- 1Department of Respiratory and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China
- 2Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Geriatrics, People Hospital of Dongying District, Dongying, China
- 4Department of Respiratory and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 5Department of Critical Care Medicine, Shandong Provincial Third Hospital, Jinan, China
- 6Department of Respiratory and Critical Care Medicine, Beijing Hospital, Beijing, China
- 7Graduate School of Peking Union Medical College, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 8First College of Clinical Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
Background: Coronavirus disease 2019 (COVID-19) and tuberculosis (TB) are two major infectious diseases posing significant public health threats, and their coinfection (aptly abbreviated COVID-TB) makes the situation worse. This study aimed to investigate the clinical features and prognosis of COVID-TB cases.
Methods: The PubMed, Embase, Cochrane, CNKI, and Wanfang databases were searched for relevant studies published through December 18, 2020. An overview of COVID-TB case reports/case series was prepared that described their clinical characteristics and differences between survivors and deceased patients. Pooled odds ratios (ORs) with 95% confidence intervals (CIs) for death or severe COVID-19 were calculated. The quality of outcomes was assessed using GRADEpro.
Results: Thirty-six studies were included. Of 89 COVID-TB patients, 19 (23.46%) died, and 72 (80.90%) were male. The median age of non-survivors (53.95 ± 19.78 years) was greater than that of survivors (37.76 ± 15.54 years) (p < 0.001). Non-survivors were more likely to have hypertension (47.06 vs. 17.95%) or symptoms of dyspnea (72.73% vs. 30%) or bilateral lesions (73.68 vs. 47.14%), infiltrates (57.89 vs. 24.29%), tree in bud (10.53% vs. 0%), or a higher leucocyte count (12.9 [10.5–16.73] vs. 8.015 [4.8–8.97] × 109/L) than survivors (p < 0.05). In terms of treatment, 88.52% received anti-TB therapy, 50.82% received antibiotics, 22.95% received antiviral therapy, 26.23% received hydroxychloroquine, and 11.48% received corticosteroids. The pooled ORs of death or severe disease in the COVID-TB group and the non-TB group were 2.21 (95% CI: 1.80, 2.70) and 2.77 (95% CI: 1.33, 5.74) (P < 0.01), respectively.
Conclusion: In summary, there appear to be some predictors of worse prognosis among COVID-TB cases. A moderate level of evidence suggests that COVID-TB patients are more likely to suffer severe disease or death than COVID-19 patients. Finally, routine screening for TB may be recommended among suspected or confirmed cases of COVID-19 in countries with high TB burden.
Introduction
Coronavirus disease 2019 (COVID-19), caused by a novel beta-coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide since December 2019, causing significant global public health and economic problems (1, 2). The World Health Organization declared COVID-19 a pandemic on March 11, 2020 (2). As of December 19, 2020, there have been over 75.7 million cases and 1.68 million deaths associated with COVID-19 worldwide (3). Nearly half of these cases involved four COVID-19 high-burden countries, including the United States (23.1%), India (13.2%), Brazil (9.5%), and Russia (3.7%) (3). Evidence to date suggests that COVID-19 patients with preexisting comorbidities such as hypertension, diabetes, and cardiovascular disease are at greater risk of death, but few studies have involved COVID-19 patients coinfected with other respiratory infectious diseases (4).
The initial signs and symptoms of COVID-19 are similar to other respiratory infections, such as tuberculosis (TB) and influenza. However, coinfections with common viral, bacterial, and fungal pathogens among COVID-19 patients are not unusual (5–7), which can interfere with the diagnosis and treatment of COVID-19. Before the COVID-19 outbreak, TB had been the most fatal infectious disease in the world for many years (8). Globally, an estimated 10 million people contracted TB and 1.4 million died from TB in 2019 (8). At present, evidence suggests that the main transmission route of both COVID-19 and TB is via respiratory droplets, and their main target are the lungs, which can lead to a worse outcome among COVID-19 and TB coinfection patients (aptly abbreviated COVID-TB) (7, 8). Therefore, due to the high prevalence of both of these infectious diseases and the potential worse prognosis of coinfection, an intensive investigation of COVID-TB cases may be of great clinical significance (3, 4, 8). However, few studies have focused on COVID-TB cases to date, and most of these are case reports involving only one patient, thus precluding systematic summaries of the clinical characteristics of coinfection cases (7, 9, 10). In addition, it is unclear whether COVID-TB patients have a worse prognosis or are more likely to develop severe disease, thus necessitating further study.
In this study, we aimed to more fully assess the impact of TB coinfection on COVID-19 patients using the following approach: (1) we present an overview of COVID-TB case reports or case series published through December 18, 2020 and describe the demographic characteristics, clinical symptoms, comorbidities, imaging features, laboratory indicators, type of TB coinfection, and treatment strategies for both COVID-TB survivors and non-survivors; (2) we performed a pooled analysis of published data regarding the odds ratios (ORs) of death or severe disease, comparing the COVID-TB and non-TB groups; and (3) we assessed the quality of outcomes using GRADEpro.
Methods
Search Strategy
An extensive search of the literature was conducted using the PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and two Chinese databases—the China National Knowledge Infrastructure (CNKI) and Wanfang databases—for articles published through December 18, 2020. The following keywords and Medical Subject Headings in partial or complete combinations were used in the search strategy of this review: “Coronavirus 2019,” “COVID-19,” “SARS CoV-2,” “COVID,” “novel coronavirus,” “2019-nCoV,” “severe acute respiratory syndrome,” “nCoV,” “CoV-2,” “SARS-2,” “new coronavirus,” and “tuberculosis.” The Chinese translations of these terms were searched in the CNKI and Wanfang databases (Table A1).
Study Selection
We identified 6,919 publications, which were imported into EndNote X9 (Clarivate Analytics, Philadelphia, PA, USA), and 508 duplicate reports were excluded. First, three authors (WM. S., YF. L., and SJ. L.) reviewed the titles and abstracts for selection. Inclusion criteria were as follows: case reports or cases series of COVID-19 and TB coinfection; or original studies (retrospective or prospective clinical studies) that described the number/percentage of TB patients among confirmed COVID-19 cases. After preliminary screening, 149 full-text records were reviewed. Exclusion criteria were as follows: (1) case report or cases series without outcomes of COVID-TB cases (discharge or death); (2) original articles without the number/percentage of death/non-death or severe/non-severe cases among COVID-TB and COVID-19 subgroups; (3) sample size < 10 patients in the cohort study; and 4) publication overlap. The definition of severe COVID-19 is as follows: SpO2 <94% on room air at sea level; ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg; respiratory frequency > 30 breaths/min; or lung infiltrates >50%.
