- 1Department of Nephrology, Juntendo University Faculty of Medicine, Tokyo, Japan
- 2Medical Technology Innovation Center, Juntendo University, Tokyo, Japan
- 3Department of Integrated Science and Engineering for Sustainable Society, Chuo University, Tokyo, Japan
- 4Division of General Medicine, Department of Internal Medicine, The Jikei University School of Medicine, Tokyo, Japan
- 5Division of Nephrology and Hypertension, Department of Internal Medicine, St. Marianna University School of Medicine, Kawasaki, Japan
- 6Innovation and Research Support Center, International University of Health and Welfare, Tokyo, Japan
- 7Intensive Care Unit, Department of Cardiology, Toranomon Hospital, Tokyo, Japan
- 8Internal Medicine, Kanto Central Hospital, Tokyo, Japan
- 9Tokyo Takanawa Hospital, Tokyo, Japan
- 10Division of Chronic Kidney Disease Therapeutics, The Jikei University, Tokyo, Japan
Background: The levels of circulating tumor necrosis factor receptor (TNFR) 1 and 2 help predict the future decline of estimated glomerular filtration rate (eGFR) chiefly in patients with diabetes. It has been recently reported that the change ratio in TNFR1 by SGLT2 inhibitor treatment is also related with future GFR decline in patients with diabetes. The aims of this study are to investigate the association between baseline TNFR levels and early change in TNFR levels by the non-purine selective xanthine oxidase inhibitor, febuxostat, and future eGFR decline chiefly in chronic kidney disease (CKD) patients without diabetes.
Methods: We conducted a post-hoc analysis of the FEATHER study on patients with asymptomatic hyperuricemia and CKD stage 3, who were randomly assigned febuxostat 40 mg/day or matched placebo. This analysis included 426 patients in whom baseline stored samples were available. Serum TNFR levels at baseline were measured using enzyme-linked immunosorbent assay. Those levels were also measured using 12-week stored samples from 197 randomly selected patients.
Results: Compared with placebo, short-term febuxostat treatment significantly decreased the median percent change from baseline in serum uric acid (−45.05, 95% CI −48.90 to −41.24 mg/dL), TNFR1 (1.10, 95% CI−2.25 to 4.40), and TNFR2 (1.66, 95% CI −1.72 to 4.93), but not TNFR levels. Over a median follow-up of 105 weeks, 30 patients (7.0%) experienced 30% eGFR decline from baseline. In the Cox multivariate model, high levels of baseline TNFR predicted a 30% eGFR decline, even after adjusting for age, sex, systolic blood pressure, high sensitivity C-reactive protein, uric acid, and presence or absence of febuxostat treatment and diabetes, in addition to baseline albumin to creatinine ratio and eGFR.
Conclusion: Early change in circulating TNFR levels failed to predict future eGFR decline; however, regardless of febuxostat treatment, the elevated baseline level of TNFR was a strong predictor of 30% eGFR decline even in chiefly non-diabetic CKD patients with asymptomatic hyperuricemia.
Introduction
Hyperuricemia was previously considered to occur because of reduced renal clearance of uric acid in patients with decreased renal function; therefore, treatment of asymptomatic hyperuricemia has received less attention from researchers. However, several recent epidemiological studies demonstrated that the high levels of serum uric acid increased the risk of renal function decline in the general population (1, 2). In patients with type 1 diabetes, high-normal serum uric acid was reported to predict estimated future decline in glomerular filtration rate (eGFR) (3). However, recent randomized controlled clinical trials (RCTs) demonstrated that the reduction of serum uric acid with allopurinol did not prevent renal function decline in patients with type 1 diabetes and mild to moderate diabetic kidney disease (DKD) or stage 3 and 4 chronic kidney disease (CKD), both of which are high risk factors for eGFR decline (4, 5). Data on halting the progression of CKD using non-purine selective xanthine oxidase inhibitor to lower uric acid have been insufficient (6, 7), although a number of studies demonstrated that uric acid was associated with the pathogenesis of kidney injury through endothelial dysfunction and inflammation (8, 9).
Studies indicated that the elevated levels of circulating tumor necrosis factor receptor (TNFR), which is one of the inflammatory markers, predicted the progression of renal function decline and mortality in a broad range of diseases (10–13). However, the mechanisms that modulate TNFR levels are not fully understood, although the post-hoc analyses of several clinical trials reported that several therapeutic agents altered serum TNFR levels (14, 15). We and other researchers previously demonstrated that serum TNFR level was associated with serum uric acid level in patients with type 2 diabetes (16, 17).
