- State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China
Mesenchymal stem cell (MSC) transplantation is a novel treatment for liver diseases due to the roles of MSCs in regeneration, fibrosis inhibition and immune regulation. However, the mechanisms are still not completely understood. Despite the significant efficacy of MSC therapy in animal models and preliminary clinical trials, issues remain. The efficacy and safety of MSC-based therapy in the treatment of liver diseases remains a challenging issue that requires more investigation. This article reviews recent studies on the mechanisms of MSCs in liver diseases and the associated challenges and suggests potential future applications.
Introduction
After Friedenstein et al. (1) first isolated and identified stromal cells from bone marrow in 1966, mesenchymal stem cells (MSCs) were isolated from various tissues, such as the umbilical cord (2, 3), placenta (4), adipose tissue (5, 6), amniotic fluid (7, 8), menstrual blood (9, 10), and dental pulp (11). The immunomodulatory properties, limited self-renewal capacity, and multi-lineage development of MSCs make these cells ideal candidates for clinical applications in different diseases (12). The low inherent immunogenicity of MSCs guarantees transplant safety (13). Even HLA-mismatched MSCs could be used for many clinical applications, especially for stem cell-based therapies (14). Moreover, the ability of these cells to home to specific organs and lesions is the key to the curative effect of MSC transplantation (13). MSC surface chemokine receptors, such as CCR1, CCR4, CCR7, CXCR5, and CCR10, are involved in the migration of MSCs into injured tissues along chemokine gradients (15). MSCs are administered to patients by various routes, such as intravascular injection and local transplantation, to alleviate diseases (16–18).
Liver diseases are a global health issue that cause a large number of deaths every year (19, 20). Liver transplantation, which is recommended as the only effective treatment method available for advanced liver diseases, is limited by high costs and a shortage of donor livers (21). MSC transplantation brings new hope to the treatment of liver diseases. Despite their different etiologies, symptoms and physiological processes, multiple kinds of life-threating liver diseases can be effectively treated by cell-based therapy, as three decades of clinical and preclinical studies proved. Among the most used source of cells for allogenic/autologous transplantation, the two most largely clinically infused cells are undoubtedly primary hepatocyte and MSCs. MSCs have been administered in hundreds of clinical trials during the past decades, and for a plethora of indications (Table 1). Although many laboratory and clinical trials have confirmed the efficacy of MSCs in a variety of diseases, there are still no guidelines to regulate MSC clinical applications (29–31).
The purpose of this review is to summarize and critically discuss the therapeutic effects and related mechanisms of MSCs derived from different sources in the treatment of liver diseases, as well as indicate present issues including efficacy, safety and available routes of administration in clinical MSC therapy trials. We hope this review will provide a reference for future clinical trials and applications.
Role of MSC in Tissue Repair and Regeneration
MSCs can be induced to differentiate into a variety of cell lineages, including adipocytes, osteoblasts, chondroblasts, and hepatocyte-like cells (HLCs), in vitro (32). This characteristic initially gave hope to regenerative medicine. Previous studies have shown that MSCs derived from different tissues, such as bone marrow (BM-MSCs) (33), adipose tissue (AT-MSCs) (34), menstrual blood (MenSCs) (35), and amniotic fluid (AF-MSCs) (34), can differentiate into HLCs in vitro. Clinical and laboratory trials suggested that MSCs could significantly improve liver cell regeneration in different kinds of liver diseases. Despeyroux et al. (36) showed that AF-MSCs improved liver regeneration and survival after 80% hepatectomy in mice. However, by tracking green fluorescent protein-expressing MenSCs, Chen et al. (37) showed that transplanted cells were recruited to injured liver sites in carbon tetrachloride (CCl4)-induced liver fibrosis mouse models, but few cells differentiated into HLCs. According to Shi et al. (38) human-derived hepatocytes constituted only 4.5% of total pig hepatocytes after intraportal vein infusion of BM-MSCs (3 × 106 cells/kg) in D-galactosamine (D-gal)-induced model pigs. von Bahr et al. (39) demonstrated that even minimally expanded BM-MSCs showed limited long-term engraftment and no ectopic tissue formation upon intravascular infusion. This means that MSCs may mainly promote liver regeneration through mechanisms other than differentiation into HLCs.
Several groups have showed that bone marrow transplants in mice led to the generation of liver cells bearing the donor marker, and demonstrated that this event might not due to transdifferentiation of MSCs into hepatic lineage cells. Moreover, this suggest is challenged by the scientists who were unable to reproduce the transdifferentiation of not only MSCs, but also hematological cells into non-hematological ones. Willenbring et al. (40) found that the transplantation into Fah(–/–) mice of lineage-committed granulocyte-macrophage progenitors or bone marrow-derived macrophages resulted in the robust production of bone marrow-derived hepatocytes by cell fusion, which provides potential for organ regeneration. Wang et al. (41) concluded that bone marrow-derived hepatocytes arised from cell fusion and rather than differentiation of hematopoietic stem cells. According to Camargo et al. (42), hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. Such melting event is not occurring only to bone marrow derived cells. Other types of cells, such as perinatal MSCs, have also been reported to fuse with hepatocytes (43). Okamura et al. (44) found that developing monkey embryonic stem cells could repopulate injured mouse liver by fusing with recipient mouse hepatocytes.
Studies have demonstrated that MSCs can stimulate liver cell proliferation and inhibit hepatocyte apoptosis. In a trial that used D-gal-induced rat models of acute liver injury, MSC therapy resulted in a 90% reduction in apoptotic hepatocellular death and a three-fold increase in the number of proliferating hepatocytes (45). Efimenko et al. (46) showed that MSC-conditioned medium (MSC-CM) attenuated CCl4-induced early apoptosis in C57/BL6 mouse hepatocytes through activation of FGL1. According to Chen et al., MSC-conditioned medium injection could prevent radiation-induced liver injury by protecting sinusoidal endothelial cells (47). These results indicated that MSCs protect liver injury and stimulated hepatocyte proliferation by paracrine effects.
Different liver diseases display unique pathophysiological manifestations according to their etiology (48). MSCs may promote liver regeneration through different mechanisms in different disease models.
Accumulating evidence supports that MSCs play therapeutic roles in a paracrine manner, especially through trophic factors (49–52). Hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) are the most widely reported trophic factors secreted by MSCs (46). Antibody array results showed that MSC-derived exosomes (MSC-EXs) contain measurable HGF (53). HGF is widely known to be a crucial factor in the positive regulation of hepatocyte proliferation (54). VEGF secreted by MSCs contributes to the recovery of liver damage (55). Recent studies have also demonstrated the role of miRNAs in MSC-induced liver regeneration. According to Kim et al. (56), rno-miR-122-5p is closely related to the therapeutic efficacy of placenta-derived mesenchymal stem cells (PD-MSCs) in liver tissues. PD-MSCs stimulate hepatocyte proliferation by activating the interleukin 6 (IL-6) signaling pathway through the regulation of rno-miR-21-5p. Hyun et al. (57) showed that microRNA125b-mediated regulation of Hh signaling contributed to liver regeneration that was promoted by chorionic plate-derived mesenchymal stem cells (CP-MSCs) in CCl4-induced rats.
Mechanism of the Antifibrotic Effect of MSC
Liver fibrosis is characterized by the deposition of extracellular matrix (ECM), including collagen I, collagen III and collagen IV (58). The activation of hepatic stellate cells (HSCs) plays a crucial role in this process (55). Activated HSCs proliferate and transform into myofibroblasts (59, 60). Myofibroblasts synthesize ECM and release large amounts of TIMPs, which can reduce ECM degradation by inhibiting interstitial collagenase activity and ultimately induce ECM accumulation (61). Multiple signaling pathways, such as TGF-β/Smad, Ras/ERK, Notch, and Wnt/β-catenin, are involved in HSC activation (62–65). Kupffer cell activation is an important factor that induces HSC activation during chronic liver injury (66). Kupffer cells are resident macrophages in the liver. Activated Kupffer cells release large amounts of soluble mediators, such as oxidants, cytokines, and proteinases, which can affect HSC proliferation, migration, and differentiation (67).
