
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 14 May 2021
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.656405
 Anat Reiner Benaim1†
Anat Reiner Benaim1† Jonathan A. Sobel2†
Jonathan A. Sobel2† Ronit Almog1
Ronit Almog1 Snir Lugassy2
Snir Lugassy2 Tsviel Ben Shabbat2
Tsviel Ben Shabbat2 Alistair Johnson3
Alistair Johnson3 Danny Eytan1,4‡
Danny Eytan1,4‡ Joachim A. Behar2*‡
Joachim A. Behar2*‡Background: COVID-19 is a newly recognized illness with a predominantly respiratory presentation. It is important to characterize the differences in disease presentation and trajectory between COVID-19 patients and other patients with common respiratory illnesses. These differences can enhance knowledge of pathogenesis and help in guiding treatment.
Methods: Data from electronic medical records were obtained from individuals admitted with respiratory illnesses to Rambam Health Care Campus, Haifa, Israel, between October 1st, 2014 and October 1st, 2020. Four groups of patients were defined: COVID-19 (693), influenza (1,612), severe acute respiratory infection (SARI) (2,292), and Others (4,054). The variable analyzed include demographics (7), vital signs (8), lab tests (38), and comorbidities (15) from a total of 8,651 hospitalized adult patients. Statistical analysis was performed on biomarkers measured at admission and for their disease trajectory in the first 48 h of hospitalization, and on comorobidity prevalence.
Results: COVID-19 patients were overall younger in age and had higher body mass index, compared to influenza and SARI. Comorbidity burden was lower in the COVID-19 group compared to influenza and SARI. Severely- and moderately-ill COVID-19 patients older than 65 years of age suffered higher rate of in-hospital mortality compared to hospitalized influenza patients. At admission, white blood cells and neutrophils were lower among COVID-19 patients compared to influenza and SARI patients, while pulse rate and lymphoctye percentage were higher. Trajectories of variables during the first 2 days of hospitalization revealed that white blood count, neutrophils percentage and glucose in blood increased among COVID-19 patients, while decreasing among other patients.
Conclusions: The intrinsic virulence of COVID-19 appeared higher than influenza. In addition, several critical functions, such as immune response, coagulation, heart and respiratory function, and metabolism were uniquely affected by COVID-19.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the virus underlying COVID-19, a newly recognized illness that initially spread throughout Wuhan (Hubei province), and from there, to other provinces in China and then across the globe. As of October 15th 2020, over 39,000,000 infections and over 1,100,000 casualties have been linked to SARS-CoV-2. The clinical spectrum of SARS-CoV-2-associated pneumonia ranges from mild to life-threatening (1, 2). Several studies have described general epidemiological findings, clinical presentation and clinical outcomes of SARS-CoV-2 pneumonia, and identified mortality risk factors (3–9). The need for detailed information on the clinical characteristics of hospitalized patients with COVID-19 and their clinical course is essential to achieve a thorough understanding of the disease development and progression. Moreover, the exact differences between the clinical presentation and illness trajectory of COVID-19 vs. other respiratory (viral) infections remain illusive. Investigating the clinical features of influenza like illness (ILI) is of paramount importance to identify COVID-19 specificities. This work questioned the virulence of COVID-19 as compared to seasonal influenza and SARI. Recognizing the characteristics discriminating COVID-19 from influenza, will be critical to support the management of the current pandemic.
There are limited works that have pursued the pathophysiological differences between ILIs and COVID-19, and have focused solely on the H1N1 influenza strain (10–13). They investigated symptoms, comorbidities, laboratory examinations, treatments and scans in relatively small cohorts of patients and consequently had limited statistical power. Hence, the relative virulence of COVID-19 vs. influenza has been and remains under debate. Discriminating biomarkers at clinical presentation and characteristics of the trajectory of COVID-19 vs. influenza are poorly characterized.
To address this knowledge gap, this retrospective analysis reviewed the electronic medical records (EMR) from the Rambam Health Care Campus, located in Haifa, Israel. Demographics, comorbidities, vital signs and laboratory tests were analyzed to identify features that can potentially discriminate between COVID-19, influenza and SARI at the time of admission to the hospital, as well as their trajectory during the first 48 h after admission.
A unique cloud-based database, named COV19, was created based on the model of MIMIC III (14). The database contained detailed de-identified clinical information. Specific views (tables) that contained multiple variables related to a given type of medical data, were created. Ethical approval for this research was provided by the local institutional review board (IRB; #0141-20). The de-identified datasets were uploaded to a Microsoft Azure cloud server, which also offers data analysis, visualization, and querying tools. The description of the tables and variables included in COV19 is available on the online resource site (https://cov19-resource.com/). Future access to the cloud can be given to interested researchers, subject to hospital IRB approval.
This single center retrospective observational cohort study uses EMR data from Rambam Health Care Campus, a 1,000-bed tertiary academic hospital in Northern Israel, during which the pandemic opened five dedicated COVID-19 departments. The hospital EMR database was queried for hospitalized adult (age 18 and above) cases admitted for COVID-19, influenza or SARI, or tested for COVID-19 during hospitalization (with either a positive or negative result), from October 1st, 2014 until October 1st, 2020.
The disease groups were defined as follows: COVID-19: At least one positive reverse transcription polymerase chain reaction (RT-PCR) test for SARS-CoV-2 in nasopharyngeal swab. Most COVID-19 cases were positive within a week before admission to the hospital or at admission, and very few of them were diagnosed a few days after admission. COVID-19 were also tested for Influenza. Influenza: tested positive for influenza A or B virus by RT-PCR test and tested negative for COVID-19, or tested positive for influenza A or B virus by RT-PCR test and admission date prior to COVID-19 emergence in Israel, on February 23, 2020. SARI only: Physician report in the EMR that matches World Health Organization (WHO) SARI case definition (15), i.e., an acute respiratory infection with history of fever or measured fever of ≥38°C, and cough with onset within the last 10 days, hospitalization, and no positive test for COVID-19 or influenza (either negative or not tested). Others: Tested negative for COVID-19 and not classified into influenza or SARI groups.
Forty percent of the SARI cases were tested for Influenza, when indicated by the physician for differential diagnosis or for the purpose of surveillance during the influenza season. As the cohort is defined by inclusion criteria as all patients tested for COVID-19, or either testing positive for influenza or having a SARI diagnosis, the “others” group is a control group inevitably generated by those with negative result for COVID-19, that are also negative for Influenza and SARI. Thus, this group contains zero cases prior to February 23, 2020, the date of COVID-19 emergence in Israel. After this date, which approaches the end of the Influenza season, only 5% of the patients in “others” group were tested for Influenza. Tests for COVID-19 after its emergence were performed for 80% of the SARI patients, and by inclusion criteria, for all patients in “others.”
Following existing guidelines within the context of COVID-19 (16–18), patients were defined as moderately ill if they were diagnosed for COVID-19 pneumonia clinically or by X-ray. Patients were defined as severely ill if either their breaths number per minute was larger than 30, their unsupported oxygen saturation was of 93% or lower, their PF ratio was below 300, or were critically ill. Critically ill patients were defined as those who either went through mechanical ventilation support (invasive or non-invasive), were hospitalized in an intensive care unit, or were administered vasopressor medications (noradrenaline and vasopressin) or inotropic medications (dopamine, dobutamine, milrinone, and adrenaline).