Therefore, in the current post-hoc analysis of the previously reported FEATHER study, we investigated whether baseline TNFR levels and early change in circulating TNFR levels predicted 30% eGFR decline chiefly in non-diabetic CKD patients with asymptomatic hyperuricemia.
Methods
Study Patients
The FEATHER trial design, baseline characteristics of the participants, and primary results have been previously described (6). This was a post-hoc analysis of a multicenter, prospective, double-blind, randomized, placebo-controlled parallel group trial to test and compare the efficacy of the xanthine oxidase inhibitor (febuxostat) with placebo in preventing the progression of CKD. Briefly, in this study, 467 patients aged ≥ 20 years, in CKD stage 3 (eGFR 30–60 mL/min/1.73 m2) with asymptomatic hyperuricemia were eligible for enrollment. Moreover, participants were required to have a uric acid concentration between 7.0 and 10.0 mg/dL. The study patients were randomized to receive febuxostat 40 mg/day or placebo for up to 108 weeks. In this study, we included 426 patients whose baseline samples were available.
Measurement of Circulating TNFR Concentrations
Direct sandwich enzyme-linked immunosorbent assay was used for quantifying TNFR1 and TNFR2 (R&D systems, Minneapolis, Minnesota), as previously described (18, 19). To assess the effect of febuxostat, we measured serum TNFR levels using baseline and 12-week stored samples from 197 randomly selected patients. Moreover, the baseline TNFR levels were measured in 426 patients to examine whether they predicted renal function decline.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD) or as median (25th−75th percentile). Categorical variables were then assessed using the chi-square test. Moreover, baseline demographics and clinical characteristics were assessed according to the baseline TNFR level. The Wilcoxon rank-sum test was also applied for comparison between–two groups.
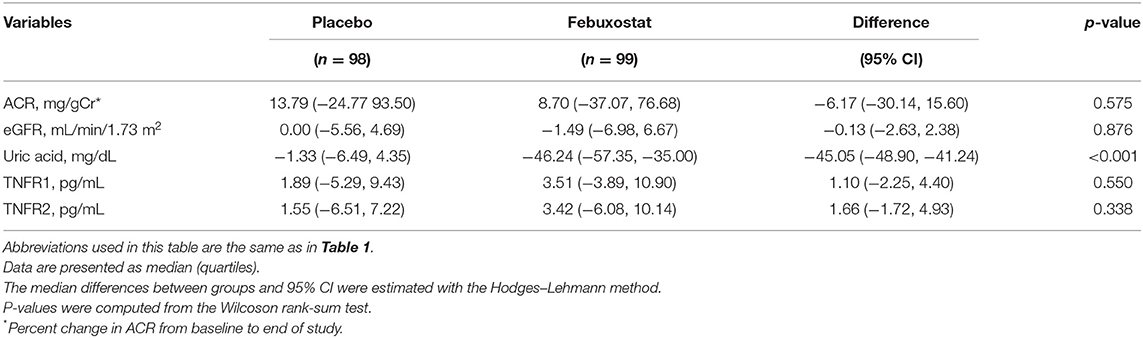
Percentages of change in uric acid, TNFR1, and TNFR2 over the intervention period from baseline to 12 weeks were assessed for the febuxostat and placebo groups. Moreover, we calculated percentages of change in the albumin to creatinine ratio (ACR) and eGFR from baseline to end of study. The effect of febuxostat compared to placebo on circulating ACR, eGFR, uric acid, and TNFR levels was determined by the Wilcoxon rank-sum test. The median differences between groups and 95% CIs were estimated with the Hodges–Lehmann method. Cumulative incidence of 30% eGFR decline was estimated using Kaplan–Meier curves. Univariate and multivariate Cox proportional hazard regression analyses were applied to analyze the association between baseline TNFR levels and ≥30% eGFR decline, with adjustments for age, sex, presence of diabetes, systolic blood pressure (BP), high sensitivity C-reactive protein (hsCRP), ACR, eGFR, uric acid, and febuxostat treatment at baseline. The slope of eGFR (mL/min/1.73 m2/year) was determined using a random coefficient model in which the regression coefficients (intercept and slope) were assumed to be a random effect. In this model, an unstructured covariance matrix was specified for random intercept and slope effects, and all measurements were used to compute regression slopes; i.e., at baseline, at 4, 8, and 12 weeks, and then every 12 weeks until week 108 or until discontinuation of treatment. Moreover, multivariate linear regression analysis was performed to evaluate the independent association between TNFR levels and the slope of eGFR change, with adjustments for age, sex, presence of diabetes, systolic BP, hsCRP, ACR, eGFR, uric acid, and febuxostat treatment at baseline.
