- Department of Dermatology, Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Psoriasis is a chronic multisystem inflammatory disease that affects ~0.1–1.5% of the world population. The classic cutaneous manifestation of psoriasis is scaly erythematous plaques, limited or widely distributed. Moreover, psoriasis could be associated with comorbidities like psoriatic arthritis, metabolic syndrome, diabetes, cardiovascular disease, nephropathy, bowel disease, and brain diseases. In this review, we suggest that psoriasis should be classified as cutaneous psoriasis or systemic psoriasis and propose the classification for distinction. This would help to better understand and manage psoriasis.
Introduction
Psoriasis is a chronic systemic inflammatory disease that affects about 0.1–1.5% of the population worldwide (1). Proinflammatory cytokines such as interleukin-23 (IL-23), IL-17, and tumor necrosis factor α (TNF-α) play critical roles in the initiation and maintenance of psoriasis (2, 3). Approximately 36% of patients with psoriasis have a family history of psoriasis, and multiple genetic susceptibility loci have been identified (4, 5). Psoriasis could be triggered by a variety of extrinsic and intrinsic risk factors (6).
The incidence of psoriasis varies greatly around the world and is related to races, geographic locations, and environment (7).
The classic cutaneous presentation of psoriasis is scaly erythematous plaques, localized or widely distributed (8). Up to 30% of patients with psoriasis could develop psoriatic arthritis (9). Moderate-to-severe psoriasis is associated with an increased risk of metabolic syndrome and cardiovascular disease (10–12).
Diagnosis of psoriasis is mainly made upon clinical findings and a skin biopsy is rarely required (13). However, immunogenic evidence challenges the traditional taxonomy of psoriasis and suggests the reclassification of psoriasis into several subtypes. Sustained remission is an ultimate goal in the management of psoriasis, especially for moderate-to-severe psoriasis. Personalized systemic medicine such as biologics, could not only treat psoriatic lesions but also prevent or improve systemic comorbidities (14). Herein, we propose new classifications of psoriasis and classification for distinction.
Classifications of Psoriasis
Psoriasis vulgaris is the most common phenotype, affecting ~85–90% of patients with psoriasis (3, 15). The most commonly affected areas of the lesions include the extensor surfaces of elbows and knees, the sacral region, and the scalp, though lesions may involve any part of the skin (1). Furthermore, growing evidence suggests that compared to the general population, patients with psoriasis have a higher prevalence in other chronic and serious health diseases, including arthritis, metabolism disease, diabetes, cardiovascular diseases, hypertension, depression or anxiety, liver disease, Crohn's disease, and lymphoma or other cancers (2, 13, 15). Therefore, we suggest psoriasis may be classified into cutaneous psoriasis and systemic psoriasis.
Cutaneous Psoriasis
Plaque Psoriasis (Psoriasis Vulgaris)
The typical lesions of plaque psoriasis are erythematous, scaly, and well-demarcated plaques (2). The psoriasis area severity index (PASI) is an index used to determine the severity of psoriasis. It combines the severity (erythema, induration, and desquamation) and the percentage of affected areas (16). Lesions begin as erythematous papules that gradually enlarge into rich red (also referred to as “salmon pink”) plaques. The shape of plaque and the amount of scaling are variable, but most lesions are covered by silvery white scaling. When gently scraping the surface, scales fall off like candle wax (wax-spot phenomenon); this is a sign of the parakeratosis and hyperkeratosis of the epidermis. When plaques are scraped deeper, a wet smooth layer can be revealed referred to as the “last membrane phenomenon.” On the background of the erythematous membrane, pinpoints-like bleeding foci appear known as “Auspitz's sign” (3).
The clinical features of scalp psoriasis vary from intermittent mild erythematous scaly plaques to total scalp involvement, usually beyond the hairline giving the appearance of bundled hair (13).
Nail psoriasis mostly demonstrates as nail pitting. Other presentations range from oil drop discoloration, splinter hemorrhaging of the nail bed to crumbling or loosening of the nail plate (17). Of note, it is an important predictor for psoriatic arthritis (PsA) (18). An estimated 50% of patients have nail psoriasis at the time of diagnosis of psoriasis, which contributes to a greater social burden and worsens the quality of life in these patients (13, 19).
Guttate Psoriasis
Guttate psoriasis is more common in children and adolescents than adults and is usually triggered by streptococcal infection. Patients were classically present with numerous, scaly, small “drop-like” papules and plaques that are 0.3–0.5 cm in diameter (2). Itch is of various levels and intensity. One-third of guttate psoriasis would develop into chronic plaque psoriasis in later life (20–22).