Demographic information, such as age, sex, ethnic group (Jewish or Arabs, which included Druze and Muslims), weight, body mass index (BMI), length of hospitalization and mortality rates were collected. In addition, comorbidities, vital signs including fever, respiratory variables (breaths count per minutes, oxygen saturation) blood pressure and tests results, such as metabolic profiles, complete blood count, and coagulation tests were collected. Comorbidities were defined by ICD-9 codes as detailed in Supplementary Table 1. A thorough analysis (see “Statistical Analysis” section) of comorbidities, demographics and mortality rate was performed to characterize predispositions and the severity and case fatality of each disease. Moreover, lab tests and vital signs were screened at admission and for the first 2 days of hospitalization in order to identify distinct signature or putative biomarkers of COVID-19, influenza, and SARI.
Demographic variables, comorbidity rate, vital signs, and lab tests were compared between disease groups at admission using the Chi-squared test or Fisher's exact test for categorical variables, and analysis of variance or Kruskal-Wallis test for continuous variables. The p-values across all tests were corrected to control the false discovery rate (FDR) criterion (19). Mortality rates were compared before and after excluding patients at mild severity level. Medians and inter-quartile range (IQR) were used to describe the continuous variables. In addition, standardized scores were calculated for scale unification across variables, using the median for centralization and the median absolute deviation (MAD) for rescaling, thereby allowing their representation in a comparative heatmap. Adjustments for confounders were performed using generalized linear models. Age-adjusted COVID-19 odds ratios for each comorbidity were calculated using multivariate logistic regression, excluding mild cases, to eliminate severity bias.
The trajectory over time was compared for each numeric measure, using a non-parametric repeated measure model for a factorial design (20). In the first stage, for each measure, we used the interaction effect in the model to test for difference in time trend between the disease groups. For variables that showed a significant effect, we conducted post hoc pairwise tests between the groups. The p-values over all tests across the two stages were corrected using a hierarchical FDR controlling procedure (21). Three time intervals were defined to follow trends during hospitalization: 0–6, 6–24, and 24–48 h from admission. Effect size was defined by the difference in slopes across time between each pair of compared disease groups. The larger slope difference among the slopes obtained for (0–6, 6–24) and (6–24, 24–48) time gaps was selected as the effect size for each pairwise comparison. The R software (22) was used for statistical analysis, including the R package nparLD (23) for applying the non-parametric model. A 0.05 threshold was used to determine significance.
A total of 9,670 hospitalizations at Rambam Health Care Campus met the initial inclusion criteria between October 1st, 2014 and October 1st, 2020 (Figure 1). Excluded were 33 non-critical COVID-19 cases admitted before April 1, 2020, a period during which all positive cases were systematically hospitalized, including very mild and asymptomatic cases, and 23 COVID-19 cases admitted for reasons unrelated to COVID-19 (e.g., women at labor, traffic accident injury). In order to remove bias and correlations due to repeated per-patient hospitalizations 963 cases, mainly non-COVID-19 hospitalization, with fewer than 30 days between consecutive hospitalization, were excluded. The remaining 8,651 cases were classified into the four disease groups. This included 693 COVID-19 patients, of whom 127 (18.3%) were classified as critical. A total of 68 variables were evaluated: demographics (7), vital signs (8), lab tests (38), and comorbidities (15).
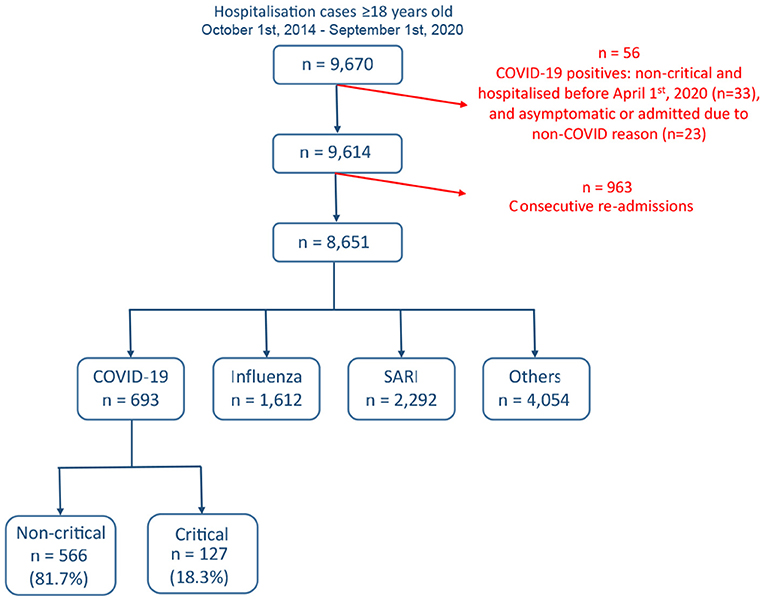
Figure 1. Cohort selection and criteria for exclusion. Data from a total of 9,670 admitted cases were extracted from the Rambam Health Care Campus electronic medical records system.
As shown in Table 1, COVID-19 patients (median age 59.8 ± 29.7 IQR years) were younger compared to influenza patients (median age 70.5 ± 21.8 IQR years) and SARI patients (median age 70.5 ± 22.9 IQR years). Patients between 18 and 44 years of age made up 25% of the COVID-19, compared to 13–14% among influenza and SARI patients. In contrast 24% of the COVID-19 patients were 75 years or older, compared to 40% among influenza and SARI patients. The sex distribution among COVID-19 patients reflected a small preference toward males (52%), compared to a more significant preference toward males among SARI patients (58.6%), and no preference among influenza patients (50%). The proportion of Arabs among the COVID-19 patients (40%) was larger compared to influenza and SARI patients (25 and 23.5%, respectively), independently of disease severity. Duration of hospitalization was shorter for COVID-19 patients (median 4 ± 7 IQR days) compared to influenza and SARI patients (respectively median 5 ± 5 IQR and median 5 ± 6 IQR days). However, for moderate to severe COVID-19 patients, a longer duration of hospitalization was observed (median 6 ± 7.75 IQR days) as compared to influenza and SARI.
BMI was higher for COVID-19 patients (median 28.7 ± IQR 7.2 kg/m2) compared to influenza (median 27.8 ± IQR 7.3 kg/m2) and SARI (median 26.6 ± 7.4 IQR kg/m2), with 35% of the COVID-19 patients being obese (BMI > 30 kg/m2), compared to 30% of the influenza patients and 24% of the SARI patients. Moreover, 48% of moderate to severe COVID-19 patients under 65 years of age with no comorbidities were obese, compared to 26% of influenza patients and 14% of SARI patients. After adjustment for age, BMI among moderate to severe COVID-19 patients was found larger by 1.7 kg/m2 compared to influenza patients, and by 2.9 kg/m2 compared to SARI patients.
Overall in-hospital mortality was similar between COVID-19 (8%), influenza (8.1%), and other (8.6%) group, and markedly higher for the SARI group (13.5%). To limit the selection bias introduced by the current pandemic situation that would result in a more permissive hospitalization policy for the COVID-19 patients, we excluded mild COVID-19 cases. This analysis resulted in an in-hospital death rate of 12.6% for moderate to severe COVID-19 patients. When focusing on age groups 65–74 and 75+ years, mortality rates of COVID-19 patients were 15.5 and 21.4%, respectively. Moreover, in-hospital mortality stratified by BMI showed that a lower proportion of patient with BMI over 30 kg/m2 died compared to the group with BMI between 20 and 25 kg/m2 (5.2 and 9.2%, respectively).
