
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 22 February 2021
Sec. Ophthalmology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.640360
This article is part of the Research Topic Update on Stevens Johnson Syndrome View all 18 articles
This review describes the current knowledge regarding genetic susceptibilities and treatment strategies for Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), with ocular complications, in Korea. In a case-control study, the gene frequencies of both HLA-A*0206 (20.0%) and HLA-Cw*0304 (15.0%) increased but the gene frequency of HLA-Cw*0303 (1.3%) decreased with cold medicine (CM)-SJS/TEN with severe ocular complications (SOCs). In a case-series, positive genotyping of HLA-B*5801 was 80.0% in allopurinol-induced SJS/TEN without SOCs. In a genome-wide association study, HLA-A*0206 was substantially related to CM-SJS/TEN with SOCs. Both HLA-A*0206 and prostaglandin-E receptor 3 (PTGER3) single nucleotide polymorphism (SNP) rs1327464 exert a synergistic effect on SOCs in CM-SJS/TEN. In the acute stage, conventional procedures, amniotic membrane transplantation or suture-less amniotic contact lenses are applied. Applications of intravenous Immunoglobulin (IVIG) or mega-dose steroids are attempted in patients with high acute ocular and systemic involvement scores. In the chronic stage, keratolimbal transplantation and penetrating keratoplasty are the standard procedures. Either autologous nasal or oral mucosal grafts, or biomaterial-free cultured oral mucosal epithelial cell sheets are transplanted as alternative therapies. Deep anterior lamellar keratoplasty is attempted. Combined photodynamic therapy with intrastromal bevacizumab injection or intense pulse laser are used to resolve chronic ocular complication. Corneoscleral contact lenses are available for a visual rehabilitation. As a last resort, Seoul-type keratoprosthesis had been transplanted. There are unmet needs to standardize nationwide ocular grading system and to correct tarsal scarring using mucosal grafting. This review provides a perspective on the current practices to treat ocular complications in SJS/TEN.
Steven-Johnson syndrome (SJS) and its severe form, toxic epidermal necrolysis (TEN), are severe, inflammatory vesiculobullous reactions of the skin and mucous membranes. The mortality rate of SJS and TEN are estimated as 1–10%, and 30%, respectively. According to the Korean National Health Insurance Database, SJS and TEN are infrequent, yet they constantly occur throughout the year by showing 3.96 and 0.94 cases per million/year for SJS and TEN, respectively (1). The management of SJS/TEN imposes a considerable clinical and financial burden, which is comparable with that of other major health problems (1, 2). SJS/TEN may permanently damage the affected mucosa, inducing severe sequelae including the lungs, genitals and eye. During primary intervention, acute ocular involvement occurs in approximately 60–100% of SJS/TEN patients (3–5). In Korea, ocular complications are reported as the most common complication related to SJS/TEN (1). Patients with ocular complications spent a considerable amount of money even after their recovery (2).
It is well known that SJS/TEN can be induced by various infections or classes of pharmacological agents, such as antibiotics, anticonvulsants, nonsteroidal anti-inflammatory drugs (NSAIDS), or allopurinol (6). Among the culprit drugs, as reported in a nationwide study, anticonvulsants are most frequent, followed by allopurinol, amoxicillin/dorzolamide, and acetaminophen (6). Previous pharmacogenomic studies demonstrated that certain human leukocyte antigen (HLA) genotypes could induce T-cell activation in response to a specific drug (7, 8). In a nationwide study that enrolled 5,802 Korean patients, allele frequencies of HLA-A*0206, HLA-B*5801, HLA-Cw*0303, and HLA-Cw*0304 were reported to be 10.3, 7.0, 10.9, and 9.1%, respectively (9). Specific genetic risk factors play an important role in the development of SJS/TEN. Recently, a genome-wide association study (GWAS) with a single nucleotide polymorphism (SNP) microarray has been employed to detect an association between SNPs and SJS/TEN (10, 11).
This review describes the current knowledge on the clinical aspect and treatment strategies for SJS/TEN, with ocular complications, in Korea. We summarized the HLA genotypes and the associated drugs for Koreans responsible for severe ocular complications (SOCs) in the acute and chronic stage of SJS/TEN, and elaborated upon the treatment strategies.
Regarding frequencies of the culprit medications related with SOCs in Korea, cold medicine is the highest frequency, but antibiotics, allopurinol, or anti-epileptic drugs are not related to SOCs (12).
Allopurinol, a xanthine oxidase inhibitor, has been widely used to manage hyperuricemia and gout. Several studies report a strong association of the HLA-B*5801 genotype and allopurinol-induced SJS/TEN among Koreans (7–13%) (13–15). A recent study revealed that HLA-B75, DR13 homozygosity, or DR- 14 increased the risk of allopurinol-induced SJS/TEN when combined with HLA-B*5801, especially in patients with impaired renal function (14, 16). Compared to other drugs, allopurinol-induced SJS/TEN was associated with longer and more severe systemic manifestations, resulting in a high mortality rate (15); however, allopurinol-induced SJS/TEN may not cause serious acute or chronic ocular surface complications (12, 17). In a case-series, HLA-B*5801 genotype was observed in 80.0% of allopurinol-induced SJS/TEN without SOCs (17).