Epithelial-to-mesenchymal transition (EMT) and mesenchymal-to-epithelial transition (MET) are important contributors to liver cirrhosis (68). Epithelial cells in the chronic injured liver undergo EMT, which makes these cells exhibit fibroblastic features and move into the hepatic mesenchyme. Then, these cells undergo MET to differentiate into hepatocytes or cholangiocytes to repair injured tissue. However, the microenvironment of the injured liver may upregulate the EMT, which stimulates fibrogenic repair and causes liver fibrosis (69).
MSCs have significant effects on liver fibrosis. In vitro and in vivo experiments demonstrated that MSCs mainly exert antifibrotic effects by paracrine mechanisms (70). Secretomes or the culture medium of MSCs could also significantly suppress liver fibrosis (71). MSC transplantation could alleviate liver fibrosis and reduce the expression of transforming growth factor-β1 (TGF-β1), Smad2, collagen type I, and α-SMA, and pathological examination showed reduced liver fibrosis areas (72). According to Jang et al. (73), BM-MSCs could reduce hepatic collagen distribution by suppressing the TGF-β/Smad signaling pathway in TAA-induced cirrhosis rat models. An et al. (74) found that the secretomes of UC-MSCs contained high levels of milk fat globule-EGF factor 8 (MFGE8). This factor could downregulate TGF-β1 receptor levels by binding to αβ integrin on hepatic stellate cells (HSCs), thereby strongly inhibiting the activation of primary human HSCs. MSCs can also exert antifibrotic effects through the Wnt/β-catenin pathway. According to Rong et al., BM-MSC-derived exosomes (BM-MSCs-Exs) could suppress HSC activation by inhibiting the expression of Wnt/β-catenin pathway components, including peroxisome proliferator activated receptor γ (PPARγ), Wnt3a, Wnt10b, β-catenin, WISP1, and Cyclin D1 (75).
Chai et al. (76) transfused UC-MSCs into dimethylnitrosamine (DMN)-induced liver fibrosis model rats and found that UC-MSCs alleviated liver fibrosis by increasing IL-4 levels and promoting the mobilization of Kupffer cells. UC-MSC-mediated regulation of Kupffer cells was demonstrated in an in vitro coculture system. According to Ohara et al. (77), amnion-derived mesenchymal stem cell-derived EVs (AMCS-EVs) could significantly inhibit HSC activation and decrease the number of Kupffer cells (KCs) in the livers of rats with liver fibrosis induced by CCl4.
On the other hand, MSCs can regulate the EMT-MET balance in fibrogenic liver tissue. According to Li et al., UC-MSC-Ex transplantation reduced the expression levels of collagen types I and III, TGF-β1 and phosphorylated Smad2 by inhibiting EMT activation in CCl4-induced liver fibrosis models (78). TGF-β has been demonstrated to activate fibrogenic EMT through the RAS and mitogen-activated protein kinase (MAPK) pathways (79, 80). To date, RAS-responsive element binding protein 1 (RREB1) has been identified as a key partner of TGF-β-activated SMAD transcription factors associated with the EMT in liver fibrosis (75). Considering the inhibitory effects of MSCs on TGF-β, MSCs may inhibit the EMT by regulating TGF-β activity, which requires further examination.
Besides, MSC applications are reported to improve liver fibrosis related complications, which are the mainly causes of death in clinic. According to Zhang et al. (22), UC-MSC transplantation could not only lead to the regression of liver fibrosis in patients, but also reduce related ascites. A meta-analysis showed that BM-MSC transplantation could improve ascites and encephalopathy in patients with chronic liver disease especially liver fibrosis (22). As to Pietrosi et al. (81), human amniotic membrane-derived mesenchymal stromal (hAMSCs) could improve hepatic microvascular dysfunction and portal hypertension, which are responsible for the complications defining clinical decompensation. Vitro experiment revealed that sinusoidal cell phenotype ameliorated when co-cultured with hAMSCs. However, the related mechanisms still need further explorations.
Mechanism of the Immunomodulatory Effects of MSC
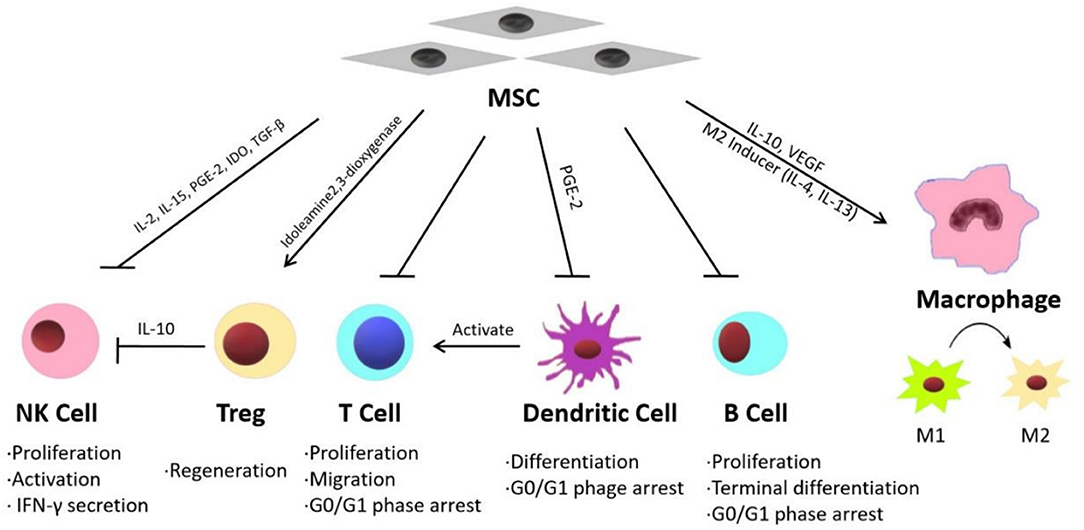
Inflammatory reactions are widely detected in injured liver tissues and are considered the primary causes of fibrosis and hepatic function failure (82–84). Recent studies have shown that MSC therapy can reduce inflammation in liver diseases through different mechanisms. Some published studies have indicated that the effects of MSC treatment on various acute and chronic liver diseases are mainly mediated by their immunomodulatory properties (Figure 1) (15).
MSCs exhibit immunomodulatory functions through paracrine mechanisms (85). MSCs can release multiple immunosuppressive factors, such as IL-10, VEGF, and TGF-β (86–88). Winkler et al. identified the factors secreted by BM-MSCs and AT-MSCs through protein arrays and identified related pathways through biomathematical analyses. The results showed that many cytokines are involved in innate immunity and inflammatory pathways, including the JAK-STAT signaling pathway and Toll-like receptor pathway (89). On the other hand, MSCs can modulate the inflammatory microenvironment by suppressing the expression of inflammatory factors such as IFN, IL-1 and TNF-α (90). Furthermore, MSCs exhibit immunoregulatory activities by balancing the functions of different innate and adaptive immune cells, including natural killer cells (NK cells), T regulatory cells (Tregs), T lymphocytes, B lymphocytes, macrophages and dendritic cells (DCs).
According to DelaRosa et al., AT-MSCs and BM-MSCs inhibit IL-2- and IL-15-induced NK cell proliferation, as well as PGE-2- and IDO-induced NK cell activation. In vitro experiments showed that MSCs could modulate the secretion of IFN-γ by NK cells through the action of soluble factors such as indoleamine 2,3-dioxygenase (91).
Tregs play an important role in fulminant hepatitis. Tregs are necessary for the suppression of immune cell-mediated hepatocyte damage during fulminant hepatitis (92). MSCs significantly promote the regeneration of Tregs both in vivo and in vitro, thereby inhibiting immune cell activation (93). According to Qu et al., BM-MSC transplantation significantly attenuated immune-mediated liver injury and controlled virus levels in hepatitis B virus (HBV)-infected mice. Their study showed that BM-MSC-derived TGF-β suppressed the expression of natural killer group 2 member D (NKG2D), an important receptor required for NK cell activation in the liver in HBV-infected mice, thereby influencing innate immunity and limiting immune-mediated liver injury (94).