Statistical analyses were performed with SAS software version 9.3 (SAS Institute, Cary, NC, USA). A P-value of <0.05 was considered to be statistically significant.
Results
Characteristics of the Study Patients According to the Baseline TNFR Level
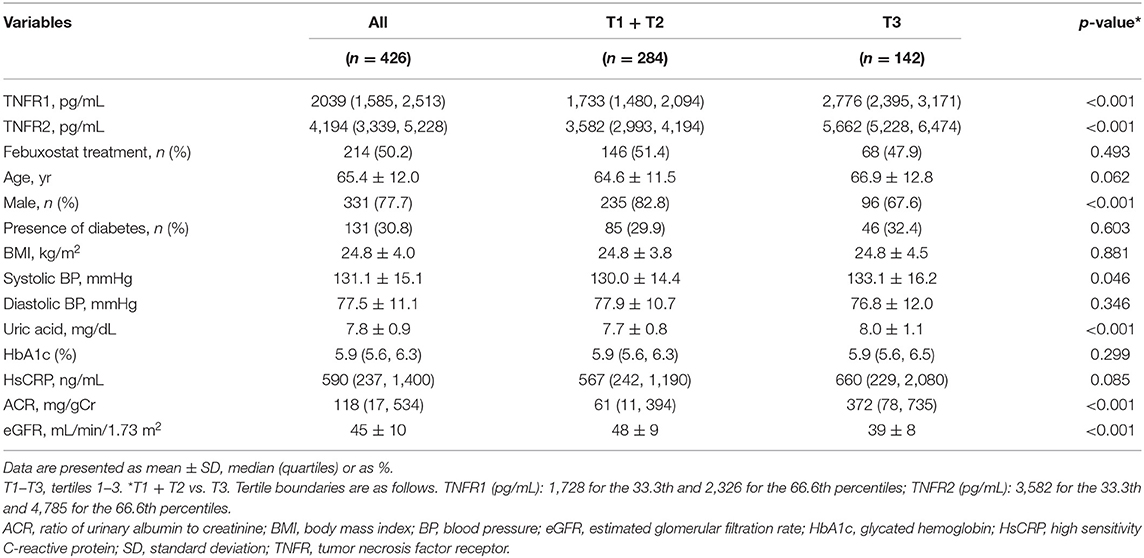
Table 1 shows the demographic and baseline characteristics. Compared with patients in the lowest tertile of TNFR2 level, those in the highest tertile of TNFR2 level comprised fewer men; had higher systolic BP, uric acid, and ACR; and had lower eGFR at baseline. However, age, body mass index (BMI), diastolic BP, glycated hemoglobin (HbA1c), hsCRP, presence of diabetes, and febuxostat treatment did not differ between both groups.
Effect of Febuxostat on Uric Acid, TNFR1, and TNFR2 Levels
The characteristics of the 197 randomly selected patients in the febuxostat and placebo groups are shown in Supplementary Table 1. Compared with patients on placebo treatment at week 12, those on febuxostat treatment had a more pronounced reduction in median uric acid level (−45.05, 95% CI −48.90 to −41.24 mg/dL) but had similar serum TNFR levels (Table 2).
Effect of TNFR Levels on Future eGFR Decline: Univariate and Multivariate Cox Proportional Hazards Regression Analyses
The mean eGFR slope significantly differed between patients with TNFR2 level in the highest tertile (−1.24 ± 2.21 mL/min/1.73 m2 per year) and those in the two lowest tertile (0.20 ± 1.91 mL/min/1.73 m2 per year) groups (difference, −1.43; 95% CI, −1.84 to −1.03; P < 0.001). A similar result was observed according to TNFR1 level (difference, −1.54; 95% CI, −1.95 to −1.14; P < 0.001) (Supplementary Table 2).
During the median follow-up period of 105 weeks, 12 (2.8%) and 30 (7.0%) patients developed the 40 and 30% eGFR decline, respectively. Therefore, we investigated the impact of circulating TNFRs on renal disease progression (i.e., 30% eGFR decline from baseline) using Cox proportional hazards regression model. In the univariate Cox regression model, high levels of TNFR2 and ACR at baseline significantly increased the incidence for 30% eGFR decline (Table 3). The cumulative incidence of 30% eGFR decline for the highest tertile of TNFRs steeply increased from start of observation (Figure 1). Overall, the risk of renal progression significantly increased in patients in the highest tertile of TNFR2 than in those in lowest two tertiles of TNFR2, after adjustment for age, sex, presence of diabetes, systolic BP, hsCRP, febuxostat treatment, ACR, and eGFR (HR 4.76, 95% CI 1.79–12.64, P = 0.002) (Table 3). Similar results were obtained when TNFR1 was included instead of TNFR2 in the model (Supplementary Table 3).
