
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 22 April 2021
Sec. Intensive Care Medicine and Anesthesiology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.648375
This article is part of the Research Topic Emerging Technology for Monitoring and Treatment in Critical Care View all 30 articles
Background: Delta shock index (SI; i.e., change in SI over time) has been shown to predict mortality and need for surgical intervention among trauma patients at the emergency department (ED). However, the usefulness of delta SI for prognosis assessment in non-traumatic critically ill patients at the ED remains unknown. The aim of this study was to analyze the association between delta SI during ED management and in-hospital outcomes in patients admitted to the intensive care unit (ICU).
Method: This was a retrospective study conducted in two tertiary medical centers in Taiwan from January 1, 2016, to December 31, 2017. All adult non-traumatic patients who visited the ED and who were subsequently admitted to the ICU were included. We calculated delta SI by subtracting SI at ICU admission from SI at ED triage, and we analyzed its association with in-hospital outcomes. SI was defined as the ratio of heart rate to systolic blood pressure (SBP). The primary outcome was in-hospital mortality, and the secondary outcomes were hospital length of stay (HLOS) and early mortality. Early mortality was defined as mortality within 48 h of ICU admission.
Result: During the study period, 11,268 patients met the criteria and were included. Their mean age was 64.5 ± 15.9 years old. Overall, 5,830 (51.6%) patients had positive delta SI. Factors associated with a positive delta SI were multiple comorbidities (51.2% vs. 46.3%, p < 0.001) and high Simplified Acute Physiology Score [39 (29–51) vs. 37 (28–47), p < 0.001). Patients with positive delta SI were more likely to have tachycardia, hypotension, and higher SI at ICU admission. In the regression analysis, high delta SI was associated with in-hospital mortality [aOR (95% CI): 1.21 (1.03–1.42)] and early mortality [aOR (95% CI): 1.26 (1.07–1.48)], but not for HLOS [difference (95% CI): 0.34 (−0.48 to 1.17)]. In the subgroup analysis, high delta SI had higher odds ratios for both mortality and early mortality in elderly [aOR (95% CI): 1.59 (1.11–2.29)] and septic patients [aOR (95% CI): 1.54 (1.13–2.11)]. It also showed a higher odds ratio for early mortality in patients with triage SBP <100 mmHg [aOR (95% CI): 2.14 (1.21–3.77)] and patients with triage SI ≥ 0.9 [aOR (95% CI): 1.62 (1.01–2.60)].
Conclusion: High delta SI during ED stay is correlated with in-hospital mortality and early mortality in patients admitted to the ICU via ED. Prompt resuscitation should be performed, especially for those with old age, sepsis, triage SBP <100 mmHg, or triage SI ≥ 0.9.
In the emergency department (ED), the survival rate of patients is mainly determined by the severity of acute illness on admission (1, 2) and the quality of care throughout the treatment process (3). Numerous scoring systems based on physiological parameters recorded in the ED have been developed for initial patient assessment and the identification of patients at risk (4–7). Nevertheless, patient deterioration and unexpected death are often preceded by abnormalities in vital signs in the ED (8, 9). It is important to document vital sign changes in the ED for physicians to provide adequate management.
Shock index (SI), calculated from the two most commonly used physiological measures [heart rate (HR) divided by systolic blood pressure (SBP)], is a simple bedside assessment originally developed to evaluate the degree of shock in hemorrhagic and septic patients (10). In recent studies, it has been used for the prediction of outcomes in other critically ill patients, including those with severe sepsis (11, 12), hemorrhagic shock (13), pulmonary embolism (14), and acute myocardial infarction (15). An SI <0.9 is considered to be associated with increased mortality risk (16). This cutoff value of the SI may help with early mobilization of resources in the ED.
Recently, it has been noted that delta SI (i.e., change of SI over time) predicts mortality in apparently hemodynamically stable trauma patients with normal traditional vital signs in the ED (17, 18). A similar result has been observed for postpartum hemorrhage in the ED; delta SI was superior in identifying the need for emergent intervention than other traditional vital signs (19).
However, research on the prognosis value of delta SI in critically ill patients in the ED is scarce. The aim of this study was to analyze the association between delta SI in the ED and in-hospital outcomes of critically ill patients who required intensive care unit (ICU) admission. Having a simple index that reliably correlates pre-ICU admission physiological parameters to mortality would be ideal to assess the quality of care in critically ill patients.