Data Extraction and Quality Evaluation
The following relevant data were extracted and collected in Excel: (1) baseline data, including the first author, country, publication date, type of study, number of patients with COVID-19, number of COVID-19 patients with TB, mean age, male/female ratio, outcome (survival or death), or clinical classification (severe or non-severe); (2) for case reports or cases series, we also collected detailed data on clinical symptoms, comorbidities, imaging features, laboratory indicators, and treatment strategies; and (3) for retrospective or prospective clinical studies, we collected the number/percentage regarding death/survival and severe/non-severe disease for the COVID-TB and COVID-19 subgroups.
The methodological quality of case reports or case series was evaluated using the Mayo Evidence-Based Practice Centre tool according to four domains (selection, ascertainment, causality, and reporting) (11). In addition, the methodological quality of other retrospective or cohort studies was assessed using the modified Newcastle–Ottawa scale (12). Two investigators (WM. S. and TT. X.) performed the analyses and summarized the scores of each study. Publications with a score ≥ 6 were considered of high quality and included in our analysis.
Statistical Analysis
Eighty-nine COVID-TB cases were divided into survival and non-survival groups. Continuous variables including hematological and biochemical indicators of each group were described as the median (P25, P75) due to their skewed distribution. The Mann–Whitney U test was used for comparing two groups of continuous data (13). The mean and standard deviation of the age variable were described. Categorical variables, including age subgroup, sex, country, symptoms at admission, type of TB, computed tomography (CT) findings, and therapy, were expressed as frequencies and proportions. Categorical variables were compared using the chi-squared test or Fisher's exact test. Forest plots were prepared to show the pooled estimated ORs and associated 95% confidence intervals (CIs) for death from COVID-TB or severe disease, respectively. The heterogeneity of studies included in the Forest plots was assessed using Cochran's Q test and the I2 statistic. A fixed-effect model (inverse variance) was used when I2 was <50%. Otherwise, a random-effect model (DerSimonian–Laird) was used (14). A visual inspection of funnel plots was conducted to evaluate publication bias (Figures A1, A2), in which an asymmetric, inverted funnel shape usually indicates publication bias. Finally, we assessed the quality of outcomes using the GRADEpro software. A two-sided p < 0.05 was considered statistically significant. All statistical analyses were carried out using Review Manager (RevMan; version 5.3), SPSS (version 22.0), and GRADEpro (version 3.6.1).
Results
Characteristics of Included Studies
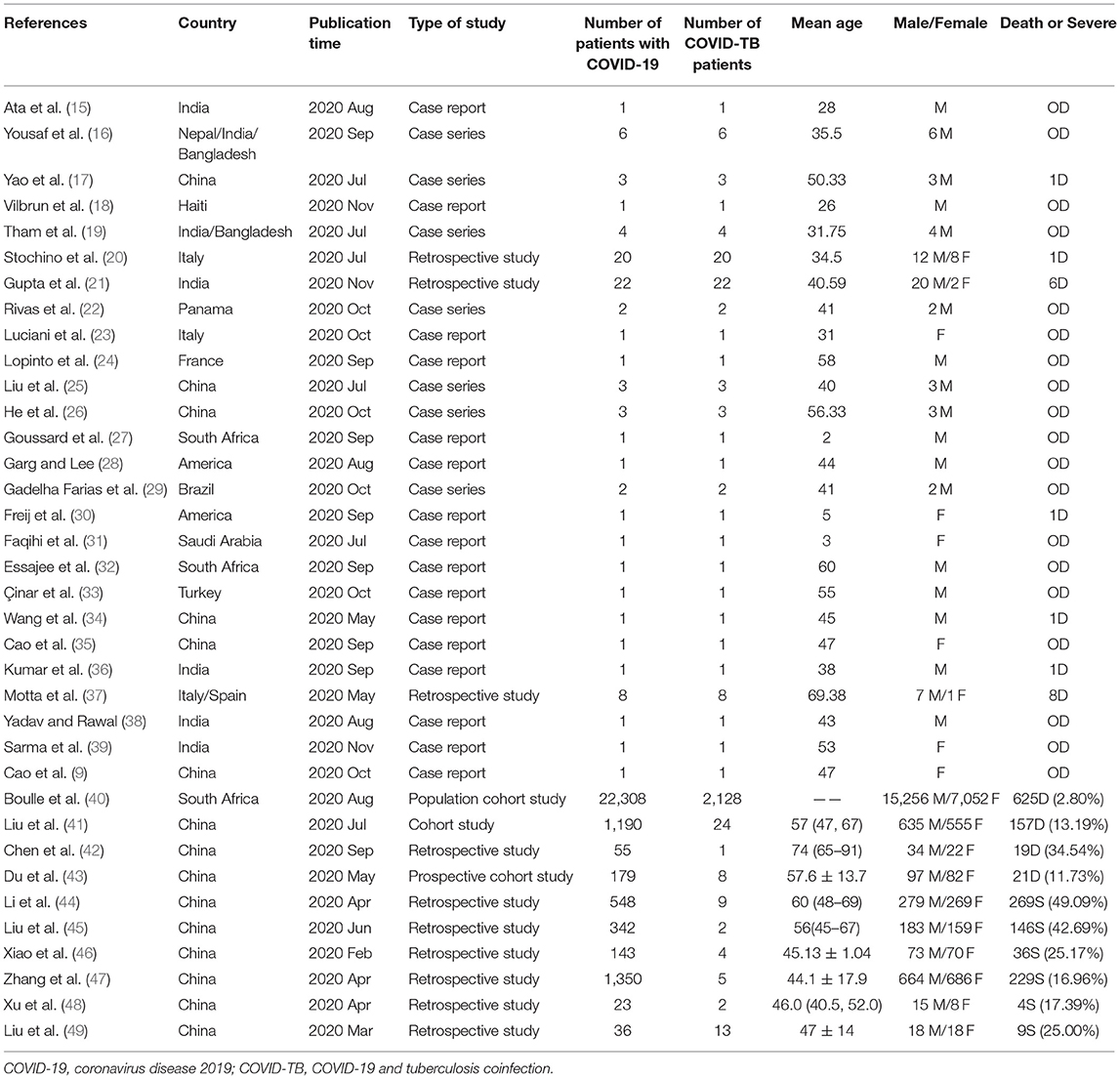
A total of 6,919 articles were retrieved from the literature, including 599 from PubMed, 1,034 from Embase, 3,523 from Cochrane, 1,660 from CNKI, and 103 from Wanfang. After removing 508 duplicates, we evaluated the eligibility of 6,411 articles by screening the title and abstract, resulting in 149 studies being enrolled for full-text screening. Ultimately, we identified 36 eligible studies for our final analysis, of which 26 studies were used for an overview of COVID-TB cases, and 10 studies were used for the estimation of pooled ORs (Figure 1, Table 1).