MSCs suppress T lymphocyte proliferation induced by mitogens, and CD3 and CD28 antibodies, as well as allogeneic antigens, both in vitro and in vivo. T lymphocytes can be arrested in the G0/G1 phase of the cell cycle by MSCs through the downregulation of cyclin D2 (95). Wang et al. (96) demonstrated that MSC treatment could reverse nonalcoholic fatty liver disease (NAFLD) by suppressing the activation of cluster of differentiation (CD) 4+ T lymphocytes in mouse spleens. MSCs have been shown to suppress B lymphocyte proliferation and terminal differentiation. According to Yi and Song MSCs inhibit B lymphocytes by arresting these cells in the G0/G1 phase of the cell cycle (92).
DCs play an important role in the initiation, maintenance and regulation of immune reactions by stimulating antigen-specific T cell activation (14). According to Ramasamy et al. (97), human BM-MSCs inhibit the differentiation of DCs by blocking the synthesis of cyclin D2 in monocytes, thereby arresting DCs in the G0/G1 phase of the cell cycle. Zhang et al. showed that MSCs effectively attenuated Propionibacterium acnes (P. acnes)-primed and lipopolysaccharide (LPS)-induced liver injury in mice and increased the survival rates of the animals by regulating DC differentiation. MSCs induced the differentiation of a distinct, functional CD11c1MHCIIhi CD80loCD86lo regulatory DC population from CD11c1B2202 DC precursors by secreting PGE2 through a PI3K-dependent pathway. This group of DCs suppressed Th1 cells while inducing Treg proliferation (98).
Two kinds of macrophages participate in the immune response during liver injury, local macrophages (Kupffer cells) and circulating macrophages. As a response to various inflammatory signals during the progression of liver injury, macrophages undergo classic activation (proinflammatory M1) and alternative activation (anti-inflammatory M2) (99). MSCs can regulate M1/M2 balance in macrophages. According to Cho et al., in bone marrow-derived macrophages co-cultured with MSCs, the M1 markers significantly reduced while the M2 markers increased. The result suggested that MSCs could promote the shift of the macrophage phenotype from M1 to M2 (100). Li et al. (101) suggested that MSCs exhibit therapeutic effect in liver sterile inflammatory injury by leading to reprograming macrophage polarization toward anti-inflammatory M2 phenotype through Hippo pathway. UC-MSC-Exs were shown to inhibit the expression of NLRP3, caspase-1, IL-1β, and IL-6 in LPS-stimulated RAW 264.7 macrophages (102).
Liver transplantation is considered the only effective treatment for end-stage liver diseases; however, subsequent rejection, especially acute graft-vs.-host disease (aGVHD), is the leading cause of surgical failure and postoperative death in patients (103). Various immune cells, including T cells, DCs, Tregs, and NK cells, mediate the occurrence and development of rejection (104). Accumulating evidence has demonstrated that MSC transplantation can significantly attenuate the severity of aGVHD due to the immunomodulatory effects of MSCs (104–106). However, the cytological and molecular mechanisms still require further exploration.
Improved MSC Treatment Efficacy (Factors Influencing the Efficacy of MSC)
Although MSC treatment has shown significant effects in liver diseases, researchers are still exploring ways to increase the efficacy of MSC applications. According to previous studies, many factors can influence the efficacy of MSCs. Regardless of expansion protocol, MSCs undergo replicative senescence in culture, with obvious repercussion on therapeutical effects. Based on the study of telomere length, Baxter et al. (107) found that even protocols that involve minimal expansion induce a rapid aging of MSCs, which may influence MSC phenotype and paracrine potential. In AD-MSCs, the expression of the anti-inflammatory cytokine IL-10 showed substantial differences between P7 and P9, with a consistent decrease in mRNA expression (77). In BM-MSCs, compared with those of P1, the expression levels of IL-6 and VEGF were much higher in P5 (108). According to Choi et al., gradual decreases in IL-6 and VEGF expression levels during the long-term culture of MSCs may be related to reductions in the differentiation potential and proliferation of MSCs. The authors suggested that MSCs at earlier passages were more suitable for therapy due to their stability and more potent anti-inflammatory properties than cells at later passages (109). Another study stated that age reduced human MSC-mediated T cell suppression (110). Although the mechanisms of the impact of senescence on the immunomodulatory activity of MSCs are still not clear, multiple studies have demonstrated that senescence due to both donor age or multiple passages impacts the immunomodulatory properties of MSCs (111, 112). These results indicated that optimizing the criteria for the selection of MSC donors and low-passage MSCs could enhance the cell transplantation efficacy.
Pretreatment before application is the most common method to improve the therapeutic efficacy of MSCs in vitro and in vivo. Culture conditions significantly influence MSC phenotype. A hypoxic culture environment can contribute to the maintenance of MSC proliferation, differentiation and metabolic balance (113). According to Kojima et al., compared with those cultured in normal conditions, BM-MSCs cultured under hypoxic conditions showed greater therapeutic effects in mice with liver cirrhosis (114). Yu et al. found that hypoxia preconditioning enhanced the expression of VEGF in BM-MSCs in vitro. These pretreated MSCs exhibited improved regenerative effects in rat massive hepatectomy models (115).
Melatonin is an endogenous indoleamine produced and released into the blood circulation by the pineal gland (116). In addition to regulating biorhythms, melatonin can also play an antiaging role due to its antioxidant effects (117). Mohsin et al. (118) found that pretreating MSCs with injured liver tissue resulted in high expression of albumin, cytokeratin 8, 18, TAT and HNF1α, thereby improving the antifibrotic effect of MSCs in CCl4-induced mice. Fang et al. (119) showed that AD-MSCs pretreated with melatonin showed enhanced beneficial effects in canine acute liver injury. In vitro experiments showed that melatonin pretreatment improved the survival of AD-MSCs by activating Nrf2 through the MT1/MT2 receptor pathway, stimulating ERAD, inhibiting NF-κB and ERS, and alleviating AD-MSC senescence (120). Another study indicated that melatonin pretreatment enhanced the homing capacity of BM-MSCs in a rat model of liver fibrosis (121).
Recently, an increasing number of studies have focused on the role of polymer materials in promoting the efficacy of MSCs in liver diseases. Salem et al. (122) reported that pretreatment with growth factors in the presence of nanofibers promoted the homing, repopulation and hepatic differentiation abilities of MSCs, thereby increasing the efficacy of MSCs in liver fibrosis. As the most frequently used polymer combinations for cell microencapsulation, hybrid poly (ethylene glycol)-alginate hydrogels have been chosen to pretreat MSCs to protect cells against larger compounds such as circulating antibodies, as well as immune and inflammatory cells, without blocking nutrient and metabolite exchange (123). Studies have shown that hybrid poly (ethylene glycol)-alginate hydrogel microencapsulation significantly improved MSC efficacy in ALF and liver fibrosis (124, 125).
In addition to MSC pretreatment, gene modification is also widely used to improve the therapeutic effects of MSCs in liver diseases. Overexpression of c-Met in BM-MSCs could improve the homing of BM-MSCs to injured liver tissue, thereby promoting the efficacy of BM-MSC therapy for ALF repair in rats (126). Various studies have demonstrated that HGF-overexpressing MSCs present an increased ability to treat liver injuries by promoting liver regeneration (127–129). On the other hand, HGF-overexpressing MSCs have longer telomeres, as well as increased mtDNA replication, which leads to increased ATP generation (130).
