard to treatment completed with clinical improvement. Similarly, statistically significant difference was found in improved with empiric therapy (i.e., p < 0.05) with regard to treatment completed with clinical improvement and death (Table 6).
Treatment modification with imipenem and amikacin + imipenem lead to death in more case (a total of 8 and 4 died, respectively) as compared to other modification. Number of deaths in culture negative patients (5 out of 48) was slightly higher than culture positive (3 out of 58) patients. Number of deaths with regard to modified treatment and number of deaths with regard to culture status is provided in Supplementary Tables 3, 4, respectively.
Factors Associated With Unsuccessful Treatment Outcomes Among the Patients
In multivariate binary logistic regression analysis, none of the patient's variables were significantly associated with unsuccessful treatment outcomes (Table 7).
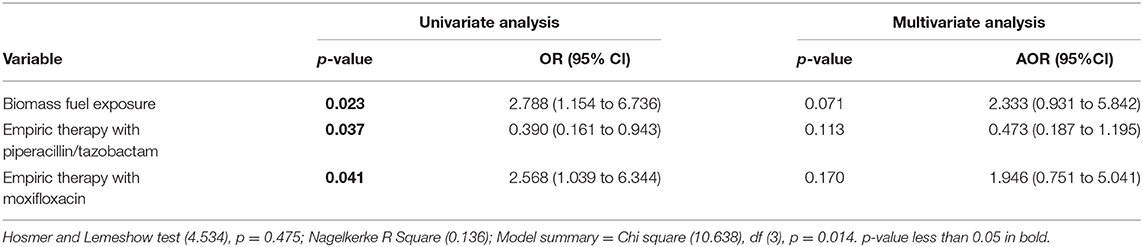
Table 7. Factors associated with unsuccessful treatment outcome: multivariate binary logistic regression analysis.
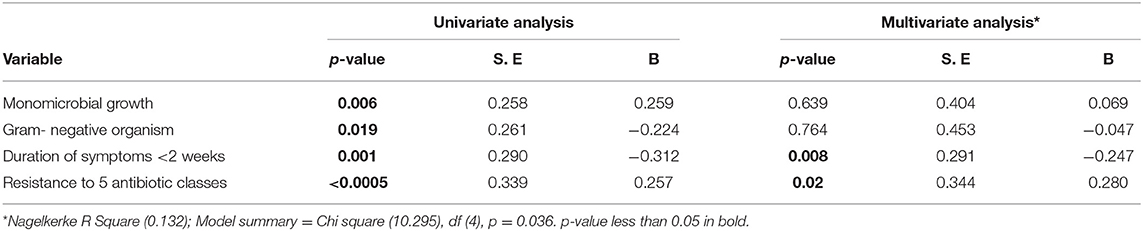
Factors Associated With Duration of Hospital Stay Among the Patients
In multivariate linear regression, the factors which were associated significantly with duration of hospital stay included; duration of symptoms <2 weeks prior to admission (beta = −0.247, p = 0.008) and resistance to five antibiotic classes (beta = 0.280, p = 0.02) (Table 8).
Discussion
Empyema thoracis is a rare complication of the pleural space of lungs (31), and there is a scarcity of data, especially from developing countries, due to unavailability of published studies (6, 8). To the extent of our knowledge, this is the first study from Pakistan that concurrently evaluated the spectrum of microorganism, antibiotic resistance pattern, duration of hospital stay, and treatment outcomes among the patients with empyema thoracis. Evaluation of antibiotic resistance pattern, spectrum of microorganisms and treatment outcomes in the empyema thoracis patients could be helpful for the clinicians in prescribing appropriate antibiotics for achieving better clinical outcomes. In this study cohort, close to 25% of the patients did not complete the treatment successfully. Most common gram- negative bacterial isolates showed high level of resistance against empiric therapy. We also analyzed the independent factors associated with unsuccessful treatment outcome and length of hospital stay.