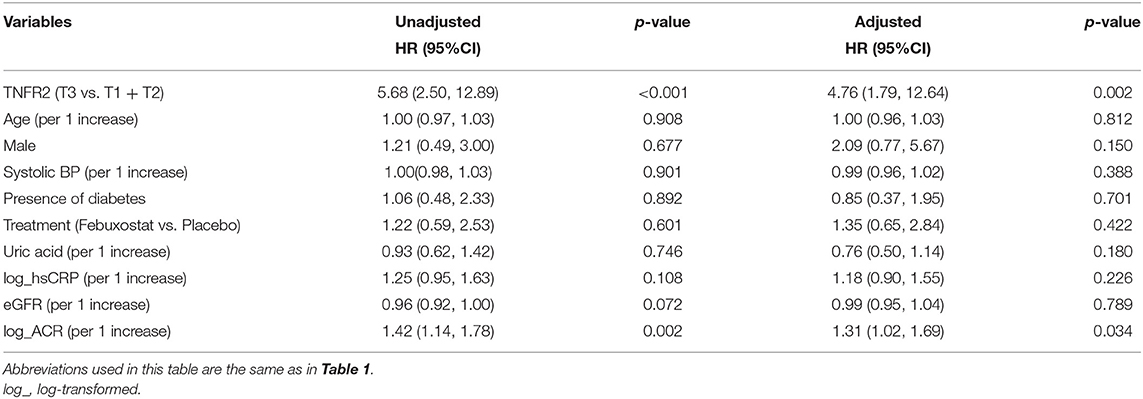
Table 3. Univariate and multivariate Cox regression analyses of the risk factors for 30% eGFR decline from baseline.
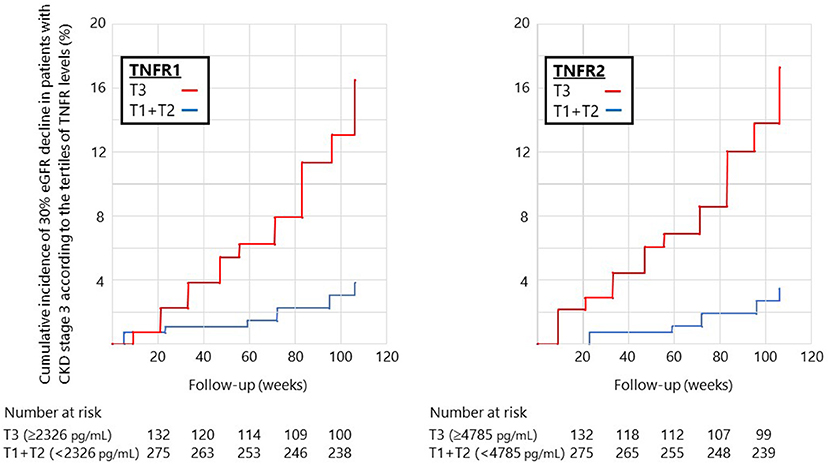
Figure 1. Cumulative incidence of 30% eGFR decline in patients with stage 3 CKD according to the tertiles of circulating TNFR levels at baseline. The cumulative incidence of 30% eGFR decline steeply increased at a constant rate from the start of observation for patients in the highest tertile of TNFR1 (left) or TNFR2 (right).
Next, multivariate regression model was used to examine the factors associated with the slope of eGFR rather than 30% eGFR decline. After adjusting all relevant variables in the multivariate regression model, baseline TNFR2, in addition to baseline eGFR and ACR, was significantly associated with the eGFR slope (Table 4). Moreover, similar results were obtained when TNFR1 was included rather than TNFR2 in the model (Supplementary Table 4).