As shown in Table 2 and depicted in Figure 2, the incidence rates for almost all analyzed comorbidities were lower among moderate to severe COVID-19 patients compared to other patients. A total of 9.5% of COVID-19 patients had cancer, compared to 17.1% of influenza patients and 23.4% of SARI patients. Accordingly, the age-adjusted odd ratio (AOR) for COVID-19 were 0.4 (95%CI: 0.27; 0.59). Similar results were obtained when separating to solid-type cancer and hematologic cancer. In total, 3.5% of moderate to severe COVID-19 patients had chronic obstructive pulmonary disease (COPD), compared to 10–11% of influenza and SARI patients and the AOR for COVID-19 was 0.29 (95%CI: 0.16; 0.53). A total of 41.7% of COVID-19 patients had hypertension, compared to 52.6% of influenza patients and 47.6% of SARI patients, and the AOR for COVID-19 was 0.66 (95%CI: 0.5; 0.87). A total of 52% of COVID-19 patients had cardiovascular disease, compared to 62–64% of influenza and SARI patients. The AOR for COVID-19 was 0.46 (95%CI: 0.34; 0.61). A total of 8.8% of COVID-19 patients smoked, compared to 20–21% of influenza and SARI patients. The AOR for COVID-19 was 0.32 (95%CI: 0.22; 0.48). Only dementia was more frequent among COVID-19 compared to other patients. A total of 10.1% of COVID-19 patients had dementia, compared to 4.9% of influenza patients and 8.2% of SARI patients. The AOR for COVID-19 was 2.82 (95%CI: 1.67; 4.76). However, an interaction effect between dementia and arrival from nursing home was observed (p-value = 0.0047). Stratified AOR led to nearly significant levels due to smaller sample size. COVID-19 patients hospitalized from nursing homes depicted an AOR of 1.82 (p-value = 0.058), while the AOR for COVID-19 patients not coming from nursing homes was 0.54 (p-value = 0.079).
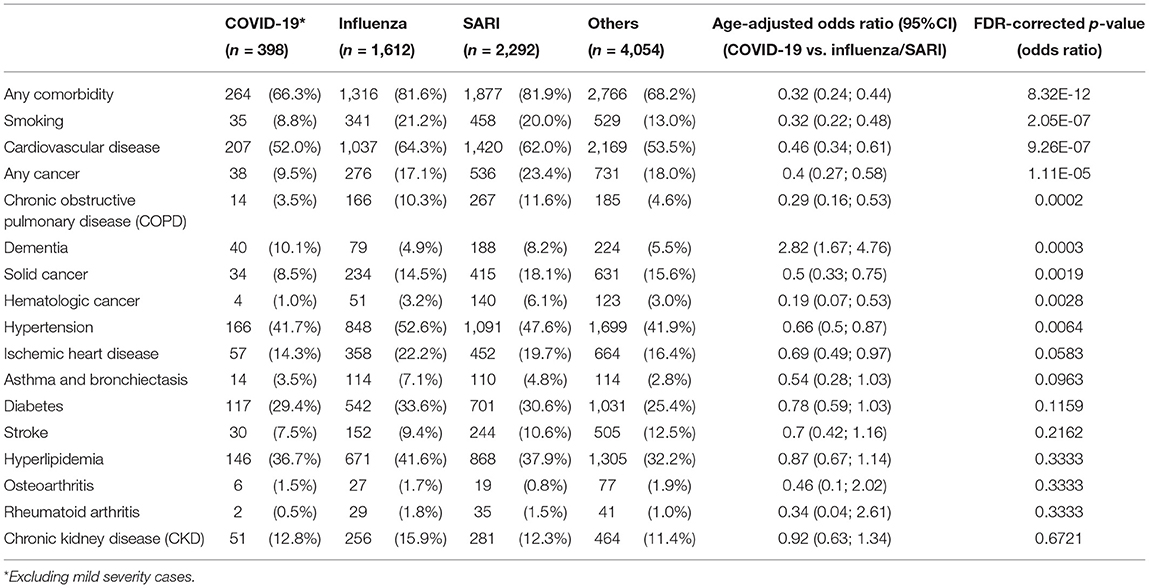
Table 2. Comorbidities prevalence and age-adjusted odds ratios among study patients at admission and comparison between disease groups.
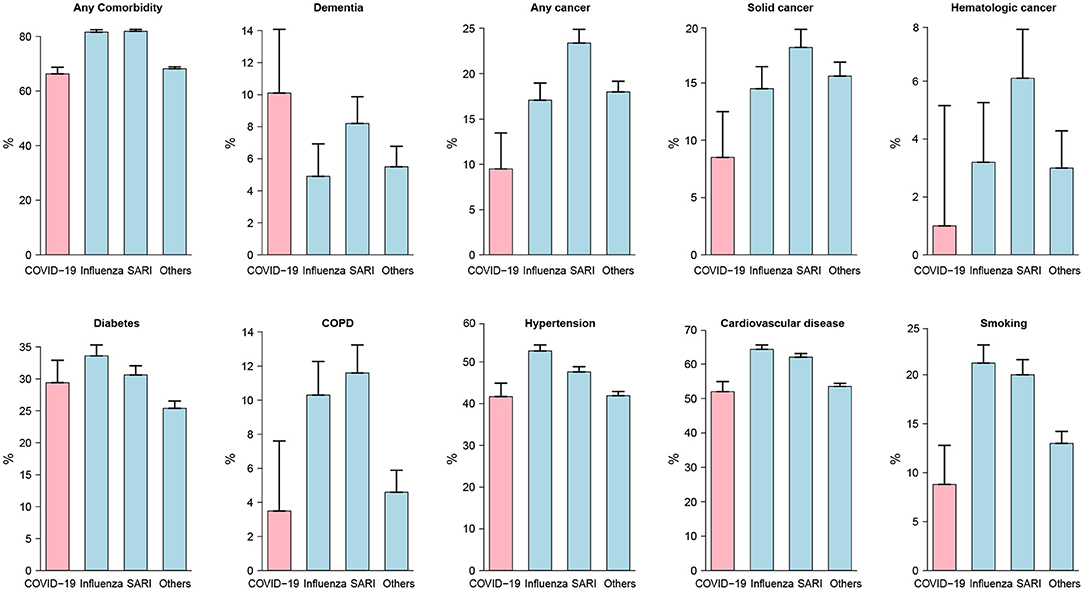
Figure 2. Comorbidity prevalence rates among study patients at admission. Moderate to severe cases were considered for COVID-19 patients. For all disease types but dementia, rates were lower among COVID-19 patients compared to other patients. The differences between disease groups were all statistically significant except for diabetes.
Lastly, diabetes was an important risk factor for COVID-19 severity, with a prevalence of nearly 30% of COVID-19 cases. Influenza and SARI depicted a similar prevalence suggesting diabetes as a common comorbidity of ILIs.
Tables 3, 4 present results of the intercohort comparison of vital signs and laboratory examinations at admission. The median standardized scores (see Methods) for each group are presented per variable within the heatmap in Figure 3.