In the present study, most of the patients with empyema thoracis (68.2%) were male. This finding is consistent with other similar studies conducted in Pakistan (66.1%), India (75.4%) and Canada (64%) (25, 32, 33). The probable reason due to which the males were more vulnerable to disease is having only one copy of X-linked genes responsible for production of immunoglobulins (24, 34). In this study, 58 (52.7%) patients were culture positive. Contrary to this, a study conducted in the US (43%) reported relatively low culture positivity rate (35). Whereas, studies conducted in India (87.2%) and China (68%) showed high percentages (36, 37). The possible reason for low culture positivity might be use of antibiotics prior to sample collection for culture sensitivity test, improper specimen transportation, wrong techniques of sample collection and/or other viral, parasitic and fungal infections (24, 38).
In this study cohort, Pseudomonas (18.1%), and Klebsiella sp. (10%) were the most commonly identified gram- negative isolates. Similarly, the same results were reported by a study conducted in India (8). For the treatment of empyema thoracis, the physicians in our study prescribed piperacillin/tazobactam alone or in combination with cephalosporins, carbapenems and fluoroquinolones which in line with AATS guidelines. But the most common bacterial isolates identified in this study showed high level of resistance against aforementioned antibiotics. Similarly, other studies conducted in Pakistan, India, Hungary, and the US also reported high level of resistance to these antibiotics (39–43). The probable reason for increased antibiotic resistance could be an excess, inappropriate use and careless selection of antibiotics (44), as a number of studies have reported irrational prescribing, dispensing and use of antibiotics in Pakistan (45–48). The rapidly growing antibiotic resistance in Pakistan demand effective implementation of antibiotic stewardship program in accordance with the recommendations of the WHO (49–51). In addition, promotion of One Health, which involves collaboration of all the healthcare professionals responsible for antibiotic use in humans, animals and environment, seems mandatory to halt gradual extinction of effective antibiotics.
It is an important point to notice that in the present study, despite of being aware of the most commonly identified bacterial isolates and their resistance pattern, the physicians neither tailored the antibiotic treatment according to the need of the patient nor according to the culture and sensitivity results. The AATS guidelines recommends piperacillin/tazobactam, cephalosporins, carbapenems and fluoroquinolones to be taken as empiric therapy for empyema thoracis (2), however, this may not be a viable choice here because the most common bacterial isolates showed high level of resistance against these antibiotics (Tables 3, 4). However, amoxicillin/clavulanic acid, ertapenem, clarithromycin, colistin, vancomycin, fosfomycin, rifampicin and tigecycline showed better sensitivity against most common bacterial isolates. Studies from other countries like the US, Italy and Germany also supported the judicious use of aforementioned antibiotics (35, 52–54). The German guideline by The Paul- Ehrlich- Gesellschaft fur Chemotherapie have also suggested the use of amoxicillin/clavulanic acid, ertapenem, clarithromycin, colistin, vancomycin, fosfomycin, rifampicin and tigecycline against most common gram- negative bacterial isolates causing empyema thoracis (54, 55). On the basis of the antibacterial spectrum of these antibiotics against most commonly identified bacterial isolates, vancomycin in combination with amoxicillin/clavulanic acid, sulbactam or clarithromycin and colistin alone or in combination with tigecycline, fosfomycin, rifampicin, sulbactam, or ertapenem seems to be the better option for most common bacterial isolates (52, 54).
With regard to final treatment outcomes, the treatment success rate in this study was 74.5%. Similarly, high success rate was reported in a study from the United Kingdom (UK) (88%) (56). The probable reason for high success rate in the UK study might be due to the less severity of the disease and use of targeted antibiotics earlier (56, 57). We found that the mortality rate in this study was 10% which was comparable with the study of Netherland (8%) (58). However, another study conducted in Pakistan reported relatively less mortality rate (1.3%) (25). Contrary to this, China reported higher mortality rate (33.3%) (59). The major reason for high mortality rate could be poor diagnosis, delay in empiric therapy, resistance to antibiotics, longer duration of hospital stay, harmful habits like smoking, and comorbidities (59). Moreover, a study conducted in the UK showed increased hospital stay (>2 weeks) (56). While, the mean length of hospital stay in our study was 2.3 weeks and these results were almost similar to a study conducted in India (2 weeks) (60). This Indian study showed that shorter duration of hospital stay was observed among those patients who responded better toward their treatment (60).