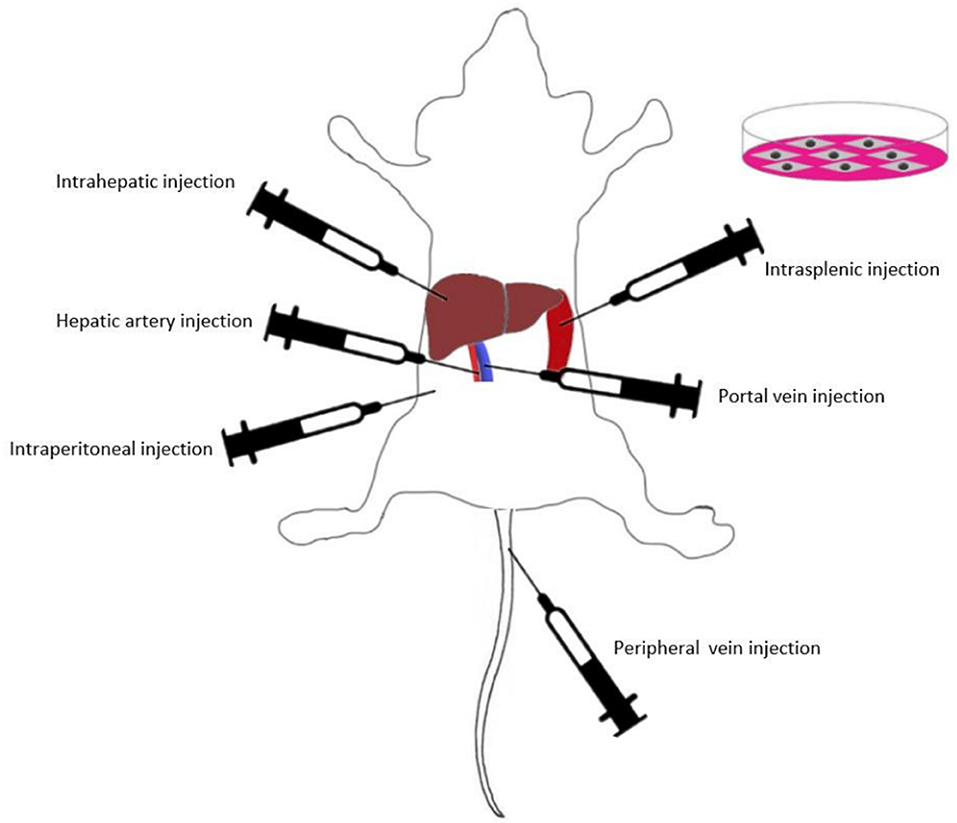
Presently, various routes of MSC transplantation have been proven to be curative in different liver injuries (Figure 2). Intravascular infusion is the most popular route for MSC transplantation in animal models and clinical trials (131). Intravascular infusion of MSCs typically proceeds via portal vein injection, hepatic artery injection or peripheral vein injection (132). In addition, local transplantation methods such as intraperitoneal injection, intrasplenic injection and intrahepatic injection are also widely used in MSC efficacy studies (16, 133–135).
To date, there have been few comparative studies on different methods of MSC transplantation. Sun et al. (132) assessed the efficacy of four BM-MSC transplantation methods (hepatic artery injection, portal vein injection, vena caudalis injection, and intraperitoneal injection) in a rat model of D-galactosamine (D-gal)/lipopolysaccharide (LPS)-induced ALF. The results showed that intravascular infusion was significantly more effective than intraperitoneal injection, while the selection of blood vessels as the implantation pathway did not affect the transplantation outcomes. Idriss et al. (16) compared the efficacy of intravenous and intrasplenic BM-MSC transplantation in CCl4-induced liver fibrosis model rats. The authors found that the intravenous route was more effective than the intrasplenic route in suppressing the gene expression levels of IL-1β, IL-6, and INF-γ. This result indicated that compared with the intrasplenic route, intravenous BM-MSC injection was an efficient and appropriate route for BM-MSC transplantation in liver fibrosis. Zhao et al. showed that compared with the intrahepatic and intraperitoneal injection groups, the BM-MSC intravenous injection group had the highest number of MSCs that migrated into liver lobules in CCl4-induced fibrosis model rats. IL-10 levels were highest in the intravenous group, whereas IL-1β, IL-6, TNF-α, and TGF-β were significantly lower than those in the other groups (136). These studies indicated that intravascular infusion was very suitable for BM-MSC transplantation. According to a study of UC-MSCs, UC-MSC transplantation via the tail vein had similar therapeutic efficacy compared with that of intrahepatic injection (137). The present study on MSC transplantation routes has some potential limitations. Further explorations are needed to determine the optimal application routes of MSCs from different sources, and the related mechanisms are still not completely understood.
Although various methods have been demonstrated to successfully improve MSC efficacy in liver diseases in animal models, current culture conditions (both in term of medium composition and supplements, adhesion to ECM selective proteins, and exposure to inflammatory signals) significantly influence MSC phenotype and paracrine potential. That's why further explorations are still needed for clinical applications in the future.
Safety Issues Associated With MSC Transplantation
Large numbers of in vitro and in vivo trials have demonstrated the capacity of MSCs to promote regeneration, antifibrosis, and immunomodulation, as well as the significant effects of MSCs in various liver injury models. These findings opened up possibilities for the clinical application of MSCs in liver diseases. A growing number of clinical trials have demonstrated the therapeutic effect of MSCs in patients with liver diseases, especially ALF, cirrhosis, and GVHD (23, 138, 139). However, safety is still the most concerning issue in MSC clinical applications. Despite rash and fever (37–38°C) in several cases that resolved without additional treatment (24), no significant adverse effects were reported in most clinical trials. A series of meta-analysis results also proved the therapeutic efficacy and safety of MSCs from different sources in patients with ALF, liver cirrhosis and end-stage liver disease associated with HBV and HCV (22, 140–142).
MSCs have been shown to have the ability to migrate and integrate into tumor tissue (143), but the effect of MSCs on hepatocellular carcinoma cells in vitro and in vivo is still controversial. According to Zhao et al. (144), AD-MSC-CM inhibited proliferation and promoted cell death in a hepatocellular carcinoma cell line in vitro. Some studies have indicated that BM-MSCs can promote the migration and invasion of hepatocellular carcinoma cells (145, 146). Moreover, investigators have documented the influence of MSC culture on genetic instability and tumorigenicity (147). Rosland et al. (148) found that malignant transformation occurred in 45.8% of the human MSCs after long-term cultures (5–106 weeks). According to Ren et al. (149), MSCs derived from adult cynomolgus monkeys could transform spontaneously into highly tumorigenic transformed mesenchymal cells (TMCs) after cultured in vitro. Although many researchers announced that MSC transplantation was not likely to cause tumors after following up with patients for up to 11 years and 5 months (150), it is still not clear how MSCs influence tumorigenesis and development in patients.
Some researchers also indicated the risk of thrombosis and embolization that occurred during intravascular MSC administration due to the incompatibility of MSCs with the innate immune cascade systems of the blood. During a clinical trial of 11 patients with liver-based metabolic disorders, one patient exhibited a thrombogenic event after MSC infusion, and four patients were observed to have significant decreases in platelet and increases in D-dimer levels at the end of MSC infusion, which spontaneously normalized after 7 days. So Coppin et al. recommend anticoagulants combined with MSC infusion to limit infusion-related thrombogenesis to subclinical levels in patients (151). Moll et al. suggested that all cellular therapies should be subjected to hemocompatibility screening before intravascular infusion to ensure patient safety (152).
These studies emphasize that the regulation of MSC clinical applications still needs further exploration, evaluation and optimization. Moll et al. (131) indicated that comprehensive safety evaluations were essential before use in humans, and new clinical guidelines were needed to standardize MSC clinical treatment strategies.
Conclusions
A large number of studies have demonstrated that MSCs exert therapeutic effects in liver diseases by promoting regeneration, regulating immunity, and inhibiting fibrosis. Further studies are ongoing to determine the related mechanisms and explore strategies to enhance MSC efficacy. In addition to pretreatment and gene modification of MSCs, the extraction and applications of MSC-CM and MSC-EV are also under intense study.
Although the application of MSCs from various tissue sources have entered clinical trials, several concerns remain, such as the low MSC survival rate, as well as the risk of carcinogenesis, thrombosis, and embolization. Furthermore, strict standards are needed to regulate MSC source selection, culture medium composition, culture conditions, delivery routes, doses, course of treatment, indications of application, and so on. In this review, we suggest that formulating and following treatment guidelines is the most effective way to avoid treatment risks and improve treatment efficacy.
Author Contributions
LL, YY, and YZ designed and wrote the manuscript. LZ and FZ collected and analyzed the references. LL, YY, and YZ revised the manuscript. All authors approved the final manuscripts as submitted.
Funding
This study was supported by the National Key Research and Development Program of China (2016YFC 1101304/3).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. (1966) 16:381–90.