In a nationwide registry-based study, the most common causative AEDs were carbamazepine, lamotrigine, and levetiracetam (18, 19). In the case of AEDs-induced SJS/TEN, aromatic AEDs (e.g., carbamazepine, lamotrigine) were greatly associated with a severe reaction than non-aromatic AEDs (e.g., valproic acids) (19). It may be caused that AEDs containing an aromatic ring can form an arene-oxide intermediate, resulting in a hypersensitivity reaction (19). HLA-B*1502, which is closely related to SJS/TEN, is very rare in Koreans (7, 20). Several HLA genes are weakly associated with AEDs-induced hypersensitivity syndrome in the Korean population, but not with SJS/TEN (7, 20). Ocular manifestations are relatively mild in AEDs-induced SJS/TEN (12).
Cold medicine (CM), including NSAIDS and acetaminophen, is relatively safe; however, it can trigger SJS/TEN in patients with suspected viral infection mediated by T-cells and monocytes (12, 21). SJS/TEN with severe ocular complications (SOCs) are commonly associated with CM in the Korean population (10, 22–25). In GWAS, HLA-A*0206 was considerably related to CM-SJS/TEN with SOCs (22). In addition, both HLA-A*0206 and prostaglandin-E receptor 3 (PTGER3) single nucleotide polymorphism (SNP) rs1327464 exert synergistic effect in CM-SJS/TEN with SOCs (23). A recent multicenter case-control study suggested that HLA-Cw*0304 may also be a positive marker for CM-SJS/TEN with SOCs; however, HLA-Cw*0303 may be an indicator of protection against this disease in the Korean population (24). In a worldwide GWAS that enrolled Korean, Japanese, Indian, and Brazilian patients, IKAROS family zinc-finger 1 (IKZF1) was revealed as a novel susceptibility gene (meta-analysis, rs4917047) for CM-SJS/TEN with severe mucosal involvement (25).
Surprisingly, both topical and oral formulations of CAIs, such as acetazolamide, methazolamide, and dorzolamide, can induce SJS/TEN (6, 26). HLA-B*5901 genotype, which occurs in 2.1% of the Korean population, has been suggested as a genetic marker for CAIs-induced SJS/TEN (27, 28). CAIs-induced SJS/TEN results in more extensive cutaneous manifestations and frequent ocular sequelae when compared with SJS/TEN due to other drugs including allopurinol, anticonvulsants, or anti-tuberculosis drugs (29).
Antibiotics such as amoxicillin/clavulanate and cephalosporin are the most common causative drugs in pediatric patients with SJS/TEN (30). However, there is no report stating that antibiotics may be related to SOCs (12). The anti-human immunodeficiency virus agents including abacavir and nevirapine could induce a hypersensitive reaction associated with HLA-B*5701 (7). However, HLA-B*5701 is not a clinically critical allele, since it is rare in Koreans (31).
General supportive care with anti-inflammatory intervention is the mainstay to restore barrier function of the skin and mucous membrane, and fluid balance, and to treat the infection (3, 10). The Korean severe cutaneous adverse drug reactions (SCARs) registry includes patients who were diagnosed with SJS and TEN (18, 32). Ocular involvement was in the ranges of 34–43% in Korea (1, 30). Therefore, therapeutic approaches to SJS/TEN should be multidisciplinary (3, 10). In Korea, patients with SJS/TEN are usually referred to the ophthalmologists upon presenting with complaints of ocular symptoms during hospitalization. Given that there is window of time within which vision-saving treatments can be applied, ophthalmologic consultation upon admission or within 24–48 h after diagnosis is critical (3, 10). The concept of a multidisciplinary approach, including eye care, should be shared with a primary physician.
There are local and systemic interventions to treat ocular complications in the acute stage of SJS/TEN (3, 10, 33, 34). As local treatments, aggressive lubrication, mechanical membrane removal/synechiolysis, bandage contact lens (CL) placement, and topical antibiotics and steroid application are implemented (3). Preservative-free artificial tears are instilled every 1–2 h and eyedrops containing hyaluronate are preferred in epithelial defected ocular surface. All membranes should be mechanically removed (33). However, there is no consensus about how often either membrane removal or synechiolysis be conducted since cotton-tip application can induce mechanical trauma. The benefits of mechanical synechiolysis should be cautiously weighed against the intervention-induced inflammation. One percentage topical prednisolone acetate combined with antiseptic eyedrops containing fluoroquinolone are preferably applied. 0.5–1.5% levofloxacin or 0.5% moxifloxacin eyedrops are administered three to four times a day. Topical 1% prednisolone acetate is administered every 2–3 h depending on the severity. High-oxygen-transmissible silicone hydrogel CL with medium water content (35–46%) such as Acuvue Oasys, Acuvue Advance, and PureVision are available to cover corneal epithelial defects (35).
Although there is no worldwide consensus on a grading system to assess severity of acute ocular involvement in SJS/TEN, new grading systems are currently being proposed (34, 36, 37). Sotozono et al. proposed a grading scale of 0–3 using three parameters, including conjunctival hyperemia, ocular surface epithelial defect, and pseudomembrane (36). Gregory et al. proposed four grading scales based on the presence of epithelial defected area with three parameters, including conjunctiva, cornea, and lid margin (37). In French, they reached a nationwide consensus on a diagnostic grading system for acute ocular complications that consists of three stages of severity using seven parameters (34). In Korea, we have not reached a consensus yet on grading system for acute ocular complications. The authors working with the international collaboration network of ocular SJS/TEN led by Kinoshita currently use the grading scales proposed by Sotozono (17, 38). Therefore, a nationwide consensus on the grading system to evaluate acute ocular complications should be established.
