- 1Charité–Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Department for Medicine (Gastroenterology, Infectious diseases, Rheumatology), Berlin, Germany
- 2Department of Medicine 1, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
- 3Department of Chemical and Biological Engineering, Institute of Medical Biotechnology, Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany
- 4Deutsches Zentrum Immuntherapie DZI, Erlangen, Germany
High-definition endoscopy is one essential step in the initial diagnosis of inflammatory bowel disease (IBD) characterizing the extent and severity of inflammation, as well as discriminating ulcerative colitis (UC) from Crohn's disease (CD). Following general recommendations and national guidelines, individual risk stratification should define the appropriate surveillance strategy, biopsy protocol and frequency of endoscopies. Beside high-definition videoendoscopy the application of dyes applied via a spraying catheter is of additional diagnostic value with a higher detection rate of intraepithelial neoplasia (IEN). Virtual chromoendoscopy techniques (NBI, FICE, I-scan, BLI) should not be recommended as a single surveillance strategy in IBD, although newer data suggest a higher comparability to dye-based chromoendoscopy than previously assumed. First results of oral methylene blue formulation are promising for improving the acceptance rate of classical chromoendoscopy. Confocal laser endomicroscopy (CLE) is still an experimental but highly innovative endoscopic procedure with the potential to contribute to the detection of dysplastic lesions. Molecular endoscopy in IBD has taken application of CLE to a higher level and allows topical application of labeled probes, mainly antibodies, against specific target structures expressed in the tissue to predict response or failure to biological therapies. First pre-clinical and in vivo data from label-free multiphoton microscopy (MPM) are now available to characterize mucosal and submucosal inflammation on endoscopy in more detail. These new techniques now have opened the door to individualized and highly specific molecular imaging in IBD in the future and pave the path to personalized medicine approaches. The quality of evidence was stated according to the Oxford Center of evidence-based medicine (March 2009). For this review a Medline search up to January 2021 was performed using the words “inflammatory bowel disease,” “ulcerative colitis,” “crohn's disease,” “chromoendoscopy,” “high-definition endoscopy,” “confocal laser endomicroscopy,” “confocal laser microscopy,” “molecular imaging,” “multiphoton microscopy.”
Introduction
Gastrointestinal endoscopy plays a crucial role in patients with inflammatory bowel disease (IBD; Crohn's disease CD; ulcerative colitis UC). The initial diagnosis, the determination of disease activity and surveillance are the key steps of rational disease management and include primarily an endoscopic approach to visualize and characterize the extent and severity of mucosal inflammation and to take targeted biopsies of inflamed and non-inflamed tissue areas. High-resolution or high-definition endoscopy should be the gold standard when examining IBD patients. In surveillance colonoscopy, a combination of high-definition endoscopy with classical dye-based chromoendoscopy (e.g., indigocarmine solution 0.1–0.5%) is of additional value to detect flat polypoid neoplastic mucosal lesions and discriminate these areas from colitis-associated pseudopolyps or other benign lesions (level 1, grade of recommendation A). The exclusion or detection of intraepithelial neoplasia (IEN) is the aim of all surveillance colonoscopies in IBD to reduce the risk of malignant transformation to colorectal cancer. Although many study data showed different results this risk seems to be lower than previously assumed (1) and is in the range of 1–7% after 10 and 30 years of UC, respectively (2), (level 2, grade B). In patients with CD the risk of developing colorectal cancer is lower than in UC, but still heightened with an incidence rate of 2% after 30 years (2), (level 2, grade B). The detection rate of IEN can be further improved by using in-vivo histology techniques. Confocal laser endomicroscopy (CLE) was introduced in 2006 and gave exclusive insight into the gastrointestinal tract on a cellular and subcellular level in a variety of gastrointestinal diseases. Initially, there were two independent in-vivo histology systems available on the market, the endoscope-based CLE (eCLE) by Pentax, Tokyo, Japan, and a probe-based CLE (pCLE) by Mauna Kea Technologies, Paris, France. A couple of years ago the technical support for eCLE was permanently discontinued and research activities with that specific system were restricted to a very small number of research centers with active running systems. In IBD, CLE was used for characterization and classification of inflammatory activity and mucosal healing in active disease as well as for the detection of IEN during surveillance. For example, a combination of chromoendoscopy with CLE can detect 5-fold higher rates of IEN compared with random biopsy protocols (3), (level 4 grade C). After evaluation of CLE as a unique tool for the characterization of normal, inflamed and pre-malignant or malignant intestinal mucosa, some research groups focused on the analysis of the intestinal barrier function for predicting clinical relapse (4), (level 3, grade C). Mucosal healing can predict response to therapy or, vice versa, ongoing mucosal or submucosal inflammation may indicate treatment failure. Kiesslich et al. published a study investigating epithelial barrier function by CLE and described leakage of fluorescein due to epithelial gaps during cell shedding (5), (level 3, grade C). Based on these and other data, highly specific fluorescein-labeled probes binding to their molecular targets on the surface of the gastrointestinal epithelium established a fascinating new era of molecular imaging studies. Molecular endoscopy allows a more specific and individual treatment by predicting the response to anti-inflammatory therapy (6), (level 3, grade C). Recently label-free multiphoton microscopy based on endogenous autofluorescence visualized mucosal inflammation in human biopsies of CD patients (7, 8).