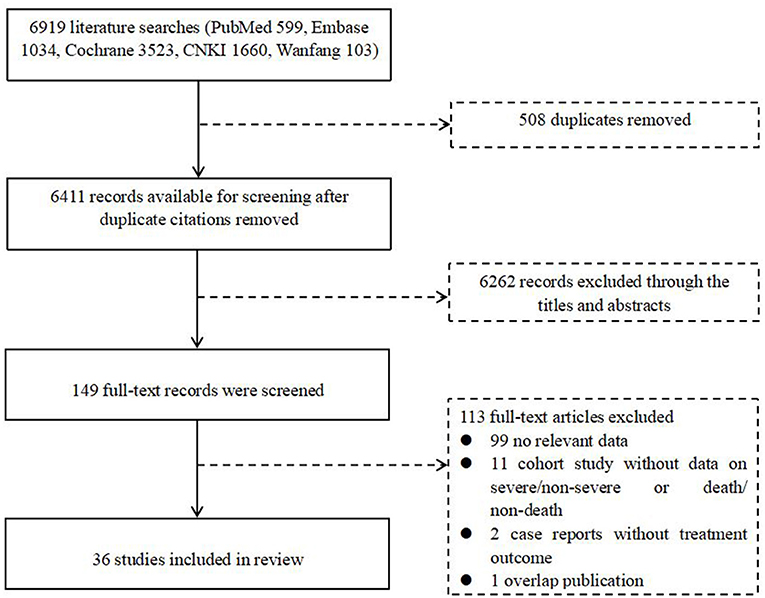
Figure 1. Flow diagram for the inclusion of studies. CNKI, Chinese National Knowledge Infrastructure.
Clinical Features of COVID-TB Cases
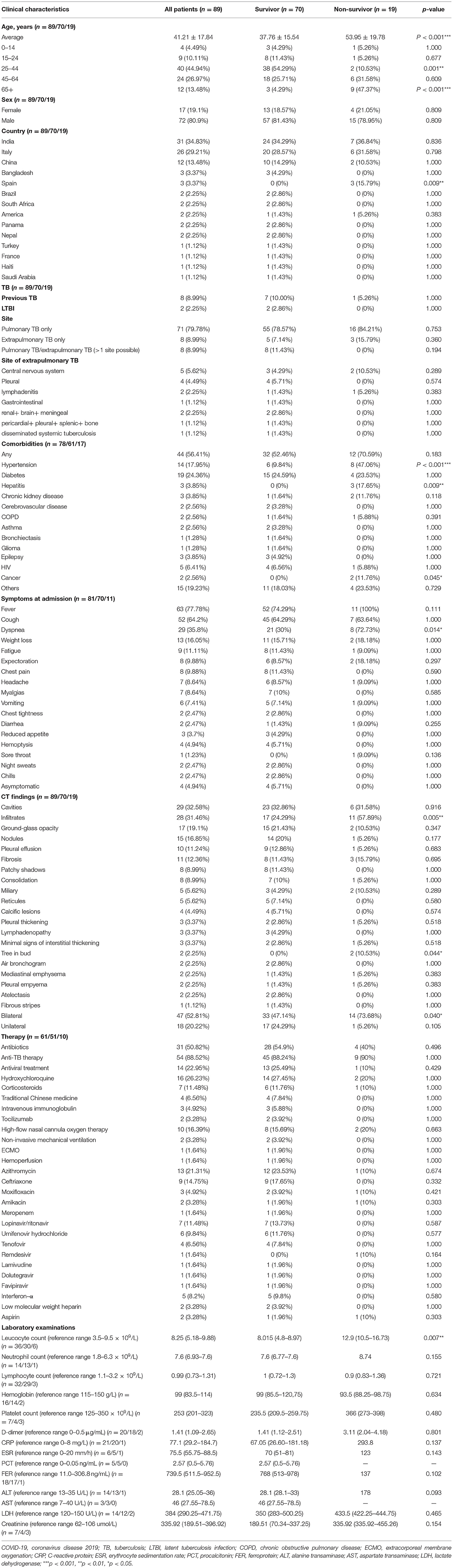
Table 2 describes the demographic characteristics, clinical symptoms, comorbidities, imaging features, laboratory indicators, treatment strategies, and type of TB among the overall group and the COVID-TB non-survivors and survivors. A total of 89 COVID-TB patients were included in the overview of case reports, of which 19 (23.46%) died, and 72 (80.9%) were male. The number and proportion of COVID-TB patients in the 0–14, 15–24, 25–44, 45–64, and 65+ years age groups were 4 (4.49%), 9 (10.11%), 40 (44.94%), 24 (26.97%), and 12 (13.48%), respectively, with an average age of 41.21 ± 17.84 years. The median age of the non-survivor group (53.95 ± 19.78 years) was older than that of the survivor group (37.76 ± 15.54 years) (p < 0.001). The non-survivors were less likely to be 25–44 years old compared with survivors (10.53 vs. 54.29%) but more likely than survivors to be in the 65+ years age group (47.37 vs. 4.29%) (p < 0.01). More than 85% of these cases were from India (n = 31, 4.83%), Italy (n = 26, 29.21%), and China (n = 12, 13.48%).
Among all 89 COVID-TB cases examined, 88.76% involved active TB, 8.99% had previous TB, and 2.25% had latent tuberculosis infection (LTBI). The proportions of pulmonary TB only, extrapulmonary TB only, and pulmonary TB/extrapulmonary TB (>1 site possible) were 79.78, 8.99, and 8.99%, respectively. Moreover, 5.62% were classified as central nervous system TB, 4.49% pleural TB, and 2.25% lymphadenitis. A total of 56.41% (44/78) of the COVID-TB patients had comorbidities, the most common of which was diabetes (24.36%, 19/78), followed by hypertension (17.95%, 14/78), HIV infection (6.41%, 5/78), hepatitis (3.85%, 3/78), epilepsy (3.85%, 3/78), chronic kidney disease (2.56%, 2/78), cerebrovascular disease (2.56%, 2/78), chronic obstructive pulmonary disease (2.56%, 2/78), asthma (2.56%, 2/78), or cancer (2.56%, 2/78). The non-survivors had more complications, such as hypertension (47.06 vs. 17.95%), hepatitis (17.65 vs. 0%), and cancer (11.76% vs. 0%), than survivors (p < 0.05). The 10 most common symptoms of COVID-TB at admission were fever (77.78%), cough (64.2%), dyspnea (35.8%), weight loss (16.05%), fatigue (11.11%), expectoration (9.88%), chest pain (9.88%), headache (8.64%), myalgia (8.64%), and vomiting (7.41%). The non-survivors had a higher percentage of dyspnea than survivors (72.73 vs. 30%) (p = 0.014).
