
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 26 October 2021
Sec. Obstetrics and Gynecological Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.663287
 Xiao Fu1,2,3
Xiao Fu1,2,3 Xiaojie Liu1,2,3
Xiaojie Liu1,2,3 Jing Li1,2,3
Jing Li1,2,3 Meng Zhang1,2,3
Meng Zhang1,2,3 Jingjing Jiang2,3
Jingjing Jiang2,3 Qianqian Chen2,3
Qianqian Chen2,3 Mei Li2,3
Mei Li2,3 Shanshan Gao1,2,3*
Shanshan Gao1,2,3* Jinlong Ma2,3*
Jinlong Ma2,3*Objective: The objective of this study was to provide a descriptive analysis of the clinical outcomes achieved in oocyte vitrification in cases where sperm was unavailable on oocyte retrieval day, and to identify predictors of oocyte survival.
Methods: This retrospective cohort study used data from a university-affiliated reproductive medical center. There were 321 cycles in which some of, or all oocytes were vitrified owing to the unavailability of sperm between March 2009 and October 2017. A descriptive analysis of the clinical outcomes including both fresh embryo transfers and cryopreserved embryo transfers was provided. The ability of an individual parameter to forecast oocyte survival per thawing cycle was assessed by binary logistic regression analysis. The cumulative probability of live birth (CPLB) was estimated by using the Kaplan-Meier method according to the total number of oocytes thawed in consecutive procedures.
Results: The average survival rate was 83.13%. High-quality embryo rate and blastocyst rate decreased significantly decreased significantly in vitrification oocyte group compared to fresh control oocytes. The comparison of sibling oocytes in part-oocyte-vitrified cycles shows fewer high-quality embryos developed in the vitrified group. The live birth rate per warmed-oocyte was 4.3%. Reasons for lack of sperm availability on oocyte retrieval day and serum cholesterol levels were found to be associated with oocyte survival rate in the present study. Kaplan-Meier analysis showed no significant difference in CPLB between patients ≤35 vs. >35 years.
Conclusions: Oocyte vitrification is an indispensable and effective alternative when sperm are not available on oocyte retrieval day. The present study provided evidence that oocytes from infertile couples were more likely to suffer oocyte/embryo vitrification injury. Clinicians need to take this into account when advising patients in similar situations. Further studies will be necessary to clarify the correlation between serum metabolism parameters and human oocyte survival after vitrification.
Oocyte freezing is no longer considered an experimental method by the American Society for Reproductive Medicine (1). Oocyte vitrification is gradually becoming a useful adjunct to routine in vitro fertilization (IVF) in various clinical scenarios such as the unavailability of sperm at the time of egg retrieval (2–4), and for couples who do not wish to cryopreserve supernumerary embryos in cases where plenty of oocytes are retrieved (5). Another indication for oocyte vitrification that has now become a reality is the establishment of donor oocyte banks (6–8). Oocyte cryopreservation for deferring child-bearing and fertility preservation in cancer patients has also entered clinical practice (9–12).
Reports of donor oocyte vitrification have so far been encouraging. In a sibling cohort study of recipient cycles, similar embryo development has been shown from fresh vs. vitrified oocytes (13). Several well-controlled studies involving donor oocytes have shown that clinical outcomes with vitrified oocytes are comparable to those with fresh oocytes (7, 14–16). A large study of a donation program reported by Cobo et al. has demonstrated comparable obstetric and perinatal outcomes from vitrified vs. fresh oocytes (17). These results have confirmed the further application of oocyte vitrification in assisted reproduction treatment for medical indications.
Although oocyte vitrification has been demonstrated as a successful and stable technique in donor programs, these results might provide overly optimistic evidence for oocyte vitrification where there are medical indications in infertile patients. Different oocyte sources may have varying inherent qualities that affect vitrification outcomes (9, 18). However, reports related to autologous oocyte vitrification in infertile patients are few and inconsistent (12, 19). A study of sibling oocytes from 44 patients undergoing IVF showed reduced rates of fertilization and embryo development after oocyte vitrification (19). Another study included 128 autologous vitrified/warmed oocyte cycles from IVF cycles and demonstrated significantly higher implantation rates (43 vs. 35%) and clinical pregnancy (57 vs. 44%) with vitrified-warmed compared to fresh oocytes (12).
This study aims to describe the outcomes we have achieved in our 8-year experience of oocyte vitrification owing to unavailable sperm on oocyte retrieval day. Analyses were performed to find relevant factors regarding oocyte survivability. This relatively large data set adds to the limited information currently available regarding the clinical application of vitrified autologous oocytes for medical indications.
The ethics committee at the Center for Reproductive Medicine, Shandong University approved this clinical application. Couples chose oocyte cryopreservation because of the unavailability of sperm at the time of oocyte retrieval as an alternative to using donor semen. The control group consisted of age and Body Mass Index (BMI)-matched patients, who were undergoing intracytoplasmic sperm injection (ICSI) treatment for male factor infertility (Figure 1).