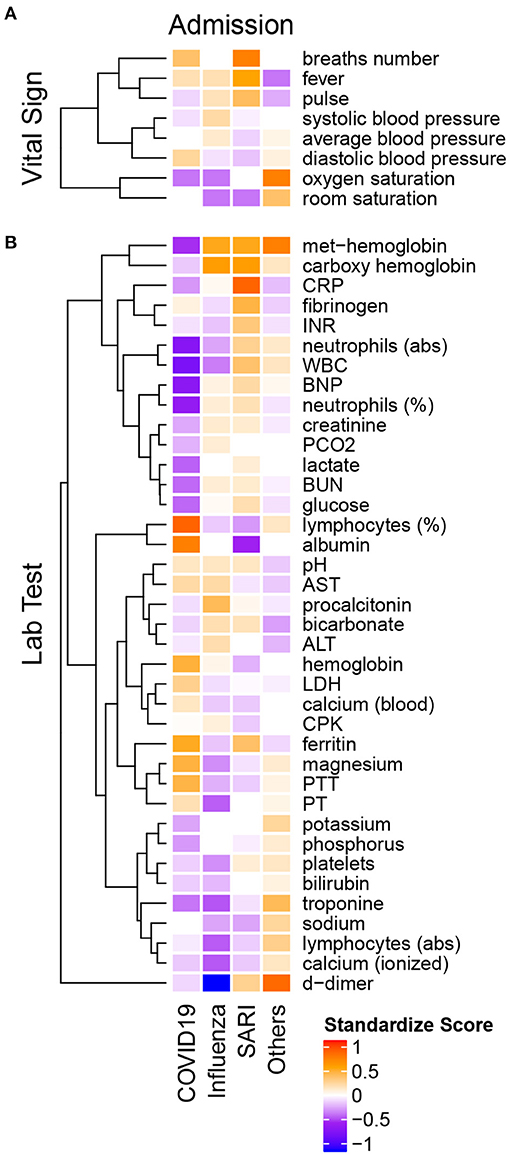
Figure 3. Heatmap of admission median standardized scores of (A) vital signs and (B) lab tests in COVID-19, influenza A/B, SARI and Others. All variables are introduced on the same scale, relative to the variable overall median. The standardized scores is positive if the group median is greater than the overall median (orange-red) and negative otherwise (purple-blue). Hierarchical clustering on the left points to groups with similar pattern across diseases.
The median standardized score (Figure 3A) of the heart rate (pulse) among COVID-19 patients (median 88 ± 23 IQR bpm) was substantially lower compared to influenza (median 93 ± 28 IQR bpm) and SARI (median 97 ± 29 IQR bpm) patients. Systolic blood pressure was lower among COVID-19 patients (median 128 ± 27 mmHg) compared to influenza patients (median 135 ± 37 mmHg), while diastolic blood pressure was higher among COVID-19 patients (median 77 ± 14 mmHg) compared to influenza (median 73 ± 19 mmHg) and SARI patients (median 72 ± 17 mmHg). The standardized scores (in Figure 3) showed that among the vital signs, blood pressure parameters (systolic, diastolic, and average) were regrouped as a single cluster. Similarly, saturation parameters (oxygen and room) formed a single group. A third group included pulse, fever, and breath number. However, neither respiratory measures nor saturation or temperature showed clinically relevant median differences between ILIs.
Laboratory examinations are presented in Table 4 and in Figure 3B. Lymphocytes percentage among COVID-19 patients (median 19.9 ± 15.5 IQR %) was substantially higher compared to influenza patients (median 11.5 ± 10.9 IQR %) and SARI patients (median 10.4 ± 11 IQR %). Similarly, albumin levels were higher among COVID-19 patients (median 3.8 ± 0.6 IQR g/dL) compared to influenza patients (median 3.4 ± 0.8 IQR g/dL) and SARI patients (median 3.1 ± 0.8 IQR g/dL). The standardized scores showed that among the lab test results, magnesium, prothrombin time (PT), partial thromboplastin time (PTT), hemoglobin, lactic dehydrogenase (LDH), and calcium, as well as of lymphocytes percentage, were regrouped as a single cluster that indicated higher levels among COVID-19 patients as compared to influenza and SARI. Conversely, neutrophils (abs), White blood count (WBC), brain natriuretic peptide (BNP) and neutrophils percentage formed a single cluster that indicated substantially lower levels in COVID-19 patients as compared to influenza and SARI. A similar effect with reduced levels in COVID-19 patients as compared to influenza and SARI was shown for the cluster containing met-hemoglobin and carboxyhemoglobin, the cluster containing procalcitonin, bicarbonate, alanine aminotransterrase (ALT), potassium and phosphorous, and the cluster containing creatinine, lactate, blood urea nitrogen (BUN), and glucose.
Trends over time of parameters that showed differences between patient groups, are presented in Table 5. The results for of all pairwise group comparisons are provided as Supplementary Table 2. For each variable and compared pair, the table provides the time gap for which the stronger difference in rank means slopes was found, either (0–6, 6–24) or (6–24, 24–48), and the difference itself. Figure 4 represents the trends for each of the variables showing a significant effect on diseases trajectories. As shown by the trends in Figure 4, both systolic and diastolic blood pressures, as well as pulse, decreased more slowly during the 6–48 h after admission among COVID-19 patients compared to influenza and SARI patients. The difference in slopes with respect to its significance level (p-value) are represented in the volcano plot in Figure 5. The left panel refers to all influenza-COVID-19 comparisons, and the right panel refers to all SARI-COVID-19 comparisons. COVID-19 patients depicted a slower decrease in fever during the 48 h after admission, compared to SARI patients. A particularly distinctive behavior among COVID-19 patients was noted for variables relating to WBC, which slightly increased among COVID-19 patients during the 24 h after admission, but declined in all other patients. COVID-19 patients, similarly to SARI patients, first showed a decrease, and then an increase in lymphocytes percentage during the first 48 h after admission, while influenza patients showed a persistent increase. Neutrophils percentage and count also showed a difference in trend across time between COVID-19 patients and influenza patients. In particular, COVID-19 patients showed a consistent increase in percentage, compared to the decrease observed in patients of other cohorts. Finally, glucose levels among COVID-19 patients were relatively stable during the first 24 h after admission, and then increased, as opposed to all other groups, where a consistent decrease was observed during the 48 h after admission.
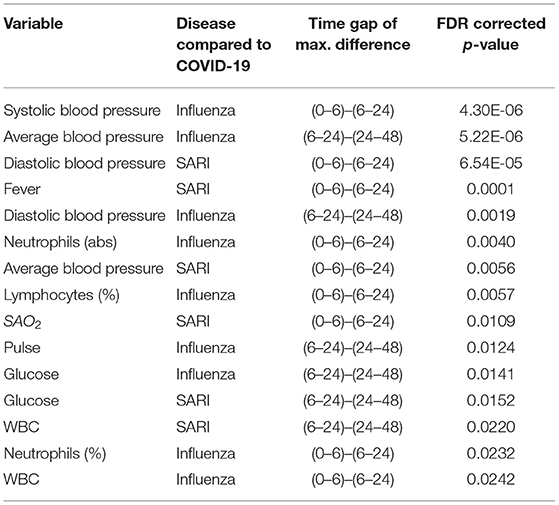
Table 5. Significant slope difference in time window during the first day or the second day of hospitalization.