In terms of treatment, 88.52% of the 61 COVID-TB patients received anti-TB therapy, 50.82% received antibiotics, 22.95% received antiviral therapy, 26.23% received hydroxychloroquine, 16.39% received oxygen therapy, 11.48% received corticosteroids, 8.2% received interferon-α, 6.56% received traditional Chinese medicine, and 4.92% received intravenous immunoglobulin. The most widely used antibiotic was azithromycin (21.31%), followed by ceftriaxone (14.75%), moxifloxacin (4.92%), amikacin (3.28%), and meropenem (1.64%). The antiviral drugs used included lopinavir/ritonavir (11.48%), umifenovir hydrochloride (9.84%), tenofovir (6.56%), remdesivir (1.64%), lamivudine (1.64%), dolutegravir (1.64%), and favipiravir (1.64%). These treatments did not differ significantly between survivors and non-survivors.
Features of lung imaging among the 89 patients were as follows: 52.81% had bilateral lesions, and 20.22% had unilateral lesions. The 10 most common imaging features included cavities (32.58%), infiltrates (31.46%), ground-glass opacity (19.1%), nodules (16.85%), pleural effusion (11.24%), fibrosis (12.36%), patchy shadows (8.99%), consolidation (8.99%), military lesions (5.62%), and reticules (5.62%). In addition, we found that non-survivors were more likely than survivors to have bilateral lesions (73.68 vs. 47.14%), infiltrates (57.89 vs. 24.29%), or tree in bud (10.53 vs. 0%) features (p < 0.05).
Elevated laboratory findings in COVID-TB patients included neutrophil count (7.60 [6.93–7.60] × 109/L), D-dimer (1.407 [1.09–2.65] μg/ml), C-reactive protein (CRP, 77.10 [29.20–184.70] mg/L), erythrocyte sedimentation rate (ESR, 75.50 [55.75–88.50] mm/h), procalcitonin (PCT, 2.57 [0.50–5.76] ng/ml), ferroprotein (FER, 739.50 [511.50–952.50] ng/ml), aspartate transaminase (46 [27.55–78.50] U/L), lactate dehydrogenase (LDH, 384 [290.25–471.75] U/L), and creatinine (335.92 [189.51–396.92] μmol/L). Reduced laboratory indicators included lymphocyte count (0.99 [0.73–1.31] × 109/L) and hemoglobin (99 [83.5–114] g/L). Leucocyte count, platelet count, and alanine transaminase levels were in the normal range. The nonsurvivors had a higher leucocyte count than the survivors (12.9 [10.5–16.73] vs. 8.015 [4.8–8.97] × 109/L, P = 0.007). There were no significant differences between the survivors and non-survivors regarding the abovementioned laboratory examinations, except for the leucocyte count.
Pooled ORs for Death or Severe COVID-TB vs. COVID-19
As shown in Figures 2, 3, Table 3, the pooled ORs for death or severe disease in the COVID-TB group compared with the non-TB group were 2.21 (95% CI: 1.80, 2.70; heterogeneity: chi-squared = 3.82, df = 3, p = 0.28; I2 = 21%; Test for overall effect: Z = 7.72, p < 0.00001) and 2.77 (95% CI: 1.33, 5.74; heterogeneity: chi-squared = 8.29, df = 5, p = 0.14; I2 = 40%; Test for overall effect: Z = 2.73, p < 0.006), with a moderate level of evidence. The percentages of deaths and cases of severe disease, respectively, were 5.69% (123/2,161) and 51.43% (18/35) in the COVID-TB group, 3.24% (699/21,571) and 28.04% (675/2,407) in the non-TB group, and 3.46% (822/23,732) and 28.38% (693/2,442) in the overall group. The proportion of TB among the non-survivor, survivor, and overall group was 14.96% (123/822), 8.90% (2038/22,910), and 9.11% (2,161/23,732), respectively. Moreover, the proportion of TB among the severe, non-severe, and overall patient groups was 2.60% (18/693), 0.97% (17/1,749), and 1.43% (35/2,442), respectively.
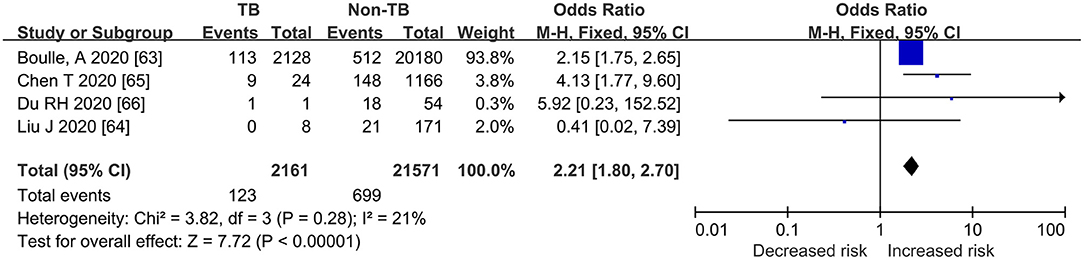
Figure 2. Forrest plot demonstrating the pooled ORs of death in COVID-19 patients with tuberculosis. ORs, odds ratios; TB, tuberculosis; CI, confidence interval; events refer to the occurrence of death.
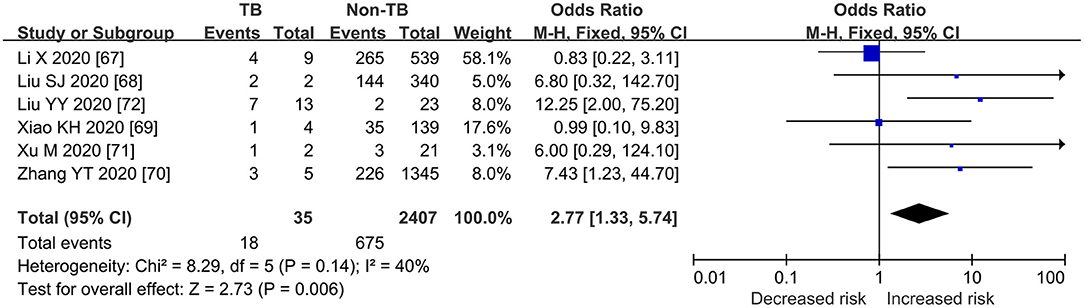
Figure 3. Forrest plot demonstrating the pooled ORs of severe cases in COVID-19 patients with tuberculosis. ORs, odds ratios; TB, tuberculosis; CI, confidence interval; events refer to the occurrence of severe cases.