This study included two commercially available kits: the MediCult (MC) kit (MediCult Vitrification Cooling, Copenhagen, Denmark) and Kitazato (KT) kit (Kitazato Biopharma Co., Ltd., Shizuoka, Japan), and one Modified kit prepared in our lab. The penetrating cryoprotectants in the MC kit were ethylene glycol (EG) and 1,2-propanediol (PROH). The KT kit included EG and dimethyl sulphoxide (DMSO). The Modified kit was made up of three types of cryoprotectants: ethylene glycol (Sigma-Aldrich, St. Louis, MO, 102466, USA), DMSO (Sigma-Aldrich, St. Louis, MO, D2650, USA), and PROH (Sigma-Aldrich, St. Louis, MO, 544324-068, USA). As for the MC kit and KT kit, equilibration solution included 7.5% EG + 7.5% PROH (DMSO), and vitrification solution included 15% EG + 15% PROH (DMSO) + 0.5 mol/L sucrose, per the instructions. The Modified kit was prepared with M-199 (Gibco Invitrogen Corp., Grand Island, NY, USA) as the basal medium. A 20% serum plasma substitute (SPS) (SAGE, Trumbull, CT, USA) was also added. The equilibration solution for the Modified kit comprised 7.5% EG + 3.75% DMSO + 3.75% PROH, and the vitrification solution comprised 15% EG + 7.5% DMSO + 7.5% PROH + 0.5 mol/L sucrose in a M-199 medium with 20% SPS (20).
Oocyte vitrification was performed at room temperature (RT). The oocytes were equilibrated in ES for 5–10 min until they recovered their shape, and then they were placed into the VS for 1 min. Finally, the vitrified oocytes were placed on a CryoLoop (Hampton Research, Laguna Niguel, CA, USA) and immediately immersed in liquid nitrogen. No more than four oocytes were loaded onto each CryoLoop. Oocyte warming was performed at RT, except for the first step. The CryoLoop with the vitrified oocytes was taken out of the liquid nitrogen and immediately placed in 1.0 mol/L sucrose in a M-199 + 20% SPS solution at 37°C for 1.5–2.0 min. Next, the oocytes were placed in 0.5 mol/L sucrose in an M-199 + 20% SPS solution for 3 min at RT, after which they were transferred into another M-199 solution with 0.25 mol/L sucrose for 3 min. Finally, they were washed in M-199 + 20% SPS for 5-10 min while the stage was warmed slowly. After warming, the surviving oocytes were cultured for 2 h in G-IVF (Vitrolife, Göteborg, Sweden) in an incubator at 37°C, 6% CO2 before being inseminated using ICSI (20). Embryo transfer was performed on Day 2 or 3 depending on embryo quality or quantity. No more than three embryos were included in each transfer. The supernumerary embryos were cultured into blastocysts, and high-quality blastocysts were vitrified.
All patients used hormone replacement therapy as the endometrial preparation protocol, which has been described in a previous study (21). In short, 4–8 mg of oral estradiol valerate (Progynova, Bayer, Germany) was administered daily for at least 10 days starting on Day 2–5 of the menstrual cycle. When the endometrial thickness reached ≥8 mm, oral progesterone (Dydrogesterone, Solvay, the Netherlands) 20 mg twice daily plus vaginal micronized progesterone (Utrogestan, Besins Manufacturing Belgium) 200 mg once daily was initiated on the day of oocyte warming. Clinical pregnancy was determined as the presence of a gestational sac identified by vaginal or abdominal ultrasound 4–5 weeks after embryo transfer (ET). Gestational age, birth weight, and congenital malformation outcomes were followed-up.
The main outcome measurements were survival rate and the cumulative live birth rate (including live birth from fresh ETs and subsequent cryo-ETs) per warming cycle. The secondary outcome measures included laboratory outcomes of vitrified-warmed oocytes, implantation, clinical pregnancy rates, and the delivery rate per fresh embryo transfer and vitrified embryo transfer, as well as gestational age, birth weight, and congenital malformation outcomes.
The difference in means and prevalence among the groups were analyzed by Student's t-test for continuous data and Chi square for categorical data. A P-value < 0.05 was considered statistically significant. A binary logistic regression model was performed to identify predictable parameters of oocyte survival per thawing cycle. The oocyte-to-baby rate was calculated by dividing the number of live births by the total number of oocytes consumed × 100. The cumulative probability of live birth (CPLB) was estimated by the Kaplan-Meier method according to the total number of oocytes thawed in consecutive procedures, including oocytes from canceled ETs and from fresh or cryo-ETs, until a live birth was achieved.
Three hundred and twenty-one vitrified-warmed-oocyte cycles were carried out from March 2009 to October 2017 owing to unavailable sperm on oocyte retrieval day. These oocytes had previously been vitrified between 2007 and 2013. The incidence rate was around 0.3–0.5% in all IVF /ICSI cycles over the time period. The majority of cases for all-oocyte-vitrification were owing to unavailable sperm on the day from ejaculated samples or surgical sperm extraction (73.23%) (Table 1). The median age of female patients at oocyte vitrification was 30.24 years (95% CI 29.82–30.89). The median preservation duration of oocyte vitrification was 6.52 months (95% CI 5.56–7.62). Overall oocyte survival rate was 83.13% (95% CI 81.81–86.35%). Data were also obtained from age and BMI matched controls undergoing fresh ICSI cycles for severe male factor with autologous oocytes. Similar fertilization rates were shown between the vitrified and fresh groups, while the high-quality embryo rate and blastocyst rate decreased significantly in the vitrified group (Table 2).