Pustular Psoriasis
Pustular psoriasis is characterized by white sterile pustules, either in generalized or localized distribution. The typical presentation is an eruption of superficial pustules with an erythematous base (23). Pustular psoriasis is further divided into generalized pustular psoriasis (GPP) and localized pustular psoriasis. Localized pustular psoriasis includes palmoplantar pustulosis (PPP) and acrodermatitis continua of Hallopeau (ACH) (23).
Generalized Pustular Psoriasis
Generalized pustular psoriasis is a neutrophilic autoinflammatory skin disease characterized by widespread sterile pustules, which can occur with or without a history of plaque psoriasis (24). GPP is often acute onset on formal psoriatic lesions or normal skin, which may be accompanied by systemic inflammation (25). Superficial aseptic small pustules appear rapidly. Densely distributed pustules often expand and coalesce, forming lakes of pus (2). Acute GPP is often associated with systemic symptoms such as chills, high fever, malaise, anorexia, nausea, and severe pain (26). Other presentations could be geographic tongue, thick and turbid nail plates, and subungual pustules (26). Generally, the pustules dry out and form crusts, and high fever relives at the same time. But pustules and high fever may recur periodically (20). Acute GPP flares may be triggered by medication withdrawal (especially systemic withdrawal of corticosteroids), infections, stress, medication, and pregnancy, causing a dramatic reduction in quality of life (27). Acute GPP could lead to mortality without appropriate treatment because of accompanied infections and multiple systemic function failures.
Palmoplantar Pustulosis
Palmoplantar pustulosis is a rare, chronic, recurrent inflammatory disease that affects palms and/or soles with sterile, symmetrically distributed, erupting pustules that appear on an erythemato-squamous background (28). Pustules are more likely to occur in the middle and inner part of palms and/or soles and may extend to the dorsal aspect of hands and fingers (or feet and toes) (2). It could persist for years and usually be resistant to treatment, with periods of partial or total remission interrupted by recurrent exacerbations (28). Nails are often affected, presented with pitting, lateral grooves, longitudinal crests, nail turbidity, nail stripping, and empyema (20).
Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau is a rare, sterile, macroscopically visible pustule affecting the nail apparatus of one or more digits (29). ACH manifests with tender pustules and underlying erythema on the tip of a finger, sometimes on the toe (30). Nails are always involved in ACH; if there is no nail involvement, then alternative diagnoses such as PPP should be considered (29). Pustules may also be present on the nail bed (under the nail plate). When patients were under unsuccessful treatment, severe complications could appear such as ongchoptosis and osteolysis, which further reduce the quality of life in these patients (30).
Erythrodermic Psoriasis
Erythrodermic psoriasis (EP) is a rare and severe variant of psoriasis, which presented with generalized (usually involved 90% or more of body surface area) erythema, edema, pruritus, scaling, exudative lesions, and palmoplantar or diffuse desquamation (31). EP is always accompanied by systemic symptoms such as chills, fever, dehydration, lymphadenopathy, gastrointestinal malaise, rarely high output heart failure, and cachexia (31). Due to the dysfunction of the skin barrier, EP patients can present with high possibilities of severe systemic infection and sepsis, which occasionally could be life-threatening (32). The course of EP is long and easy to relapse (2, 20).
Inverse Psoriasis
Inverse psoriasis (IP) is also named intertriginous or flexural psoriasis (33). The lesions typically present as smooth, moist, scaly-less, dark-red patches in the folds or rubbing areas, including the inguinal folds, axillaes, inframammary folds, anogenital areas, umbilicus, and retro-auricular areas such as hip groove, armpit, groin, and under the breast; retro-auricular regions and glands. It is common to see patients with IP have typically plaque psoriasis lesions localized in other body areas (33).
Stages of Cutaneous Psoriasis
The cutaneous psoriasis process could be categorized into three stages: progressive stage, stationary stage, and regressive stage.
Progressive Stage
New inflammatory lesions continue to appear, presenting as erythematous thick-scaly plaques. A local cutaneous trauma caused by acupuncture, scratching or surgery may lead to typical psoriatic lesions. This phenomenon is named isomorphism or the KÖbner phenomenon (34).
Stationary Stage
Lesions become stable, demonstrating as light-red scaly plaque, and new lesions barely appear.
Regressive Stage
Lesions are flattening of infiltration with slight or no scaling. Some lesions could leave hypopigmentation or pigmentation marks.
The Severity of Cutaneous Psoriasis
Cutaneous psoriasis severity categories are important for clinicians to not only make treatment decisions but also to identify eligibility criteria for clinical studies (35). Combining body surface area (BSA), psoriasis area and severity index (PASI), and investigator's global assessment (IGA), cutaneous psoriasis are divided into three categories: mild, moderate, and severe (36) (as shown in Table 1).