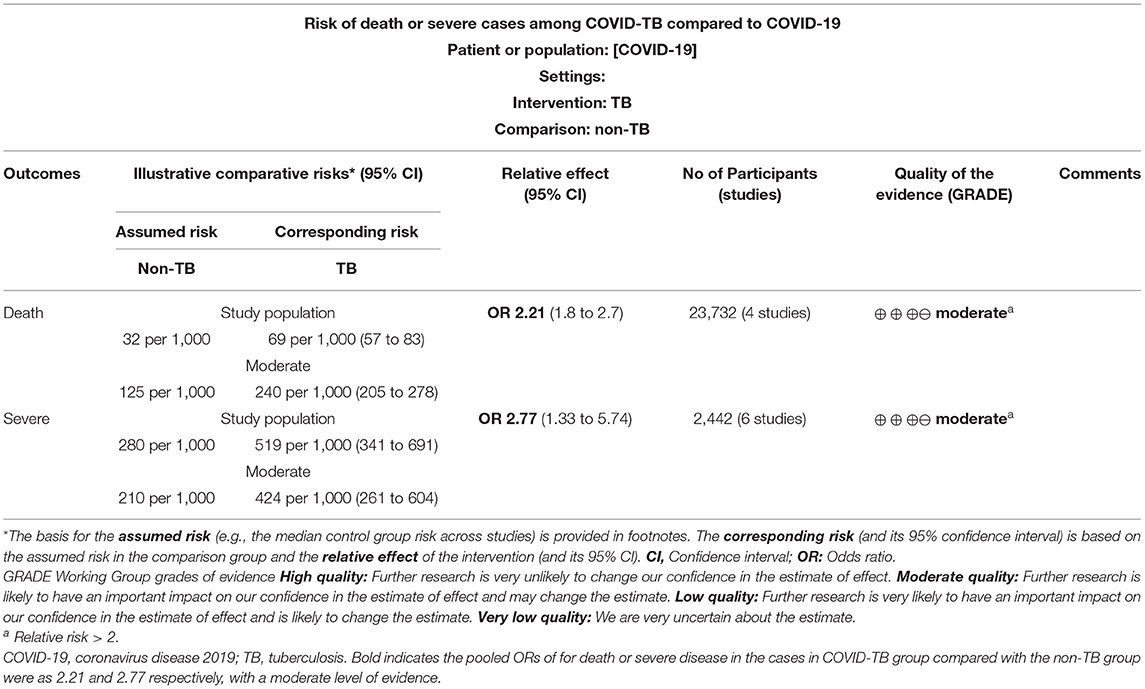
Table 3. GRADEpro assessment of methodologic quality of included studies examining the ability of TB to increase the risk of death or severe cases among COVID-19.
Discussion
This meta-analysis of 36 studies provided a summary of demographic, radiological, and laboratory characteristics as well as symptoms at admission, comorbidities, therapy, and outcomes of COVID-TB cases and investigated the impact of TB on the prognosis of COVID-19 patients. Our study showed that non-survivors were older and had more complications associated with hypertension, hepatitis, and cancer, had more symptoms of dyspnea, and were more likely to have CT imaging features of bilateral lesions, infiltrates, tree in bud, and higher leucocyte count than survivors. We also found a moderate level of evidence indicating that COVID-TB patients are at higher risk of death or serious illness than COVID-19 patients without TB.
Older age, especially >65 years, may be a risk factor for death from COVID-TB, consistent with previous findings indicating that the mortality rate from COVID-19 increases exponentially with age (50, 51). According to a model-based analysis, the estimated overall death rate for COVID-19 was 0.66%, but increasing to 7.8% among patients aged >80 years and decreasing to 0.0016% among children aged <9 years (52). There are several primary reasons for these differences, including more preexisting comorbidities, dysregulation in the immune response, and chronic subclinical systemic inflammation (inflammaging) among older adults than younger persons (53). Thus, the elderly should be the primary focus of both COVID-19 and COVID-TB mitigation efforts due to its much higher mortality risk in that group.
COVID-TB patients had a much higher rate of comorbidities than COVID-19 patients (56.41 vs. 25.1%) (54). The most prevalent comorbidities among COVID-19 patients were hypertension (21.1, 95% CI: 13.0–27.2%), diabetes (9.7%, 95 CI: 7.2–12.2%), cardiovascular disease (8.4%, 95% CI: 3.8–13.8%), and respiratory system disease (1.5%, 95% CI: 0.9–2.1%), whereas the most common comorbidities among COVID-TB patients were diabetes (24.36%), hypertension (17.95%), HIV infection (6.41%), hepatitis (3.85%), epilepsy (3.85%), and cancer (2.56%) (54). Indeed, both HIV infection and diabetes are important risk factors for TB infection (55). Interestingly, we found that COVID-TB patients who died had a much higher proportion of hypertension (47.06 vs. 9.84%) and cancer (11.76 vs. 0%) than those who survived. A previous study also indicated that underlying diseases such as hypertension (OR = 2.72, 95% CI: 1.60, 4.64) and diabetes (OR = 3.68, 95% CI: 2.68, 5.03) are risk factors for critical disease/mortality (56). Early publications reported a “harmful hypothesis” that SARS-CoV-2 binds to target cells via angiotensin-converting enzyme 2 (ACE2), and patients with hypertension usually have increased expression of ACE2 due to the use of renin angiotensin system inhibitors (57). Although some studies indicated that ACE inhibitors (angiotensin converting enzyme inhibitors) and ARB (angiotensin-receptor blockers) therapy was harmful in COVID-19 patients, an updated meta-analysis concluded that ACEI/ARB therapy does not contribute to increased risk of mortality or severe manifestations among COVID-19 patients (58, 59). It was recommended that ACEI/ARB therapy be continued among patients with coexisting hypertension (60). However, whether the guidelines regarding ACEI/ARB therapy among COVID-19 patients are equally applicable to COVID-TB patients remains to be determined.
The most common clinical manifestations of COVID-TB are fever, cough, dyspnea, weight loss, fatigue, and expectoration (13, 61). Existing evidence indicates that the features of lung imaging among COVID-19 patients include bilateral involvement, peripheral distribution, mixed ground-glass opacity and consolidation, and vascular thickening (62), whereas the most common CT findings of COVID-TB include bilateral lesions, cavities, infiltrates, ground-glass opacity, nodules, pleural effusion, and fibrosis. Thus, clinicians should take COVID-TB coinfection into consideration upon encountering the above CT imaging features in the future instead of just focusing on one disease. The increased prevalence of dyspnea and CT findings including bilateral lesions, infiltrates, and tree in bud among COVID-TB patients who died suggests that they may be good predictors of disease severity, in line with the findings of previous studies (13, 61).
Markedly elevated levels of inflammatory markers, including CRP, ESR, PCT, FER, and LDH, slightly increased neutrophil count and D-dimer level, and decreased lymphocyte count and hemoglobin level were observed in COVID-TB patients. However, we did not find any significant differences in these indexes (except the leucocyte count) between the survivors and non-survivors, which was inconsistent with previous findings that inflammatory markers were elevated in severe disease and critically ill groups (63, 64). The findings regarding the characteristics of COVID-TB biomarkers may provide references for conventional hematological and inflammatory examinations for disease severity classification, and early warning of progression (65).