Amniotic membrane transplantation (AMT) is a standardized procedure for the severe acute ocular complication. AMT within the first 7–10 days can potentially avoid vision-threatening chronic complications (33, 37, 39). The indication of AMT includes (1) any corneal epithelial defect, (2) staining of the eyelid margin > 1/3 of its length, or (3) any conjunctival staining >1 cm at its greatest diameter and/or (4) pseudomembrane formation (33, 37, 39). There are two studies reporting the effect of AMT on visual improvement or SOCs in SJS/TEN in Korea (38, 40). One of the reports had presented the beneficial effect of AMT on visual improvement and SOCs (40). On the contrary, the other report showed that AMT was related with a poor final visual outcome; however, it did not mention when the AM was transplanted (38). AMT in the latter study may not be a timely treatment. The AMT technique to cover the whole ocular surface including fornix and the eyelid was recently standardized using the symblepharon ring and lid bolsters (39, 41, 42). In Korea, a similar technique of AMT was adapted. For a bedside application, ProKera is available in western countries (43), whereas suture-less amniotic membrane patch with a silicone ring (44) or suture-less amniotic CL is available in Korea. Effect of amniotic CL on wound healing was comparable to that of AMT in vivo study (Figure 1A, Supplementary Video 1) (45). However, the size of the amniotic CL is just enough to cover the cornea.
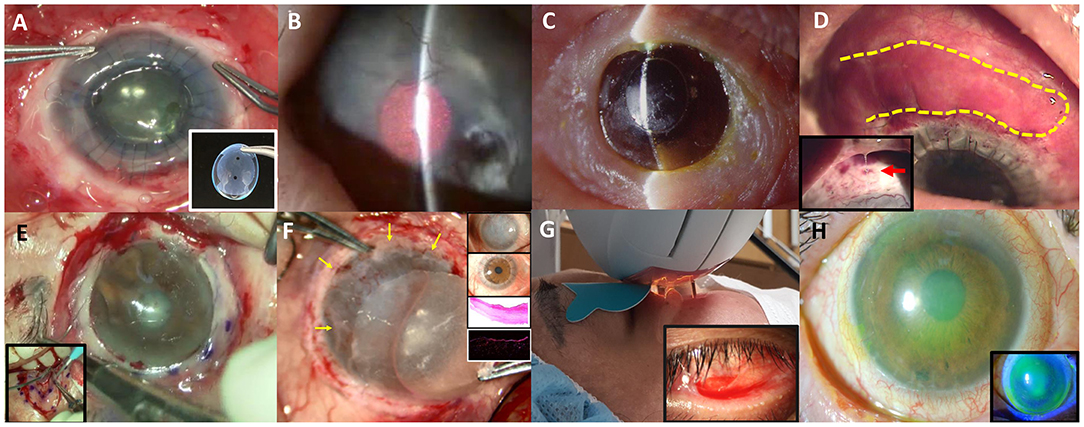
Figure 1. Various non-surgical and surgical modalities to treat ocular complications in Stevens-Johnson syndrome or Toxic epidermal necrosis are introduced. (A) Commercially available suture-less amniotic contact lens. (B) Photodynamic therapy with verteporfin combined with intrastromal bevacizumab injection. (C) Seoul-type keratoprosthesis. (D) Autologous nasal mucosal grafting (yellow-highlighted) combined with penetrating keratoplasty and keratolimbal allografting. A thick mucus is often secreted by the nasal mucosal graft (red arrow).* (E) Circumferential autologous oral labial mucosal grafting. (F) Biomaterial-free cultured oral mucosal epithelial cell sheets (COMECs) transplantation (yellow arrows) with H&E and K13 immunofluorescent staining. (G) Intense pulsed light. (H) Corneoscleral contact lens.
Systemic anti-inflammatory treatment is of utmost importance in reducing inflammation for both the body and the eye. So far, the effect of systemic intravenous immunoglobulin (IVIG) or mega-dose steroids on SOCs has been the subject of debate (38). In Korea, IVIG or mega-dose steroids are sometimes used for treatment of patients with high acute ocular and systemic involvement (38). A recent SCAR registry-based study showed that most of the patients have been treated with systemic steroid with an average maximal dose of 60 mg/day (18). Additionally, 87.5, 0.6, and 11.8% of the patients were treated using systemic steroids, IVIG, and both systemic steroids and IVIG, respectively (18); whereas, 49, 17, and 28% of the pediatric patients received systemic steroids, IVIG, and both systemic steroids and IVIG, respectively (30). It revealed that pediatric patients were more treated with IVIG compared to adults (18, 30). Considering that children with SJS/TEN have higher ocular and systemic complications than adults do (46, 47), such a treatment pattern may be reasonable. Combined treatment of IVIG and systemic steroids may be beneficial to reduce both SOCs and systemic complications.
Complex ocular sequelae, such as symblepharon, lid malformation, trichiasis, conjunctival keratinization, limbal stem cell deficiency, corneal pannus, and dry eye occur in the chronic stage in patients with SJS/TEN. The goal of treatment in the chronic stage is the preservation of visual function, and reduction of the inflammation and persistent discomfort. Herein, surgical and medical interventions that have been currently practiced in Korea are presented (Table 1 and Figure 1).