This was a retrospective database study conducted in two tertiary medical centers in Taiwan from January 1, 2016, to December 31, 2017. One hospital was located in northern Taiwan and the other in southern Taiwan, and they were both the largest medical centers in their metropolitan areas. The study protocol was approved by the institutional review board of both hospitals (IRB number 202002043B0; date of approval, December 1, 2020). All patients' and physicians' records and information were anonymized and de-identified before analysis.
All adult non-traumatic patients visiting the ED and subsequently admitted to the ICU were included. Patients with uncertain outcomes (discharged against medical advice and transferred to another hospital), transferred from other hospitals, presented with out-of-hospital cardiac arrest, or deceased at the ED were excluded (Figure 1). Patients' demographic data (age and sex), underlying comorbidities, vital signs at triage and at ICU admission, laboratory tests, and diagnosis at ICU admission were extracted from the electronic medical records of the studied hospitals for analysis. The Simplified Acute Physiology Score (SAPS) was computed based on the collected parameters for severity evaluation (20).
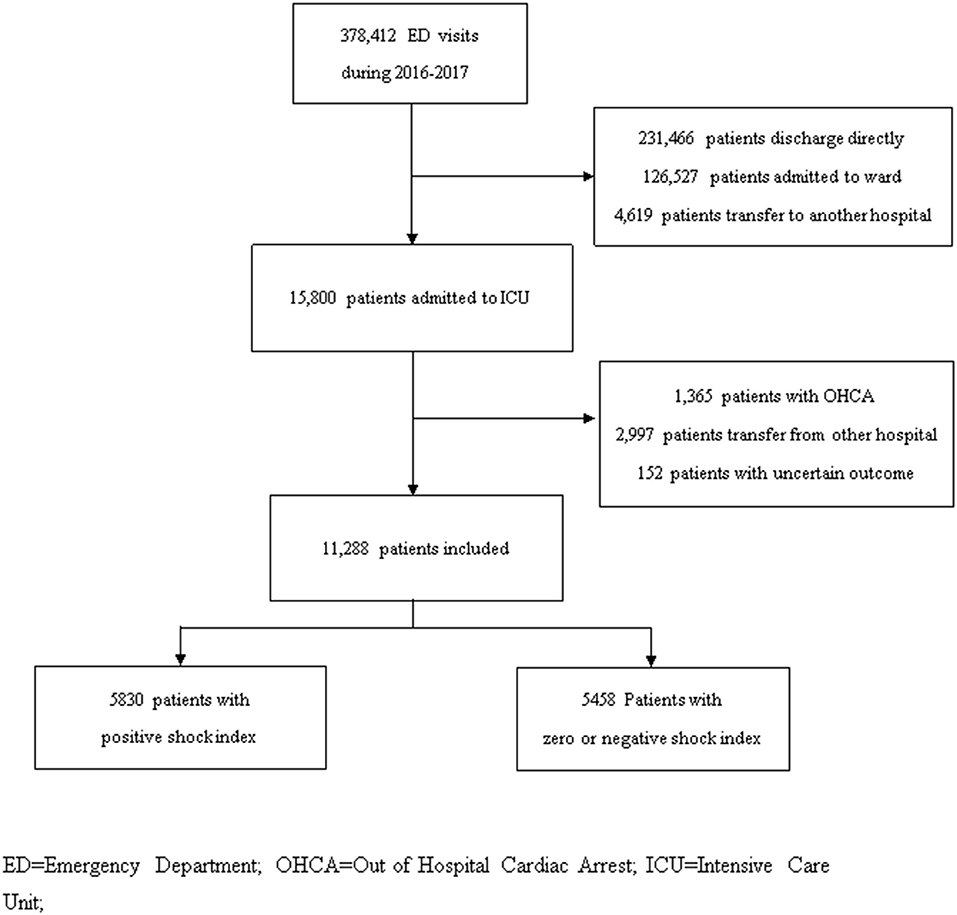
Figure 1. Patient inclusion flowchart in studied hospital during 2016–2017. ED, Emergency Department; OHCA, Out of Hospital Cardiac Arrest; ICU, Intensive Care Unit.