2. Ji W, Chen Y, Wang L, Xu Z, Ahmed J, Ge R, et al. Differentiation of human umbilical cord mesenchymal stem cells into Leydig-like cells with defined molecular compounds. Hum Cell. (2020) 33:318–29. doi: 10.1007/s13577-020-00324-y
3. Lelek J, Zuba-Surma EK. Perspectives for future use of extracellular vesicles from umbilical cord- and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies-synthetic review. Int J Mol Sci. (2020) 21:799. doi: 10.3390/ijms21030799
4. Abumaree MH, Abomaray FM, Alshabibi MA, AlAskar AS, Kalionis B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta. (2017) 59:87–95. doi: 10.1016/j.placenta.2017.04.003
5. Wang Z, Sun D. Adipose-derived mesenchymal stem cells: a new tool for the treatment of renal fibrosis. Stem Cells Dev. (2018) 27:1406–11. doi: 10.1089/scd.2017.0304
6. Gaur M, Dobke M, Lunyak VV. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int J Mol Sci. (2017) 18:208. doi: 10.3390/ijms18010208
7. Joerger-Messerli MS, Marx C, Oppliger B, Mueller M, Surbek DV, Schoeberlein A. Mesenchymal stem cells from Wharton's Jelly and amniotic fluid. Best Pract Res Clin Obstet Gynaecol. (2016) 31:30–44. doi: 10.1016/j.bpobgyn.2015.07.006
8. Loukogeorgakis SP De Coppi P. Stem cells from amniotic fluid–Potential for regenerative medicine. Best Pract Res Clin Obstet Gynaecol. (2016) 31:45–57. doi: 10.1016/j.bpobgyn.2015.08.009
9. Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther. (2013) 13:1387–400. doi: 10.1517/14712598.2013.826187
10. Chen L, Qu J, Cheng T, Chen X, Xiang C. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. (2019) 10:406. doi: 10.1186/s13287-019-1503-7
11. Cui D, Li H, Wan M, Peng Y, Xu X, Zhou X, et al. The origin and identification of mesenchymal stem cells in teeth: from Odontogenic to Non-odontogenic. Curr Stem Cell Res Ther. (2018) 13:39–5. doi: 10.2174/1574888X12666170913150403
12. Klingemann H, Matzilevich D, Marchand J. Mesenchymal stem cells - sources and clinical applications. Transfus Med Hemother. (2008) 35:272–7. doi: 10.1159/000142333
13. Karp JM, Leng Teo SG. Mesenchymal stem cell homing. the devil is in the details. Cell Stem Cell. (2009) 4:206–16. doi: 10.1016/j.stem.2009.02.001
14. Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. (2012) 18:128–34. doi: 10.1016/j.molmed.2011.10.004
15. Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. (2014) 32:2818–23. doi: 10.1002/stem.1818
16. Idriss NK, Sayyed HG, Osama A, Sabry D. Treatment efficiency of different routes of bone marrow-derived mesenchymal stem cell injection in rat liver fibrosis model. Cell Physiol Biochem. (2018) 48:2161–71. doi: 10.1159/000492558
17. Antunes MA, Abreu SC, Cruz FF, Teixeira AC, Lopes-Pacheco M, Bandeira E, et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res. (2014) 15:118. doi: 10.1186/s12931-014-0118-x
18. Sang JF, Shi XL, Han B, Huang T, Huang X, Ren HZ, et al. Intraportal mesenchymal stem cell transplantation prevents acute liver failure through promoting cell proliferation and inhibiting apoptosis. Hepatobiliary Pancreat Dis Int. (2016) 15:602–611. doi: 10.1016/S1499-3872(16)60141-8
19. Bernal W, Wendon J. Acute liver failure. N Engl J Med. (2013) 369:2525–34. doi: 10.1056/NEJMra1208937
20. Global Burden of Disease Cancer Collaboration Fitzmaurice C Abate D Abbasi N Abbastabar H Abd-Allah F . Global regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups:1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
21. Kang SH, Kim MY, Baik SK. Novelties in the pathophysiology and management of portal hypertension: new treatments on the horizon. Hepatol Int. (2018) 12(Suppl 1):112–21. doi: 10.1007/s12072-017-9806-1
22. Zhang Z, Lin H, Shi M, Xu RN, Fu JL, Lv JY, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. (2012) 27:112–20. doi: 10.1111/j.1440-1746.2011.07024.x
23. Shi M, Liu Z, Wang Y, Xu R, Sun Y, Zhang M, et al. A Pilot Study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Transl Med. (2017) 6:2053–61. doi: 10.1002/sctm.17-0134
24. Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. (2017) 66:209–19. doi: 10.1002/hep.29189
25. Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, et al. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. (2014) 5:70. doi: 10.1186/scrt459
26. Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis. Phase 2 trial. Hepatology. (2016) 64:2185–97. doi: 10.1002/hep.28693
27. Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. (2013) 33:1490–6. doi: 10.1111/liv.12228
28. Zhang YC, Liu W, Fu BS, Wang GY, Li HB, Yi HM, et al. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. (2017) 19:194–9. doi: 10.1016/j.jcyt.2016.11.005
29. Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, et al. Mesenchymal stem versus stromal cells. International Society for Cell and Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. (2019) 21:1019–24. doi: 10.1016/j.jcyt.2019.08.002
30. Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. (2019) 6:34. doi: 10.21037/sci.2019.08.11
31. Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. (2015) 8:54–68. doi: 10.15283/ijsc.2015.8.1.54
32. Wang YH, Wu DB, Chen B, Chen EQ, Tang H. Progress in mesenchymal stem cell-based therapy for acute liver failure. Stem Cell Res Ther. (2018) 9:227. doi: 10.1186/s13287-018-0972-4
33. Shi D, Xin J, Lu Y, Ding W, Jiang J, Zhou Q, et al. Transcriptome profiling reveals distinct phenotype of human bone marrow mesenchymal stem cell-derived hepatocyte-like cells. Int J Med Sci. (2020) 17:263–73. doi: 10.7150/ijms.36255
34. Coronado RE, Somaraki-Cormier M, Ong JL, Halff GA. Hepatocyte-like cells derived from human amniotic epithelial, bone marrow, and adipose stromal cells display enhanced functionality when cultured on decellularized liver substrate. Stem Cell Res. (2019) 38:101471. doi: 10.1016/j.scr.2019.101471
35. Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. (2013) 14:961–72. doi: 10.1631/jzus.B1300081
36. Despeyroux A, Duret C, Gondeau C, Perez-Gracia E, Chuttoo L, de Boussac H, et al. Mesenchymal stem cells seeded on a human amniotic membrane improve liver regeneration and mouse survival after extended hepatectomy. J Tissue Eng Regen Med. (2018) 12:1062–73. doi: 10.1002/term.2607
37. Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, et al. Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl Med. (2017) 6:272–84. doi: 10.5966/sctm.2015-0265
38. Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L, et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut. (2017) 66:955–64. doi: 10.1136/gutjnl-2015-311146
39. von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. (2012) 30:1575–8. doi: 10.1002/stem.1118
40. Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. (2004) 10:744–8. doi: 10.1038/nm1062
41. Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. (2003) 422:897–901. doi: 10.1038/nature01531
42. Camargo FD, Finegold M, Goodell MA. Hematopoietic myelomonocytic cells are the major source of hepatocyte fusion partners. J Clin Invest. (2004) 113:1266–70. doi: 10.1172/JCI21301
43. Fujino H, Hiramatsu H, Tsuchiya A, Niwa A, Noma H, Shiota M, et al. Human cord blood CD34(+) cells develop into hepatocytes in the livers of NOD/SCID/gamma(null)(c) mice through cell fusion. Faseb J. (2007) 21:3499–510. doi: 10.1096/fj.06-6109com