Systemic Psoriasis
In addition to psoriatic lesions, other systemic diseases can occur first, simultaneously or sequentially. Evidence indicates that psoriasis is an important systemic inflammatory disease (2, 13, 37, 38), sharing manifestations with other chronic inflammatory diseases (15). After treatment, psoriatic lesions improve and the associated systemic symptoms generally improve (39). Herein, we propose the name “systemic psoriasis” to emphasize the characteristic of systemic-effect of psoriasis. According to different combordities, a personalized clinician team should be referred to make a correct diagnosis and provide optimized treatment.
Psoriatic Arthritis
Nearly 30% of patients with psoriasis progress to psoriatic arthritis (PsA) (40). In addition to psoriatic lesions, PsA may affect any joint in the body, from large joints like elbows and knees, to small joints like fingers and toes, the spine and the sacroiliac joints (41). It is progressive and could cause the affected joints to become swollen and painful resulting in either oligoarticular or polyarticular arthritis, restricting mobility, and resulting in joint destruction and deformity in severe cases (2, 42). It is important to note that the blood test of the rheumatoid factor is often negative (2, 42). X-ray characteristic features of PsA include soft tissue swelling, varying degrees of joint erosions, joint space narrowing, and osseous proliferation, including periarticular and shaft periostitis, as well as osteolysis (43). Up to 90% of patients with PsA have nail psoriasis involvement (44, 45).
Psoriatic arthritis may be further divided into several subtypes: distal subtype (damage of proximal and distal interphalangeal joints of the hands and feet), oligoarthritis (arthritis involving four joints at most), polyarthritis (arthritis affecting five or more joints), arthritis mutilans (resorption and shortening of finger bones), axial/ankylosing spondylitis, enthesitis, and dactylitis (46).
Psoriasis With Metabolic Syndrome
Moderate to severe psoriasis is frequently associated with metabolic disorders, especially metabolic syndrome (MetS) (47). MetS could combine various interrelated metabolic disorders including obesity, insulin resistance, dysglycemia, atherogenic dyslipidemia, and hypertension (10–12, 14).
Psoriasis With Cardiovascular Disease
Psoriasis is an independent risk factor in cardiovascular diseases, including hypertension, hyperlipidemia, major adverse cardiovascular events, and myocardial infarction (42, 48–51).
Psoriasis With Nephropathy
In addition to psoriasis cutaneous lesions, psoriasis patients could have kidney damage, or confirmed immune-related kidney disease (42, 52–54). Psoriasis is considered to be an independent risk factor of chronic kidney disease and end-stage renal disease (55).
Psoriasis With Bowel Disease
Patients with inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colon disease, share similarities in genetic susceptibilities and immune-mediated inflammation with psoriasis (56–59). There are significant bi-directional associations between psoriasis and IBD (58).
Psoriasis With Brain Diseases
Psoriasis has an extensive emotional and psychosocial effect on patients. Patients with psoriasis are accompanied by depression/mania, multiple sclerosis, or other mental symptoms, and may also have a significantly decreased quality of life and psychological burden including anxiety, depression, and suicidal thoughts and behavior (60–62).
Psoriasis With Pulmonary Disease
Interstitial lung disease or chronic obstructive pulmonary disease (COPD) can be seen in some patients with psoriasis (63–65).
Psoriasis With Liver Disease
Psoriasis is commonly accompanied by non-alcoholic fatty liver disease (66), liver fibrosis (67), or abnormal liver function.
Psoriasis With Uveitis
Uveitis is a known ophthalmologic manifestation of inflammation of the iris, ciliary body, and choroidal tissues. It is characterized by redness of the conjunctiva, eye pain, blurred vision, and flying mosquitoes. A significantly increased risk of both prevalent and incident uveitis is observed among patients with psoriasis (68).
Psoriasis With Lupus Erythematosus
It is rare for patients with psoriasis to have lupus erythematosus at the same time (14). Serologically positive lupus erythematosus patients are associated with psoriasis or are induced with psoriasis-treatment drugs.
Psoriasis With Malignancy
Psoriasis has also been associated with a low but elevated risk of malignant tumors of the skin or internal organs (42).
Classification Criteria for Psoriasis
Based on the clinical manifestations of psoriasis, we propose a new classification criteria for psoriasis (as shown in Table 2): cutaneous psoriasis and systemic psoriasis, according to the following classification criteria (Table 2). A psoriatic family history should be taken into account. If there is still doubt about the diagnosis, a simple punch biopsy can be performed.
Therapy for Psoriasis
Choice of treatment depends on many factors, mainly including patients and agendas, the former including the onset age, the duration, extent of disease, site of the lesions, the age of the patient, the type of psoriasis (cutaneous or systemic), pregnant or not, infection (especially tuberculosis or hepatitis B) or not, medical insurance covered or not, past therapy history and treatment willingness of the patients. The latter includes the efficacy, safety, price, response time, maintenance, frequency, and resistance of the drug. For moderate-to-severe cutaneous psoriasis and systemic psoriasis, biological therapies could be considered (37). Biologics could target specific molecules which could be essential in psoriasis pathogenesis, such as tumor necrosis factor (TNF) α, interleukin (IL)-17, IL-23, and IL-12 (72). Cutaneous psoriasis may resolve entirely after appropriate therapies targeting specific immune molecules.