We calculated SI, defined as the ratio of HR to SBP, from vital signs at ED triage and at ICU admission. Delta SI was calculated by subtracting SI at ICU admission from SI at ED triage. Patients were then divided into two groups (patients with a positive delta SI and patients with zero or negative delta SI), and their demographics and clinical characteristics were compared. The primary outcome was in-hospital mortality, and the secondary outcomes were hospital length of stay (HLOS) and early mortality. Early mortality was defined as mortality within 48 h of ICU admission. We also performed subgroup analysis based on patient's age, comorbidity, vital signs, and diagnostic categories to clarify the association of delta SI to patient's outcome in different clinical conditions. Age older than 65 years was considered as elderly.
Data are presented as the mean (standard deviation) for continuous variables, proportions for nominal variables, and median (interquartile range) for ordinal variables. We performed Student's t-test and chi square analysis to determine the parameters that correlated with positive delta SI and with zero or negative SI. Logistic regressions assessing the association of clinical outcomes with delta SI were performed after adjusting for confounding factors. A two-sided p < 0.05 was considered statistically significant. Stratified regression analyses assessing the relationship between delta SI and clinical outcomes in different ages, comorbidities, vital signs, and diagnosis categories were also performed. All statistical analyses were conducted using IBM SPSS Statistics for Mac (Version 26).
During the study period, 11,268 patients who met the criteria were included. Their mean age was 64.5 ± 15.9 years, and 64.3% were male. The average SAPS was 38 (29–49). Of all patients, 5,830 (51.6%) had a positive delta SI. The parameters significantly associated with positive delta SI were multiple comorbidities (51.2% vs. 46.3%, p < 0.001) and high SAPS [39 (29–51) vs. 37 (28–47), p < 0.001]. In addition, compared with patients with zero or negative delta SI, patients with positive delta SI were less likely to have tachycardia, hypotension, and high SI at ED triage. Conversely, they were more likely to present with tachycardia, hypotension, and high SI at ICU admission (Table 1). Regarding prognosis at the ICU (Table 2), positive delta SI was significantly associated with higher mortality (20.3 vs. 18.9%, p = 0.032) and early mortality (6.5 vs. 5.2%, p = 0.005) than was zero or negative delta SI, while no significant relationship was observed with HLOS (13 vs. 13 days, p = 0.277).
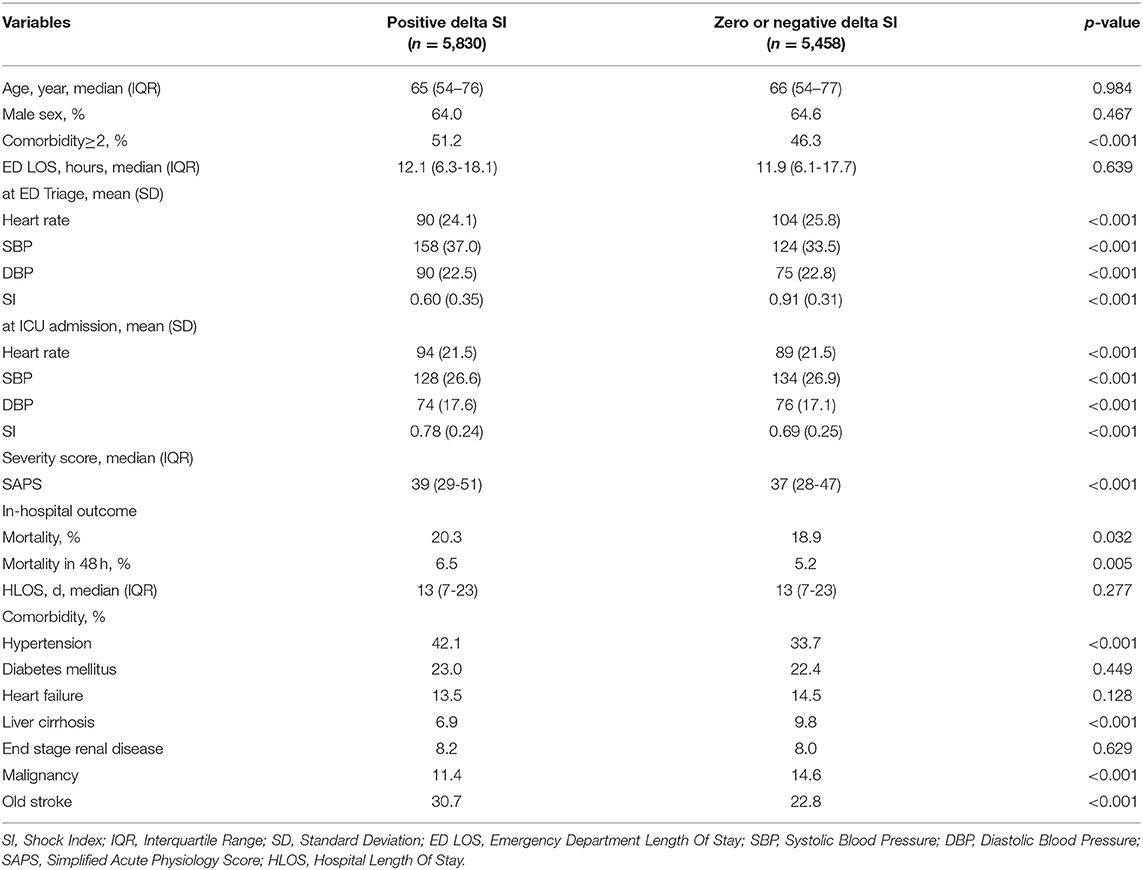
Table 1. Demographic and clinical characteristics in comparison of positive delta SI with zero or negative delta SI.