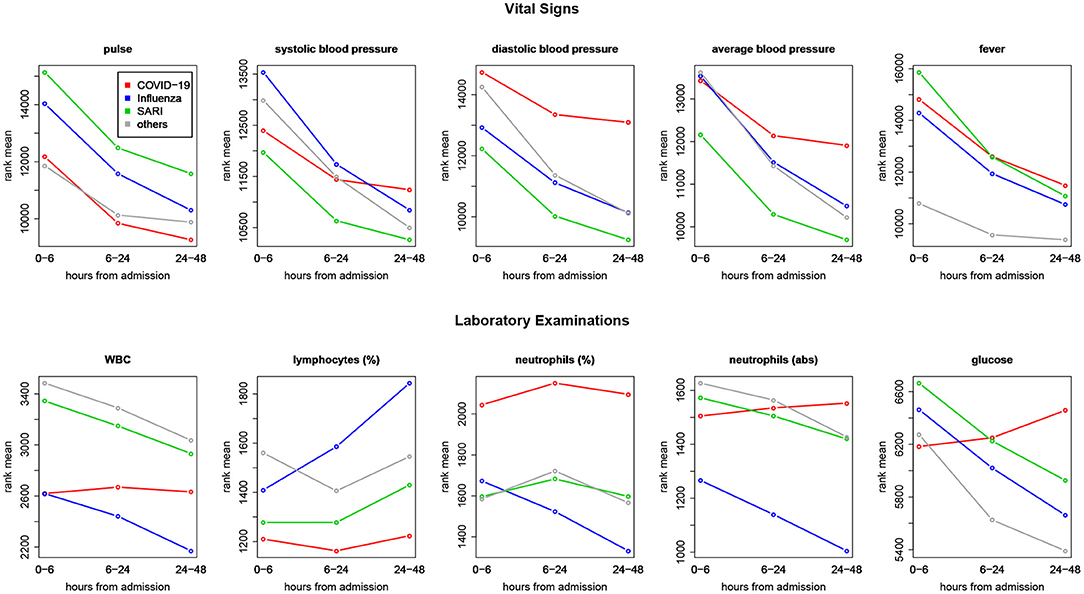
Figure 4. Comparing trends across time—ranks means vs. time intervals. Blood pressure and pulse were decreasing more slowly during 6–48 h after admission among COVID-19 patients compared to influenza and SARI patients. Glucose and measures related to white blood cells (white blood count, lymphocytes, and neutrophils) among COVID-19 patients showed distinct trajectories, with respect to other patients.
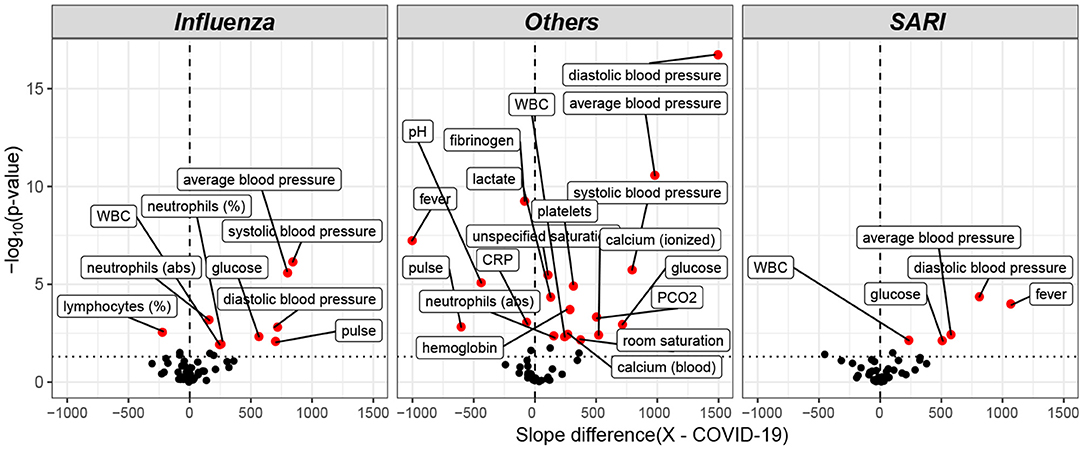
Figure 5. Volcano plot of pairwise post hoc analysis of patients trajectory of COVID-19, influenza, SARI, and others for each lab tests. The level of significance is shown vs. the effect size, namely the slope difference (x—COVID-19). The most highly significant result was for average blood pressure, as seen at the left window, which refers to all influenza-COVID-19 comparisons, and at the right window, which refers to all SARI-COVID-19 comparisons. Trends for WBC, glucose, and diastolic blood pressure for COVID-19 patients were found to be different from both influenza and SARI.
The relative virulence of COVID-19 vs. influenza has been and is still debated. Therefore, our focus was to assess COVID-19 mortality and virulence compared to other ILIs. In-hospital death rates were investigated. While COVID-19 depicted a lower overall mortality, stratification by age showed an increased fraction of casualty among elderly persons consistent with other reports (24). Age has been associated with COVID-19 severity and mortality in plethora of studies (1, 2, 4–7, 9). The presented comorbidity analysis exposed striking differences between disease groups. COVID-19 patients depicted substantially fewer “overall comorbidities” than influenza or SARI patients (66.3 vs. 81.6 and 81.9%). The difference remained after age adjustment and excluding mild severity, suggesting that COVID-19 patients required hospitalization more frequently even if they were healthier before their infection. Hypertension, cardiovascular disease and diabetes depicted a large prevalence in COVID-19, similarly to previous findings (3, 6, 25), and represented common risk factors for ILIs. However, only hypertension and cardiovascular disease showed a significantly lower prevalence in COVID-19 patients compared to other ILIs. Interestingly, cancer prevalence was lower among COVID-19 patients, although it remains unclear how different cancers and/or cancer therapies affect COVID-19 severity or SARS-CoV2 infectivity (26). A recent study suggested that the differential immune cell profiles of cancer patients treated with immunomodulatory agents, may impact the host response to the SARS-COV2 diminish disease severity (27). The lower prevalence can also be explained by higher self COVID-19 risk perception among cancer patients that leads to higher adherence to isolation methods. Taken together, the present investigation demonstrates that COVID-19 hospitalized patients present a significantly lower amount of comorbidities and are younger. COVID-19 patients hospitalized with moderate to severe disease had a significantly higher mortality rate than hospitalized influenza patients. Overall, this suggests that the intrinsic virulence of COVID-19 is higher than influenza.
Our analysis of vital signs revealed that pulse, blood pressure and temperature (fever) were significantly different between ILIs at admission and showed different patterns in the 2 days post-admission despite the small effect size on fever. An independent study on acute respiratory syndrome (10) comparing Influenza and COVID-19 patients (severe) showed that partial pressure of oxygen in arterial blood (PaO2) was remarkably low for COVID-19 patients compared to influenza. Hence, oxygen saturation, heartbeat, temperature, and others continuous measures (i.e., blood glucose, activity) may serve as informative parameters to be continuously monitored in COVID-19 patients. For example, such measurements could be used to anticipate hospitalization, at the point of care, or to predict potential readmission (28–35). Monitoring vital signs at the hospital is critical for following patient disease trajectory (36) and for guiding physicians on clinical interventions, such as initiation of ventilation assistance or pharmaceutical treatment.