High-Definition Endoscopy and Chromoendoscopy in IBD
Lower optical resolution of previous endoscope generations and random biopsy protocols in all patients were central elements in the surveillance of IBD during the first decade of this century. The lower image quality might be one reason for the increased rate of colorectal cancers described earlier in UC patients (1). High-definition endoscopes have an average diameter of 9–13 mm, a field of view between 140 and 170°, an optical resolution up to 2 million pixels and a 4-way angulation and the newest generation of endoscopes is mostly equipped with bright LEDs (9). Over the last 10 years a more specific and, moreover, individual endoscopic strategy was implemented in national IBD guidelines focusing on defined risk factors. In Germany, surveillance colonoscopy in UC starts 6–8 years after initial diagnosis and should be performed between each year in high-risk patients and every 4 year in patients with low-risk conditions (10), (level 1, grade A). Recently patients with primary sclerosing cholangitis (PSC), a tubular colon and those with a history of neoplasia were identified as having a higher risk for developing colorectal cancer and in these patients targeted and additional random biopsies were recommended during chromoendoscopy (11), (level 1, grade A). For classical chromoendoscopy in the colon, either indigo carmine as a contrast enhanced dye or methylene blue as an absorptive dye can be used, for both agents a 0.1–0.5% working solution is recommended and should be applied with slight pressure via a spraying catheter to the mucosal surface to ensure optimal distribution throughout the entire colon. An adequate withdrawal time and sufficient bowel preparation (Boston Preparation Scale ≥6) is mandatory for an optimal view of the complete colonic mucosal surface. Huge efforts were made to investigate if virtual chromoendoscopy techniques (NBI, FICE, I-scan) are able to replace classical dye-based chromoendoscopy. NBI can characterize histological inflammation by the determination of mucosal vascular pattern (12), (level 4, grade C). This was recently confirmed and prediction of mucosal proliferation can be helpful in the diagnosis of IEN (13), (level 4, grade C). However, the inconsistent results of various studies currently do not justify the application of virtual chromoendoscopy as a single surveillance strategy (14, 15), (level 3, grade D). Studies favoring virtual chromoendoscopy found that the examination time and the technical efforts were significantly lower and therefore more user-friendly compared to the application of classical dyes via spraying catheter (16), (level 1, grade A). A new meta-analysis identified 11 randomized-controlled trials with a total of 1328 patients and concluded that virtual chromoendoscopy is as good as high-definition endoscopy with dye-based chromoendoscopy (17). This indicates that probably in a couple of years both techniques can be applied equally depending on the local expertise of the respective endoscopy unit. As we already know from our daily practice, the acceptance rate of classical chromoendoscopy among physicians is low. Therefore, a promising future perspective for any screening colonoscopy might be the pre-interventional intake of oral chromoendoscopy dye. Recently, the results of a phase 3 trial found an increase in the adenoma detection rate of 8.5% when peroral methylene blue tablets (MMX®) were administered together with bowel preparation (18), (level 1, grade A). Further studies will evaluate oral chromoendoscopy in patients with the need for recurring endoscopic surveillance colonoscopies.