Our study found that resistance to multiple antibiotics increased the duration of hospital stay and these results were comparable with a study conducted in Vietnam (61). The possible reason for prolonged hospital stay due to multidrug resistance may be due to the fact that such patients have lesser treatment options and clinicians have to repeatedly switch the antibiotic therapy in search of appropriate antibiotics. This study further showed that patients with duration of symptoms <2 weeks prior to hospital visit had less stay in hospital. The probable reasons for lesser stay in hospital in such patients could be the early diagnosis of the disease, and the start of antibiotics (57, 60).
This study has some limitations. First, this study was a single centered study and did not describe the data of the whole country. However, it describes the antibiotic use and resistance pattern, and treatment outcomes in a tertiary care hospital which serves a large population living in the Southern part of the Punjab province of Pakistan. We assume that antibiotic resistance and sensitivity pattern may be similar for whole of the Punjab province of Pakistan. However, multicenter studies with larger sample size are required to warrant this statement. Second, because empyema thoracis is a rare disease, we were unable to gather data from a larger sample. Third, though a valid and widely used statistical analysis was performed to answer the study objectives, a hierarchical cluster analysis was not performed due to unavailability of data in required format which is recommended in future studies.
Conclusion
Close to one fourth of the patients did not complete their treatment successfully. Pseudomonas aeruginosa, Klebsiella sp., and E. coli were the most common bacterial isolates identified in the study cohort. Most of the bacterial isolates showed resistance against empiric treatment. Given the antibacterial spectrum of the tested antibiotics, the combination of vancomycin with amoxicillin/clavulanic acid, sulbactam or clarithromycin, and colistin alone or in combination with other antibiotics like tigecycline, sulbactam, rifampicin, fosfomycin or ertapenem may be considered as the treatment options.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MA, MN, SS, and SM: conceptualization, formal analysis, methodology, and validation. MN: data curation. MA: supervision. MA, MN, SS, SM, MH, IM, MNI, and NA: writing—original draft and writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.665963/full#supplementary-material
References
1. Iguina MM, Danckers M. Thoracic Empyema. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2021).
2. Shen KR, Bribriesco A, Crabtree T, Denlinger C, Eby J, Eiken P, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thoracic Cardiovasc Surg. (2017) 153:e129–46. doi: 10.1016/j.jtcvs.2017.01.030
4. Jaffé A, Cohen G. Thoracic empyema. Arch Dis Childhood. (2003) 88:839–41. doi: 10.1136/adc.88.10.839
5. Lehtomäki A, Nevalainen R, Ukkonen M, Nieminen J, Laurikka J, Khan J. Trends in the incidence, etiology, treatment, and outcomes of pleural infections in adults over a decade in a Finnish University Hospital. Scand J Surg. (2020) 109:127–32. doi: 10.1177/1457496919832146