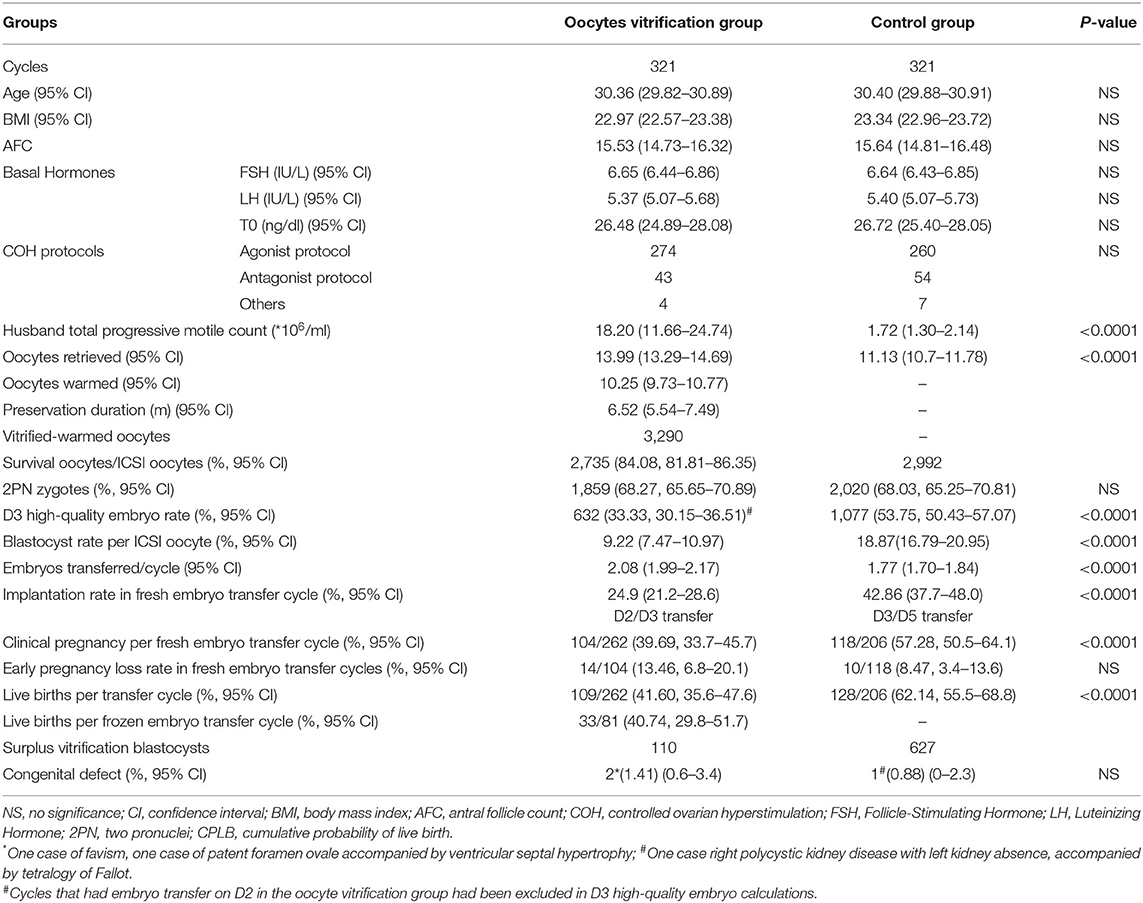
Table 2. Comparison of baseline characteristics, laboratory outcome, and clinical outcome between the oocyte vitrification group and control group.
Table 3 shows information from the two groups divided by median survival rate (91.67%). Serum total cholesterol in the ≥91.67% survival group was higher. The blood glucose level was also higher in the ≥91.67% survival group. There were more cycles owing to absolute male factors included in the ≥91.67% survival group. And finally, the preservation time was longer in the <91.67% survival group.