We suggest therapeutics for mild, moderate, and severe adult cutaneous psoriasis as shown in Figure 1. For mild cutaneous psoriasis, topical agents are recommended, or just a wait-and-see approach. For moderate and severe cutaneous psoriasis, topical agents, phototherapy, systemic non-biological therapy, and/or biologics could be chosen (14, 37, 73).
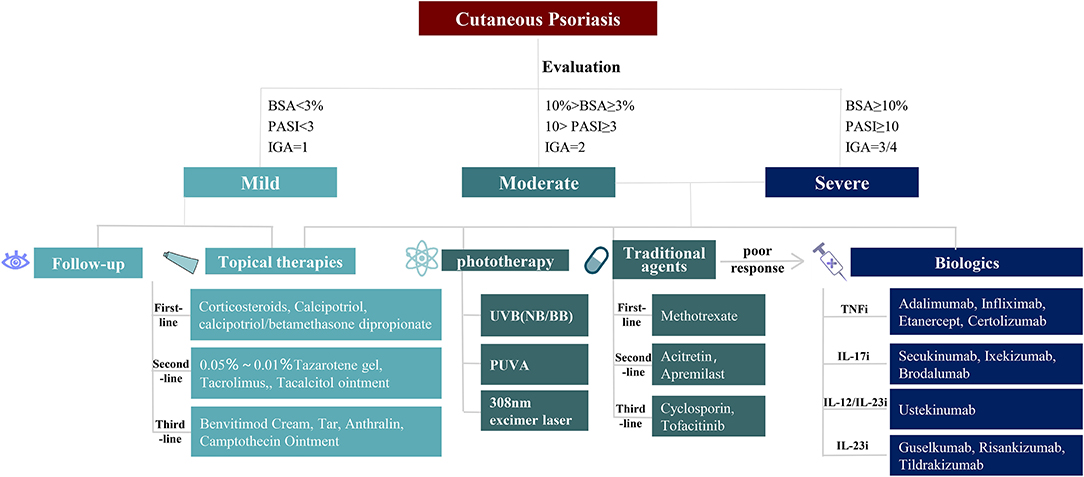
Figure 1. Therapies for cutaneous psoriasis. Different treatments for mild, moderate, and severe patients with cutaneous psoriasis. For mild cutaneous psoriasis, follow-up and topical therapies are advised choice. For moderate to severe cutaneous psoriasis, topical therapies, phototherapy, traditional agents, and biologics can be selected one by one. BSA, body surface area; PASI, psoriasis area and severity index; IGA, investigator's global assessment; UVB, ultraviolet radiation b; NB, narrow bound; BB, broad bound; PUVA, psoralen plus ultraviolet radiation a.
Comorbidities could influence the treatment strategy (14). Figure 2 lists selected therapeutics for adult systemic psoriasis with comorbidities (74–77). Treatment should be tailored to meet the needs of patients (Figure 2). It is recommended to select treatments from top to bottom for each category of systemic psoriasis.
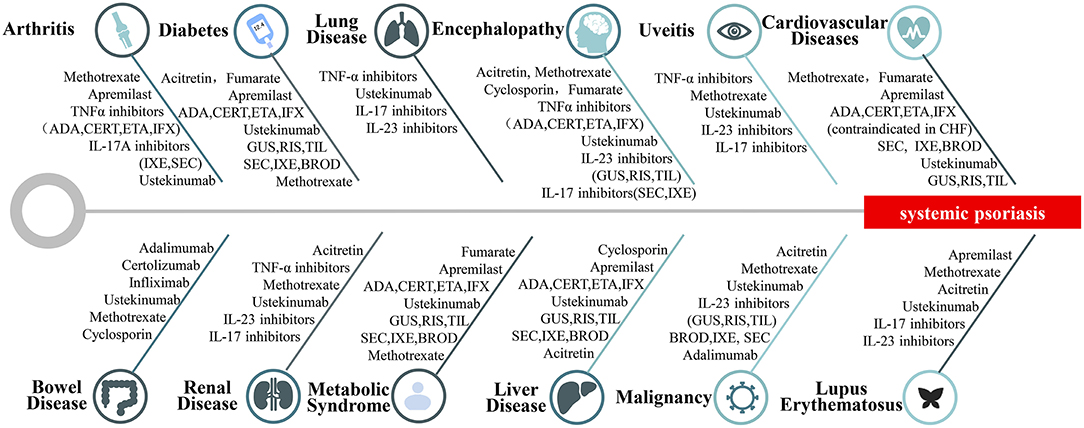
Figure 2. Therapies for systemic psoriasis. Selection of treatments for different types of systemic psoriasis includes psoriatic arthritis, psoriasis with diabetes, psoriasis with pulmonary disease, psoriasis with brain diseases, psoriasis with uveitis, psoriasis with cardiovascular disease, psoriasis with bowel disease, psoriasis with nephropathy, psoriasis with metabolic syndrome, psoriasis with liver disease, psoriasis with malignancy, psoriasis with lupus erythematosus.