6. Shankar G, Sahadev R, Santhanakrishnan R. Pediatric empyema thoracis management: should the consensus be different for the developing countries? J Pediatric Surg. (2020) 55:513–7. doi: 10.1016/j.jpedsurg.2019.08.009
7. Pogorelić Z, Bjelanović D, Gudelj R, Jukić M, Petrić J, Furlan D. Video-assisted thoracic surgery in early stage of pediatric pleural empyema improves outcome. Thorac Cardiovasc Surg. (2020) doi: 10.1055/s-0040-1708475
8. Das NN, Lakhotia S, Verma A. Surgical outcome of empyema thoracis patients with special correlation to pre-operative contrast-enhanced computerized tomography (CECT) thorax morphometry Indian J Thorac Cardiovasc Surg. (2020) 37:164–74. doi: 10.1007/s12055-020-01053-5
9. Ahmad R, Bhatti DS, Bokhari MHT, Asad A. A university hospital based study on thoracic trauma: life threatening event, its etiology, presentation, and management. Cureus. (2019) 11:e6306. doi: 10.7759/cureus.6306
10. Kang J, Sickbert-Bennett EE, Brown VM, Weber DJ, Rutala WA. Changes in the incidence of health care-associated pathogens at a university hospital from 2005 to 2011. Am J Infect Control. (2014) 42:770–5. doi: 10.1016/j.ajic.2014.03.019
11. Matta R, Hallit S, Hallit R, Bawab W, Rogues A-M, Salameh P. Epidemiology and microbiological profile comparison between community and hospital acquired infections: a multicenter retrospective study in Lebanon. J Infect Public Health. (2018) 11:405–11. doi: 10.1016/j.jiph.2017.09.005
12. Ungureanu A, Zlatian O, Mitroi G, Drocaş A, Tîrcă T, Călina D, et al. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol Med Rep. (2017) 16:8771–80. doi: 10.3892/mmr.2017.7746
13. Zlatian O, Balasoiu AT, Balasoiu M, Cristea O, Docea AO, Mitrut R, et al. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp Ther Med. (2018) 16:4499–510. doi: 10.3892/etm.2018.6737
14. Ansarie M, Kasmani AJ. Community acquired pneumonia in Pakistan: an analysis on the literature published between 2003 and 2013. J Pak Med Assoc. (2014) 64:1405–9.
15. Iqbal N, Irfan M, Siddique F, Arshad V, Zubairi ABS. Factors predicting in-hospital mortality among patients admitted with community acquired pneumonia at a tertiary care hospital Karachi, Pakistan. Clinic Respir J. (2020). 14:328–34. doi: 10.1111/crj.13137
16. DeSuza K, Bush LM. Empyema necessitans due to methicillin-sensitive staphylococcus aureus: case report and review. Infect Dis Clin Pract. (2020) 28:130–3. doi: 10.1097/IPC.0000000000000839
17. Watkins RR, Lemonovich TL. Diagnosis and management of community-acquired pneumonia in adults. Am Fam Physician. (2011) 83:1299–306.
18. Reta A, Bitew Kifilie A, Mengist A. Bacterial infections and their antibiotic resistance pattern in ethiopia: a systematic review. Adv Preventive Med. (2019) 2019. doi: 10.1155/2019/4380309
19. Towe CW, Srinivasan S, Ho VP, Bachmann K, Worrell SG, Perry Y, et al. Antibiotic resistance is associated with morbidity and mortality after decortication for empyema. Ann Thorac Surg. (2020) 111:206–13. doi: 10.1016/j.athoracsur.2020.06.056
20. Ullah K, Baloch M, Saleem F, Khan MA, Saeed H, Hashmi FK, et al. Patterns of physicians' knowledge, attitude and prescribing trends against upper respiratory tract infections in Lahore, Pakistan. Pak J Pharm Sci. (2020) 33:1889–98.