According to the COVID-19 treatment guidelines, the main treatments include antiviral therapy, immune-based therapy, and adjunctive therapy (66). Antiviral therapies may have a greater effect in the early course of COVID-19, whereas immunosuppressive/anti-inflammatory therapies will be more beneficial in the later stages (66, 67). Although previous studies indicated that corticosteroids are associated with a reduction in short-term mortality and the need for mechanical ventilation in COVID-19 patients, whether immunosuppressive therapies such as dexamethasone, a corticosteroid, can be used in COVID-TB patients as well has not been investigated (68). In our study, although there was no statistically significant difference in the proportion of corticosteroid therapy among COVID-TB survivors and non-survivors, we still recommend a more cautious use of corticosteroids in COVID-TB patients because of the potential increased risk of active or severe TB infection associated with corticosteroid use. Studies involving larger samples are needed to explore the impact of corticosteroid therapy on the prognosis of COVID-TB patients (69). It is also worthwhile to explore whether COVID-19 patients with active TB, LTBI, or previous TB should receive standard anti-TB treatment.
Based on this meta-analysis, we found that COVID-TB patients were 2.21 and 2.27 times more likely to die or develop severe COVID-19, respectively. In many countries, the ongoing COVID-19 pandemic coincides with other major public health problems, especially TB, and the impact of the COVID-19 pandemic may be ameliorated if we continue to implement health-care services and key prevention measures (70, 71). COVID-TB infection is a novel disease that remains to be further explored and needs more attention in high-TB burden countries such as India, Indonesia, and China (8). Encouragingly, it has been reported that use of the GeneXpert MTB/RIF platform for the surveillance of COVID-19 is relevant and achievable, especially in low-income and middle-income countries without sufficient classical real-time PCR capabilities but with an already existing GeneXpert MTB/RIF network (72).
Our study has some strengths. First, detailed information was collected in our study, including data regarding demographic characteristics, imaging findings, symptoms at admission, comorbidities, therapies, and outcomes, and the synthesis of these characteristics may provide further guidance for clinicians in terms of diagnosis and treatment of COVID-TB. Second, a comprehensive literature search of both Chinese and English language databases were performed, resulting in a more accurate evaluation of summary estimates with higher precision. Third, the studies included in our meta-analysis had relatively low levels of heterogeneity.
Our study also has several limitations. First, although we performed an extensive search of the literature, most of the eligible studies included in the Forest plots were Chinese. Second, some detailed patient information was not available due to publication bias or no relevant laboratory tests having been performed. Finally, the sample size of our case overview was still limited; thus, further large cohort studies of COVID-TB are needed.
Conclusion
In summary, older age, complications including hypertension, hepatitis, cancer, symptoms of dyspnea at admission, CT imaging features of bilateral lesions, infiltrates, tree in bud, and higher leucocyte count may be predictors for poor prognosis of COVID-TB patients. Furthermore, a moderate level of evidence suggests that people with COVID-TB are 2.21 and 2.27 times more likely to die or develop severe disease, respectively, than COVID-19 patients. Finally, routine screening for Mycobacterium tuberculosis is recommended among suspected or confirmed cases of COVID-19 in high–TB burden countries due to the worse prognosis of COVID-TB and the confounding clinical symptoms of these two diseases.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
H-cL, W-mS, and Y-fL conceived and designed the study. H-cL, X-hZ, Q-qA, S-qL, and YL directed its implementation including the data analysis and writing of the paper. W-mS and Y-fL analyzed the data. W-mS, YL, Q-yZ, J-yL, T-tX, S-jL, X-hZ, and N-nT contributed to data collection, materials, and analytic tools. W-mS and H-cL wrote and revised the manuscript. All authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Funding
This work was supported by the Department of Science & Technology of Shandong Province (CN) (Nos. 2007GG30002033 and 2017GSF218052), Natural Science Foundation of Shandong Province (CN) (No. ZR2020KH013), and Jinan Science and Technology Bureau (CN) (No. 201704100).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all individuals who contributed to this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.657006/full#supplementary-material
Table A1. Search strategies for PubMed, Embase, Cochrane, CNKI, and Wanfang. CNKI, Chinese National Knowledge Infrastructure.
Figure A1. Funnel plot to detect publication bias.
Figure A2. Funnel plot to detect publication bias.
References
1. Perico L, Benigni A, Casiraghi F, Ng L, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. (2021) 17:46–64. doi: 10.1038/s41581-020-00357-4
2. World Health Organization (WHO) Director-General's Opening Remarks at the Media Briefing on COVID-19. (2020). Available online at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19 (accessed March 11, 2020).
3. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online at: https://covid19.who.int/table (accessed December 19, 2020).
4. Callender L, Curran M, Bates S, Mairesse M, Weigandt J, Betts C. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Front Immunol. (2020) 11:1991. doi: 10.3389/fimmu.2020.01991
5. Rawson T, Moore L, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support covid-19 antimicrobial prescribing. Clin Infect Dis. (2020) 71:2459–68. doi: 10.1093/cid/ciaa530
6. Lin D, Liu L, Zhang M, Hu Y, Yang Q, Guo J, et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci. (2020) 63:606–9. doi: 10.1007/s11427-020-1668-5
7. Tadolini M, Codecasa L, García-García J, Blanc F, Borisov S, Alffenaar J, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. (2020) 56:2002328. doi: 10.1183/13993003.02328-2020
8. World Health Organization. Global Tuberculosis Reports. (2020). Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports (accessed December 19, 2020).