Confocal Laser Endomicroscopy (eCLE, pCLE)
Today we can look back on 15 years of confocal laser endomicroscopy (CLE). This exciting technique was originally designed to allow virtual histology on a cellular and subcellular level with the potential to at least partially replace classical histology. The procedure, however, is time-consuming, technically challenging and intravenous applied fluorescein is necessary for each procedure to generate high-resolution images. This and the fact that no reimbursement was provided by health care authorities restricted the running CLE systems to large research units in University centers. The acquisition of targeted biopsies became reality and a large number of clinical studies investigating a variety of gastrointestinal diseases were published between 2005 and 2012. Most of these studies characterized pre-malignant or inflammatory lesions in Barrett's esophagus (19), gastric cancer (20), celiac disease (21), IBD (22), graft-vs. host disease (23) or adenomatous polyps (24) in the upper and lower gastrointestinal tract (level 3, grade C). A fascinating overview of different cellular and subcellular pathologies was provided and after an initial characterization period the next level of CLE research was reached by explaining functional dynamic changes within the intestinal mucosa. The identification of epithelial gaps during cell shedding and the increase in gaps after stimulation with tumor necrosis factor (TNF) alpha caused loss of barrier function and integrity (5), (level 3, grade C). In IBD patients in clinical remission, increased cell shedding with fluorescein leakage was observed and associated with subsequent relapse 12 months after initial CLE (22) indicating that CLE is able to relapse or can define a stable disease when the barrier function is intact (level C, grade C). These observations were in accordance with electrophysiological measurements in human biopsies of patients with CD as described earlier. After anti-TNF treatment the upregulation of epithelial apoptotic cells in active disease restored to normal and barrier dysfunction completely recovered (25). In vivo histology was also able to contribute to the diagnosis and detection of IEN during surveillance colonoscopy. For CLE a meta-analysis revealed a pooled sensitivity and specificity of 91 and 97% for the differentiation of neoplastic from non-neoplastic lesions (26). Data of chromoendoscopy-guided CLE showed inconsistent results (level 4, grade C). Whereas, some studies describe a higher detection rate of IEN (3) in UC patients, other working groups did not observe a benefit over chromoendoscopy alone (27). However, the general use of this approach for surveillance cannot be recommended. Ongoing study activities with eCLE were hampered by the missing combination of the initial confocal microscope device with a newer high-definition endoscope technology due to several, unfortunately also economic reasons. Currently there is only pCLE available on the market and although there are technical and optical differences between the two systems, the usefulness of pCLE in predicting postoperative recurrence in patients with CD was shown recently (28), (level 4, grade C). Now there is a possibility to apply pCLE with nearly any commercial endoscope independent of the manufacturer. For further characterization of intestinal barrier function in IBD in vivo by pCLE, reliable and reproducible diagnostic criteria should be defined. The quantification of gaps, fluorescein leakage and cell shedding (5, 29) (level 3–4, grade C) are encouraging first candidates for the measurement of barrier function in vivo and may act as main criteria. Crypt tortuosity, distortion of crypt openings and decreased crypt density were additional observations in UC patients (30) and could potentially act as minor criteria (level 3, grade C). A number of CLE-based rating systems and scores have been published so far taking into account the degree of inflammation and the prediction of relapse (31). For the assessment of clinical outcomes or the determination of relapse rates in IBD patients under immunosuppressive therapy further research is necessary. The number of research projects investigating CLE in IBD is currently decreasing. One reason for this may be the introduction of emerging artificial intelligence systems (32) on the market, which will be part of future detection of IEN. However, for the determination of disease activity to predict relapse or therapy response in IBD there is an ongoing need for further CLE evaluation.