Obesity has been reported as a major risk factor for COVID-19 and type 2 diabetes. Furthermore, impaired metabolic health (i.e., dyslipidemia and insulin resistance) is linked with a higher risk of COVID-19-associated pneumonia (37–39). The presented analysis showed that BMI was indeed significantly higher in COVID-19 patients with respect to SARI and Influenza, while diabetes prevalence was high but similar to other ILIs (~30%). The lower BMI group depicted a higher mortality as compared to obese patients. However, due to the high proportion of missing data on BMI among the patients that died and the relatively small sample size, the observed differences warrant further investigations. This obesity paradox has been reported elsewhere (40) and seems to be common between ILIs (41). Several hypothesis have been proposed to explain why obese patients are more affected by COVID-19 without an increased mortality (42). For instance, Adipocytes contains the ACE2 receptor that enable the entrance of the COVID-19 virus, which turns adipose tissue into a potential target and viral reservoir (43). On the other side, Influenza infection relies on a different mechanism, through hemagglutinin onto sialic acid sugars on the surfaces of epithelial cells (44) which could explain a higher severity of COVID-19 disease in overweight patients. Conversely, in obese patients, an increased circulating levels of adipokines and inflammatory cytokines, such as TNFα, IL-6, or C-Reactive Protein (CRP) was observed (45). This chronic low-grade inflammation may impair the adaptive immune responses to viral infections (46) and consequently reduce the probability of a lethal cytokine storm. However, it remains controversial for the COVID-19 disease and the underlying mechanisms remains unclear (47).
At the laboratory examination level, several metabolic intermediates and enzymes were significantly different between COVID-19, SARI, and influenza patients, particularly, lactate, LDH and glucose. Intriguingly, glucose levels in COVID-19 patients were lower at admission but took an opposite trajectory in the first 2 days of admission, compared to SARI and influenza. Impaired glucose homeostasis is associated with poor COVID-19 prognosis and has been hypothesized as an underlying trigger of the cytokine storm in COVID-19 patients (48–51). In severe cases of COVID-19, a hyperinflammatory response (cytokine storm) is correlated with poor outcome (52–54). Several studies reported an association between the neutrophils-to-lymphocyte ratio (NLR) and the severity of COVID-19 (55–57). Accordingly, our investigation of lab examination revealed that COVID-19 have a prominent effect on blood cell-types counts and proportions, such as lymphocytes, neutrophils, WBCs at admission and along the first 2 days of hospitalization. The present analysis reveal that the trajectories of glucose and immune cells are affected by the clinical management of COVID-19 patients, probably through corticosteroid treatments (58).
Ferritin is a key player of immune dysregulation through its immune-suppressive and pro-inflammatory effects, supporting the possibility of cytokine storm (59, 60). We observed that ferritin levels were more elevated among COVID-19 patients as compared to SARI or influenza patients at admission. However, here the ferritin test was performed only for a small number (~1.5%) of severe COVID-19 patients.
A non-neglectable risk of thrombosis or disseminated intravascular clot formation has been described for COVID-19 patients (53, 61). Histopathological studies in post-mortem lung tissue revealed pulmonary microthrombi in 57% of COVID-19 and 58% of SARS as compared to 24% of H1N1 influenza patients (62). However, the underlying mechanisms remain to be fully characterized. It has been hypothesized that the dysregulated immune responses orchestrated by inflammatory cytokines is involved. Interestingly, our analysis of laboratory tests at admission confirmed higher level of PT and PTT in COVID-19 patients while platelet levels did not show extreme values for COVID patients with respect to influenza or SARI patients. In addition, D-dimers depicted a much lower value in influenza compared to COVID-19 or SARI patients. However, this observation might be biased due to the low number of tests made for influenza patients (~5%). As the risk due to thrombosis has been described early in the pandemic (63), D-dimer quantification is currently performed routinely for COVID-19 patients.
The diagnosis of dementia has been reported as an important risk factor for mortality in COVID-19 patients (64–66). The prevalence of demented patient found in the COVID-19 cohort (~10%) was lower than previous estimates, which vary from 13 to 42% (67). According to our analysis, dementia was found to be more prevalent among COVID-19 patients as compared to patients of the other cohorts. In order to determine if this finding can be explained by the higher proportion of COVID-19 patients arriving from nursing homes, the variable of nursing home residence was added to the model. A significant interaction between the nursing home residence variable and dementia in COVID-19 patients was observed. The results can be potentially explained by a higher exposure and infection risk of demented nursing home residents caused by a lower ability to adhere to the needed isolation behaviors (68). COVID-19 exposure in nursing home has been reported by others and is a common issue in several countries (24, 69). More than 20% of all reported COVID-19-associated deaths occurring in nursing homes in countries, such as Canada, Sweden, and the UK. For instance, nursing home population depicted a 1.70-fold higher infection attack rate than the general population in France (24, 69). Thus, COVID-19 exposure in nursing home has been reported by others and is a common issue in several countries.
The above findings point to vital signs and lab results, as well as to comorbidities, that can be helpful in discriminating between COVID-19 and other ILI's at presentation. We plan to pursue a study to develop data-driven tools to efficiently identify COVID-19 cases and predict their disease trajectory at the point of care.
Haifa and the Northern district of Israel are a multicultural region that encompasses several ethnic groups, who have distinct cultural agendas and socio-economic characteristics. The data collected from the largest regional hospital provided were of value for assessment of differential COVID-19 manifestations and risk factors in various ethnic groups. A striking enrichment of all COVID-19 cases in Arab communities accounting for about 40% of COVID-19 cases was observed, as opposed to roughly 25% for the influenza and SARI cases, similar to the Arab proportion in the Haifa area. Higher exposure risk and infection rates of COVID-19 at Arab settlements may account for this effect as no differences in terms of severity between Arabs and Jews were observed. Further stratification of the Jewish population could not be performed for a more detailed analysis of the orthodox or secular fraction. However, it was previously reported that a similar effect is likely in the orthodox Jewish communities (70–72).
Other studies that compared COVID-19 to Influenza did not refer to associations between variables, which may be attributed to systematic activity, and to their clinical trajectory, and used limited cohorts of patients or focused only on acute respiratory distress syndrome, for instance (10, 11). Furthermore, they did not account for multiple testing of variables, which may lead to increased overall type I error (19). In this study, a comprehensive approach was applied to accommodate simultaneous testing of multiple biomarkers. Heterogeneity in distribution between variables was addressed by unifying their scale through robust standardization, thereby allowing comparison of their effects and detection of common patterns through clustering. Non-parametric repeated measures model was performed to allow for time-course analysis under various forms of non-symmetric, long-tailed distributions.
The present study has few important limitations. Firstly, this study is based on a single medical center. Secondly this analysis did not include important information, such as symptoms, images (scans, X-rays), waveforms (e.g., electrocardiogram, oxygen measurements in blood, respiration, ventilation), omics data (e.g., genomics, epi-genomics, transcriptomics, lipidomics, and metabolomics) and pharmacological interventions (apart from the severe cases definition). We decided not to include oxygen measurement because of the uncertainly as per whether the measurement was taken on an arterial or venous line. Thirdly, potential selection bias, information bias, or non-differential misclassification associated with the use of EMR data are existing in the presented dataset. Variables were collected from a unique EMR sources and using a single query tools. These EMR were curated similarly for each groups which allowed to observe clinically relevant significant differences. Differences in the prevalence of personal characteristics between the diseases among hospitalized patients can be related to differences in exposure risk, infection susceptibility, disease severity and admission to hospital policies and biases. To address the effect of selection bias related to differences in hospitalization admission policy of COVID-19 patients compared to the other diseases, asymptomatic and mild cases were excluded in part of the analysis. Asymptomatic and mild cases are usually not hospitalized in influenza and SARI groups unless combined with other risk factors (Berkson's bias) (73).