21. Khan FU, Khan FU, Hayat K, Chang J, Saeed A, Khan Z, et al. Knowledge, attitude and practices among consumers toward antibiotics use and antibiotic resistance in Swat, Khyber-Pakhtunkhwa, Pakistan. Expert Rev Anti Infect Ther. (2020) 18:937-46. doi: 10.1080/14787210.2020.1769477
22. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS ONE. (2017) 12:e0189621. doi: 10.1371/journal.pone.0189621
23. Aku FY, Akweongo P, Nyarko K, Sackey S, Wurapa F, Afari EA, et al. Bacteriological profile and antibiotic susceptibility pattern of common isolates of neonatal sepsis, Ho Municipality, Ghana-2016. Maternal Health, Neonatol Perinatol. (2018) 4:2. doi: 10.1186/s40748-017-0071-z
24. Atif M, Zia R, Malik I, Ahmad N, Sarwar S. Treatment outcomes, antibiotic use and its resistance pattern among neonatal sepsis patients attending Bahawal Victoria Hospital, Pakistan. PLoS ONE. (2021) 16:e0244866. doi: 10.1371/journal.pone.0244866
25. Majeed FA, Zafar U, Chatha SS, Ali A, Raza A. Decortication as an option for empyema thoracis. J College Phys SurgPakistan. (2020) 30:313–7. doi: 10.29271/jcpsp.2020.03.313
26. Saleem AF, Shaikh AS, Khan RS, Khan F, Faruque AV, Khan MAM. Empyema thoracis in children: clinical presentation, management and complications. J Coll Physicians Surg Pak. (2014) 24:573–6.
27. Atif M, Ahmad W, Ahmad N, Malik I, Sarwar S. Treatment outcomes among multidrug-resistant TB patients in Bahawal Victoria Hospital, Bahawalpur, Pakistan: a retrospective record review. Transact R Soc Trop Med Hygiene. (2020) 114:733–41. doi: 10.1093/trstmh/traa040
28. Menzies SM, Rahman NM, Wrightson JM, Davies HE, Shorten R, Gillespie SH, et al. Blood culture bottle culture of pleural fluid in pleural infection. Thorax. (2011) 66:658–62. doi: 10.1136/thx.2010.157842
29. Walters J, Foley N, Molyneux M. Pus in the thorax: management of empyema and lung abscess. BJA education. (2011) 11:229–33. doi: 10.1093/bjaceaccp/mkr036
30. Calik M, Calik SG, Dagli M, Kesli R, Esme H. Pleural fluid penetration of moxifloxacin and doripenem: An experimental model of empyema. Northern Clin Istanbul. (2020) 7:99. doi: 10.14744/nci.2019.05902
31. Brims FJ, Lansley SM, Waterer GW, Lee YC. Empyema thoracis: new insights into an old disease. Eur Respir Rev. (2010) 19:220–8. doi: 10.1183/09059180.00005610
32. Pulle MV, Asaf BB, Kumar A, Puri HV, Vijay C, Bishnoi S. Microbiological profile of tubercular and nontubercular empyemas and its impact on clinical outcomes: a retrospective analysis of 285 consecutively operated cases. Lung India. (2020) 37:389–93. doi: 10.4103/lungindia.lungindia_553_19
33. Ayala A, Uribe J, Mitchell M, Majid A, Parikh M, Chee A. Institutional empyema trend analysis: an increasing concern. A59 clinical diagnosis, prediction and outcomes of lung infections. Am Thorac Soc. (2020) A2153-A. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2153
34. Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. (2020) 34: 339–43. doi: 10.23812/Editorial-Conti-3
35. Towe CW, Srinivasan S, Ho VP, Bachmann K, Worrell SG, Perry Y, et al. Antibiotic Resistance Is Associated With Morbidity and Mortality After Decortication for Empyema. Ann Thorac Surg. (2021) 111:206–13.
36. Karmakar S, Karmakar S, Prasad R, Kant S, Nath A, Mahdi F. Clinical and microbiological characteristics of thoracic empyema: retrospective analysis in a tertiary care centre. Int J Adv Med. (2017) 4:1309. doi: 10.18203/2349-3933.ijam20174166
37. Tsang K, Leung W, Chan VL, Lin AW, Chu C. Complicated parapneumonic effusion and empyema thoracis: microbiology and predictors of adverse outcomes. Hong Kong Med J. (2007) 13:178–8.