Molecular Imaging
Fluorescence endoscopy (33), near-infrared fluorescence endoscopy (34) and autofluorescence endoscopy were often subsumed under molecular imaging devices. These techniques can be combined with virtual chromoendoscopy (35). However, these technologies were rather classical “red flag” technologies than real molecular imaging techniques. The years of research of the newer in-vivo histology techniques deliver the basis for a more detailed analysis of the underlying molecular pathways. More specific and distinct molecular imaging in advanced gastrointestinal endoscopy is the real-time visualization and binding of labeled-molecules to targeted structures on the surface of epithelial cells and the detection of this conjunction by in vivo histology. Probes usable for molecular imaging could be labeled antibodies, peptides, enzymes, affibodies or lectins, respectively (36). Molecular imaging is far away from widespread clinical use. However, it potentially allows a highly-individualized and specific characterization of mucosal inflammatory diseases in the future. In vivo studies with labeled antibodies imply a long-lasting and extensive process of approval and fulfillment of strict requirements before the use in humans is approved by regulatory authorities. The first in vivo application of fluorescein-labeled heptapeptides during a colonoscopy detecting colonic dysplasia was in 2008 (37). Two years later, targeting of epidermal growth factor receptor (EGFR) in colorectal cancer allowed the discrimination of neoplastic and non-neoplastic tissue areas in living animals and human tissue samples (38). One underlying signaling pathway identified a link between inflammation and tumorigenesis and was described in colitis-associated cancer (39). After demonstrating the feasibility and safety of molecular imaging in pre-malignant or malignant disease in vivo, further research focused on inflammatory disease with the goal to predict therapy response or relapse. The first molecular target of interest in IBD was TNF. A landmark study detecting the binding of membrane-bound TNF (mTNF) by a fluorescent-labeled adalimumab anti-TNF antibody showed that high numbers of mTNF-positive cells correlated with higher short-term response rates to treatment with the TNF-neutralizing antibody adalimumab. Patients with high numbers of mTNF-expressing cells demonstrated a higher probability of clinical response than patients with low numbers of mTNF+ cells (92 vs. 15%). The sensitivity, specificity and accuracy for the prediction of therapeutic responses were 92, 85, and 88%, respectively. Positive and negative predictive values were 85 and 92% (40). Recently, first data presented the detection of mucosal α4β7 integrin ex vivo with a fluorescent labeled anti-adhesion antibody vedolizumab in CD (41). In the clinical management of IBD patients, early prediction of response or failure of a planned therapy would be of utmost clinical importance. Consequently, a prompt adjustment of planned immunosuppressive therapy would be possible (42).
Multiphoton Microscopy
Multiphoton microscopy (MPM) is one of the emerging innovative imaging technologies with the potential to visualize intestinal epithelial cells under normal and inflamed conditions without the addition of exogenous fluorescent dyes (43). The first data with MPM as a promising imaging technology in IBD revealed a clear discrimination of epithelial and immune cells and the amount of extracellular matrix (7). This label-free imaging of intestinal cellular and subcellular structures based on autofluorescence and second harmonic generation signals has therefore some advantages compared to CLE and was further developed for in vivo use. Recently, the first experiments in normal and inflamed murine colonic mucosa in a dextran-sulfate sodium-induced colitis model showed feasibility and a gradually deformation of the crypt architecture depending on the activity of the colitis (8). A future perspective would be the combination of MPM with a high-definition endoscope to enable the use during routine gastrointestinal endoscopy without the requirement of any exogenous labeling.
Future Perspectives
On the way to an individualized endoscopic approach, a large number of technical improvements are nowadays available for patients with IBD. These include mainly high-definition endoscopy with nearly comparable efficiency compared to dye-based and virtual chromoendoscopy techniques. If upcoming clinical studies with oral intake of methylene blue prior to surveillance colonoscopy become available and confirm the additional benefit, the reservations against classical dye spraying would finally come to an end. Although CLE as the most widely used in vivo histology method brought extensive insight and understanding of gastrointestinal mucosal pathology, its widespread use in routine endoscopy is hampered by the lack of reimbursement and additional examination time (Figure 1). However, CLE opened the field for molecular endoscopy allowing specific targeting of surface molecules. The prediction of therapeutic response followed by prompt adjustment of targeted therapeutic strategies improve clinical decisions in complex IBD courses. MPM is an emerging new technology and the first data are now available showing in vivo use in an animal model. Label-free high-resolution endomicroscopy would be the logical consequence and a perfect long-term perspective for the use in patients with IBD.