44. Okamura K, Asahina K, Fujimori H, Ozeki R, Shimizu-Saito K, Tanaka Y, et al. Generation of hybrid hepatocytes by cell fusion from monkey embryoid body cells in the injured mouse liver. Histochem Cell Biol. (2006) 125:247–57. doi: 10.1007/s00418-005-0065-1
45. van Poll Biju Parekkadan D, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. (2008) 47:1634–43. doi: 10.1002/hep.22236
46. Efimenko A, Starostina E, Kalinina N, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. (2011) 9:10. doi: 10.1186/1479-5876-9-10
47. Chen YX, Zeng ZC, Sun J, Zeng HY, Huang Y, Zhang ZY. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J Radiat Res. (2015) 56:700–8. doi: 10.1093/jrr/rrv026
48. Forbes SJ, Newsome PN. New horizons for stem cell therapy in liver disease. J Hepatol. (2012) 56:496–9. doi: 10.1016/j.jhep.2011.06.022
49. Zhang S, Yang Y, Fan L, Zhang F, Li L. The clinical application of mesenchymal stem cells in liver disease: the current situation and potential future. Ann Transl Med. (2020) 8:565. doi: 10.21037/atm.2020.03.218
50. Xie Q, Liu R, Jiang J, Peng J, Yang C, Zhang W, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. (2020) 11:519. doi: 10.1186/s13287-020-02011-z
51. Fiore EJ, Dominguez LM, Bayo J, Garcia MG, Mazzolini GD. Taking advantage of the potential of mesenchymal stromal cells in liver regeneration. Cells and extracellular vesicles as therapeutic strategies. World J Gastroenterol. (2018) 24:2427–40. doi: 10.3748/wjg.v24.i23.2427
52. Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. (2014) 12:260. doi: 10.1186/s12967-014-0260-8
53. Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. (2017) 8:9. doi: 10.1186/s13287-016-0453-6
54. Gui Y, Yeganeh M, Ramanathan S, Leblanc C, Pomerleau V, Ferbeyre G, et al. SOCS1 controls liver regeneration by regulating HGF signaling in hepatocytes. J Hepatol. (2011) 55:1300–8. doi: 10.1016/j.jhep.2011.03.027
55. Feng J, Yao W, Zhang Y, Xiang AP, Yuan D, Hei Z. Intravenous anesthetics enhance the ability of human bone marrow-derived mesenchymal stem cells to alleviate hepatic ischemia-reperfusion injury in a receptor-dependent manner. Cell Physiol Biochem. (2018) 47:556–66. doi: 10.1159/000489989
56. Kim JY, Jun JH, Park SY, Yang SW, Bae SH, Kim GJ. Dynamic regulation of miRNA expression by functionally enhanced placental mesenchymal stem cells promoteshepatic regeneration in a rat model with bile duct ligation. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20215299
57. Hyun J, Wang S, Kim J, Kim GJ, Jung Y. MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci Rep. (2015) 5:14135. doi: 10.1038/srep14135
58. Martinez-Hernandez A, Amenta PS. The hepatic extracellular matrix. II. Ontogenesis, regeneration and cirrhosis. Virchows Arch A Pathol Anat Histopathol. (1993) 423:77–84. doi: 10.1007/BF01606580
59. Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. (1993) 328:1828–35. doi: 10.1056/NEJM199306243282508
60. Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. (2014) 14:181–94. doi: 10.1038/nri3623
61. Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. (2017) 121:27–42. doi: 10.1016/j.addr.2017.05.007
62. Ni MM, Wang YR, Wu WW, Xia CC, Zhang YH, Xu J, et al. Novel Insights on Notch signaling pathways in liver fibrosis. Eur J Pharmacol. (2018) 826:66–74. doi: 10.1016/j.ejphar.2018.02.051
63. Wang JN, Li L, Li LY, Yan Q, Li J, Xu T. Emerging role and therapeutic implication of Wnt signaling pathways in liver fibrosis. Gene. (2018) 674:57–69. doi: 10.1016/j.gene.2018.06.053
64. Yoshida K, Matsuzaki K, Murata M, Yamaguchi T, Suwa K, Okazaki K. Clinico-pathological importance of TGF-beta/phospho-smad signaling during human hepatic fibrocarcinogenesis. Cancers (Basel). (2018) 10:183. doi: 10.3390/cancers10060183
65. Yoshiji H, Kuriyama S, Fukui H. Blockade of renin-angiotensin system in antifibrotic therapy. J Gastroenterol Hepatol. (2007) 22(Suppl 1):S93–5. doi: 10.1111/j.1440-1746.2006.04663.x
66. Sato K, Hall C, Glaser S, Francis H, Meng F, Alpini G. Pathogenesis of Kupffer cells in cholestatic liver injury. Am J Pathol. (2016) 186:2238–47. doi: 10.1016/j.ajpath.2016.06.003
67. Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. (2017) 66:1300–12. doi: 10.1016/j.jhep.2017.02.026
68. Cicchini C, Amicone L, Alonzi T, Marchetti A, Mancone C, Tripodi M. Molecular mechanisms controlling the phenotype and the EMT/MET dynamics of hepatocyte. Liver Int. (2015) 35:302–10. doi: 10.1111/liv.12577
69. Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. (2009) 50:2007–13. doi: 10.1002/hep.23196
70. Berardis S, Dwisthi Sattwika P, Najimi M, Sokal EM. Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World J Gastroenterol. (2015) 21:742–58. doi: 10.3748/wjg.v21.i3.742
71. Hu C, Zhao L, Zhang L, Bao Q, Li L. Mesenchymal stem cell-based cell-free strategies: safe and effective treatments for liver injury. Stem Cell Res Ther. (2020) 11:377. doi: 10.1186/s13287-020-01895-1
72. Xuan J, Feng W, An ZT, Yang J, Xu HB, Li J, et al. Anti-TGFβ-1 receptor inhibitor mediates the efficacy of the human umbilical cord mesenchymal stem cells against liver fibrosis through TGFbeta-1/Smad pathway. Mol Cell Biochem. (2017) 429:113–22. doi: 10.1007/s11010-017-2940-1
73. Jang YO, Cho MY, Yun CO, Baik SK, Park KS, Cha SK, et al. Effect of function-enhanced mesenchymal stem cells infected with decorin-expressing adenovirus on hepatic fibrosis. Stem Cells Transl Med. (2016) 5:1247–56. doi: 10.5966/sctm.2015-0323
74. An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, et al. Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology. (2017) 152:1174–86. doi: 10.1053/j.gastro.2016.12.003
75. Rong XL, Liu JZ, Yao X, Jiang TC, Wang YM, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/beta-catenin pathway. Stem Cell Res Ther. (2019) 10:98. doi: 10.1186/s13287-019-1204-2
76. Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol. (2016) 22:6036–48. doi: 10.3748/wjg.v22.i26.6036
77. Ohara M, Ohnishi S, Hosono H, Yamamoto K, Yuyama K, Nakamura H, et al. Extracellular vesicles from amnion-derived mesenchymal stem cells ameliorate hepatic inflammation and fibrosis in rats. Stem Cells Int. (2018) 2018:3212643. doi: 10.1155/2018/3212643
78. Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. (2013) 22:845–54. doi: 10.1089/scd.2012.0395
79. Ding ZY, Jin GN, Liang HF, Wang W, Chen WX, Datta PK, et al. Transforming growth factor beta induces expression of connective tissue growth factor in hepatic progenitor cells through Smad independent signaling. Cell Signal. (2013) 25:1981–92. doi: 10.1016/j.cellsig.2013.05.027
80. Park JH, Yoon J, Lee KY, Park B. Effects of geniposide on hepatocytes undergoing epithelial-mesenchymal transition in hepatic fibrosis by targeting TGFbeta/Smad and ERK-MAPK signaling pathways. Biochimie. (2015) 113:26–34. doi: 10.1016/j.biochi.2015.03.015
81. Pietrosi G, Fernández-Iglesias A, Pampalone M, Ortega-Ribera M, Lozano JJ, García-Calderó H, et al. Human amniotic stem cells improve hepatic microvascular dysfunction and portal hypertension in cirrhotic rats. Liver Int. (2020) 40:2500–14. doi: 10.1111/liv.14610
82. Zhangdi HJ, Su SB, Wang F, Liang ZY, Yan YD, Qin SY, et al. Crosstalk network among multiple inflammatory mediators in liver fibrosis. World J Gastroenterol. (2019) 25:4835–49. doi: 10.3748/wjg.v25.i33.4835