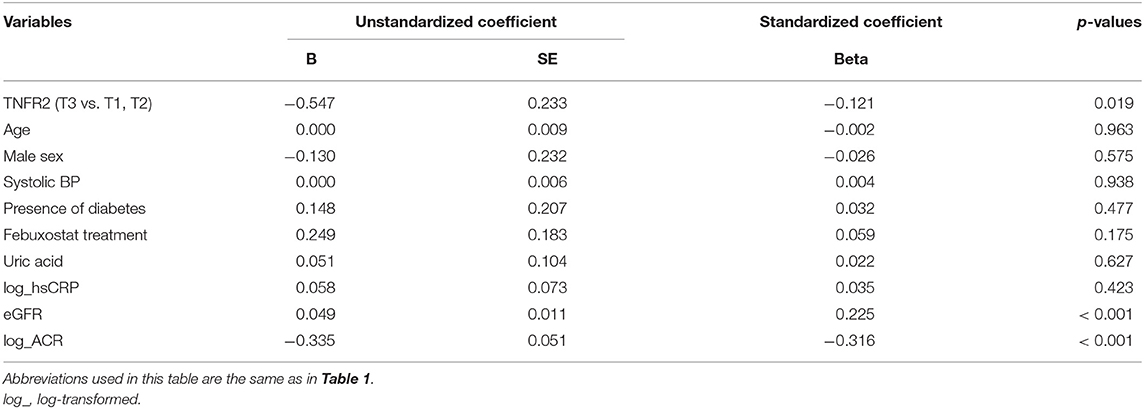
Table 4. Multivariate regression analysis of the factors associated with eGFR slope in the study patients.
Discussion
In this post-hoc analysis, we tested the hypothesis that treatment with the xanthine oxidase inhibitor (febuxostat) would lower serum TNFR levels, and early change in circulating TNFR levels predicts future eGFR decline in patients with CKD stage 3. Our hypothesis was derived from the subsequent results of previous clinical and experimental studies: (1) uric acid stimulated renal inherent cells, such as mesangial cell, tubular cell, and endothelial cells, to produce inflammatory cytokines (20–23); (2) xanthine oxidase inhibitor treatment decreased uric acid level, resulting in attenuating macrophage infiltration to the kidney in diabetic rat (24); and (3) there was positive correlation between serum uric acid and circulating TNFR levels in patients with IgA nephropathy and diabetes (16, 17). In this study, serum TNFR level was not altered after 12 weeks of febuxostat treatment, although the baseline circulating TNFR level predicted the risk for 30% eGFR decline and was associated with eGFR slope, even after adjustment for relevant factors, including baseline eGFR and ACR.
A number of studies have shown the association of uric acid with inflammation, oxidative stress, and endothelial dysfunction in experimental models (20, 24, 25). Recently, Zhou et al. (26) showed that, compared with healthy individuals, even young patients with asymptomatic hyperuricemia had significantly higher inflammation and oxidative stress. Chronic inflammation plays a critical role in the development and/or progression of CKD. In human kidney biopsy samples, independent of crystal deposition, hyperuricemia was shown to be associated with tubular atrophy and interstitial inflammation and fibrosis (27). In animal models of several kidney diseases, hyperuricemia was demonstrated to induce macrophage infiltration, which was alleviated by treatment with xanthine oxidase inhibitor (24, 28). Although several studies indicated that treatment with allopurinol attenuated the increase in intercellular adhesion molecule-1 (ICAM-1) levels after acute ischemic stroke or decreased hsCRP in asymptomatic hyperuricemia (29, 30), recent placebo-controlled RCTs demonstrated that treatment with allopurinol did not improve endothelial function, oxidative stress, and inflammation in patients with stage 3 CKD (31). We previously demonstrated that serum uric acid is negatively associated with eGFR and positively associated with TNF-related markers such as TNFα and TNFRs in patients with IgA nephropathy and type 2 diabetes (16, 17). To date, therapeutic agents that modulate TNFR levels remain to be completely elucidated; however, several studies have revealed that treatment with sodium glucose cotransporter (SGLT2) inhibitor or Janus kinase (JAK) 1/2 inhibitor may decrease serum TNFR levels in patients with early and advanced DKD, respectively, whereas renin–angiotensin system inhibitor treatment did not alter TNFR levels in patients with early DKD (15). In this study, there were no differences in the TNFR levels between the intervention and non-intervention group.
Many observational studies demonstrated that high levels of uric acid predicted eGFR decline in patients with diabetes, CKD, and the general population (1–3). However, the FEATHER study reported that urate-lowering therapy failed to prevent renal function decline in patients with hyperuricemia and stage 3 CKD (6). One of the limitations of this study is that the eGFR decline in patients who received placebo is less (−0.47 ± 4.48 mL/min/1.73 m2), making it difficult to confirm the effect on renal function. Therefore, if we restrict the study patients whose baseline TNFR levels are high (i.e., patients whose eGFR steeply declines), the urate-lowering therapy may prevent renal function decline. However, in this study, use of febuxostat fails to prove this hypothesis (Supplementary Table 5).