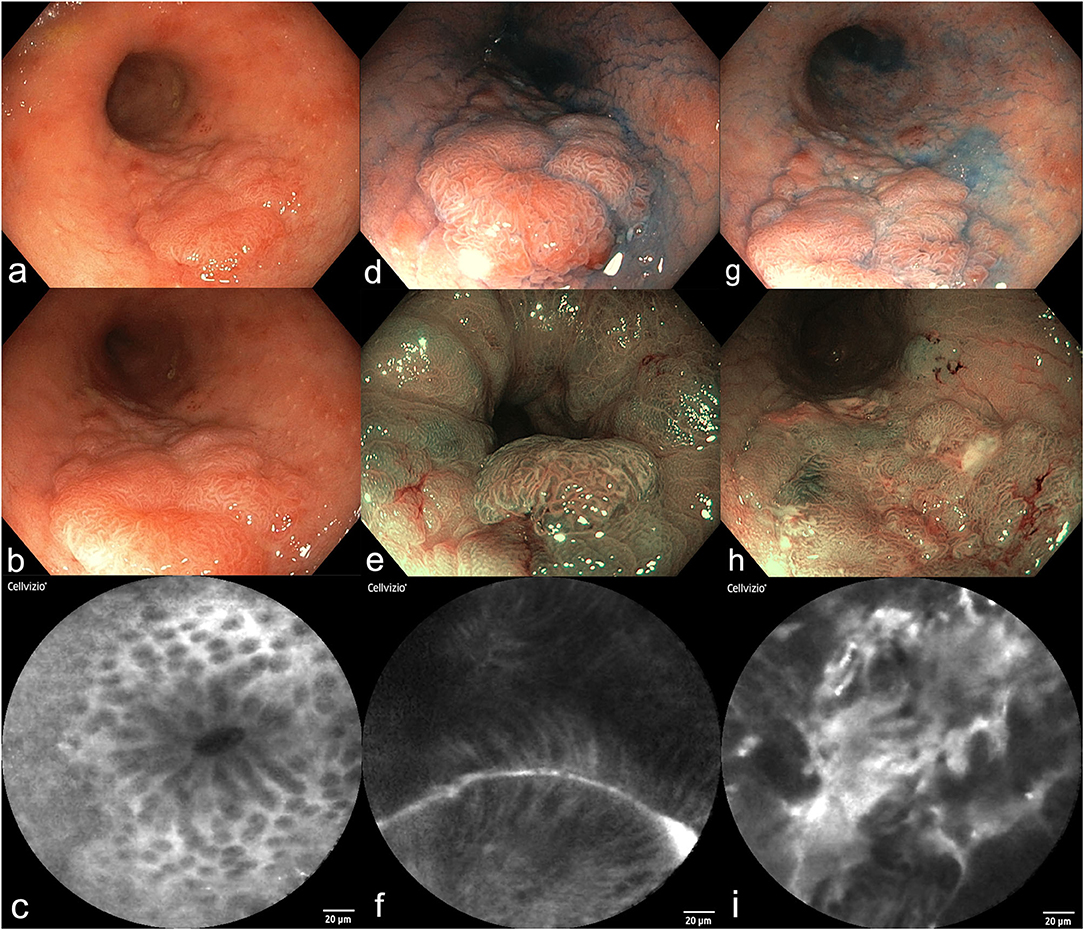
Figure 1. Surveillance colonoscopy in a female patient (64y) with a history of UC for 15 years (a–i). (a,b) High-resolution video endoscopy shows a flat polypoid lesion in the sigmoid colon, size 4 × 2 cm, Paris Classification IIa+c. (c) Probe-based confocal laser endomicroscopy of the surrounding mucosa revealed mild inflammation and normal crypts. (d–f) Dye-based chromoendoscopy with indigocarmine (d), narrow-band imaging [NBI, (e)], and pCLE (f) of the distal border of the polyp. A tubular structure and distorted mucosal epithelial cells become visible. (g–i) Dye-based chromoendoscopy with indigocarmine (g), NBI (h), and pCLE (i) of the proximal part of the polyp. (i) Shows high-grade intraepithelial neoplasia. Final histology of this lesion after proctocolectomy revealed well-differentiated intramucosal cancer without invasion.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (INST 335/534-1 FUGG) and is part of the Transregio TRR241.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. (2001) 48:526–35. doi: 10.1136/gut.48.4.526
2. Selinger CP, Andrews JM, Titman A, Norton I, Jones DB, McDonald C, et al. Long-term follow-up reveals low incidence of colorectal cancer, but frequent need for resection, among Australian patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. (2014) 12:644–50. doi: 10.1016/j.cgh.2013.05.017
3. Gunther U, Kusch D, Heller F, Burgel N, Leonhardt S, Daum S, et al. Surveillance colonoscopy in patients with inflammatory bowel disease: comparison of random biopsy vs. targeted biopsy protocols. Int J Colorectal Dis. (2011) 26:667–72. doi: 10.1007/s00384-011-1130-y
4. Karstensen JG, Saftoiu A, Brynskov J, Hendel J, Klausen P, Cartana T, et al. Confocal laser endomicroscopy: a novel method for prediction of relapse in Crohn's disease. Endoscopy. (2016) 48:364–72. doi: 10.1055/s-0034-1393314
5. Kiesslich R, Goetz M, Angus EM, Hu Q, Guan Y, Potten C, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. (2007) 133:1769–78. doi: 10.1053/j.gastro.2007.09.011
6. Atreya R, Goetz M. Molecular imaging in gastroenterology. Nat Rev Gastroenterol Hepatol. (2013) 10:704–12. doi: 10.1038/nrgastro.2013.125