On binary logistic regression analysis, highly positive delta SI was an independent risk factor for in-hospital mortality [adjusted odds ratio (95% CI): 1.21 (1.03–1.42)] and early mortality [adjusted odds ratio (95% CI): 1.26 (1.07–1.48)]. On the other hand, the linear regression analysis on the association of delta SI with HLOS showed no significant difference [difference (95% CI): 0.34 (−0.48 to 1.17); Table 3].
In the subgroup analysis, high delta SI had higher odds ratios for mortality in elderly patients [adjusted odds ratio (95% CI): 1.59 (1.11–2.29)] and in patients with a diagnosis of sepsis [adjusted odds ratio (95% CI): 1.54 (1.13–2.11)], with respect to other age ranges and diagnoses, respectively. The analysis also showed higher odds ratios for early mortality in elderly patients [adjusted odds ratio (95% CI): 1.66 (1.06–2.38)], in patients with triage SBP <100 [adjusted odds ratio (95% CI): 2.14 (1.21–3.77)], in patients with triage SI ≥ 0.9 [adjusted odds ratio (95% CI): 1.62 (1.01–2.60)], and in patients with a diagnosis of sepsis [adjusted odds ratio (95% CI): 1.46 (1.03–1.94)], compared with the other possibilities of each category. There were no statistical differences in the regression analyses regarding patients with a diagnosis of respiratory failure and heart failure in either of the two in-hospital outcomes with previous significant relationships.
The aim of this study was to determine the relationship between delta SI during ED management and in-hospital outcomes in patients admitted to the ICU via ED. In this study, we found that positive delta SI is more likely to occur in patients with multiple comorbidities and in patients who present at the ED with high SAPS, as shown in Table 1. There is no doubt that comorbidity is an important factor in estimating a patient's outcome; and in some cases, the patient's comorbid condition presents a greater risk than the index disease (21). Therefore, the association between delta SI and comorbidity was foreseeable: patients presenting to the ED with more comorbidities are at greater risk of deterioration. A similar conclusion can be drawn for patients with scores that indicate severe conditions, which were often admitted to the ICU. SAPS (20), which includes items such as age, physiological parameters, type of admission, and chronic diseases, is a reliable indicator of the risk of death upon ICU admission. Patients with high SAPS, which theoretically indicates poor outcome for patients admitted to the ICU, tended to have positive delta SI.
Moreover, the positive delta SI group had better initial vital signs than the zero or negative delta SI group. For this paradoxical phenomenon, ED clinicians usually spend more time and effort managing patients with worse vital signs. And this condition may lead to delayed assessment and treatment or to relatively conservative treatment in this group of patients with initially better vital signs at ED assessment (22, 23). In addition, patients in the negative delta SI group presented with higher SI (mean: 0.91) at ED triage, so they required aggressive and fast management due to their initially unstable conditions. Thus, more effort (continuous bedside evaluation, resuscitation, and re-evaluation) was devoted to them (24–26). Intubation, ventilation, volume support, and even vasoactive therapy were initiated earlier in the group of patients with worse vital signs, leading to negative delta SI in this group of patients.