For the therapy of pediatric psoriasis for patients under 18 years, AAD/NPF jointly published a comprehensive review recently (38). Briefly, pediatrics failure to topical therapy may undergo phototherapy and systemic therapy (Figure 3) (78). Long-term maintenance at the lowest effective dose with the least toxic therapy is the preferred approach (38). Till now, four biologics, etanercept, adalimumab, ustekinumab, and ixekizumab have been approved by FDA or EMA for pediatric psoriasis, which may be considered as first-line systemic agents (38).
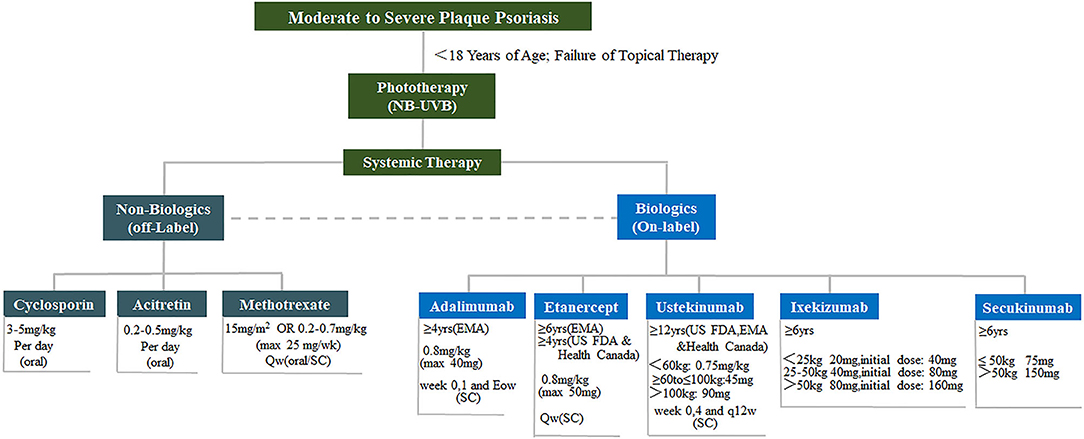
Figure 3. Therapies for pediatric psoriasis. Treatment of moderate to severe pediatric psoriasis. Non-biologics include cyclosporin, acitretin and methotrexate. Biologics include adalimumab, etanercept, ustekinumab, and ixekizumab. NB-UVB, narrow bound ultra violet B light.
Conclusions
Psoriasis can be classified as cutaneous psoriasis and systemic psoriasis. Cutaneous psoriasis can be subdivided into plaque, inverse, erythrodermic, pustular, and guttate forms. In addition to cutaneous manifestations, systemic cormobidities may present in systemic psoriasis. Psoriasis patients should undergo a complete history query and a thorough physical examination, including joints, and other systemic diseases. Optimal management of psoriasis depends on the type of psoriasis and the severity of the disease.