7. Schurmann S, Foersch S, Atreya R, Neumann H, Friedrich O, Neurath MF, et al. Label-free imaging of inflammatory bowel disease using multiphoton microscopy. Gastroenterology. (2013) 145:514–6. doi: 10.1053/j.gastro.2013.06.054
8. Dilipkumar A, Al-Shemmary A, Kreiss L, Cvecek K, Carle B, Knieling F, et al. Label-Free multiphoton endomicroscopy for minimally invasive in vivo imaging. Adv Sci (Weinh). (2019) 6:1801735. doi: 10.1002/advs.201801735
9. Tang Y, Anandasabapathy S, Richards-Kortum R. Advances in optical gastrointestinal endoscopy: a technical review. Mol Oncol. (2020). doi: 10.1002/1878-0261.12792. [Epub ahead of print].
10. Kucharzik T, Dignass AU, Atreya R, Bokemeyer B, Esters P, Herrlinger K, et al. Aktualisierte S3-leitlinie colitis ulcerosa – living guideline. Z Gastroenterol. (2020) 58:e241–326. doi: 10.1055/a-1296-3444
11. Moussata D, Allez M, Cazals-Hatem D, Treton X, Laharie D, Reimund JM, et al. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut. (2018) 67:616–24. doi: 10.1136/gutjnl-2016-311892
12. Kudo T, Matsumoto T, Esaki M, Yao T, Iida M. Mucosal vascular pattern in ulcerative colitis: observations using narrow band imaging colonoscopy with special reference to histologic inflammation. Int J Colorectal Dis. (2009) 24:495–501. doi: 10.1007/s00384-008-0631-9
13. Guo T, Qian JM, Yang AM, Li Y, Zhou WX. Predicting mucosal proliferation in ulcerative colitis by assessing mucosal vascular pattern under narrow band imaging colonoscopy. Turk J Gastroenterol. (2021) 32:203–8. doi: 10.5152/tjg.2021.20256
14. Bisschops R, Bessissow T, Joseph JA, Baert F, Ferrante M, Ballet V, et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut. (2018) 67:1087–94. doi: 10.1136/gutjnl-2016-313213
15. Leifeld L, Rogler G, Stallmach A, Schmidt C, Zuber-Jerger I, Hartmann F, et al. White-Light or narrow-band imaging colonoscopy in surveillance of ulcerative colitis: a prospective multicenter study. Clin Gastroenterol Hepatol. (2015) 13:1776–81.e1. doi: 10.1016/j.cgh.2015.04.172
16. Gonzalez-Bernardo O, Riestra S, Vivas S, de Francisco R, Perez-Martinez I, Castano-Garcia A, et al. Chromoendoscopy with indigo carmine vs virtual chromoendoscopy (iSCAN 1) for neoplasia screening in patients with inflammatory bowel disease: a prospective randomized study. Inflamm Bowel Dis. (2020). doi: 10.1093/ibd/izaa291. [Epub ahead of print].