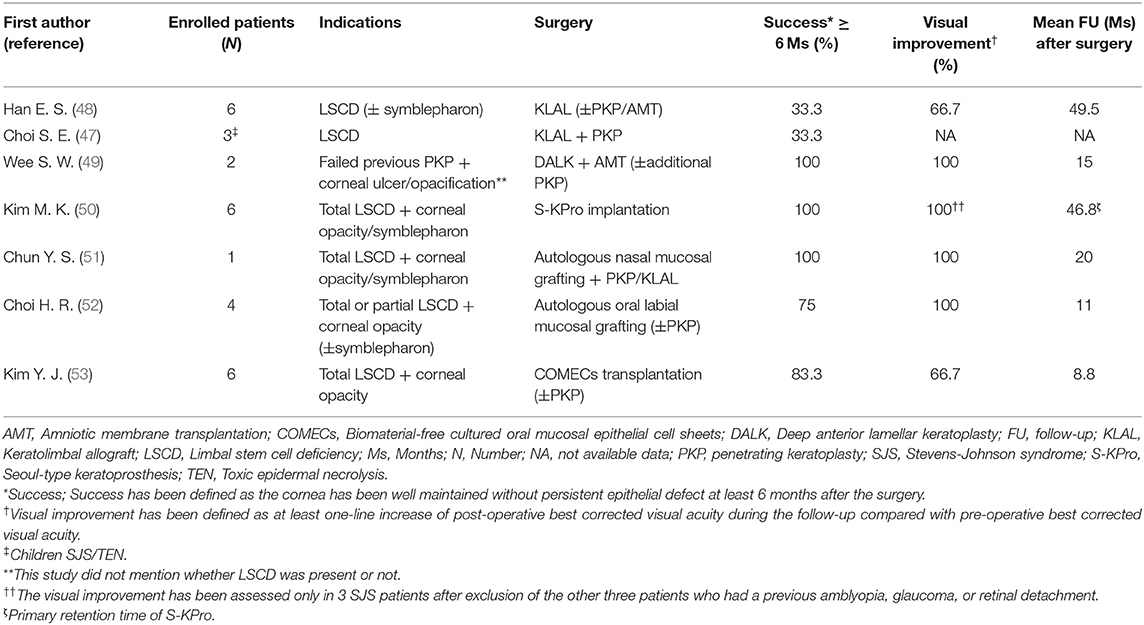
Table 1. The clinical outcomes of various surgical interventions to treat chronic limbal stem cell deficiency in Korean SJS/TEN patients.
Chronic inflammation has been controlled by various medical interventions, such as serum eyedrops, topical or systemic steroid, and immunosuppressants. Autologous serum eye drops contain many anti-inflammatory molecules and are effective in reducing inflammation on the ocular surface (54); however, the components of serum eye drops may differ depending on the general condition of the patient. Steroid is effective but has undesirable adverse effects including glaucoma, cataract, infection, and delayed wound healing (55). Topical 0.02% tacrolimus ointment, originally approved for dermatologic purpose, is a good alternative to topical steroid (56). Topical 0.02% tacrolimus ointment was added to treat refractory chronic conjunctival inflammation in six SJS patients with tapering of the topical steroid (56). Topical tacrolimus decreased surface inflammation, corneal neovascularization, and intraocular pressure within 4 weeks (56).
Additionally, infection of the ocular surface should be closely monitored especially in SJS/TEN with SOCs. Unlike healthy people who show a high diversity of ocular microbiomes with prevalent streptococcus and lactobacillus, staphylococcus is a predominant bacteria with a less diversity with SJS, which can become easily pathogenic (57). A report in Korea presented higher rate of infective keratitis (35%) in LSCD with SJS than in those with a chemical burn (18%) (58). The higher the score of chronic ocular complications is, the more frequently infective keratitis develops in SJS (58).
Besides, tear film is unstable and meibomian gland is dysfunctional by the sequelae of SJS, and the degrees of meibomian gland disease tend to be correlated with the severity of SJS (59). Intense pulsed light is used to improve meibum expressibility (Figure 1G) (60). It contributes to decreased inflammatory cytokines such as IL-4,−6,−10, 17A, and TNF-α. IPL can be applied to stabilize tear film and reduce inflammatory cytokines, thereby treating severe meibomian gland obstruction in SJS.
Finally, corneoscleral CL with a total diameter of 14.0 mm is available for non-surgical visual rehabilitation in Korea (61). Fitting of a corneoscleral CL improved the vision by reducing corneal punctate erosions and reconstructing a new optical surface in six of eight SJS patients (Figure 1H) (61).
Intense immunologic reactions destruct limbal stem cells. Subsequently, corneal pannus occurs due to the loss of the limbal barrier function. Despite a high risk of rejection, keratolimbal allograft (KLAL) and penetrating keratoplasty (PKP) are standard procedures for visual rehabilitation. The clinical outcomes of various surgical interventions to treat chronic limbal stem cell deficiency (LSCD) in Korean SJS/TEN patients are shown in Table 1.