Furthermore, some of the lab tests are not part of the routine work, hence selection bias by indication is likely. Not all patients within the SARI group were tested for Influenza, COVID-19 or other viruses, thus the SARI group potentially contains undetected Influenza cases, and less likely, COVID-19 cases, as the vast majority patients with Influenza or SARI in our cohort were diagnosed prior to COVID-19 emergence. Nevertheless, the SARI group is heterogeneous in diagnoses, while the Influenza group, which is the focus in this paper for comparison to the COVID-19 group, remains homogeneous and clearly defined. Potential misclassification of influenza within the “others” group is expected to be negligible. This group contains cases with a negative COVID-19 test result, at a period starting at the emergence of COVID-19 in Israel, which was the end of the 2020 influenza season, and ending before the beginning of the next influenza season (74).
The intrinsic virulence of COVID-19 appears higher than influenza. Several critical functions, such as immune response, coagulation, heart and respiratory function and metabolism were markedly impacted by the COVID-19 disease, despite some similarities observed with influenza and SARI. Moreover, COVID-19 seems to differently affect specific segments of the population, potentially due to increased exposure in localized communities or in nursing homes.
The datasets presented in this article are not readily available because of privacy restrictions. Requests to access the datasets should be directed to Dr. Ronit Almog, r_almog@rambam.health.gov.il.
The studies involving human participants were reviewed and approved by the Rambam Health Care Campus Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AR: statistical approach and methodology, data analysis, visualization and interpretation, data curation, manuscript writing, and revision. JS: data analysis, visualization, and interpretation, manuscript writing, and revision. RA: study conception, medical and epidemiological interpretation, data curation, and manuscript revision. SL, TB, and AJ: software/infrastructure development. DE: study conception, medical interpretation, and manuscript revision. JB: study conception, supervision, funding, and manuscript revision. All authors contributed to the article and approved the submitted version.
This research was partially supported by The Milner Foundation, founded by Yuri Milner and his wife Julia. We were grateful to the Placide Nicod foundation for their financial support (JS). We acknowledge the financial support of the Technion Machine Learning & Intelligent Systems Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.656405/full#supplementary-material
1. Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:458–64. doi: 10.15585/mmwr.mm6915e3
2. COVID TC, Team R. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)-United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:343–6. doi: 10.15585/mmwr.mm6912e2
3. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. (2020) 94:91–5. doi: 10.1016/j.ijid.2020.03.017
4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
5. Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. (2020) 92:568–76. doi: 10.1002/jmv.25748
6. Yang L, Liu J, Zhang R, Li M, Li Z, Zhou X, et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: a descriptive study. J Clin Virol. (2020) 129:104475. doi: 10.1016/j.jcv.2020.104475
7. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
8. Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. (2020) 71:2027–34. doi: 10.1093/cid/ciaa344
9. Ioannou GN, Locke E, Green P, Berry K, O'Hare AM, Shah JA, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10,131 US veterans with SARS-CoV-2 infection. JAMA Network Open. (2020) 3:e2022310. doi: 10.1001/jamanetworkopen.2020.22310
10. Tang X, Du R, Wang R, Sun B, Peng P, Shi HZ, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. (2020) 158:195–205. doi: 10.1016/j.chest.2020.03.032
11. Zayet S, Lepiller Q, Zahra H, Royer PY, Toko L, Gendrin V, et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. (2020) 22:481–8. doi: 10.1016/j.micinf.2020.05.016
12. Shen C, Tan M, Song X, Zhang G, Liang J, Yu H, et al. Comparative analysis of early-stage clinical features between COVID-19 and influenza A H1N1 virus pneumonia. Front Public Health. (2020) 8:206. doi: 10.3389/fpubh.2020.00206
13. Mei Y, Weinberg SE, Zhao L, Frink A, Qi C, Behdad A, et al. Risk stratification of hospitalized COVID-19 patients through comparative studies of laboratory results with influenza. EClinicalMedicine. (2020) 26:100475. doi: 10.1016/j.eclinm.2020.100475
14. Johnson AE, Pollard TJ, Shen L, Li-Wei HL, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:1–9. doi: 10.1038/sdata.2016.35
15. Fitzner J, Qasmieh S, Mounts AW, Alexander B, Besselaar T, Briand S, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ. (2018) 96:122. doi: 10.2471/BLT.17.194514
16. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. (2020) 383:2451–60. doi: 10.1056/NEJMcp2009575
17. Management of Persons with COVID-19. (2020). Available online at: https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19
18. WHO. Clinical Management of COVID-19: Interim Guidance. Geneva: World Health Organization (2020).
19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
20. Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. New York, NY: John Wiley & Sons (2002).
21. Reiner-Benaim A, Yekutieli D, Letwin NE, Elmer GI, Lee NH, Kafkafi N, et al. Associating quantitative behavioral traits with gene expression in the brain: searching for diamonds in the hay. Bioinformatics. (2007) 23:2239–46. doi: 10.1093/bioinformatics/btm300
22. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2013).
23. Noguchi K, Gel YR, Brunner E, Konietschke F. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw. (2012) 50:1–23. doi: 10.18637/jss.v050.i12
24. O'Driscoll M, Dos Santos GR, Wang L, Cummings DA, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2 infection in 45 countries. medRxiv. (2020). doi: 10.1101/2020.08.24.20180851
25. Guan W, Liang W, Zhao Y, Liang H, Chen Z, Li Y, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. (2020) 55:2000547. doi: 10.1183/13993003.01227-2020
26. Lee LY, Cazier JB, Starkey T, Briggs SE, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. (2020) 21:1309–16. doi: 10.1016/S1470-2045(20)30442-3
27. Goshen-Lago T, Szwarcwort-Cohen M, Benguigui M, Almog R, Turgeman I, Zaltzman N, et al. The potential role of immune alteration in the cancer-COVID-19 equation—a prospective longitudinal study. Cancers. (2020) 12:2421. doi: 10.3390/cancers12092421
28. Behar J, Liu C, Kotzen K, Tsutsui K, Corino VD, Singh J, et al. Remote health diagnosis and monitoring in the time of COVID-19. Physiol Meas. (2020) 41:10TR01. doi: 10.1088/1361-6579/abba0a
29. Seshadri DR, Davies EV, Harlow ER, Hsu JJ, Knighton SC, Walker TA, et al. Wearable sensors for covid-19: A call to action to harness our digital infrastructure for remote patient monitoring and virtual assessments. Front Digit Health. (2020) 2:8. doi: 10.3389/fdgth.2020.00008
30. Wong CK, Ho DTY, Tam AR, Zhou M, Lau YM, Tang MOY, et al. Artificial intelligence mobile health platform for early detection of COVID-19 in quarantine subjects using a wearable biosensor: protocol for a randomised controlled trial. BMJ Open. (2020) 10:e038555. doi: 10.1136/bmjopen-2020-038555
31. Teo J. Early detection of silent hypoxia in COVID-19 pneumonia using smartphone pulse oximetry. J Med Syst. (2020) 44:1–2. doi: 10.1007/s10916-020-01587-6
32. Michard F, Shelley K, L'Her E. COVID-19: Pulse Oximeters in the Spotlight. J Clin Monit Comput. (2021) 35:11–4. doi: 10.1007/s10877-020-00550-7
33. Mishra T, Wang M, Metwally AA, Bogu GK, Brooks AW, Bahmani A, et al. Early detection of covid-19 using a smartwatch. medRxiv. (2020). doi: 10.1101/2020.07.06.20147512
34. Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. (2020) 368:m1182. doi: 10.1136/bmj.m1182
35. Shah S, Majmudar K, Stein A, Gupta N, Suppes S, Karamanis M, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med. (2020) 27:681–92. doi: 10.1111/acem.14053
36. Möhlenkamp S, Thiele H. Ventilation of COVID-19 patients in intensive care units. Herz. (2020) 1–3. doi: 10.1007/s00059-020-04923-1
37. Sattar N, McInnes IB, McMurray JJ. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. (2020) 142:1–6. doi: 10.1161/CIRCULATIONAHA.120.047659
38. Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. (2020) 8:562–4. doi: 10.1016/S2213-8587(20)30160-1
39. Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. (2020).