Regarding the association between delta SI and in-hospital outcomes, there was a statistically significant difference between positive delta SI (worsened SI) and zero or negative delta SI (improved SI) in mortality and early mortality. Previous studies have demonstrated that positive delta SI during ED management is a strong predictor of mortality and of need for blood product transfusion in trauma patients (27). Regarding the connection between delta SI and HLOS, there was no statistically significant difference between the positive and negative delta SI groups based on our data (Table 2). Similar results were obtained after adjusting for confounding factors: high delta SI was an independent risk factor for both mortality and early mortality. Delta SI appeared to be an effective and efficient index of great relevance in rapid deterioration after ED admission. Conversely, high delta SI was not related to long HLOS in the regression analysis.
In this study, we further separated participants into subgroups for stratified analysis. High delta SI had higher odds ratios for mortality and early mortality in the elderly. Since progressive decline in various physiological functions has been noted in the elderly (28, 29), physiological stresses that were not serious at young ages can be life-threatening in old age (30). Fluctuations in HR and SBP are key factors for mortality among critically ill elderly individuals. Therefore, closely monitoring vital signs and of changes in delta SI is more important in elderly patients in situations of illness deterioration.
The subgroup analysis of vital signs highlights the importance of altered SBP and SI at triage; in patients with these parameters, high delta SI has higher odds ratio for early mortality. Patients with SBP > 100 mmHg at triage and with high delta SI present higher incidence of early mortality after ICU admission. This is consistent with previous studies that revealed that SI may be a predictor of mortality (31, 32) in critically ill patients. Since mortality in patients with shock with hypoperfusion remains high, as reported previously (19), we offer a more robust dynamic index to ensure that early intervention and management are available to these critically ill patients. While a high SI at ED triage was a predictor of mortality in previous studies (31, 33), aggressive resuscitation and close monitoring before ICU admission should be performed to avoid adverse outcomes.
Concerning the stratified analysis per diagnoses, we found that high delta SI in patients with sepsis is correlated with high mortality and early mortality. This corroborates the results of previous studies on the association between high SI and outcome in septic patients (12, 32). Similar results were not found for patients with diagnoses of respiratory failure or heart failure. Respiratory distress and respiratory failure could be unrelated to hypotension and HR alterations (34). In addition, patients with respiratory failure may need advanced airway ventilation or even mechanical ventilation, and the hemodynamic effects of mechanical breathing are quite complex (35). These factors might affect delta SI during ED management. Moreover, heart failure is caused by structural and functional defects in the myocardium, which result in impairment of ventricular filling or ejection of blood. Early stages of heart failure often lack specific signs, such as tachycardia or other classic presentations (36–38). Therefore, less association of delta SI with clinical outcomes can be presumed in heart failure patients who were admitted to the ICU.
Our study has several limitations. First, retrospective studies rely mostly on administrative data, which is limited by the information documented on medical records. Second, the study was conducted in two tertiary care EDs with similar systems; thus, the generalizability of these findings may be limited to comparable institutions. However, we believe that the number of patients analyzed in our article is enough to support our conclusions, and both studied hospitals nearly meet the highest medical standards in Taiwan. Further prospective studies should be conducted for a more precise analysis of our results; nevertheless, we believe that our research has laid good foundations for this research field. In conclusion, our results indicate that high delta SI may be greatly related to poor prognosis among critically ill patients, especially to early mortality. Elderly critically ill patients with poor vital signs at ED triage and a diagnosis of sepsis should be carefully monitored and assisted with prompt resuscitation and intensive treatment before admission to the ICU. We believe that it is crucial to monitor delta SI while managing patients and that delta SI could play an important role in clinical practice.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study protocol was approved by the institutional review board of both hospitals. All patients' and physicians' records and information were anonymized and de-identified before analysis.