Eyes with SJS demonstrated a 33.3% of short-term success rate (≥6 months) and 16.7% of long-term success rate (≥2 years) in KLAL, which showed the least success rate among patients with LSCD (48). In children with SJS/TEN, LSCD developed in 32%, and combined PKP with KLAL failed in two (67%) out of three children (47). Deep anterior lamellar keratoplasty was attempted using acellular cornea with AMT in two eyes with previous failed PKP (49). One of them kept the cornea clear with epithelization. The other eye which needed additional PKP showed no additional corneal opacity (49). Photodynamic therapy with verteporfin combined with intrastromal bevacizumab injection was also applied to reduce corneal neovascularization (Figure 1B) (62). Within 6 months, five of eight eyes showed complete regression and the remaining eyes showed partial regression (62). In a few cases, Seoul-type keratoprosthesis (S-KPro) had been transplanted (Figure 1C) (50, 63). In the six S-KPro-implanted eyes of SJS, mean retention and visual preservation time was 46.8 and 35 months, respectively. To correct conjunctival keratinization with symblepharon or a LSCD, mucosal grafting has been attempted. A report presented successful reconstruction of the ocular surface and visual improvement by autologous nasal mucosal grafting accompanied with PKP and KLAL in a patient with SJS (Figure 1D) (51). Another report revealed visual improvement with a stable ocular surface by circumferential autologous oral labial mucosal grafting at the limbus in all four SJS patients (Figure 1E) (52). Recently, biomaterial-free cultured oral mucosal epithelial cell sheets (COMECs) transplantation has proven some efficacy on an LSCD in a clinical trial (Figure 1F) (53). Although the initial migration of the oral mucosal epithelial cells harvested from SJS patients (SJS-cells) was delayed with lower levels of epidermal growth factor and higher levels of vascular endothelial growth factor, compared to those of non-SJS cells, in vivo transplanted SJS-COMECs revealed similar expression of cytokeratin and stem cell markers as in non-SJS COMECs (64, 65). COMECs were transplanted in six SJS patients, and five eyes achieved complete reepithelization in a mean follow-up of 10.2 months (53). Among those five eyes, visual acuity was improved in four eyes with/without PKP (53).
Although the outcomes of various surgical interventions to treat LSCD cannot be directly compared due to different indications and follow-up periods (Table 1), autologous nasal or oral mucosal grafting, COMEC transplantation seem to show better successful outcome with visual improvement compared with that in KLAL. Meanwhile, first S-KPro implantation showed long-term successful outcome with visual improvement (50). However, due to skirt exposure, secondary exchange of S-KPro was mandatory in all S-KPro implanted patients (50). Given that retinal detachment developed in all S-KPro-exchanged eyes within 2 months (50), S-KPro implantation can be considered as a last resort. In Korea, less attention is paid to correction of scarring of the tarsal conjunctiva and lid malformation in SJS patients. There have been no reports regarding the reconstruction of tarsal scarring using a mucosal grafting in SJS yet.
In Korea, about 40% of the SJS/TEN patients suffer from chronic ocular complications. HLA-A*0206 combined with PTGER3 SNP rs1327464 enhances genetic susceptibility in CM-SJS/TEN with SOCs, whereas HLA-C*03:03 may be an indicator of protection against CM-SJS/TEN with SOCs. For the timely treatment of acute ocular complications, a nationwide consensus on ocular grading system should be reached, and a multidisciplinary approach including ophthalmologists should be standardized in Korea. In the chronic stage, various innovative surgical and medical modalities have been attempted to restore vision and stable ocular surface. Notably, both oral and nasal mucosal grafting as well as COMECs transplantation hold the most promise in the treatment of LSCD of Korean patients with SJS/TEN at present. However, the enrolled patient numbers were too small and the follow-up was too short to verify the long-term clinical efficacy. Therefore, large scale study with long-term follow-up should be further conducted. This review provides insightful information about genetic predisposition and current strategies to treat ocular complications of SJS/TEN in Korean population and gives us a perspective on how to improve current practice.
MK: conceptualization, data curation, formal analysis, investigation, methodology, resources, visualization, and roles/writing—original draft. KY: conceptualization, data curation, formal analysis, investigation, methodology, resources, and roles/writing—original draft. SY: data curation, formal analysis, investigation, visualization, and writing—editing. KS: conceptualization, formal analysis, supervision, validation, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This research was supported by Korea Mouse Phenotyping Project (NRF-2013M3A9D5072551) from the Ministry of Science and ICT through the National Research Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with several of the authors MK, KY, and KS.