40. Biscarini S, Colaneri M, Ludovisi S, Seminari E, Pieri TC, Valsecchi P, et al. The obesity paradox: analysis from the SMAtteo COvid-19 REgistry (SMACORE) cohort. Nutr Metab Cardiovasc Dis. (2020) 30:1920–5. doi: 10.1016/j.numecd.2020.07.047
41. Ball L, Neto AS, Pelosi P. Obesity and survival in critically ill patients with acute respiratory distress syndrome: a paradox within the paradox. Crit Care. (2017) 21:114. doi: 10.1186/s13054-017-1682-5
42. Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. (2020) 16:341–2. doi: 10.1038/s41574-020-0364-6
43. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity. (2020) 28:1187–90. doi: 10.1002/oby.22856
44. Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. (2002) 12:159–66. doi: 10.1002/rmv.352
45. Richard C, Wadowski M, Goruk S, Cameron L, Sharma AM, Field CJ. Individuals with obesity and type 2 diabetes have additional immune dysfunction compared with obese individuals who are metabolically healthy. BMJ Open Diabetes Res Care. (2017) 5:379. doi: 10.1136/bmjdrc-2016-000379
46. Ahn SY, Sohn SH, Lee SY, Park HL, Park YW, Kim H, et al. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ Toxicol Pharmacol. (2015) 40:924–30. doi: 10.1016/j.etap.2015.09.020
47. Jose RJ, Manuel A. Does coronavirus disease 2019 disprove the obesity paradox in acute respiratory distress syndrome? Obesity. (2020) 28:1007. doi: 10.1002/oby.22835
48. Piarulli F, Lapolla A. COVID 19 and low-glucose levels: is there a link? Diabetes Res Clin Pract. (2020) 166:108283. doi: 10.1016/j.diabres.2020.108283
49. Zhang J, Kong W, Xia P, Xu Y, Li L, Li Q, et al. Impaired Fasting Glucose and Diabetes are related to higher risks of complications and mortality among patients with coronavirus disease 2019. Front Endocrinol. (2020) 11:525. doi: 10.3389/fendo.2020.00525
50. Singh AK, Singh R. Does poor glucose control increase the severity and mortality in patients with diabetes and COVID-19? Diabetes Metab Syndr. (2020) 14:725–7. doi: 10.1016/j.dsx.2020.05.037
51. Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. (2020) 31:1068–77.e3. doi: 10.1016/j.cmet.2020.04.021
52. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033. doi: 10.1016/S0140-6736(20)30628-0
53. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. (2020) 8:e46–7. doi: 10.1016/S2213-2600(20)30216-2
54. Sun X, Wang T, Cai D, Hu Z, Liao H, Zhi L, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. (2020) 53:38–42. doi: 10.1016/j.cytogfr.2020.04.002
55. Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. (2020) 18:1–12. doi: 10.1186/s12967-020-02374-0
56. Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. (2020) 7:157. doi: 10.3389/fmolb.2020.00157
57. Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. (2020) 92:1733–4. doi: 10.1002/jmv.25819
58. Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: a systemic review and a Clinician's perspective. Diabetes Metab Syndr. (2020) 14:971–8. doi: 10.1016/j.dsx.2020.06.054
59. Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. (2020) 44:e72. doi: 10.26633/RPSP.2020.72
60. Gómez-Pastora J, Weigand M, Kim J, Wu X, Strayer J, Palmer AF, et al. Hyperferritinemia in critically ill COVID-19 patients-Is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. (2020) 509:249–51. doi: 10.1016/j.cca.2020.06.033
61. Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. (2020) 18:2103–9. doi: 10.1111/jth.14975
62. Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, et al. Lung histopathology in COVID-19 as compared to SARS and H1N1 influenza: a systematic review. Chest. (2020) 159:73–84. doi: 10.1016/j.chest.2020.09.259
63. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. (2020) 135:2033–40. doi: 10.1182/blood.2020006000
64. Hariyanto TI, Putri C, Situmeang RFV, Kurniawan A. Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID-19) infection. Eur Archiv Psychiatry Clin Neurosci. (2021) 271:393–5. doi: 10.1007/s00406-020-01205-z
65. Miyashita S, Yamada T, Mikami T, Miyashita H, Chopra N, Rizk D. Impact of dementia on clinical outcomes in elderly patients with coronavirus 2019 (COVID-19): an experience in New York. Geriatr Gerontol Int. (2020) 20:732. doi: 10.1111/ggi.13942
66. Hariyanto TI, Putri C, Arisa J, Situmeang RFV, Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Archiv Gerontol Geriatr. (2020) 93:104299. doi: 10.1016/j.archger.2020.104299
67. Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. (2020) 24:560–2. doi: 10.1007/s12603-020-1389-1
68. Keng A, Brown EE, Rostas A, Rajji TK, Pollock BG, Mulsant BH, et al. Effectively caring for individuals with behavioral and psychological symptoms of dementia during the COVID-19 pandemic. Front Psychiatry. (2020) 11:573367. doi: 10.3389/fpsyt.2020.573367
69. Cipriani G, Di Fiorino M. Access to care for dementia patients suffering from COVID-19. Am J Geriatr Psychiatry. (2020) 28:796–7. doi: 10.1016/j.jagp.2020.04.009
70. Schattner A, Klepfish A. Orthodox Judaism as a risk factor of Covid-19 in Israel. Am J Med Sci. (2020) 360:304. doi: 10.1016/j.amjms.2020.05.037
71. Slobodin O, Cohen O. A culturally-competent approach to emergency management: What lessons can we learn from the COVID-19? Psychol Trauma. (2020) 12:470. doi: 10.1037/tra0000790
72. Birenbaum-Carmeli D, Chassida J. Covid-19 in Israel: socio-demographic characteristics of first wave morbidity in Jewish and Arab communities. Int J Equity Health. (2020) 19:1–13. doi: 10.1186/s12939-020-01269-2
73. Westreich D. Berkson's bias, selection bias, and missing data. Epidemiology. (2012) 23:159. doi: 10.1097/EDE.0b013e31823b6296
Keywords: COVID-19, influenza, SARI, biomarkers, disease trajectory
Citation: Reiner Benaim A, Sobel JA, Almog R, Lugassy S, Ben Shabbat T, Johnson A, Eytan D and Behar JA (2021) Comparing COVID-19 and Influenza Presentation and Trajectory. Front. Med. 8:656405. doi: 10.3389/fmed.2021.656405
Received: 20 January 2021; Accepted: 13 April 2021;
Published: 14 May 2021.
Edited by:
Yimin Zhang, Zhejiang University, ChinaReviewed by:
Jianzhou Li, The First Affiliated Hospital of Xi'an Jiaotong University, ChinaCopyright © 2021 Reiner Benaim, Sobel, Almog, Lugassy, Ben Shabbat, Johnson, Eytan and Behar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joachim A. Behar, jbehar@technion.ac.il
†These authors share first authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.