Author Contributions
B-XY, X-YC, and L-RY: conceptualization, formal analysis, resources, and writing the original draft. J-QC, MZ, and X-YM: conceptualization, funding acquisition, and writing review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81930089).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. (2021) 397:1301–15. doi: 10.1016/S0140-6736(20)32549-6
2. Nestle FO, Kaplan DH, Barker J. Mechanisms of disease: psoriasis. New Engl J Med. (2009) 361:496–509. doi: 10.1056/NEJMra0804595
3. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. (2007) 445:866–73. doi: 10.1038/nature05663
4. Elder JT. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes Immun. (2009) 10:201–9. doi: 10.1038/gene.2009.11
5. Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, et al. Molecular dissection of psoriasis: integrating genetics and biology. J Investigat Dermatol. (2010) 130:1213–26. doi: 10.1038/jid.2009.319
6. Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. (2019) 20:4347. doi: 10.3390/ijms20184347
7. Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. (2020) 369:m1590. doi: 10.1136/bmj.m1590
8. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. (2020) 323:1945–960. doi: 10.1001/jama.2020.4006
9. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. (2005) 64(Suppl. 2):ii14–17. doi: 10.1136/ard.2004.032482
10. Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. (2012) 132:556–62. doi: 10.1038/jid.2011.365
11. Mallbris L, Granath F, Hamsten A, Stahle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J Am Acad Dermatol. (2006) 54:614–21. doi: 10.1016/j.jaad.2005.11.1079
12. Tom WL, Playford MP, Admani S, Natarajan B, Joshi AA, Eichenfield LF, et al. Characterization of lipoprotein composition and function in pediatric psoriasis reveals a more atherogenic profile. J Invest Dermatol. (2016) 136:67–73. doi: 10.1038/JID.2015.385
14. Kaushik SB, Lebwohl MG. Psoriasis: Which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. (2019) 80:27–40. doi: 10.1016/j.jaad.2018.06.057
15. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. (2007) 370:263–71. doi: 10.1016/S0140-6736(07)61128-3
16. Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. (1978) 157:238–44. doi: 10.1159/000250839
17. Hadeler E, Mosca M, Hong J, Brownstone N, Bhutani T, Liao W. Nail psoriasis: a review of effective therapies and recommendations for management. Dermatol Ther. (2021) 11:799–831. doi: 10.1007/s13555-021-00523-x
18. Kaeley GS, Eder L, Aydin SZ, Rich P, Bakewell CJ. Nail psoriasis: diagnosis, assessment, treatment options, and unmet clinical needs. J Rheumatol. (2021) 48:1208–20. doi: 10.3899/jrheum.201471
19. Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. (2009) 23(Suppl. 1):15–21. doi: 10.1111/j.1468-3083.2009.03364.x
20. Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. (2017) 63:278–85.
21. Brandon A, Mufti A, Gary Sibbald R. Diagnosis and management of cutaneous psoriasis: a review. Adv Skin Wound Care. (2019) 32:58–69. doi: 10.1097/01.ASW.0000550592.08674.43
22. Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. (1996) 132:717–8. doi: 10.1001/archderm.1996.03890300147032
23. Benjegerdes KE, Hyde K, Kivelevitch D, Mansouri B. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis. (2016) 6:131–44. doi: 10.2147/PTT.S98954
24. Choon SE, Lebwohl MG, Marrakchi S, Burden AD, Tsai TF, Morita A, et al. Study protocol of the global Effisayil 1 Phase II, multicentre, randomised, double-blind, placebo-controlled trial of spesolimab in patients with generalized pustular psoriasis presenting with an acute flare. BMJ Open. (2021) 11:e043666. doi: 10.1136/bmjopen-2020-043666
25. Hoegler KM, John AM, Handler MZ, Schwartz RA. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. (2018) 32:1645–51. doi: 10.1111/jdv.14949
26. Robinson A, Van Voorhees AS, Hsu S, Korman NJ, Lebwohl MG, Bebo BF, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. (2012) 67:279–88. doi: 10.1016/j.jaad.2011.01.032
27. Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. (2014) 53:676–84. doi: 10.1111/ijd.12070
28. Freitas E, Rodrigues MA, Torres T. Diagnosis, screening and treatment of Patients with Palmoplantar Pustulosis (PPP): a review of current practices and recommendations. Clin Cosmet Investig Dermatol. (2020) 13:561–78. doi: 10.2147/CCID.S240607
29. Navarini AA, Burden AD, Capon F, Mrowietz U, Puig L, Köks S, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. (2017) 31:1792–9. doi: 10.1111/jdv.14386
30. Smith MP, Ly K, Thibodeaux Q, Bhutani T, Liao W, Beck KM. Acrodermatitis continua of Hallopeau: clinical perspectives. Psoriasis. (2019) 9:65–72. doi: 10.2147/PTT.S180608