Yeoun Sook Chun provided a photograph in Figure 1D. A photograph (Figure 1A, bottom) and the Supplementary Video 1 were provided by MS bio Inc. (Seoungnam-si, Korea).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.640360/full#supplementary-material
1. Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al. Incidence of Stevens-Johnson syndrome and toxic epidermal necrolysis: a nationwide population-based study using national health insurance database in Korea. PLoS ONE. (2016) 11:e0165933. doi: 10.1371/journal.pone.0165933
2. Yang MS, Kim JY, Kang MG, Lee SY, Jung JW, Cho SH, et al. Direct costs of severe cutaneous adverse reactions in a tertiary hospital in Korea. Korean J Intern Med. (2019) 34:195–201. doi: 10.3904/kjim.2015.365
3. Shanbhag SS, Chodosh J, Fathy C, Goverman J, Mitchell C, Saeed HN. Multidisciplinary care in Stevens-Johnson syndrome. Ther Adv Chronic Dis. (2020) 11:2040622319894469. doi: 10.1177/2040622319894469
4. Morales ME, Purdue GF, Verity SM, Arnoldo BD, Blomquist PH. Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthalmol. (2010) 150:505–10.e1. doi: 10.1016/j.ajo.2010.04.026
5. Yip LW, Thong BY, Lim J, Tan AW, Wong HB, Handa S, et al. Ocular manifestations and complications of Stevens-Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy. (2007) 62:527–31. doi: 10.1111/j.1398-9995.2006.01295.x
6. Yang MS, Lee JY, Kim J, Kim GW, Kim BK, Kim JY, et al. Searching for the culprit drugs for Stevens-Johnson syndrome and toxic epidermal necrolysis from a nationwide claim database in Korea. J Allergy Clin Immunol Pract. (2020) 8:690–5.e2. doi: 10.1016/j.jaip.2019.09.032
7. Jung JW, Kim JY, Park IW, Choi BW, Kang HR. Genetic markers of severe cutaneous adverse reactions. Korean J Intern Med. (2018) 33:867–75. doi: 10.3904/kjim.2018.126
8. Jee YK, Kim S, Lee JM, Park HS, Kim SH. CD8(+) T-cell activation by methazolamide causes methazolamide-induced Stevens-Johnson syndrome and toxic epidermal necrolysis. Clin Exp Allergy. (2017) 47:972–4. doi: 10.1111/cea.12919
9. Park HJ, Kim YJ, Kim DH, Kim J, Park KH, Park JW, et al. HLA allele frequencies in 5802 Koreans: varied allele types associated with SJS/TEN according to culprit drugs. Yonsei Med J. (2016) 57:118–26. doi: 10.3349/ymj.2016.57.1.118
10. Chang WC, Abe R, Anderson P, Anderson W, Ardern-Jones MR, Beachkofsky TM, et al. SJS/TEN 2019: from science to translation. J Dermatol Sci. (2020) 98:2–12. doi: 10.1016/j.jdermsci.2020.02.003
11. Ueta M, Sawai H, Shingaki R, Kawai Y, Sotozono C, Kojima K, et al. Genome-wide association study using the ethnicity-specific Japonica array: identification of new susceptibility loci for cold medicine-related Stevens-Johnson syndrome with severe ocular complications. J Hum Genet. (2017) 62:485–9. doi: 10.1038/jhg.2016.160
12. Lee HK, Yoon KC, Seo KY, Ueta M, Kim MK. Chronic ocular complications of Stevens-Johnson syndrome associated with causative medications in Korea. J Allergy Clin Immunol Pract. (2018) 6:700–2.e2. doi: 10.1016/j.jaip.2017.09.001
13. Kim EY, Seol JE, Choi JH, Kim NY, Shin JG. Allopurinol-induced severe cutaneous adverse reactions: a report of three cases with the HLA-B(*)58:01 allele who underwent lymphocyte activation test. Transl Clin Pharmacol. (2017) 25:63–6. doi: 10.12793/tcp.2017.25.2.63
14. Jung JW, Song WJ, Kim YS, Joo KW, Lee KW, Kim SH, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. (2011) 26:3567–72. doi: 10.1093/ndt/gfr060
15. Park HJ, Yun J, Kang DY, Park JW, Koh YI, Kim S, et al. Unique clinical characteristics and prognosis of allopurinol-induced severe cutaneous adverse reactions. J Allergy Clin Immunol Pract. (2019) 7:2739–49.e3. doi: 10.1016/j.jaip.2019.05.047
16. Shim JS, Yun J, Kim MY, Chung SJ, Oh JH, Kang DY, et al. The presence of HLA-B75, DR13 homozygosity, or DR14 additionally increases the risk of allopurinol-induced severe cutaneous adverse reactions in HLA-B*58:01 Carriers. J Allergy Clin Immunol Pract. (2019) 7:1261–70. doi: 10.1016/j.jaip.2018.11.039
17. Lee HS, Ueta M, Kim MK, Seo KY, Sotozono C, Kinoshita S, et al. Analysis of ocular manifestation and genetic association of allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in South Korea. Cornea. (2016) 35:199–204. doi: 10.1097/ICO.0000000000000708
18. Park CS, Kang DY, Kang MG, Kim S, Ye YM, Kim SH, et al. Severe cutaneous adverse reactions to antiepileptic drugs: a nationwide registry-based study in Korea. Allergy Asthma Immunol Res. (2019) 11:709–22. doi: 10.4168/aair.2019.11.5.709
19. Kim HK, Kim DY, Bae EK, Kim DW. Adverse skin reactions with antiepileptic drugs using Korea adverse event reporting system database, 2008-2017. J Korean Med Sci. (2020) 35:e17. doi: 10.3346/jkms.2020.35.e17
20. Kim SH, Lee KW, Song WJ, Kim SH, Jee YK, Lee SM, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. (2011) 97:190–7. doi: 10.1016/j.eplepsyres.2011.08.010