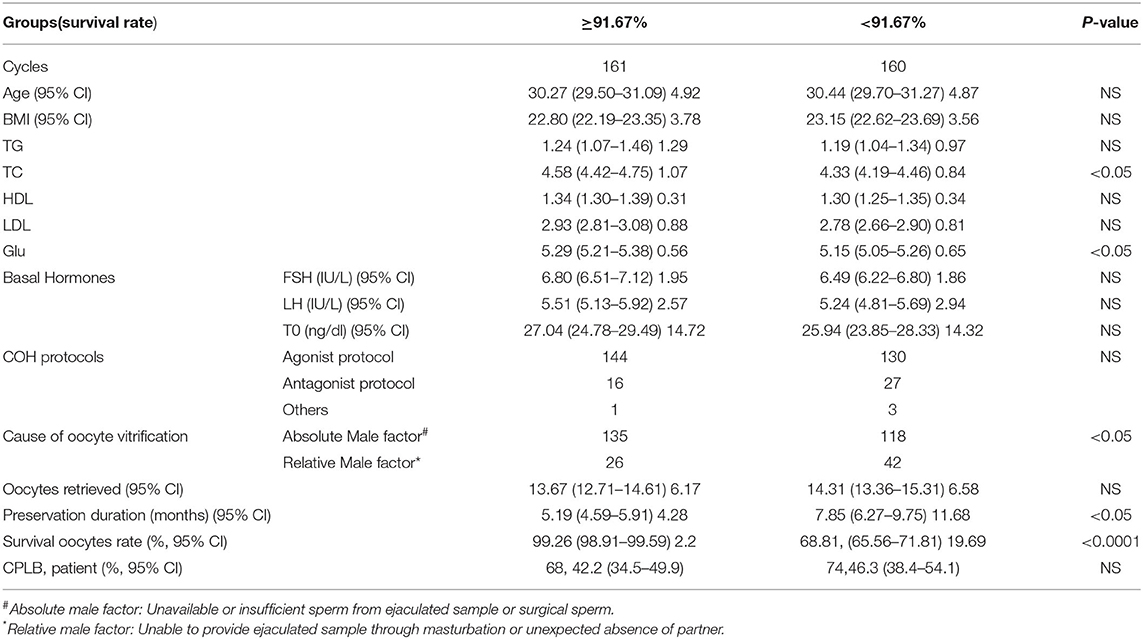
Table 3. Patient and cycle characteristics and CPLB in the two oocyte vitrification groups divided by the median survival rate (91.67%).
Table 4 gives a comparison between the two oocyte vitrification groups divided by different reasons for lack of sperm availability on oocyte retrieval day. The relative male factor group (owing to an inability to provide an ejaculated sample through masturbation or unexpected absence of partner) presented a higher serum triglyceride level. Oocyte survival rate was higher in the absolute male factor group (owing to unavailable or insufficient sperm from an ejaculated sample or surgical collection).
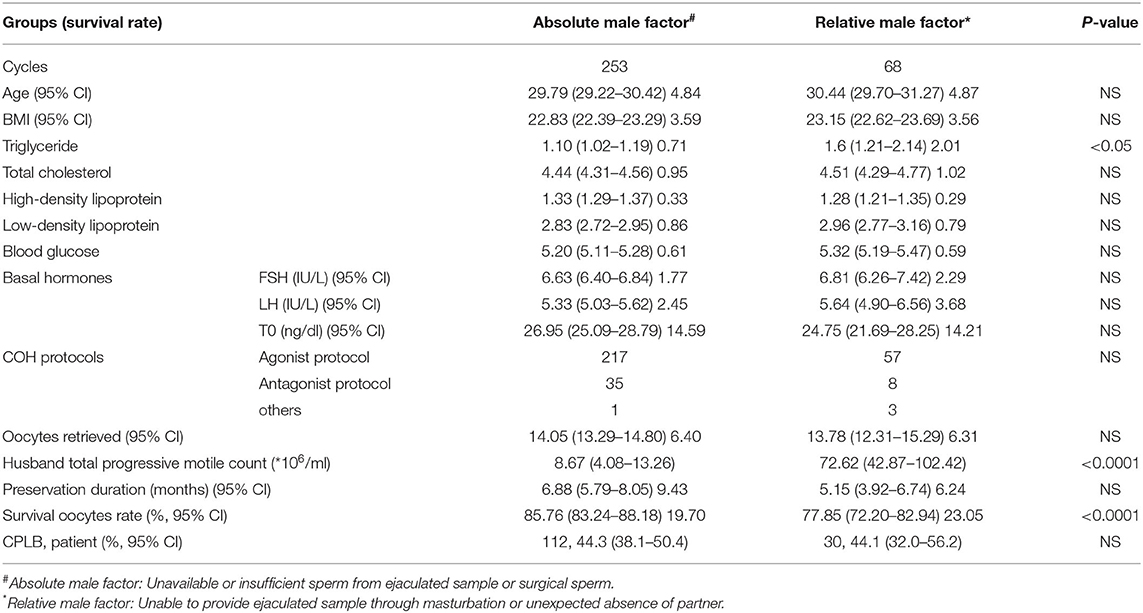
Table 4. Patient and cycle characteristics and CPLB in the two oocyte vitrification groups sorted by different reasons for lack of sperm availability on oocyte retrieval day.
Table 5 shows the comparison of sibling oocytes between fresh and vitrified groups in part-oocyte-vitrified cycles. A total of 67 cycles had a portion of oocytes vitrified because of male factors. Forty-one cases were inseminated with the husband's sperm in both fresh oocytes and vitrified oocytes. No significant difference was found between the vitrification and fresh groups of sibling oocytes in fertilization rate (66.93 vs. 59.77%), but fewer high-quality embryos developed in the vitrified group (27.68 vs. 53.46%).
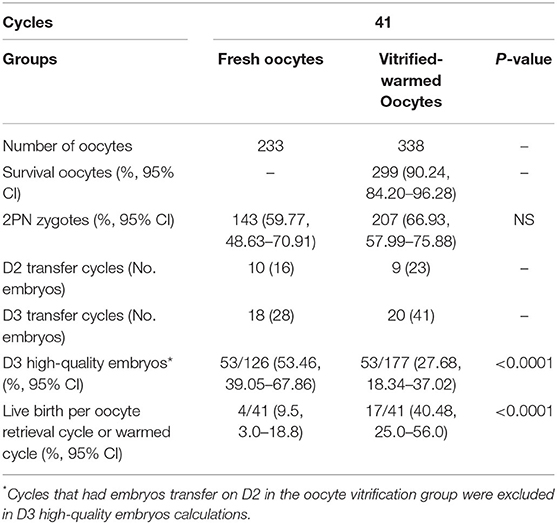
Table 5. Sibling oocytes compared in part-oocyte-vitrified cycles (survival of oocytes inseminated with sperm from the husband).