9. Cao B, Wei M, Du Y, Xiao K, Li Q, Lu W, et al. Coronavirus disease 2019 with comorbid pulmonary tuberculosis: a case report. Iranian Red Cresc Med J. (2020) 22:e196. doi: 10.32592/ircmj.2020.22.10.196
10. Martínez Orozco JA, Sánchez Tinajero Á, Becerril Vargas E, Delgado Cueva AI, Reséndiz Escobar H, Vázquez Alcocer E, et al. COVID-19 and tuberculosis coinfection in a 51-year-old taxi driver in Mexico city. Am J Case Rep. (2020) 21:e927628. doi: 10.12659/AJCR.927628
11. Murad M, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. (2018) 23:60–3. doi: 10.1136/bmjebm-2017-110853
12. Wells G, Shea B, O Connell DL, Peterson J, Welch, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2014). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
13. Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. (2020) 21:893–903. doi: 10.1016/S1470-2045(20)30309-0
14. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
15. Ata F, Yousaf Q, Veliyankodan Parambil J, Parengal J, Mohamedali MG, Yousaf Z. A 28-year-old man from India with SARS-Cov-2 and pulmonary tuberculosis co-infection with central nervous system involvement. Am J Case Rep. (2020) 21:e926034. doi: 10.12659/AJCR.926034
16. Yousaf Z, Khan AA, Chaudhary HA, Mushtaq K, Parengal J, Aboukamar M, et al. Cavitary pulmonary tuberculosis with COVID-19 coinfection. IDCases. (2020) 22:e00973. doi: 10.1016/j.idcr.2020.e00973
17. Yao Z, Chen J, Wang Q, Liu W, Zhang Q, Nan J, et al. Three patients with COVID-19 and pulmonary tuberculosis, Wuhan, China, January-February, 2020. Emerg Infect Dis. (2020) 26:2755–8. doi: 10.3201/eid2611.201536
18. Vilbrun SC, Mathurin L, Pape JW, Fitzgerald D, Walsh KF. Case report: multidrug-resistant tuberculosis and COVID-19 coinfection in Port-au-Prince, Haiti. Am J Trop Med Hyg. (2020) 103:1986–8. doi: 10.4269/ajtmh.20-0851
19. Tham SM, Lim WY, Lee CK, Loh J, Premkumar A, Yan B, et al. Four Patients with COVID-19 and Tuberculosis, Singapore, April-May, 2020. Emerg Infect Dis. (2020) 26:2764–6. doi: 10.3201/eid2611.202752
20. Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione M. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. (2020) 56:2001708. doi: 10.1183/13993003.01708-2020
21. Gupta N, Ish P, Gupta A, Malhotra N, Caminero JA, Singla R, et al. A profile of a retrospective cohort of 22 patients of COVID-19 with active/treated tuberculosis. Eur Respir J. (2020) 56:2003408. doi: 10.1183/13993003.03408-2020
22. Rivas N, Espinoza M, Loban A, Luque O, Jurado J, Henry-Hurtado N, et al. Case report: COVID-19 recovery from triple infection with Mycobacterium tuberculosis, HIV, and SARS-CoV-2. Am J Trop Med Hyg. (2020) 103:1597–9. doi: 10.4269/ajtmh.20-0756
23. Luciani M, Bentivegna E, Spuntarelli V, Amoriello Lamberti P, Guerritore L, Chiappino D, et al. Coinfection of tuberculosis pneumonia and COVID-19 in a patient vaccinated with Bacille Calmette-Guérin (BCG): case report. SN Comprehen Clin Med. (2020) 1–4. doi: 10.21203/rs.3.rs-59744/v1
24. Lopinto J, Teulier M, Milon A, Voiriot G, Fartoukh M. Severe hemoptysis in post-tuberculosis bronchiectasis precipitated by SARS-CoV-2 infection. BMC Pulmon Med. (2020) 20:244. doi: 10.1186/s12890-020-01285-6
25. Liu C, Yu Y, Fleming J, Wang T, Shen S, Wang Y, et al. Severe COVID-19 cases with a history of active or latent tuberculosis. Int J Tubercul Lung Dis. (2020) 24:747–9. doi: 10.5588/ijtld.20.0163
26. He G, Wu J, Shi J, Dai J, Gamber M, Jiang X, et al. COVID-19 in tuberculosis patients: a report of three cases. J Med Virol. (2020) 92:1802–6. doi: 10.1002/jmv.25943
27. Goussard P, Solomons RS, Andronikou S, Mfingwana L, Verhagen LM, Rabie H. COVID-19 in a child with tuberculous airway compression. Pediatr Pulmonol. (2020) 55:2201–3. doi: 10.1002/ppul.24927
28. Garg N, Lee YI. Reactivation TB with severe Covid-19. Chest. (2020) 158:A777. doi: 10.1016/j.chest.2020.08.724
29. Gadelha Farias LAB, Gomes Moreira AL, Austregésilo Corrêa E, Landim de Oliveira Lima CA, Lopes IMP, de Holanda PEL, et al. Case report: coronavirus disease and pulmonary tuberculosis in patients with human immunodeficiency virus: report of two cases. Am J Trop Med Hyg. (2020) 103:1593–6. doi: 10.4269/ajtmh.20-0737
30. Freij B, Gebara B, Tariq R, Wang A, Gibson J, El-Wiher N, et al. Fatal central nervous system co-infection with SARS-CoV-2 and tuberculosis in a healthy child. BMC Pediatr. (2020) 20:429. doi: 10.1186/s12887-020-02308-1
31. Faqihi F, Alharthy A, Noor A, Balshi A, Balhamar A, Karakitsos D. COVID-19 in a patient with active tuberculosis: a rare case-report. Respir Med Case Rep. (2020) 31:101146. doi: 10.1016/j.rmcr.2020.101146
32. Essajee F, Solomons R, Goussard P, Van Toorn R. Child with tuberculous meningitis and COVID-19 coinfection complicated by extensive cerebral sinus venous thrombosis. BMJ Case Rep. (2020) 13:e238597. doi: 10.1136/bcr-2020-238597
33. Çinar O, Sayinalp B, Aladag Karakulak E, Avşar Karataş A, Velet M, Inkaya A, Ersoy Ortaç N, et al. Convalescent (immune) plasma treatment in a myelodysplastic COVID-19 patient with disseminated tuberculosis. Transfusion Apheresis Sci. (2020) 59:102821. doi: 10.1016/j.transci.2020.102821
34. Wang LCJ, Luo HT, Guan HZ, Wang ZH, Huang C, Zhou FC. A case of COVID-19 with tuberculous meningitis. Chin J Neurol. (2020) 5:361–4.