21. Ban GY, Ahn SJ, Yoo HS, Park HS, Ye YM. Stevens-Johnson syndrome and toxic epidermal necrolysis associated with acetaminophen use during viral infections. Immune Netw. (2016) 16:256–60. doi: 10.4110/in.2016.16.4.256
22. Ueta M, Kannabiran C, Wakamatsu TH, Kim MK, Yoon KC, Seo KY, et al. Trans-ethnic study confirmed independent associations of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe ocular surface complications. Sci Rep. (2014) 4:5981. doi: 10.1038/srep05981
23. Ueta M, Tokunaga K, Sotozono C, Sawai H, Yoon KC, Kum Kim M, et al. HLA-A*02:06 and PTGER3 polymorphism exert additive effects in cold medicine-related Stevens-Johnson syndrome with severe ocular complications. Hum Genome Var. (2015) 2:15023. doi: 10.1038/hgv.2015.23
24. Jun I, Rim JH, Kim MK, Yoon KC, Joo CK, Kinoshita S, et al. Association of human antigen class I genes with cold medicine-related Stevens-Johnson syndrome with severe ocular complications in a Korean population. Br J Ophthalmol. (2019) 103:573–6. doi: 10.1136/bjophthalmol-2018-313263
25. Ueta M, Sawai H, Sotozono C, Hitomi Y, Kaniwa N, Kim MK, et al. IKZF1, a new susceptibility gene for cold medicine-related Stevens-Johnson syndrome/toxic epidermal necrolysis with severe mucosal involvement. J Allergy Clin Immunol. (2015) 135:1538–45.e17. doi: 10.1016/j.jaci.2014.12.1916
26. Chun JS, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. Toxic epidermal necrolysis induced by the topical carbonic anhydrase inhibitors brinzolamide and dorzolamide. Ann Dermatol. (2008) 20:260–2. doi: 10.5021/ad.2008.20.4.260
27. Kim SH, Kim M, Lee KW, Kim SH, Kang HR, Park HW, et al. HLA-B*5901 is strongly associated with methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics. (2010) 11:879–84. doi: 10.2217/pgs.10.54
28. Shu C, Shu D, Tie D, Yu M, Zhang R, Wang T, et al. Toxic epidermal necrolysis induced by methazolamide in a Chinese-Korean man carrying HLA-B*59:01. Int J Dermatol. (2015) 54:1242–5. doi: 10.1111/ijd.12651
29. Kim S, Yun J, Kang DY, Park HJ, Jo EJ, Jung JW, et al. Carbonic anhydrase inhibitor-induced Stevens-Johnson syndrome/toxic epidermal necrolysis leads to extensive cutaneous involvement. J Allergy Clin Immunol Pract. (2019) 7:2851–3.e2. doi: 10.1016/j.jaip.2019.05.010
30. Oh HL, Kang DY, Kang HR, Kim S, Koh YI, Kim SH, et al. Severe cutaneous adverse reactions in Korean pediatric patients: a study from the Korea SCAR registry. Allergy Asthma Immunol Res. (2019) 11:241–53. doi: 10.4168/aair.2019.11.2.241
31. Lee KW, Oh DH, Lee C, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. (2005) 65:437–47. doi: 10.1111/j.1399-0039.2005.00386.x
32. Kang DY, Yun J, Lee SY, Koh YI, Sim DW, Kim S, et al. A nationwide study of severe cutaneous adverse reactions based on the multicenter registry in Korea. J Allergy Clin Immunol Pract. (2021) 9:929–36. doi: 10.1016/j.jaip.2020.09.011
33. Saeed HN, Chodosh J. Ocular manifestations of Stevens-Johnson syndrome and their management. Curr Opin Ophthalmol. (2016) 27:522–9. doi: 10.1097/ICU.0000000000000312
34. Thorel D, Ingen-Housz-Oro S, Royer G, Delcampe A, Bellon N, Bodemer C, et al. Management of ocular involvement in the acute phase of Stevens-Johnson syndrome and toxic epidermal necrolysis: french national audit of practices, literature review, and consensus agreement. Orphanet J Rare Dis. (2020) 15:259. doi: 10.1186/s13023-020-01538-x
35. Efron N, Morgan PB, Cameron ID, Brennan NA, Goodwin M. Oxygen permeability and water content of silicone hydrogel contact lens materials. Optom Vis Sci. (2007) 84:328–37. doi: 10.1097/OPX.0b013e31804375ed
36. Sotozono C, Ueta M, Nakatani E, Kitami A, Watanabe H, Sueki H, et al. Predictive factors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. (2015) 160:228–37.e2. doi: 10.1016/j.ajo.2015.05.002
37. Gregory DG. New grading system and treatment guidelines for the acute ocular manifestations of Stevens-Johnson syndrome. Ophthalmology. (2016) 123:1653–8. doi: 10.1016/j.ophtha.2016.04.041
38. Kim DH, Yoon KC, Seo KY, Lee HS, Yoon SC, Sotozono C, et al. The role of systemic immunomodulatory treatment and prognostic factors on chronic ocular complications in Stevens-Johnson syndrome. Ophthalmology. (2015) 122:254–64. doi: 10.1016/j.ophtha.2014.08.013
39. Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis - a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf. (2016) 14:168–88. doi: 10.1016/j.jtos.2016.02.001
40. Kim KH, Park SW, Kim MK, Wee WR. Effect of age and early intervention with a systemic steroid, intravenous immunoglobulin or amniotic membrane transplantation on the ocular outcomes of patients with Stevens-Johnson syndrome. Korean J Ophthalmol. (2013) 27:331–40. doi: 10.3341/kjo.2013.27.5.331
41. Shay E, Kheirkhah A, Liang L, Sheha H, Gregory DG, Tseng SC. Amniotic membrane transplantation as a new therapy for the acute ocular manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis. Surv Ophthalmol. (2009) 54:686–96. doi: 10.1016/j.survophthal.2009.03.004
42. Ma KN, Thanos A, Chodosh J, Shah AS, Mantagos IS. A novel technique for amniotic membrane transplantation in patients with acute Stevens-Johnson syndrome. Ocul Surf. (2016) 14:31–6. doi: 10.1016/j.jtos.2015.07.002