38. Mohsen L, Ramy N, Saied D, Akmal D, Salama N, Haleim MMA, et al. Emerging antimicrobial resistance in early and late-onset neonatal sepsis. Antimicrob Resist Infect Control. (2017) 6:63. doi: 10.1186/s13756-017-0225-9
39. Afzal M. Antibiotic resistance pattern of escherichia coli and klebsiella species in pakistan: a brief overview. J Microb Biochem Technol. (2017) 9:277–9.
40. MS D, Mansuri F, Chaudhari M, Marathe A, Vaghasiya J, Nath M. Development of antibiogram for evaluation of antibiotic resistance pattern in tertiary care teaching hospital: a cross sectional study (2020).
41. Tóth H, Fésus A, Kungler-Gorácz O, Balázs B, Majoros L, Szarka K, et al. Utilization of vector autoregressive and linear transfer models to follow up the antibiotic resistance spiral in Gram-negative bacteria from cephalosporin consumption to colistin resistance. Clin Infect Dise. (2019) 69:1410–21. doi: 10.1093/cid/ciy1086
42. Eichenberger EM, Thaden JT. Epidemiology and mechanisms of resistance of extensively drug resistant Gram-negative bacteria. Antibiotics. (2019) 8:37. doi: 10.3390/antibiotics8020037
43. Maddocks S, Fabijan AP, Ho J, Lin RC, Ben Zakour NL, Dugan C, et al. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by Pseudomonas aeruginosa. Am J Respir Critical Care Med. (2019) 200:1179–81. doi: 10.1164/rccm.201904-0839LE
44. Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. (2012) 25:450–70. doi: 10.1128/CMR.05041-11
45. Atif M, Ihsan B, Malik I, Ahmad N, Saleem S, Sehar A, et al. Antibiotic stewardship program in Pakistan: a multicenter qualitative study exploring doctors' knowledge, perception and practices. BMC Infect Dis. (2021) 21:374. doi: 10.1186/s12879-021-06043-5
46. Atif M, Asghar S, Mushtaq I, Malik I. Community pharmacists as antibiotic stewards: a qualitative study exploring the current status of Antibiotic Stewardship Program in Bahawalpur, Pakistan. J Infect Public Health. (2020) 13:118–24. doi: 10.1016/j.jiph.2019.07.003
47. Malik I, Atif M, Riaz F, Asghar S, Ahmad N. Pediatric antibiotic pack size compliance with the dosage regimen: a descriptive study. Therap Innovat Regul Sci. (2020) 54:492–506. doi: 10.1007/s43441-019-00081-7
48. Malik I, Atif M. Global menace of superbugs: time to consider a “Pharmacist led One Health Approach” to counteract the crisis. Res Social Adm Pharm. (2020) 16:848–9. doi: 10.1016/j.sapharm.2020.02.018
49. Asghar S, Atif M, Mushtaq I, Malik I, Hayat K. Factors associated with inappropriate dispensing of antibiotics among non-pharmacist pharmacy workers. Res Soc Admin Pharm. (2019)16:805–11. doi: 10.1016/j.sapharm.2019.09.003
50. Atif M, Asghar S, Mushtaq I, Malik I, Amin A, Babar Z-U-D, et al. What drives inappropriate use of antibiotics? A mixed methods study from Bahawalpur, Pakistan. Infect Drug Resist. (2019) 12:687–99. doi: 10.2147/IDR.S189114
51. Malik I, Atif M, Scahill SL, Babar ZU. Pharmacy Practice and policy research in pakistan: a review of literature between 2014 and 2019. In: ZUD. B, editor. Global Pharmaceutical Policy. Singapore: Palgrave Macmillan. (2020) p. 139–75. doi: 10.1007/978-981-15-2724-1_6
52. Grabein B, Ebenhoch M, Kühnen E, Thalhammer F. Calculated parenteral initial treatment of bacterial infections: Infections with multi-resistant Gram-negative rods–ESBL producers, carbapenemase-producing Enterobacteriaceae, carbapenem-resistant Acinetobacter baumannii. GMS Infect Dis. (2020) 8:Doc04. doi: 10.3205/id000048