35. Cao BC WM, Wu G, Xiao K, LI Q, Lu W, Huang YM, et al. Coronavirus disease 2019 complicated with pulmonary tuberculosis: a case report. Chin J Infect Chemother. (2020) 20:546–8. doi: 10.16718/j.1009-7708.2020.05.017
36. Kumar D, Bhattacharya D, Meena D, Soneja D, Wig D. COVID-19 and TB co-infection - 'FINISHING touch” in perfect recipe to 'severity' or 'death'. J Infection. (2020) 81:e39–40. doi: 10.1016/j.jinf.2020.06.062
37. Motta I, Centis R, D'Ambrosio L, García-García J, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. (2020) 26:233–40. doi: 10.1016/j.pulmoe.2020.05.002
38. Yadav S, Rawal G. The case of pulmonary tuberculosis with COVID-19 in an Indian male-a first of its type case ever reported from South Asia. Pan Afr Med J. (2020) 36:374. doi: 10.11604/pamj.2020.36.374.24260
39. Sarma U, Mishra V, Goel J, Yadav S, Sharma S, Sherawat RK. Covid-19 pneumonia with delayed viral clearance in a patient with active drug-resistant pulmonary tuberculosis. Indian J Crit Care Med. (2020) 24:1132–4. doi: 10.5005/jp-journals-10071-23662
40. Boulle A, Davies M, Hussey H, Ismail M, Morden E, Vundle Z, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. (2020) ciaa1198. doi: 10.1093/cid/ciaa1198
41. Liu J, Zhang S, Wu Z, Shang Y, Dong X, Li G, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intens Care. (2020) 10:99. doi: 10.1186/s13613-020-00706-3
42. Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with Coronavirus Disease, 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. Js Gerontol Ser A Biol Sci Med Sci. (2020) 75:1788–95. doi: 10.1093/gerona/glaa089
43. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. (2020) 55:2000524. doi: 10.1183/13993003.00524-2020
44. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. (2020) 146:110–18. doi: 10.1016/j.jaci.2020.04.006
45. Liu SJ CF, Yang XY, He J, Li H, Zhang W, Zhang JB, et al. A study of laboratory confirmed cases between laboratory indexes and clinical classification of 342 cases with corona virus disease 2019 in Ezhou. Lab Med. (2020) 6:551–6. doi: 10.3969/j.issn.1673-8640.2020.06.008
46. Xiao KH SL, Pang XH, Mu HM, Wang JB, Lang CH, Lv JL, et al. The clinical features of the 143 patients with COVID-19 in North-East of Chongqing. J Third Military Med Univ. (2020) 42:549–54. doi: 10.16016/j.1000-5404.202002097
47. Zhang Y, Deng A, Hu T, Chen X, Zhuang Y, Tan X, et al. Clinical outcomes of COVID-19 cases and influencing factors in Guangdong province. Chin J Epidemiol. (2020) 41:1999–2004. doi: 10.3760/cma.j.cn112338-20200318-00378
48. Xu M LM, Zhan WQ, Han T, Liu LT, Zhang GS, Lu YB. Clinical analysis of 23 patients with coronavirus disease 2019 in Xinyang City of Henan Province. Chin Crit Care Med. (2020) 4:421–5. doi: 10.3760/cma.j.cn121430-20200301-00153
49. Liu Y, Bi L, Chen Y, Wang Y, Fleming J, Yu Y, et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv. (2020). doi: 10.1101/2020.03.10.20033795
50. Promislow DEL. A geroscience perspective on COVID-19 mortality. Js Gerontol Ser A Biol Sci Med Sci. (2020) 75:e30–3. doi: 10.1093/gerona/glaa094
51. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
52. Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. (2020) 20:669–77. doi: 10.1016/S1473-3099(20)30243-7
53. Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. (2020) 52:154–64. doi: 10.3947/ic.2020.52.2.154
54. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. (2020) 55:2001227. doi: 10.1183/13993003.01227-2020
55. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. (2020) 94:91–5. doi: 10.1016/j.ijid.2020.03.017
56. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. (2020) 81:e16–25. doi: 10.1016/j.jinf.2020.04.021
57. Warner FJ, Rajapaksha H, Shackel N, Herath CB. ACE2: from protection of liver disease to propagation of COVID-19. Clin Sci. (2020) 134:3137–58. doi: 10.1042/CS20201268
58. Lee HW, Yoon CH, Jang EJ, Lee CH. Renin-angiotensin system blocker and outcomes of COVID-19: a systematic review and meta-analysis. Thorax. (2021) 76:479–86. doi: 10.1136/thoraxjnl-2020-215322
59. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease, 2019 (COVID-19). JAMA Cardiol. (2020) 5:811–18. doi: 10.1001/jamacardio.2020.1017
60. Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. (2020) 22:31. doi: 10.1007/s11886-020-01291-4
61. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
62. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. (2020) 214:1072–7. doi: 10.2214/AJR.20.22976
63. Sun Y, Dong Y, Wang L, Xie H, Li B, Chang C, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun. (2020) 112:102473. doi: 10.1016/j.jaut.2020.102473
64. Liu SL, Wang SY, Sun YF, Jia QY, Yang CL, Cai PJ, et al. Expressions of SAA, CRP, and FERR in different severities of COVID-19. Eur Rev Med Pharmacol Sci. (2020) 24:11386–94. doi: 10.26355/eurrev_202011_23631
65. Fu J, Kong J, Wang W, Wu M, Yao L, Wang Z, et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D-dimer in COVID-19: A retrospective study in Suzhou China. Thromb Res. (2020) 192:3–8. doi: 10.1016/j.thromres.2020.05.006
66. Therapeutic Management of Patients With COVID-19. (2020). Available online at: https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ (accessed December 26, 2020).
67. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. (2020) 383:1813–26. doi: 10.1056/NEJMc2022236
68. van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. (2020) 24:696. doi: 10.1186/s13054-020-03400-9
69. Lai CC, Lee MT, Lee SH, Lee SH, Chang SS, Lee CC. Risk of incident active tuberculosis and use of corticosteroids. Int J Tuberculosis Lung Dis. (2015) 19:936–42. doi: 10.5588/ijtld.15.0031
70. Homolka S, Paulowski L, Andres S, Hillemann D, Jou R, Günther G, et al. Two pandemics, one challenge-leveraging molecular test capacity of tuberculosis laboratories for rapid COVID-19 case-finding. Emerg Infect Dis. (2020) 26:2598–606. doi: 10.3201/eid2611.202602
71. Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Global Health. (2020) 8:e1132–41. doi: 10.1016/S2214-109X(20)30288-6
Keywords: COVID-19, tuberculosis, co-infection, clinical features, risk factors
Citation: Song W-m, Zhao J-y, Zhang Q-y, Liu S-q, Zhu X-h, An Q-q, Xu T-t, Li S-j, Liu J-y, Tao N-n, Liu Y, Li Y-f and Li H-c (2021) COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front. Med. 8:657006. doi: 10.3389/fmed.2021.657006
Received: 22 January 2021; Accepted: 26 July 2021;
Published: 24 August 2021.
Edited by:
Susan Christina Welburn, University of Edinburgh, United KingdomReviewed by:
Zhichun Gu, Shanghai JiaoTong University, ChinaZohaib Yousaf, Hamad Medical Corporation, Qatar
Copyright © 2021 Song, Zhao, Zhang, Liu, Zhu, An, Xu, Li, Liu, Tao, Liu, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huai-chen Li, bGlodWFpY2hlbkAxNjMuY29t; Yi-fan Li, MTAzNTMxMjI4OUBxcS5jb20=
 Wan-mei Song
Wan-mei Song Jing-yu Zhao3
Jing-yu Zhao3 Ning-ning Tao
Ning-ning Tao Yao Liu
Yao Liu Huai-chen Li
Huai-chen Li