83. Donnelly MC, Hayes PC, Simpson KJ. Role of inflammation and infection in the pathogenesis of human acute liver failure. Clinical implications for monitoring and therapy. World J Gastroenterol. (2016) 22:5958–70. doi: 10.3748/wjg.v22.i26.5958
84. Chen P, Wang YY, Chen C, Guan J, Zhu HH, Chen Z. The immunological roles in acute-on-chronic liver failure: an update. Hepatobiliary Pancreat Dis Int. (2019) 18:403–11. doi: 10.1016/j.hbpd.2019.07.003
85. Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. (2019) 8:1025. doi: 10.3390/jcm8071025
86. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. (2002) 99:3838–43. doi: 10.1182/blood.V99.10.3838
87. Adas G, Koc B, Adas M, Duruksu G, Subasi C, Kemik O, et al. Effects of mesenchymal stem cells and VEGF on liver regeneration following major resection. Langenbecks Arch Surg. (2016) 401:725–40. doi: 10.1007/s00423-016-1380-9
88. Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. (2013) 4:65. doi: 10.1186/scrt216
89. Winkler S, Hempel M, Bruckner S, Tautenhahn HM, Kaufmann R, Christ B. Identification of pathways in liver repair potentially targeted by secretory proteins from human mesenchymal stem cells. Int J Mol Sci. (2016) 17:1099. doi: 10.3390/ijms17071099
90. Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom HJ, et al. Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response. Transpl Immunol. (2011) 25:7–15. doi: 10.1016/j.trim.2011.05.004
91. DelaRosa O, Sanchez-Correa B, Morgado S C. Ramirez, del Rio B, Menta R, Lombardo E, Tarazona R, Casado JG. Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev. (2012) 21:1333–43. doi: 10.1089/scd.2011.0139
92. Yi T, Song SU. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharm Res. (2012) 35:213–21. doi: 10.1007/s12272-012-0202-z
93. Gazdic M, Markovic BS, Arsenijevic A, Jovicic N, Acovic A, Harrell CR, et al. Crosstalk between mesenchymal stem cells and T regulatory cells is crucially important for the attenuation of acute liver injury. Liver Transpl. (2018) 24:687–702. doi: 10.1002/lt.25049
94. Qu M, Yuan X, Liu D, Ma Y, Zhu J, Cui J, et al. Bone marrow-derived mesenchymal stem cells attenuate immune-mediated liver injury and compromise virus control during acute hepatitis b virus infection in mice. Stem Cells Dev. (2017) 26:818–27. doi: 10.1089/scd.2016.0348
95. Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. (2003) 101:2999–3001. doi: 10.1182/blood-2002-06-1830
96. Wang H, Zhang H, Huang B, Miao G, Yan X, Gao G, et al. Mesenchymal stem cells reverse highfat dietinduced nonalcoholic fatty liver disease through suppression of CD4+ T lymphocytes in mice. Mol Med Rep. (2018) 17:3769–74. doi: 10.3892/mmr.2017.8326
97. Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. (2007) 83:71–6. doi: 10.1097/01.tp.0000244572.24780.54
98. Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, Huang J, et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology. (2014) 59:671–82. doi: 10.1002/hep.26670
99. Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, et al. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. (2010) 55:1629–38. doi: 10.1016/j.jacc.2009.08.089
100. Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. (2014) 46:e70. doi: 10.1038/emm.2013.135
101. Li CY, Jin YT, Wei S, Sun YS, Jiang LF, Zhu Q, et al. Hippo signaling controls NLR family pyrin domain containing 3 activation and governs immunoregulation of mesenchymal stem cells in mouse liver injury. Hepatology. (2019) 70:1714–31. doi: 10.1002/hep.30700
102. Jiang L, Zhang S, Hu H, Yang J, Wang X, Ma Y, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun. (2019) 508:735–41. doi: 10.1016/j.bbrc.2018.11.189
103. Hu C, Li L. The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation. J Transl Med. (2019) 17:412. doi: 10.1186/s12967-019-02167-0
104. Amorin B, Alegretti AP, Valim V, Pezzi A, Laureano AM, da Silva MA, et al. Mesenchymal stem cell therapy and acute graft-versus-host disease: a review. Hum Cell. (2014) 27:137–50. doi: 10.1007/s13577-014-0095-x
105. Yang D, Wang LP, Zhou H, Cheng H, Bao XC, Xu S, et al. Inducible costimulator gene-transduced bone marrow-derived mesenchymal stem cells attenuate the severity of acute graft-versus-host disease in mouse models. Cell Transplant. (2015) 24:1717–31. doi: 10.3727/096368914X684592
106. Zhao K, Lou R, Huang F, Peng Y, Jiang Z, Huang K, et al. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2015) 21:97–104. doi: 10.1016/j.bbmt.2014.09.030
107. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. (2004) 22:675–82. doi: 10.1634/stemcells.22-5-675
108. Moghadam M, Tokhanbigli S, Baghaei K, Farivar S, Asadzadeh Aghdaei H, Zali MR. Gene expression profile of immunoregulatory cytokines secreted from bone marrow and adipose derived human mesenchymal stem cells in early and late passages. Mol Biol Rep. (2020) 47:1723–32. doi: 10.1007/s11033-020-05264-2
109. Choi MR, Kim HY, Park JY, Lee TY, Baik CS, Chai YG, et al. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci Lett. (2010) 472:94–8. doi: 10.1016/j.neulet.2010.01.054
110. Kizilay Mancini O, Shum-Tim D, Stochaj U, Correa JA, Colmegna I. Age, atherosclerosis and type 2 diabetes reduce human mesenchymal stromal cell-mediated T-cell suppression. Stem Cell Res Ther. (2015) 6:140. doi: 10.1186/s13287-015-0127-9
111. Gnani D, Crippa S, Della Volpe L, Rossella V, Conti A, Lettera E, et al. An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell. (2019) 18:e12933. doi: 10.1111/acel.12933
112. Huang R, Qin C, Wang J, Hu Y, Zheng G, Qiu G, et al. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY). (2019) 11:7996–8014. doi: 10.18632/aging.102314
113. Hu C, Wu Z, Li L. Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med. (2020) 24:40–9. doi: 10.1111/jcmm.14788
114. Kojima Y, Tsuchiya A, Ogawa M, Nojiri S, Takeuchi S, Watanabe T, et al. Mesenchymal stem cells cultured under hypoxic conditions had a greater therapeutic effect on mice with liver cirrhosis compared to those cultured under normal oxygen conditions. Regen Ther. (2019) 11:269–81. doi: 10.1016/j.reth.2019.08.005
115. Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, et al. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. (2013) 4:83. doi: 10.1186/scrt234
116. Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. (2015) 61:77–84. doi: 10.1016/j.neuchi.2015.03.002
117. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B. Mitochondria: central organelles for melatonin's antioxidant and anti-aging actions. Molecules. (2018) 23:509. doi: 10.3390/molecules23020509
118. Mohsin S, Shams S, Ali Nasir G, Khan M, Javaid Awan S, Khan SN, et al. Enhanced hepatic differentiation of mesenchymal stem cells after pretreatment with injured liver tissue. Differentiation. (2011) 81:42–8. doi: 10.1016/j.diff.2010.08.005
119. Fang J, Yan Y, Teng X, Wen X, Li N, Peng S, et al. Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging (Albany NY). (2018) 10:2954–72. doi: 10.18632/aging.101602
120. Phonchai R, Phermthai T, Kitiyanant N, Suwanjang W, Kotchabhakdi N, Chetsawang B. Potential effects and molecular mechanisms of melatonin on the dopaminergic neuronal differentiation of human amniotic fluid mesenchymal stem cells. Neurochem Int. (2019) 124:82–93. doi: 10.1016/j.neuint.2018.12.012