To date, a growing body of evidence, including our research, demonstrated that circulating TNFR levels predict future eGFR decline chiefly in patients with diabetes. Similar to previous studies, our study expanded the prognostication value of TNFR on the renal function decline in part in non-diabetic population (32, 33). It is important to consider whether elevated circulating TNFR levels only reflect impaired renal processing of these proteins. Indeed, these levels are elevated in patients with advanced renal failure or hemodialysis patients (12, 13, 34). We demonstrated that increased TNFR production of any tissue, including kidneys, partly contributes to the elevation of those levels considering the measurements of fractional excretion TNFR (35). On the other hand, Niewczas et al. (15) demonstrated that overproduction of TNFR occurs chiefly outside the kidneys and suggests that leukocytes are one plausible non-kidney source of TNFR from elegant studies (36, 37).
The limitations of the study should be acknowledged. First, it is not clear whether long-term febuxostat treatment would lower serum TNFR levels, although those levels did not change after a relatively short-term (12 weeks) treatment. Second, our multivariate model may not be appropriately adjusted because inflammatory markers other than hsCRP are not measured. Finally, we use 30% decline in eGFR from baseline as alternative end point to ESRD or 57% decline in eGFR from baseline (doubling of serum creatinine level). Therefore, this endpoint may not accurately reflect future renal function decline. Nevertheless, the strength of the study is that baseline TNFR levels are shown to predict eGFR reduction despite limited reduction in eGFR in the study period.
Conclusions
Elevated baseline TNFR levels were associated with eGFR decline; however, early change in circulating TNFR levels failed to predict future eGFR decline chiefly in non-diabetic CKD patients with asymptomatic hyperuricemia. Therefore, additional studies are required to identify treatments that modulate circulating TNFR levels.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Juntendo University Faculty of Medicine, Tokyo, Japan (No. H18-0204). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
TG and NY helped to design the study, oversee its execution, and write the manuscript. YO, IO, YSh, NI, SI, HH, KK, and TH helped to provide clinical data and write the manuscript. MM, YN, SN, SU, and YSu helped to interpret data. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by Grant-in-Aid for Scientific Research (C) Nos. 17K09711 and 20K08617 to TG.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all FEATHER investigators for recruitment of patients.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.634932/full#supplementary-material
Abbreviations
ACR, albumin to creatinine ratio; ANCOVA, analysis of covariance; BMI, body mass index; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; DKD, diabetic kidney disease; eGFR, estimated glomerular filtration rate; ELISA, enzyme-linked immunosorbent assay; FEATHER, Febuxostat vs. Placebo Randomized Controlled Trial Regarding Reduced Renal Function in Patients With Hyperuricemia Complicated by Chronic Kidney Disease Stage 3; HbA1c, glycated hemoglobin; hsCRP, high sensitivity C-reactive protein; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; JAK, janus kinase; NLRP3, NOD, LRR, and pyrin domain-containing protein 3; PERL, Preventing Early Renal Loss in Diabetes; RCT, randomized controlled trial; SD, standard deviation; SGLT, sodium glucose cotransporter; TNF, tumor necrosis factor; TNFR, TNF receptor.