17. El-Dallal M, Chen Y, Lin Q, Rakowsky S, Sattler L, Foromera J, et al. Meta-analysis of virtual-based chromoendoscopy compared with dye-spraying chromoendoscopy standard and high-definition white light endoscopy in patients with inflammatory bowel disease at increased risk of colon cancer. Inflamm Bowel Dis. (2020) 26:1319–29. doi: 10.1093/ibd/izaa011
18. Repici A, Wallace MB, East JE, Sharma P, Ramirez FC, Bruining DH, et al. Efficacy of per-oral methylene blue formulation for screening colonoscopy. Gastroenterology. (2019) 156:2198–207.e1. doi: 10.1053/j.gastro.2019.02.001
19. Dunbar KB, Okolo P, 3rd, Montgomery E, Canto MI. Confocal laser endomicroscopy in barrett's esophagus and endoscopically inapparent barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. (2009) 70:645–54. doi: 10.1016/j.gie.2009.02.009
20. Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, Iguchi Y, et al. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. (2006) 38:1110–4. doi: 10.1055/s-2006-944855
21. Gunther U, Daum S, Heller F, Schumann M, Loddenkemper C, Grunbaum M, et al. Diagnostic value of confocal endomicroscopy in celiac disease. Endoscopy. (2010) 42:197–202. doi: 10.1055/s-0029-1243937
22. Kiesslich R, Duckworth CA, Moussata D, Gloeckner A, Lim LG, Goetz M, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. (2012) 61:1146–53. doi: 10.1136/gutjnl-2011-300695
23. Bojarski C, Gunther U, Rieger K, Heller F, Loddenkemper C, Grunbaum M, et al. In vivo diagnosis of acute intestinal graft-versus-host disease by confocal endomicroscopy. Endoscopy. (2009) 41:433–8. doi: 10.1055/s-0029-1214604
24. Sanduleanu S, Driessen A, Gomez-Garcia E, Hameeteman W, de Bruine A, Masclee A. In vivo diagnosis and classification of colorectal neoplasia by chromoendoscopy-guided confocal laser endomicroscopy. Clin Gastroenterol Hepatol. (2010) 8:371–8. doi: 10.1016/j.cgh.2009.08.006
25. Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. (2004) 53:1295–302. doi: 10.1136/gut.2003.036632
26. Lord R, Burr NE, Mohammed N, Subramanian V. Colonic lesion characterization in inflammatory bowel disease: a systematic review and meta-analysis. World J Gastroenterol. (2018) 24:1167–80. doi: 10.3748/wjg.v24.i10.1167
27. Freire P, Figueiredo P, Cardoso R, Donato MM, Ferreira M, Mendes S, et al. Surveillance in ulcerative colitis: is chromoendoscopy-guided endomicroscopy always better than conventional colonoscopy? A randomized trial. Inflamm Bowel Dis. (2014) 20:2038–45. doi: 10.1097/MIB.0000000000000176
28. Auzoux J, Boschetti G, Anon B, Aubourg A, Caulet M, Poisson L, et al. Usefulness of confocal laser endomicroscopy for predicting postoperative recurrence in patients with Crohn's disease: a pilot study. Gastrointest Endosc. (2019) 90:151–7. doi: 10.1016/j.gie.2019.02.030
29. Lim LG, Neumann J, Hansen T, Goetz M, Hoffman A, Neurath MF, et al. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. (2014) 20:892–900. doi: 10.1097/MIB.0000000000000027
30. Karstensen JG, Saftoiu A, Brynskov J, Hendel J, Ciocalteu A, Klausen P, et al. Confocal laser endomicroscopy in ulcerative colitis: a longitudinal study of endomicroscopic changes and response to medical therapy (with videos). Gastrointest Endosc. (2016) 84:279–86.e1. doi: 10.1016/j.gie.2016.01.069
31. Buchner AM. Confocal laser endomicroscopy in the evaluation of inflammatory bowel disease. Inflamm Bowel Dis. (2019) 25:1302–12. doi: 10.1093/ibd/izz021