53. Ott SR, Bodmann K-F, Grabein B, Höffken G, Kolditz M, Lode H, et al. Calculated parenteral initial treatment of bacterial infections: respiratory infections. GMS Infect Dis. (2020) 8:Doc15. doi: 10.3205/id000059
54. Bassetti M, Righi E. New antibiotics and antimicrobial combination therapy for the treatment of gram-negative bacterial infections. Curr Opin Critic Care. (2015) 21:402–11. doi: 10.1097/MCC.0000000000000235
55. Bodmann K-F, Grabein B, Kresken M. S2k guideline “Calculated parenteral initial treatment of bacterial infections in adults–update 2018”, 2nd updated version: Foreword. GMS Infect Dis. (2020) 8:Doc20. doi: 10.3205/id000064
56. Peters RT, Parikh DH, Singh M. Thoracoscopic debridement for empyema thoracis. J Pediatric Surg. (2020) 55:2187–90. doi: 10.1016/j.jpedsurg.2020.02.004
57. Whitehouse A, Thavagnanam S, Nwokoro C, Brown S, Pao C. Rapid identification of bacterial infection in children with empyema. D24 lung infection. Am Thorac Soc. (2020) A6341-A. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A6341
58. van Middendorp LB, Franssen S, Gillissen S, Maessen JG, Hulsewé KW, Vissers YL, et al. Uniportal video-assisted thoracoscopy is a safe approach in patients with empyema requiring surgery. J Thorac Dis. (2020) 12:1460–6. doi: 10.21037/jtd.2020.02.29
59. Fhong E, Qian H, Hou C, Li J, Qian X, Zou L, et al. Clinical characteristics and related factors of fungal empyema thoracis. Preprint. (2021) doi: 10.21203/rs.3.rs-43519/v1
60. Giron Matute W, Dominguez Zabaleta I, Suarez Escudero S, Recio Moreno B, De Miguel Diez J, Puente-Maestu L. Pleural empyema in hospitalization and mortality risk: application of RAPID score. C52 studies in bronchiectasis, HIV, and Lung Microbiome, Am Thorac Soc. (2020) A5423-A. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A5423
61. Peters L, Olson L, Khu DT, Linnros S, Le NK, Hanberger H, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS ONE. (2019) 14:e0215666. doi: 10.1371/journal.pone.0215666
Keywords: drug resistance, antimirobial, antibiotic resisitance, infectious disease, respiratory disease
Citation: Atif M, Naseem M, Sarwar S, Mukhtar S, Malik I, Hassan MRu, Iqbal MN and Ahmad N (2021) Spectrum of Microorganisms, Antibiotic Resistance Pattern, and Treatment Outcomes Among Patients With Empyema Thoracis: A Descriptive Cross-Sectional Study From the Bahawal Victoria Hospital Bahawalpur, Punjab, Pakistan. Front. Med. 8:665963. doi: 10.3389/fmed.2021.665963
Received: 09 February 2021; Accepted: 28 June 2021;
Published: 06 August 2021.
Edited by:
Walid Alali, Kuwait University, KuwaitReviewed by:
Daniela Calina, University of Medicine and Pharmacy of Craiova, RomaniaHaroon Ahmed, COMSATS University, Islamabad Campus, Pakistan
Liliane Okdah, King Abdullah International Medical Research Center (KAIMRC), Saudi Arabia
Copyright © 2021 Atif, Naseem, Sarwar, Mukhtar, Malik, Hassan, Iqbal and Ahmad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Atif, cGhhcm1hY2lzdF9hdGlmQHlhaG9vLmNvbQ==
 Muhammad Atif
Muhammad Atif Mehwish Naseem
Mehwish Naseem Sajjad Sarwar2
Sajjad Sarwar2 Iram Malik
Iram Malik Nafees Ahmad
Nafees Ahmad