References
1. Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. (2004) 44:642–50. doi: 10.1016/S0272-6386(04)00934-5
2. Tseng WC, Chen YT, Lin YP, Ou SM, Yang CY, Lin CH, et al. Hyperuricemia predicts an early decline in renal function among older people: a community-based cohort study. Sci Rep. (2019) 9:980. doi: 10.1038/s41598-018-37529-z
3. Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. (2010) 33:1337–43. doi: 10.2337/dc10-0227
4. Doria A, Galecki AT, Spino C, Pop-Busui R, Cherney DZ, Lingvay I, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. New Engl J Med. (2020) 382:2493–503. doi: 10.1056/NEJMoa1916624
5. Badve SV, Pascoe EM, Tiku A, Boudville N, Brown FG, Cass A, et al. Effects of allopurinol on the progression of chronic kidney disease. New Engl J Med. (2020) 382:2504–13. doi: 10.1056/NEJMoa1915833
6. Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. (2018) 72:798–810. doi: 10.1053/j.ajkd.2018.06.028
7. Lin TC, Hung LY, Chen YC, Lo WC, Lin CH, Tam KW, et al. Effects of febuxostat on renal function in patients with chronic kidney disease: a systematic review and meta-analysis. Medicine. (2019) 98:e16311. doi: 10.1097/md.0000000000016311
8. Kang DH. Hyperuricemia and progression of chronic kidney disease: role of phenotype transition of renal tubular and endothelial cells. Contrib Nephrol. (2018) 192:48–55. doi: 10.1159/000484278
9. Jung SW, Kim SM, Kim YG, Lee SH, Moon JY. Uric acid and inflammation in kidney disease. Am J Physiol Renal Physiol. (2020) 18:F1327–40. doi: 10.1152/ajprenal.00272.2019
10. Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. (2012) 23:516–24. doi: 10.1681/ASN.2011060628
11. Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. (2012) 23:507–15. doi: 10.1681/ASN.2011060627
12. Gohda T, Maruyama S, Kamei N, Yamaguchi S, Shibata T, Murakoshi M, et al. Circulating TNF receptors 1 and 2 predict mortality in patients with end-stage renal disease undergoing dialysis. Sci Rep. (2017) 7:43520. doi: 10.1038/srep43520
13. Neirynck N, Glorieux G, Schepers E, Verbeke F, Vanholder R. Soluble tumor necrosis factor receptor 1 and 2 predict outcomes in advanced chronic kidney disease: a prospective cohort study. PLoS ONE. (2015) 10:e0122073. doi: 10.1371/journal.pone.0122073
14. Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, et al. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. (2019) 62:1154–66. doi: 10.1007/s00125-019-4859-4
15. Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. (2019) 25:805–13. doi: 10.1038/s41591-019-0415-5
16. Sonoda Y, Gohda T, Suzuki Y, Omote K, Ishizaka M, Matsuoka J, et al. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PLoS ONE. (2015) 10:e0122212. doi: 10.1371/journal.pone.0122212
17. Kamei N, Yamashita M, Nishizaki Y, Yanagisawa N, Nojiri S, Tanaka K, et al. Association between circulating tumor necrosis factor-related biomarkers and estimated glomerular filtration rate in type 2 diabetes. Sci Rep. (2018) 8:15302. doi: 10.1038/s41598-018-33590-w
18. Murakoshi M, Gohda T, Sonoda Y, Suzuki H, Tomino Y, Horikoshi S, et al. Effect of tonsillectomy with steroid pulse therapy on circulating tumor necrosis factor receptors 1 and 2 in IgA nephropathy. Clin Exp Nephrol. (2017) 21:1068–74. doi: 10.1007/s10157-017-1408-7
19. Gohda T, Nishizaki Y, Murakoshi M, Nojiri S, Yanagisawa N, Shibata T, et al. Clinical predictive biomarkers for normoalbuminuric diabetic kidney disease. Diabetes Res Clin Pract. (2018) 141:62–8. doi: 10.1016/j.diabres.2018.04.026
20. Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. (2005) 16:3553–62. doi: 10.1681/ASN.2005050572
21. Liang WY, Zhu XY, Zhang JW, Feng XR, Wang YC, Liu ML. Uric acid promotes chemokine and adhesion molecule production in vascular endothelium via nuclear factor-kappa B signaling. Nutr Metab Cardiovasc Dis. (2015) 25:187–94. doi: 10.1016/j.numecd.2014.08.006
22. Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, et al. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS ONE. (2012) 7:e39738. doi: 10.1371/journal.pone.0039738
23. Xiao J, Fu C, Zhang X, Zhu D, Chen W, Lu Y, et al. Soluble monosodium urate, but not its crystal, induces toll like receptor 4-dependent immune activation in renal mesangial cells. Mol Immunol. (2015) 66:310–8. doi: 10.1016/j.molimm.2015.03.250