Table 6 shows the clinical outcomes according to different sperm sources after oocyte warming in all-oocyte-vitrified cycles. Among 254 cycles, the warmed oocytes were fertilized with the husbands' sperm, testicular sperm aspiration/percutaneous epididymal sperm aspiration (PESA/TESA) sperm, and frozen donor sperm in 150, 46, and 58 cycles, respectively. The frozen donor sperm group showed a better high-quality-embryo rate than the husbands' sperm and the PESA/TESA sperm group (40.94 vs. 32.76%; 40.94 vs. 31.95%).
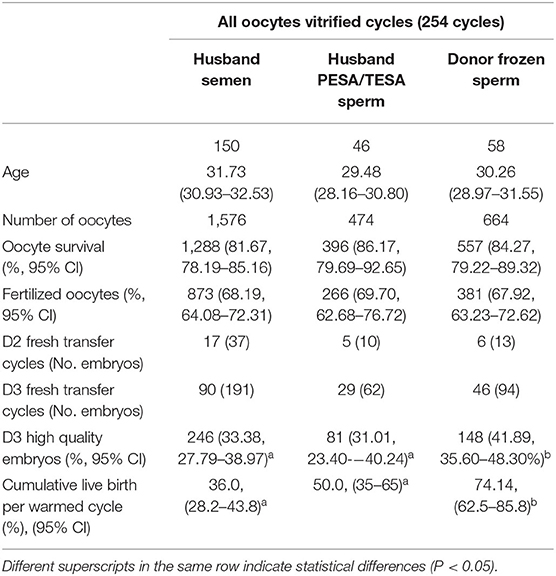
Table 6. Clinical outcomes according to different sperm sources after oocyte warming in all-oocyte-vitrified cycle groups.
There were 81 vitrified embryo transfer cycles, including 53 double frozen transfers (i.e., vitrified oocyte and vitrified embryo) cycles and 28 triple frozen (i.e., vitrified oocyte, frozen sperm, and vitrified embryo) transfer cycles, which yielded 22 and 11 neonates, respectively. The delivery rate per transfer, gestational age, birth weight, and congenital malformation outcomes were similar among groups.
One hundred and forty-two babies were born as a result of 262 fresh ETs and 81 subsequent cryo-ETs. The cumulative live birth rate per warming cycle was 41.40%. The oocyte-to-baby rate was 4.3%. At the end of the present study, 110 blastocysts remained cryopreserved from the oocyte warming cycles included in this work. Assuming that the delivery rates are maintained with this cohort, a rough estimation after their use could yield an outcome of 36 additional babies, which would enhance the oocyte-to-baby rate to 5.4%. The Kaplan-Meier analysis showed no significantly different CPLB between patients ≤35 vs. >35 years (Log-rank (Mantel-Cox); P = 0.231; Breslow (generalized-Wilcoxon); P = 0.458; and Tarone–Ware; P = 0.388). The CPLB improved when more oocytes were warmed and the curve for older patients reached a plateau earlier (with 15 oocytes) than those for young women (with 23 oocytes) (Figure 2).

Figure 2. The cumulative probability of live birth according to age ( ≤35 vs. >35 year) and number of oocytes thawed.
A binary logistic regression model was performed to find predictable parameters of oocyte survival per thawing cycle. Several parameters were introduced into the initial model as predictors, including age, BMI, metabolic indicators (including triglyceride, total cholesterol, high density lipoprotei, etc.), basic hormones, infertility years, polycystic ovary syndrome (PCOS)/non-PCOS, endometriosis/non-endometriosis, ovarian stimulation protocols, reason for lack of sperm availability, vitrification kits, and storage duration. As shown by the odds ratio (OR), the effect of reason for lack of sperm availability was acknowledged, and the effect of serum TC on survival was suggested (Supplementary Table 2).
Given that oocyte cryopreservation techniques have changed from slow freezing to vitrification according to the safety and efficacy of reports over the past decade (22), oocyte vitrification has been gradually introduced into assisted reproduction treatment in various clinical scenarios. In particular, oocyte vitrification is becoming an indispensable alternative technique for couples who do not have sufficient available sperm at the time of egg retrieval (18). Our study represents the findings of the largest data set from a single center in China of vitrified autologous oocytes, which were obtained from couples who lacked available sperm at the time of egg retrieval. This report comprises 321 oocyte-vitrification-warming cycles. A total of 142 healthy babies born from fresh and frozen embryo transfer and the cumulative live birth rate per warming cycle was 44.24%. We found that oocyte survival was better in those couples with absolute male factors.