121. Mortezaee K, Pasbakhsh P, Ragerdi Kashani I, Sabbaghziarani F, Omidi A, Zendedel A, et al. Melatonin pretreatment enhances the homing of bone marrow-derived mesenchymal stem cells following transplantation in a rat model of liver fibrosis. Iran Biomed J. (2016) 20:207–16. doi: 10.7508/ibj.2016.04.004
122. Salem NA, Ahmed HH, Aglan HA, ElShebiney SA. Nanofiber-expanded stem cells mitigate liver fibrosis: Experimental study. Tissue Cell. (2016) 48:544–51. doi: 10.1016/j.tice.2016.06.005
123. Meier RP, Montanari E, Morel P, Pimenta J, Schuurman HJ, Wandrey C, et al. Microencapsulation of hepatocytes and mesenchymal stem cells for therapeutic applications. Methods Mol Biol. (2017) 1506:259–71. doi: 10.1007/978-1-4939-6506-9_18
124. Sgroi A, Mai G, Morel P, Baertschiger RM, Gonelle-Gispert C, Serre-Beinier V, et al. Transplantation of encapsulated hepatocytes during acute liver failure improves survival without stimulating native liver regeneration. Cell Transplant. (2011) 20:1791–803. doi: 10.3727/096368911X564976
125. Meier RP, Mahou R, Morel P, Meyer J, Montanari E, Muller YD, et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol. (2015) 62:634–41. doi: 10.1016/j.jhep.2014.10.030
126. Wang K, Li Y, Zhu T, Zhang Y, Li W, Lin W, et al. Overexpression of c-Met in bone marrow mesenchymal stem cells improves their effectiveness in homing and repair of acute liver failure. Stem Cell Res Ther. (2017) 8:162. doi: 10.1186/s13287-017-0614-2
127. Seo KW, Sohn SY, Bhang DH, Nam MJ, Lee HW, Youn HY. Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats. Cell Biol Int. (2014) 38:106–16. doi: 10.1002/cbin.10186
128. Moon SH, Lee CM, Park SH, Jin Nam M. Effects of hepatocyte growth factor gene-transfected mesenchymal stem cells on dimethylnitrosamine-induced liver fibrosis in rats. Growth Factors. (2019) 37:105–19. doi: 10.1080/08977194.2019.1652399
129. Zhang J, Zhou S, Zhou Y, Feng F, Wang Q, Zhu X, et al. Hepatocyte growth factor gene-modified adipose-derived mesenchymal stem cells ameliorate radiation induced liver damage in a rat model. PLoS One. (2014) 9:e114670. doi: 10.1371/journal.pone.0114670
130. Lee EJ, Hwang I, Lee JY, Park JN, Kim KC, Kim GH, et al. Hepatocyte growth factor improves the therapeutic efficacy of human bone marrow mesenchymal stem cells via RAD51. Mol Ther. (2018) 26:845–59. doi: 10.1016/j.ymthe.2017.12.015
131. Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringden O, Volk HD, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: Time for New Clinical Guidelines. Trends Mol Med. (2019) 25:149–63. doi: 10.1016/j.molmed.2018.12.006
132. Sun L, Fan X, Zhang L, Shi G, Aili M, Lu X, et al. Bone mesenchymal stem cell transplantation via four routes for the treatment of acute liver failure in rats. Int J Mol Med. (2014) 34:987–96. doi: 10.3892/ijmm.2014.1890
133. Ohshima M, Taguchi A, Tsuda H, Sato Y, Yamahara K, Harada-Shiba M, et al. Intraperitoneal and intravenous deliveries are not comparable in terms of drug efficacy and cell distribution in neonatal mice with hypoxia-ischemia. Brain Dev. (2015) 37:376–86. doi: 10.1016/j.braindev.2014.06.010
134. Li H, Guo ZK, Li XS, Hou CM, Tang PH, Mao N. Functional and phenotypic alteration of intrasplenic lymphocytes affected by mesenchymal stem cells in a murine allosplenocyte transfusion model. Cell Transplant. (2007) 16:85–95. doi: 10.3727/000000007783464470
135. Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clement S, et al. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. (2009) 4:e6657. doi: 10.1371/journal.pone.0006657
136. Zhao W, Li JJ, Cao DY, Li X, Zhang LY, He Y, et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. (2012) 18:1048–58. doi: 10.3748/wjg.v18.i10.1048
137. Zheng S, Yang J, Yang J, Tang Y, Shao Q, Guo L, et al. Transplantation of umbilical cord mesenchymal stem cells via different routes in rats with acute liver failure. Int J Clin Exp Pathol. (2015) 8:15854–62.
138. Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. (2012) 1:725–31. doi: 10.5966/sctm.2012-0034
139. Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. (2014) 34:33–41. doi: 10.1111/liv.12218
140. Chen B, Wang YH, Qian JQ, Wu DB, Chen EQ, Tang H. Human mesenchymal stem cells for hepatitis B virus-related acute-on-chronic liver failure: a systematic review with meta-analysis. Eur J Gastroenterol Hepatol. (2018) 30:1224–9. doi: 10.1097/MEG.0000000000001156
141. Sang W, Lv B, Li K, Lu Y. Therapeutic efficacy and safety of umbilical cord mesenchymal stem cell transplantation for liver cirrhosis in Chinese population: A meta-analysis. Clin Res Hepatol Gastroenterol. (2018) 42:193–204. doi: 10.1016/j.clinre.2017.11.003
142. Pan XN, Zheng LQ, Lai XH. Bone marrow-derived mesenchymal stem cell therapy for decompensated liver cirrhosis: a meta-analysis. World J Gastroenterol. (2014) 20:14051–7. doi: 10.3748/wjg.v20.i38.14051
143. Ong HT, Federspiel MJ, Guo CM, Ooi LL, Russell SJ, Peng KW, et al. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J Hepatol. (2013) 59:999–1006. doi: 10.1016/j.jhep.2013.07.010
144. Zhao W, Ren G, Zhang L, Zhang Z, Liu J, Kuang P, et al. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother Radiopharm. (2012) 27:606–13. doi: 10.1089/cbr.2011.1150
145. Pelagalli A, Nardelli A, Fontanella R, Zannetti A. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int J Mol Sci. (2016) 17:1102. doi: 10.3390/ijms17071102
146. Fontanella R, Pelagalli A, Nardelli A, D'Alterio C, Ierano C, Cerchia L, et al. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. (2016) 370:100–7. doi: 10.1016/j.canlet.2015.10.018
147. Dahl JA, Duggal S, Coulston N, Millar D, Melki J, Shahdadfar A, et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. (2008) 52:1033–42. doi: 10.1387/ijdb.082663jd
148. Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation (this article contains errors due to a cross contamination of the cell lines we used. To correct this issue we published a letter in Cancer Res. 2010 Aug 1, 70:6393–6). Cancer Res. (2009) 69:5331–9. doi: 10.1158/0008-5472.CAN-08-4630
149. Ren ZH, Wang JY, Zhu WW, Guan YQ, Zou CL, Chen ZG, et al. Spontaneous transformation of adult mesenchymal stem cells from cynomolgus macaques in vitro. Exp Cell Res. (2011) 317:2950–7. doi: 10.1016/j.yexcr.2011.09.008
150. Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. (2011) 5:146–50. doi: 10.1002/term.299
151. Coppin LCF, Smets F, Ambroise J, Sokal EEM, Stephenne X. Infusion-related thrombogenesis by liver-derived mesenchymal stem cells controlled by anticoagulant drugs in 11 patients with liver-based metabolic disorders. Stem Cell Res Ther. (2020) 11:51. doi: 10.1186/s13287-020-1572-7
Keywords: mesenchymal stem cells, liver diseases, mechanism, efficacy, safety
Citation: Yang Y, Zhao Y, Zhang L, Zhang F and Li L (2021) The Application of Mesenchymal Stem Cells in the Treatment of Liver Diseases: Mechanism, Efficacy, and Safety Issues. Front. Med. 8:655268. doi: 10.3389/fmed.2021.655268
Received: 18 January 2021; Accepted: 15 April 2021;
Published: 31 May 2021.
Edited by:
Yafeng Zhu, Sun Yat-Sen Memorial Hospital, ChinaReviewed by:
Roberto Gramignoli, Karolinska Institutet (KI), SwedenAnabel Fernández-Iglesias, Institut de Recerca Biomèdica August Pi i Sunyer (IDIBAPS), Spain
Copyright © 2021 Yang, Zhao, Zhang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanjuan Li, bGpsaUB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Ya Yang†
Ya Yang† Lanjuan Li
Lanjuan Li