31. Singh RK, Lee KM, Ucmak D, Brodsky M, Atanelov Z, Farahnik B, et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis. (2016) 6:93–104. doi: 10.2147/PTT.S101232
32. Prystowsky JH, Cohen PR. Pustular and erythrodermic psoriasis. Dermatol Clin. (1995) 13:757–70. doi: 10.1016/S0733-8635(18)30040-8
33. Micali G, Verzì AE, Giuffrida G, Panebianco E, Musumeci ML, Lacarrubba F. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. (2019) 12:953–9. doi: 10.2147/CCID.S189000
34. Ahad T, Agius E. The Koebner phenomenon. Br J Hosp Med. (2015) 76:C170–2. doi: 10.12968/hmed.2015.76.11.C170
35. Strober B, Ryan C, van de Kerkhof P, van der Walt J, Kimball AB, Barker J, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. (2020) 82:117–22. doi: 10.1016/j.jaad.2019.08.026
36. Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. (2005) 64(Suppl. 2):ii65–68; discussion ii69-73. doi: 10.1136/ard.2004.031237
37. Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. (2019) 80:1029–72. doi: 10.1016/j.jaad.2018.11.057
38. Menter A, Cordoro KM, Davis DMR, Kroshinsky D, Paller AS, Armstrong AW, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. (2020) 82:161–201. doi: 10.1016/j.jaad.2019.08.049
39. Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. (2013) 149:1173–9. doi: 10.1001/jamadermatol.2013.5015
40. Henes JC, Ziupa E, Eisfelder M, Adamczyk A, Knaudt B, Jacobs F, et al. High prevalence of psoriatic arthritis in dermatological patients with psoriasis: a cross-sectional study. Rheumatol Int. (2014) 34:227–34. doi: 10.1007/s00296-013-2876-z
41. Kamata M, Tada Y. Efficacy and safety of biologics for psoriasis and psoriatic arthritis and their impact on comorbidities: a literature review. Int J Mol Sci. (2020) 21:1690. doi: 10.3390/ijms21051690
42. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases Epidemiology. J Am Acad Dermatol. (2017) 76:377–90. doi: 10.1016/j.jaad.2016.07.064
44. van der Velden HM, Klaassen KM, van de Kerkhof PC, Pasch MC. Fingernail psoriasis reconsidered: a case-control study. J Am Acad Dermatol. (2013) 69:245–52. doi: 10.1016/j.jaad.2013.02.009
45. Bardazzi F, Starace M, Bruni F, Magnano M, Piraccini BM, Alessandrini A. Nail psoriasis: an updated review and expert opinion on available treatments, including biologics. Acta Derm Venereol. (2019) 99:516–23. doi: 10.2340/00015555-3098
46. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. (2017) 376:957–70. doi: 10.1056/NEJMra1505557
47. Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol. (2018) 36:21–8. doi: 10.1016/j.clindermatol.2017.09.005
48. Egeberg A, Thyssen JP, Jensen P, Gislason GH, Skov L. Risk of myocardial infarction in patients with psoriasis and psoriatic arthritis: a nationwide cohort study. Acta Derm Venereol. (2017) 97:819–24. doi: 10.2340/00015555-2657
49. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. (2006) 296:1735–41. doi: 10.1001/jama.296.14.1735
50. Persson R, Hagberg KW, Qian Y, Vasilakis-Scaramozza C, Jick S. The risk of myocardial infarction, stroke, and revascularization among patients with psoriasis treated with apremilast compared with biologics and disease-modifying antirheumatic drugs: A cohort study in the US MarketScan database. J Am Acad Dermatol. (2020) 83:271–4. doi: 10.1016/j.jaad.2020.03.043
51. Shiba M, Kato T, Izumi T, Miyamoto S, Nakane E, Haruna T, et al. Risk of myocardial infarction in patients with psoriasis: a cross-sectional patient-population study in a Japanese hospital. J Cardiol. (2019) 73:276–9. doi: 10.1016/j.jjcc.2018.10.008
52. Grewal SK, Wan J, Denburg MR, Shin DB, Takeshita J, Gelfand JM. The risk of IgA nephropathy and glomerular disease in patients with psoriasis: a population-based cohort study. Brit J Dermatol. (2017) 176:1366–9. doi: 10.1111/bjd.14961
53. Kreimer J, Kogan N, Gusis S, Veira R. Nephropathy in patients with psoriasis. J Eur Acad Dermatol. (2016) 30:41–2.
54. Wan J, Wang SW, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. Bmj-Brit Med J. (2013) 347:f5961. doi: 10.1136/bmj.f5961
55. Chi CC, Wang J, Chen YF, Wang SH, Chen FL, Tung TH. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: a nationwide population-based cohort study. J Dermatol Sci. (2015) 78:232–8. doi: 10.1016/j.jdermsci.2015.03.012
56. Li WQ, Han JL, Chan AT, Qureshi AA. Psoriasis, psoriatic arthritis and increased risk of incident Crohn's disease in US women. Ann Rheum Dis. (2013) 72:1200–5. doi: 10.1136/annrheumdis-2012-202143
57. Wright S, Alloo A, Strunk A, Garg A. Real-world risk of new onset inflammatory bowel disease among psoriasis patients exposed to interleukin 17 inhibitors. J Investigat Dermatol. (2020) 140:S62. doi: 10.1016/j.jid.2020.03.473