Different oocyte sources, including cancer patients, women desiring fertility preservation, oocyte donors, or infertile patients, may exist with different inherent qualities that influence vitrification outcomes (3, 18, 21, 23). The current study added more information, which is not optimistic, regarding oocyte vitrification in infertile patients. Inconsistent with previous reports regarding recipient oocyte vitrification cycles (13, 17, 24), in the present study, the high-quality embryo rate and blastocyst rate in vitrified oocytes decreased significantly compared with fresh oocytes (Table 2). Similar outcomes were found in the sibling oocyte comparison from part-oocyte-vitrified cycles (Table 5). Further evidence in the present study was from the comparison between groups with different reasons for the lack of sperm availability. Survival rate was significantly higher in the absolute male factor group; this may be because the women in this group were relatively “fertile” and might have had higher quality oocytes (Table 4). In the all-oocyte-vitrified cycles, which were divided into three groups depending on the sperm resource used after oocyte warming, the donor sperm group showed better embryo quality compared with the husband sperm groups. The explanation here may be that both oocytes and sperm in this group were from relatively “fertile” individuals (Table 6). Literature also reports similar results; that vitrification could damage oocyte potential from infertile women in egg-sharing or autologous oocyte vitrification programs (25, 26). All these outcomes demonstrate that oocytes from infertile women are more vulnerable to vitrification injury and might not easily survive the vitrification-warming procedure.
In order to obtain more referential information for clinical work, we tried to find some useful predictors of successful outcome following oocyte vitrification. Age was firstly taken into consideration. However, in the present study, no significant difference was discovered between the two age groups (≤35 vs. >35 years) in survival rate, fertilization rate, and high-quality embryo rate (Supplementary Table 1). These results were confirmed by the Kaplan-Meier analysis of CPLB according to different age groups. No significant difference was observed between the two age groups (Figure 1). This result was inconsistent with previous studies (7, 9, 13, 17), most probably owing to the small sample size and characteristics of the older patients involved in the present work. Only 50 (15.68%) patients >35 years old were included, because most advanced age couples are more inclined to choose donor sperm in cases of unavailable sperm on oocyte retrieval day. The average number of retrieved oocytes in this older age group was 11.46 (95% CI 9.70–13.22), which indicated a better ovarian reserve of these patients than their peers and further explained the insignificant difference between the two age groups. However, we observed that the older patients' curve reached a plateau earlier than for younger women, which agreed with other studies (9, 13, 17).
The average survival rate in the present study was 83.13% (95% CI 81.81–86.35%), comparable to the published data range from 68.6 to 96.8% (12, 26–29) in the literature. We compared two groups divided by the median survival rate (91.67%). Statistical differences were found in the serum total cholesterol, the proportion of different reasons for lack of sperm availability, and preservation time. The outcomes were partially consistent with multiple logistic regression analysis. As shown by the adjusted OR, the effect of the reason for lack of sperm availability was reassuring. Another parameter entered in the model was serum TC, which had not previously been analyzed in human oocyte vitrification studies. A higher serum TC level was found to be favorable for oocyte survivability after vitrification. Cholesterol is known to be the major non-polar lipid in mammalian cell membranes (30). Modulation of plasma membrane cholesterol to increase post-cryopreservation survival is currently a new topic in mammalian oocyte vitrification (31–33). Large prospective research studies are needed to confirm whether serum lipid levels or other metabolic parameters, are relevant to oocyte survivability after vitrification.
Oocyte vitrification efficiency could be defined as the route to a live birth using the lowest number of vitrified oocytes. Although we have obtained a cumulative live birth rate per warming cycle of 44.24%, the oocyte-to-baby rate was only 4.3% in the present study. About one third of couples (36.76%) had successfully taken babies home. Other studies addressing oocyte vitrification for medical indications have reported quite different outcomes of oocyte-to-baby rate. Kara et al. reported the live-birth rate per mature oocyte was 3.0% in an oocyte cryopreservation group (<35 years old) (25). Doyle et al. estimated live birth per warmed oocyte as 6.5% (including predicted live birth from remaining cryopreserved blastocysts) (12). The data herein provides more information for clinicians to advise patients faced with the situation of unavailable sperm on oocyte retrieval day.
The outcomes of live delivery, including gestational age, birth weight, and live birth congenital defects were compared with the fresh control group, and no significant differences were noted. The limited data we collected showed that double vitrification (oocyte and embryo vitrification) or triple-cryopreservation (oocyte/embryo vitrification and sperm cryopreservation) had no adverse effects on perinatal outcomes.
The population in our study was very special and it is impossible to carry out a prospectively randomized controlled trial in such situations for ethical and legal reasons. The long duration of this retrospective study might have added some variations that may have affected the presented data. However, we have a relatively stable laboratory team with experienced technicians trained in oocyte vitrification. Furthermore, we included stimulation protocols and vitrification kit parameters that changed through time in the regression model as potential confounders. Another drawback was the relatively limited sample size, for the incidence rate was only 0.3–0.5% in all IVF/ICSI cycles during data collection years in our hospital. Finally, the couples in the present study mostly exhibited severe male factors, which could influence subsequent embryo development and pregnancy outcomes. Therefore, the outcome results might not represent the entire medical indications for oocyte preservation.