24. Kim SM, Lee SH, Kim YG, Kim SY, Seo JW, Choi YW, et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am J Physiol Renal Physiol. (2015) 308:F993–1003. doi: 10.1152/ajprenal.00637.2014
25. Lee HJ, Jeong KH, Kim YG, Moon JY, Lee SH, Ihm CG, et al. Febuxostat ameliorates diabetic renal injury in a streptozotocin-induced diabetic rat model. Am J Nephrol. (2014) 40:56–63. doi: 10.1159/000363421
26. Zhou Y, Zhao M, Pu Z, Xu G, Li X. Relationship between oxidative stress and inflammation in hyperuricemia: analysis based on asymptomatic young patients with primary hyperuricemia. Medicine. (2018) 97:e13108. doi: 10.1097/MD.0000000000013108
27. Myllymaki J, Honkanen T, Syrjanen J, Helin H, Rantala I, Pasternack A, et al. Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant. (2005) 20:89–95. doi: 10.1093/ndt/gfh584
28. Kamijo-Ikemori A, Sugaya T, Hibi C, Nakamura T, Murase T, Oikawa T, et al. Renoprotective effect of the xanthine oxidoreductase inhibitor topiroxostat on adenine-induced renal injury. Am J Physiol Renal Physiol. (2016) 310:F1366–76. doi: 10.1152/ajprenal.00517.2015
29. Muir SW, Harrow C, Dawson J, Lees KR, Weir CJ, Sattar N, et al. Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. (2008) 39:3303–7. doi: 10.1161/STROKEAHA.108.519793
30. Takir M, Kostek O, Ozkok A, Elcioglu OC, Bakan A, Erek A, et al. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Investig Med. (2015) 63:924–9. doi: 10.1097/JIM.0000000000000242
31. Jalal DI, Decker E, Perrenoud L, Nowak KL, Bispham N, Mehta T, et al. Vascular function and uric acid-lowering in stage 3 CKD. J Am Soc Nephrol. (2017) 28:943–52. doi: 10.1681/ASN.2016050521
32. Greenberg JH, Abraham AG, Xu Y, Schelling JR, Feldman HI, Sabbisetti VS, et al. Plasma biomarkers of tubular injury and inflammation are associated with CKD progression in children. J Am Soc Nephrol. (2020) 31:1067–77. doi: 10.1681/ASN.2019070723
33. Bhatraju PK, Zelnick LR, Shlipak M, Katz R, Kestenbaum B. Association of soluble TNFR-1 concentrations with long-term decline in kidney function: the multi-ethnic study of atherosclerosis. J Am Soc Nephrol. (2018) 29:2713–21. doi: 10.1681/ASN.2018070719
34. Mikami R, Mizutani K, Gohda T, Gotoh H, Matsuyama Y, Aoyama N, et al. Association between circulating tumor necrosis factor receptors and oral bacterium in patients receiving hemodialysis: a cross-sectional study. Clin Exp Nephrol. (2020) 25:58–65. doi: 10.1007/s10157-020-01952-2
35. Gohda T, Kamei N, Kubota M, Tanaka K, Yamashita Y, Sakuma H, et al. Fractional excretion of tumor necrosis factor receptor 1 and 2 in patients with type 2 diabetes and normal renal function. J Diabetes Investig. (2021) 12:382–9. doi: 10.1111/jdi.13351
36. Wheelock KM, Saulnier PJ, Tanamas SK, Vijayakumar P, Weil EJ, Looker HC, et al. White blood cell fractions correlate with lesions of diabetic kidney disease and predict loss of kidney function in Type 2 diabetes. Nephrol Dial Transplant. (2017) 32:2145. doi: 10.1093/ndt/gfx303
Keywords: TNF receptor, CKD, uric acid, xanthine oxidase inhibitor, eGFR
Citation: Gohda T, Yanagisawa N, Murakoshi M, Ueda S, Nishizaki Y, Nojiri S, Ohashi Y, Ohno I, Shibagaki Y, Imai N, Iimuro S, Kuwabara M, Hayakawa H, Kimura K, Hosoya T and Suzuki Y (2021) Association Between Kidney Function Decline and Baseline TNFR Levels or Change Ratio in TNFR by Febuxostat Chiefly in Non-diabetic CKD Patients With Asymptomatic Hyperuricemia. Front. Med. 8:634932. doi: 10.3389/fmed.2021.634932
Received: 29 November 2020; Accepted: 18 June 2021;
Published: 12 July 2021.
Edited by:
Michael L. Moritz, University of Pittsburgh, United StatesReviewed by:
Shunya Uchida, Teikyo University, JapanHoon Young Choi, Yonsei University, South Korea
Monika Niewczas, Joslin Diabetes Center and Harvard Medical School, United States
Copyright © 2021 Gohda, Yanagisawa, Murakoshi, Ueda, Nishizaki, Nojiri, Ohashi, Ohno, Shibagaki, Imai, Iimuro, Kuwabara, Hayakawa, Kimura, Hosoya and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomohito Gohda, Z29kYUBqdW50ZW5kby5hYy5qcA==
†These authors have contributed equally to this work
 Tomohito Gohda
Tomohito Gohda Naotake Yanagisawa2†
Naotake Yanagisawa2† Yuji Nishizaki
Yuji Nishizaki Shuko Nojiri
Shuko Nojiri Masanari Kuwabara
Masanari Kuwabara Yusuke Suzuki
Yusuke Suzuki