43. Suri K, Kosker M, Raber IM, Hammersmith KM, Nagra PK, Ayres BD, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. (2013) 39:341–7. doi: 10.1097/ICL.0b013e3182a2f8fa
44. Yang J, Sim H, Park DJ. Efficacy of the sutureless amniotic membrane patch for the treatment of ocular surface disorders. J Korean Ophthalmol Soc. (2012) 53:27–36. doi: 10.3341/jkos.2012.53.1.27
45. Seo J, Ko B, Lee D, Park W. The effects of amniotic membrane contact lens for cornea wound healing. J Korean Ophthalmol Soc. (2009) 50:989–95. doi: 10.3341/jkos.2009.50.7.989
46. Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. (2018) 189:17–28. doi: 10.1016/j.ajo.2018.01.028
47. Choi SH, Kim MK, Oh JY. Corneal limbal stem cell deficiency in children with Stevens-Johnson syndrome. Am J Ophthalmol. (2019) 199:1–8. doi: 10.1016/j.ajo.2018.10.016
48. Han ES, Wee WR, Lee JH, Kim MK. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefes Arch Clin Exp Ophthalmol. (2011) 249:1697–704. doi: 10.1007/s00417-011-1760-3
49. Wee SW, Choi SU, Kim JC. Deep anterior lamellar keratoplasty using irradiated acellular cornea with amniotic membrane transplantation for intractable ocular surface diseases. Korean J Ophthalmol. (2015) 29:79–85. doi: 10.3341/kjo.2015.29.2.79
50. Kim MK, Lee SM, Lee JL, Chung TY, Kim YH, Wee WR, et al. Long-term outcome in ocular intractable surface disease with Seoul-type keratoprosthesis. Cornea. (2007) 26:546–51. doi: 10.1097/ICO.0b013e3180415d35
51. Chun YS, Park IK, Kim JC. Technique for autologous nasal mucosa transplantation in severe ocular surface disease. Eur J Ophthalmol. (2011) 21:545–51. doi: 10.5301/EJO.2011.6336
52. Choe HR, Yoon CH, Kim MK. Ocular surface reconstruction using circumferentially-trephined autologous oral mucosal graft transplantation in limbal stem cell deficiency. Korean J Ophthalmol. (2019) 33:16–25. doi: 10.3341/kjo.2018.0111
53. Kim YJ, Lee HJ, Ryu JS, Kim YH, Jeon S, Oh JY, et al. Prospective clinical trial of corneal reconstruction with biomaterial-free cultured oral mucosal epithelial cell sheets. Cornea. (2018) 37:76–83. doi: 10.1097/ICO.0000000000001409
54. Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. (2004) 88:1467–74. doi: 10.1136/bjo.2004.044347
55. Tomlins PJ, Parulekar MV, Rauz S. “Triple-TEN” in the treatment of acute ocular complications from toxic epidermal necrolysis. Cornea. (2013) 32:365–9. doi: 10.1097/ICO.0b013e318243fee3
56. Lee YJ, Kim SW, Seo KY. Application for tacrolimus ointment in treating refractory inflammatory ocular surface diseases. Am J Ophthalmol. (2013) 155:804–13. doi: 10.1016/j.ajo.2012.12.009
57. Zilliox MJ, Gange WS, Kuffel G, Mores CR, Joyce C, de Bustros P, et al. Assessing the ocular surface microbiome in severe ocular surface diseases. Ocul Surf. (2020) 18:706–12. doi: 10.1016/j.jtos.2020.07.007
58. Kang BS, Kim MK, Wee WR, Oh JY. Infectious keratitis in limbal stem cell deficiency: Stevens-Johnson syndrome versus chemical burn. Cornea. (2016) 35:51–5. doi: 10.1097/ICO.0000000000000677
59. Shrestha T, Moon HS, Choi W, Yoon HJ, Ji YS, Ueta M, et al. Characteristics of meibomian gland dysfunction in patients with Stevens-Johnson syndrome. Medicine. (2019) 98:e16155. doi: 10.1097/MD.0000000000016155
60. Choi M, Han SJ, Ji YW, Choi YJ, Jun I, Alotaibi MH, et al. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci Rep. (2019) 9:7648. doi: 10.1038/s41598-019-44000-0
61. Lee SM, Kim YJ, Choi SH, Oh JY, Kim MK. Long-term effect of corneoscleral contact lenses on refractory ocular surface diseases. Cont Lens Anterior Eye. (2019) 42:399–405. doi: 10.1016/j.clae.2018.10.011
62. Yoon HJ, Kim MK, Seo KY, Ueta M, Yoon KC. Effectiveness of photodynamic therapy with verteporfin combined with intrastromal bevacizumab for corneal neovascularization in Stevens-Johnson syndrome. Int Ophthalmol. (2019) 39:55–62. doi: 10.1007/s10792-017-0786-x
63. Kim MK, Lee JL, Wee WR, Lee JH. Seoul-type keratoprosthesis: preliminary results of the first 7 human cases. Arch Ophthalmol. (2002) 120:761–6. doi: 10.1001/archopht.120.6.761
64. Kim YH, Kim DH, Shin EJ, Lee HJ, Wee WR, Jeon S, et al. Comparative analysis of substrate-free cultured oral mucosal epithelial cell sheets from cells of subjects with and without Stevens-Johnson syndrome for use in ocular surface reconstruction. PLoS ONE. (2016) 11:e0147548. doi: 10.1371/journal.pone.0147548
Keywords: HLA-A*0206, HLA-B*5801, HLA-Cw*0303, HLA-Cw*0304, South Korea, Stevens-Johnson syndrome
Citation: Kim MK, Yoon KC, Yoon SH and Seo KY (2021) Clinical Aspects of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis With Severe Ocular Complications in South Korea. Front. Med. 8:640360. doi: 10.3389/fmed.2021.640360
Received: 11 December 2020; Accepted: 22 January 2021;
Published: 22 February 2021.
Edited by:
Mayumi Ueta, Kyoto Prefectural University of Medicine, JapanReviewed by:
Chie Sotozono, Kyoto Prefectural University of Medicine, JapanCopyright © 2021 Kim, Yoon, Yoon and Seo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyoung Yul Seo, c2Vva3lAeXVocy5hYw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.