Chi-FL: conceptualization and supervision. Y-SH, Chu-FL, I-MC, and Chi-FL: data curation and methodology. M-TT and Y-SH: formal analysis. M-TT, Y-SH, and Chi-FL: investigation. I-MC and Chi-FL: validation, writing—review, and editing. Y-SH and Chi-FL: visualization. Y-SH, I-MC, and Chi-FL: writing—original draft. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Nguyen HB, Banta JE, Cho TW, Van Ginkel C, Burroughs K, Wittlake WA, et al. Mortality predictions using current physiologic scoring systems in patients meeting criteria for early goal-directed therapy and the severe sepsis resuscitation bundle. Shock. (2008) 30:23–8. doi: 10.1097/SHK.0b013e3181673826
2. Jones AE, Fitch MT, Kline JA. Operational performance of validated physiologic scoring systems for predicting in-hospital mortality among critically ill emergency department patients. Crit Care Med. (2005) 33:974–8. doi: 10.1097/01.CCM.0000162495.03291.C2
3. McQuillan P, Pilkington S, Allan A, Taylor B, Short A, Morgan G, et al. Confidential inquiry into quality of care before admission to intensive care. BMJ. (1998) 316:1853–8. doi: 10.1136/bmj.316.7148.1853
4. Rees JE, Mann C. Use of the patient at risk scores in the emergency department: a preliminary study. Emerg Med J. (2004) 21:698–9. doi: 10.1136/emj.2003.006197
5. Subbe CP, Slater A, Menon D, Gemmell L. Validation of physiological scoring systems in the accident and emergency department. Emerg Med J. (2006) 23:841–5. doi: 10.1136/emj.2006.035816
6. Brabrand M, Folkestad L, Clausen NG, Knudsen T, Hallas J. Risk scoring systems for adults admitted to the emergency department: a systematic review. Scand J Trauma Resusc Emerg Med. (2010) 18:8. doi: 10.1186/1757-7241-18-8
7. Prytherch DR, Smith GB, Schmidt PE, Featherstone PI. ViEWS–Towards a national early warning score for detecting adult inpatient deterioration. Resuscitation. (2010) 81:932–7. doi: 10.1016/j.resuscitation.2010.04.014
8. Lovett PB, Massone RJ, Holmes MN, Hall RV, Lopez BL. Rapid response team activations within 24 hours of admission from the emergency department: an innovative approach for performance improvement. Acad Emerg Med. (2014) 21:667–72. doi: 10.1111/acem.12394
9. Walston JM, Cabrera D, Bellew SD, Olive MN, Lohse CM, Bellolio MF. Vital signs predict rapid-response team activation within twelve hours of emergency department admission. West J Emerg Med. (2016) 17:324–30. doi: 10.5811/westjem.2016.2.28501
10. Allgower M, Burri C. [“Shock index”]. Dtsch Med Wochenschr. (1967) 92:1947–50. doi: 10.1055/s-0028-1106070
11. Rousseaux J, Grandbastien B, Dorkenoo A, Lampin ME, Leteurtre S, Leclerc F. Prognostic value of shock index in children with septic shock. Pediatr Emerg Care. (2013) 29:1055–9. doi: 10.1097/PEC.0b013e3182a5c99c
12. Yussof SJ, Zakaria MI, Mohamed FL, Bujang MA, Lakshmanan S, Asaari AH. Value of Shock Index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia. (2012) 67:406–11.
13. Olaussen A, Blackburn T, Mitra B, Fitzgerald M. Review article: shock index for prediction of critical bleeding post-trauma: a systematic review. Emerg Med Australas. (2014) 26:223–8. doi: 10.1111/1742-6723.12232
14. Toosi MS, Merlino JD, Leeper KV. Prognostic value of the shock index along with transthoracic echocardiography in risk stratification of patients with acute pulmonary embolism. Am J Cardiol. (2008) 101:700–5. doi: 10.1016/j.amjcard.2007.10.038
15. Zhang X, Wang Z, Wang Z, Fang M, Shu Z. The prognostic value of shock index for the outcomes of acute myocardial infarction patients: a systematic review and meta-analysis. Medicine (Baltimore). (2017) 96:e8014. doi: 10.1097/MD.0000000000008014
16. Montoya KF, Charry JD, Calle-Toro JS, Núñez LR, Poveda G. Shock index as a mortality predictor in patients with acute polytrauma. J Acute Disease. (2015) 4:202–4. doi: 10.1016/j.joad.2015.04.006
17. Joseph B, Haider A, Ibraheem K, Kulvatunyou N, Tang A, Azim A, et al. Revitalizing vital signs: the role of delta shock index. Shock. (2016) 46(3 Suppl. 1):50–4. doi: 10.1097/SHK.0000000000000618
18. Bruijns SR, Guly HR, Bouamra O, Lecky F, Wallis LA. The value of the difference between ED and prehospital vital signs in predicting outcome in trauma. Emerg Med J. (2014) 31:579–82. doi: 10.1136/emermed-2012-202271