32. Le Berre C, Sandborn WJ, Aridhi S, Devignes MD, Fournier L, Smail-Tabbone M, et al. Application of artificial intelligence to gastroenterology and hepatology. Gastroenterology. (2020) 158:76–94.e2. doi: 10.1053/j.gastro.2019.08.058
33. Tjalma JJ, Garcia-Allende PB, Hartmans E, Terwisscha van Scheltinga AG, Boersma-van Ek W, Glatz J, et al. Molecular fluorescence endoscopy targeting vascular endothelial growth factor a for improved colorectal polyp detection. J Nucl Med. (2016) 57:480–5. doi: 10.2967/jnumed.115.166975
34. Gounaris E, Ishihara Y, Shrivastrav M, Bentrem D, Barrett TA. Near-Infrared fluorescence endoscopy to detect dysplastic lesions in the mouse colon. Methods Mol Biol. (2016) 1422:137–47. doi: 10.1007/978-1-4939-3603-8_13
35. van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, et al. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. (2008) 57:1083–9. doi: 10.1136/gut.2007.144097
36. Rath T, Kiesslich R, Neurath MF, Atreya R. Molecular imaging within the lower gastrointestinal tract: from feasibility to future. Dig Endosc. (2018) 30:730–8. doi: 10.1111/den.13251
37. Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. (2008) 14:454–8. doi: 10.1038/nm1692
38. Goetz M, Ziebart A, Foersch S, Vieth M, Waldner MJ, Delaney P, et al. In vivo molecular imaging of colorectal cancer with confocal endomicroscopy by targeting epidermal growth factor receptor. Gastroenterology. (2010) 138:435–46. doi: 10.1053/j.gastro.2009.10.032
39. Waldner MJ, Wirtz S, Jefremow A, Warntjen M, Neufert C, Atreya R, et al. VEGF receptor signaling links inflammation and tumorigenesis in colitis-associated cancer. J Exp Med. (2010) 207:2855–68. doi: 10.1084/jem.20100438
40. Atreya R, Neumann H, Neufert C, Waldner MJ, Billmeier U, Zopf Y, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn's disease. Nat Med. (2014) 20:313–8. doi: 10.1038/nm.3462
41. Rath T, Bojarski C, Neurath MF, Atreya R. Molecular imaging of mucosal alpha4beta7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn's disease. Gastrointest Endosc. (2017) 86:406–8. doi: 10.1016/j.gie.2017.01.012
42. Digby-Bell JL, Atreya R, Monteleone G, Powell N. Interrogating host immunity to predict treatment response in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:9–20. doi: 10.1038/s41575-019-0228-5
Keywords: high-definition endoscopy, confocal laser microscopy, chromoendoscopy, molecular endoscopy, multiphoton microscopy
Citation: Bojarski C, Waldner M, Rath T, Schürmann S, Neurath MF, Atreya R and Siegmund B (2021) Innovative Diagnostic Endoscopy in Inflammatory Bowel Diseases: From High-Definition to Molecular Endoscopy. Front. Med. 8:655404. doi: 10.3389/fmed.2021.655404
Received: 18 January 2021; Accepted: 22 June 2021;
Published: 21 July 2021.
Edited by:
Xingshun Qi, General Hospital of Shenyang Military Command, ChinaReviewed by:
John Gásdal Karstensen, Hvidovre Hospital, DenmarkYf Gu, Zhejiang University, China
Dong Wu, Peking Union Medical College Hospital (CAMS), China
Copyright © 2021 Bojarski, Waldner, Rath, Schürmann, Neurath, Atreya and Siegmund. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Bojarski, Y2hyaXN0aWFuLmJvamFyc2tpQGNoYXJpdGUuZGU=
 Christian Bojarski
Christian Bojarski Maximilian Waldner
Maximilian Waldner Timo Rath2
Timo Rath2 Sebastian Schürmann
Sebastian Schürmann Markus F. Neurath
Markus F. Neurath Raja Atreya
Raja Atreya Britta Siegmund
Britta Siegmund