Oocyte vitrification is an indispensable and effective alternative when there is a lack of available sperm on oocyte retrieval day. The present study showed that oocyte survival was better in couples with absolute male factors and this suggested that oocytes from infertile women were more likely to suffer from vitrification injury. Further studies will be necessary to clarify the correlation between serum metabolism parameters and human oocyte survival after vitrification. Our study has preliminarily contributed to the important question for clinical practice of how to distinguish the female population who have oocytes with better survivability after vitrification. We hope more data from autologous oocyte vitrification studies with a large-scale and controlled variable design could add to and clarify our results.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SG and JM contributed to the study concept and design of this study. XF analyzed data and drafted the paper. XL, JL, and MZ contributed to the review and the revision of the manuscript. All authors approved the final submitted and published versions.
This study was supported by the National Key Research and Development Program of China (2016YFC1000202 and 2018YFC1002804).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the medical workers in the research group at the Reproductive Hospital of Shandong University for allowing this study and also thank the information engineer for assembling the data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.663287/full#supplementary-material
1. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. (2013) 99:37–43. doi: 10.1016/j.fertnstert.2012.09.028
2. Song W-Y, Sun Y-P, Jin H-X, Xin Z-M, Su Y-C, Chian R-C. Clinical outcome of emergency egg vitrification for women when sperm extraction from the testicular tissues of the male partner is not successful. Syst Biol Reprod Med. (2011) 57:210–3. doi: 10.3109/19396368.2011.566666
3. Virant-Klun I, Bacer-Kermavner L, Tomazevic T, Vrtacnik-Bokal E. Slow oocyte freezing and thawing in couples with no sperm or an insufficient number of sperm on the day of in vitro fertilization. Reprod Biol Endocrinol. (2011) 9:19. doi: 10.1186/1477-7827-9-19
4. Harrison KL, Lane MT, Osborn JC, Kirby CA, Jeffrey R, Esler JH, et al. Oocyte cryopreservation as an adjunct to the assisted reproductive technologies. Med J Aust. (2007) 186:379. doi: 10.5694/j.1326-5377.2007.tb00946.x
5. Lucena E, Bernal DP, Lucena C, Rojas A, Moran A, Lucena A. Successful ongoing pregnancies after vitrification of oocytes. Fertil Steril. (2006) 85:108–11. doi: 10.1016/j.fertnstert.2005.09.013
6. Cobo A, Garrido N, Pellicer A, Remohí J. Six years' experience in ovum donation using vitrified oocytes: report of cumulative outcomes, impact of storage time, and development of a predictive model for oocyte survival rate. Fertil Steril. (2015) 104:1426–34.e1-8. doi: 10.1016/j.fertnstert.2015.08.020
7. Cai L-B, Qian X-Q, Wang W, Mao Y-D, Yan Z-J, Liu C-Z, et al. Oocyte vitrification technology has made egg-sharing donation easier in China. Reprod Biomed. (2012) 24:186–90. doi: 10.1016/j.rbmo.2011.11.002
8. Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update. (2016) 22:440–9. doi: 10.1093/humupd/dmw007
9. Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J. Elective and Onco-fertility preservation: factors related to IVF outcomes. Hum Reprod. (2018) 33:2222–31. doi: 10.1093/humrep/dey321
10. Dolinko AV, Farland LV, Missmer SA, Srouji SS, Racowsky C, Ginsburg ES. Responses to fertility treatment among patients with cancer: a retrospective cohort study. Fertil Res Pract. (2018) 4:3. doi: 10.1186/s40738-018-0048-2
11. Domingo J, Garcia-Velasco JA. Oocyte cryopreservation for fertility preservation in women with cancer. Curr Opin Endocrinol Diabetes Obes. (2016) 23:465–9. doi: 10.1097/MED.0000000000000295
12. Doyle JO, Richter KS, Lim J, Stillman RJ, Graham JR, Tucker MJ. Successful elective and medically indicated oocyte vitrification and warming for autologous in vitro fertilization, with predicted birth probabilities for fertility preservation according to number of cryopreserved oocytes and age at retrieval. Fertil Steril. (2016) 105:459–66.e2. doi: 10.1016/j.fertnstert.2015.10.026
13. Cobo A, Kuwayama M, Pérez S, Ruiz A, Pellicer A, Remohí J. Comparison of concomitant outcome achieved with fresh and cryopreserved donor oocytes vitrified by the Cryotop method. Fertil Steril. (2008) 89:1657–64. doi: 10.1016/j.fertnstert.2007.05.050
14. Seshadri S, Saab W, Exeter H, Drew E, Petrie A, Davies M, et al. Clinical outcomes of a vitrified donor oocyte programme: a single UK centre experience. Eur J Obstet Gynecol Reprod Biol. (2018) 225:136–40. doi: 10.1016/j.ejogrb.2018.04.017