19. Kohn JR, Dildy GA, Eppes CS. Shock index and delta-shock index are superior to existing maternal early warning criteria to identify postpartum hemorrhage and need for intervention. J Matern Fetal Neonatal Med. (2019) 32:1238–44. doi: 10.1080/14767058.2017.1402882
20. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. (1993) 270:2957–63. doi: 10.1001/jama.270.24.2957
21. Piccirillo JF, Costas I. The impact of comorbidity on outcomes. ORL J Otorhinolaryngol Relat Spec. (2004) 66:180–5. doi: 10.1159/000079875
22. Asplin BR, Magid DJ, Rhodes KV, Solberg LI, Lurie N, Camargo CA Jr. A conceptual model of emergency department crowding. Ann Emerg Med. (2003) 42:173–80. doi: 10.1067/mem.2003.302
23. Morley C, Unwin M, Peterson GM, Stankovich J, Kinsman L. Emergency department crowding: a systematic review of causes, consequences and solutions. PLoS ONE. (2018) 13:e0203316. doi: 10.1371/journal.pone.0203316
24. Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. (1994) 331:1105–9. doi: 10.1056/NEJM199410273311701
25. Holmes CL, Walley KR. The evaluation and management of shock. Clin Chest Med. (2003) 24:775–89. doi: 10.1016/S0272-5231(03)00107-2
26. Richards JB, Wilcox SR. Diagnosis and management of shock in the emergency department. Emerg Med Pract. (2014) 16:1–22; quiz 22–23. doi: 10.1111/acem.12556
27. Schellenberg M, Strumwasser A, Grabo D, Clark D, Matsushima K, Inaba K, et al. Delta shock index in the emergency department predicts mortality and need for blood transfusion in trauma patients. Am Surg. (2017) 83:1059–62. doi: 10.1177/000313481708301009
28. Gimbrone MA Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. (2000) 902:230–9; discussion 239–240. doi: 10.1111/j.1749-6632.2000.tb06318.x
29. Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. (1988) 112:1018–31.
30. Horiuchi S, Finch CE, Mesle F, Vallin J. Differential patterns of age-related mortality increase in middle age and old age. J Gerontol A Biol Sci Med Sci. (2003) 58:495–507. doi: 10.1093/gerona/58.6.B495
31. Torabi M, Mirafzal A, Rastegari A, Sadeghkhani N. Association of triage time shock index, modified shock index, and age shock index with mortality in emergency severity index level 2 patients. Am J Emerg Med. (2016) 34:63–8. doi: 10.1016/j.ajem.2015.09.014
32. Althunayyan SM, Alsofayan YM, Khan AA. Shock index and modified shock index as triage screening tools for sepsis. J Infect Public Health. (2019) 12:822–6. doi: 10.1016/j.jiph.2019.05.002
33. Koch E, Lovett S, Nghiem T, Riggs RA, Rech MA. Shock index in the emergency department: utility and limitations. Open Access Emerg Med. (2019) 11:179. doi: 10.2147/OAEM.S178358
34. Tulaimat A, Gueret RM, Wisniewski MF, Samuel J. Association between rating of respiratory distress and vital signs, severity of illness, intubation, and mortality in acutely ill subjects. Respir Care. (2014) 59:1338–44. doi: 10.4187/respcare.02650
35. Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. (2005) 103:419–28; quiz 449–415. doi: 10.1097/00000542-200508000-00026
36. Inamdar AA, Inamdar AC. Heart failure: diagnosis, management and utilization. J Clin Med. (2016) 5:62. doi: 10.3390/jcm5070062
37. Heart Failure Society of A Lindenfeld J Albert NM Boehmer JP Collins SP Ezekowitz JA . (2010). Comprehensive heart failure practice guideline. J Card Fail. (2010) 16:e1–194. doi: 10.1016/j.cardfail.2010.04.004
Keywords: delta shock index, emergency department, mortality, critical ill, intensive care unit
Citation: Huang Y-S, Chiu I-M, Tsai M-T, Lin C-F and Lin C-F (2021) Delta Shock Index During Emergency Department Stay Is Associated With in Hospital Mortality in Critically Ill Patients. Front. Med. 8:648375. doi: 10.3389/fmed.2021.648375
Received: 31 December 2020; Accepted: 01 March 2021;
Published: 22 April 2021.
Edited by:
Koichiro Shinozaki, Feinstein Institute for Medical Research, United StatesReviewed by:
Romain Jouffroy, Assistance Publique Hopitaux De Paris, FranceCopyright © 2021 Huang, Chiu, Tsai, Lin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Fu Lin, ZmFudGFjcmF6eTE5QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.