58. Alinaghi F, Tekin HG, Burisch J, Wu JJ, Thyssen JP, Egeberg A. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease-a systematic review and meta-analysis. J Crohns Colitis. (2020) 14:351–60. doi: 10.1093/ecco-jcc/jjz152
59. Schreiber S, Colombel JF, Feagan BG, Reich K, Deodhar AA, McInnes IB, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. (2019) 78:473–9. doi: 10.1136/annrheumdis-2018-214273
60. Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. (2014) 134:1542–51. doi: 10.1038/jid.2013.508
61. Singh S, Taylor C, Kornmehl H, Armstrong AW. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. (2017) 77:425–40 e422. doi: 10.1016/j.jaad.2017.05.019
62. Chi CC, Chen TH, Wang SH, Tung TH. Risk of suicidality in people with psoriasis: a systematic review and meta-analysis of cohort studies. Am J Clin Dermatol. (2017) 18:621–7. doi: 10.1007/s40257-017-0281-1
63. Ungprasert P, Srivali N, Thongprayoon C. Association between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Dermatol Treat. (2016) 27:316–21. doi: 10.3109/09546634.2015.1107180
64. Li X, Kong LJ, Li FL, Chen C, Xu R, Wang HS, et al. Association between psoriasis and chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. (2015) 10:e0145221. doi: 10.1371/journal.pone.0145221
65. Chiang YY, Lin HW. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol. (2012) 26:59–65. doi: 10.1111/j.1468-3083.2011.04009.x
66. Gisondi P, Targher G, Zoppini G, Girolomoni G. Non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J Hepatol. (2009) 51:758–64. doi: 10.1016/j.jhep.2009.04.020
67. Gisondi P, Barba E, Girolomoni G. Non-alcoholic fatty liver disease fibrosis score in patients with psoriasis. J Eur Acad Dermatol. (2016) 30:282–7. doi: 10.1111/jdv.13456
68. Chaiyabutr C, Ungprasert P, Silpa-archa N, Wongpraparut C, Chularojanamontri L. Psoriasis and risk of uveitis: a systematic review and meta-analysis. Biomed Res Int. (2020) 2020:9308341. doi: 10.1155/2020/9308341
69. Van den Bosch F, Coates L. Clinical management of psoriatic arthritis. Lancet. (2018) 391:2285–94. doi: 10.1016/S0140-6736(18)30949-8
70. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. (2014) 70:512–6. doi: 10.1016/j.jaad.2013.11.013
71. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. (2019) 71:1400–12. doi: 10.1002/art.40930
72. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. (2017) 140:645–53. doi: 10.1016/j.jaci.2017.07.004
73. Smith CH, Yiu ZZN, Bale T, Burden AD, Coates LC, Edwards W, et al. British Association of Dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. (2020) 183:628–37. doi: 10.1111/bjd.19039
74. Lambert JLW, Segaert S, Ghislain PD, Hillary T, Nikkels A, Willaert F, et al. Practical recommendations for systemic treatment in psoriasis in case of coexisting inflammatory, neurologic, infectious or malignant disorders (BETA-PSO: Belgian Evidence-based Treatment Advice in Psoriasis; part 2). J Eur Acad Dermatol Venereol. (2020) 34:1914–23. doi: 10.1111/jdv.16683
75. Lambert JLW, Segaert S, Ghislain PD, Hillary T, Nikkels A, Willaert F, et al. Practical recommendations for systemic treatment in psoriasis according to age, pregnancy, metabolic syndrome, mental health, psoriasis subtype and treatment history (BETA-PSO: Belgian Evidence-based Treatment Advice in Psoriasis; part 1). J Eur Acad Dermatol Venereol. (2020) 34:1654–65. doi: 10.1111/jdv.16684
76. Yan D, Blauvelt A, Dey AK, Golpanian RS, Hwang ST, Mehta NN, et al. New frontiers in psoriatic disease research, part ii: comorbidities and targeted therapies. J Invest Dermatol. (2021) 141:2328–37. doi: 10.1016/j.jid.2021.02.743
77. Schwade MJ, Tien L, Waller JL, Davis LS, Baer SL, Mohammed A, et al. Treatment of psoriasis in end-stage renal disease patients is associated with decreased mortality: a retrospective cohort study. Am J Med Sci. (2021) 362:24–33. doi: 10.1016/j.amjms.2021.03.009
Keywords: psoriasis, cutaneous and systemic, classification criteria, therapy, diagnosis, biologics
Citation: Yan B-X, Chen X-Y, Ye L-R, Chen J-Q, Zheng M and Man X-Y (2021) Cutaneous and Systemic Psoriasis: Classifications and Classification for the Distinction. Front. Med. 8:649408. doi: 10.3389/fmed.2021.649408
Received: 04 January 2021; Accepted: 16 September 2021;
Published: 13 October 2021.
Edited by:
Robert Gniadecki, University of Alberta, CanadaReviewed by:
Takashi Hashimoto, Osaka City University, JapanCurdin Conrad, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2021 Yan, Chen, Ye, Chen, Zheng and Man. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Yong Man, bWFueHlAemp1LmVkdS5jbg==
 Bing-Xi Yan
Bing-Xi Yan Xue-Yan Chen
Xue-Yan Chen Li-Ran Ye
Li-Ran Ye Jia-Qi Chen
Jia-Qi Chen Min Zheng
Min Zheng Xiao-Yong Man
Xiao-Yong Man