15. Gala A, Ferrières-Hoa A, Loup-Cabaniols V, Fournier A, Anav M, Brunet C, et al. Closed vitrification system and egg donation: predictive factors of oocyte survival and pregnancy. J Gynecol Obstet Hum Reprod. (2020) 49:101687. doi: 10.1016/j.jogoh.2020.101687
16. De Munck N, Belva F, Van de Velde H, Verheyen G, Stoop D. Closed oocyte vitrification and storage in an oocyte donation programme: obstetric and neonatal outcome. Hum Reprod. (2016) 31:1024–33. doi: 10.1093/humrep/dew029
17. Cobo A, Serra V, Garrido N, Olmo I, Pellicer A, Remohí J. Obstetric and perinatal outcome of babies born from vitrified oocytes. Fertil Steril. (2014) 102:1006–1015.e4. doi: 10.1016/j.fertnstert.2014.06.019
18. Cil AP, Seli E. Current trends and progress in clinical applications of oocyte cryopreservation. Curr Opin Obstet Gynecol. (2013) 25:247–54. doi: 10.1097/GCO.0b013e32836091f4
19. Forman EJ Li X, Ferry KM, Scott K, Treff NR, Scott RT. Oocyte vitrification does not increase the risk of embryonic aneuploidy or diminish the implantation potential of blastocysts created after intracytoplasmic sperm injection: a novel, paired randomized controlled trial using DNA fingerprinting. Fertil Steril. (2012) 98:644–9. doi: 10.1016/j.fertnstert.2012.04.028
20. Li M, Wang M-M, Liu H, Wu K-L, Ma S-Y, Li C, et al. Comparison of the developmental potential and clinical results of in vivo matured oocytes cryopreserved with different vitrification media. Chin Med J. (2015) 128:3029–34. doi: 10.4103/0366-6999.169052
21. Shanshan G, Mei L, Keliang W, Yan S, Rong T, Zi-Jiang C. Effect of different rehydration temperatures on the survival of human vitrified-warmed oocytes. J Assist Reprod Genet. (2015) 32:1197–203. doi: 10.1007/s10815-015-0480-8
22. Boldt J. Current results with slow freezing and vitrification of the human oocyte. Reprod Biomed Online. (2011) 23:314–22. doi: 10.1016/j.rbmo.2010.11.019
23. De Santis L, Cino I, Coticchio G, Fusi FM, Papaleo E, Rabellotti E, et al. Objective evaluation of the viability of cryopreserved oocytes. Reprod Biomed Online. (2007) 15:338–45. doi: 10.1016/S1472-6483(10)60348-3
24. Eaton JL, Truong T, Li Y-J, Polotsky AJ. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. (2020) 135:709–16. doi: 10.1097/AOG.0000000000003695
25. Goldman KN, Noyes NL, Knopman JM, McCaffrey C, Grifo JA. Oocyte efficiency: does live birth rate differ when analyzing cryopreserved and fresh oocytes on a per-oocyte basis? Fertil Steril. (2013) 100:712–7. doi: 10.1016/j.fertnstert.2013.04.040
26. Braga DPAF, Setti AS, Figueira RCS, Azevedo MdC, Iaconelli A, Lo Turco EG, et al. Freeze-all, oocyte vitrification, or fresh embryo transfer? Lessons from an egg-sharing donation program. Fertil Steril. (2016) 106:615–22. doi: 10.1016/j.fertnstert.2016.05.004
27. Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. (2003) 79:1323–6. doi: 10.1016/S0015-0282(03)00258-9
28. Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, et al. Embryo development of fresh 'versus' vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. (2010) 25:66–73. doi: 10.1093/humrep/dep346
29. Chang C-C, Elliott TA, Wright G, Shapiro DB, Toledo AA, Nagy ZP. Prospective controlled study to evaluate laboratory and clinical outcomes of oocyte vitrification obtained in in vitro fertilization patients aged 30 to 39 years. Fertil Steril. (2013) 99:1891–7. doi: 10.1016/j.fertnstert.2013.02.008
30. Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. (2008) 9:125–38. doi: 10.1038/nrm2336
31. Chen X, Dong H, Cheng M, Wang Q, Jin Y. Addition of cholesterol loaded cyclodextrin prior to GV-phase vitrification improves the quality of mature porcine oocytes in vitro. Cryobiology. (2019) 90:54–62. doi: 10.1016/j.cryobiol.2019.08.006
32. Arcarons N, Morató R, Vendrell M, Yeste M, López-Bejar M, Rajapaksha K, et al. Cholesterol added prior to vitrification on the cryotolerance of immature and in vitro matured bovine oocytes. PLoS ONE. (2017) 12:e0184714. doi: 10.1371/journal.pone.0184714
Keywords: oocyte vitrification, survival rate, live birth, obstetric outcomes, blood lipid level
Citation: Fu X, Liu X, Li J, Zhang M, Jiang J, Chen Q, Li M, Gao S and Ma J (2021) An Eight Year Experience of Autologous Oocyte Vitrification for Infertile Patients Owing to Unavailability of Sperm on Oocyte Retrieval Day. Front. Med. 8:663287. doi: 10.3389/fmed.2021.663287
Received: 02 February 2021; Accepted: 23 August 2021;
Published: 26 October 2021.
Edited by:
Ferdinando Antonio Gulino, Garibaldi Hospital, ItalyReviewed by:
Salim Alfred Bassil, Al-Arz Hospital, LebanonCopyright © 2021 Fu, Liu, Li, Zhang, Jiang, Chen, Li, Gao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanshan Gao, c2Rzemdhb3NoYW5zaGFuQDE2My5jb20=; Jinlong Ma, bWFqaW5sb25nX3NkdUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.