
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 25 May 2021
Sec. Nephrology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.632097
This article is part of the Research Topic Insights in Nephrology: 2021 View all 14 articles
 Ji Eun Kim1
Ji Eun Kim1 Sang Joon Park2
Sang Joon Park2 Yong Chul Kim3
Yong Chul Kim3 Sang-Il Min4
Sang-Il Min4 Jongwon Ha4
Jongwon Ha4 Yon Su Kim3
Yon Su Kim3 Soon Ho Yoon2,5*
Soon Ho Yoon2,5* Seung Seok Han3*
Seung Seok Han3*Background: Because obesity is associated with the risk of posttransplant diabetes mellitus (PTDM), the precise estimation of visceral fat mass before transplantation may be helpful. Herein, we addressed whether a deep-learning based volumetric fat quantification on pretransplant computed tomographic images predicted the risk of PTDM more precisely than body mass index (BMI).
Methods: We retrospectively included a total of 718 nondiabetic kidney recipients who underwent pretransplant abdominal computed tomography. The 2D (waist) and 3D (waist or abdominal) volumes of visceral, subcutaneous, and total fat masses were automatically quantified using the deep neural network. The predictability of the PTDM risk was estimated using a multivariate Cox model and compared among the fat parameters using the areas under the receiver operating characteristic curves (AUROCs).
Results: PTDM occurred in 179 patients (24.9%) during the median follow-up period of 5 years (interquartile range, 2.5–8.6 years). All the fat parameters predicted the risk of PTDM, but the visceral and total fat volumes from 2D and 3D evaluations had higher AUROC values than BMI did, and the best predictor of PTDM was the 3D abdominal visceral fat volumes [AUROC, 0.688 (0.636–0.741)]. The addition of the 3D abdominal VF volume to the model with clinical risk factors increased the predictability of PTDM, but BMI did not.
Conclusions: A deep-learning based quantification of visceral fat volumes on computed tomographic images better predicts the risk of PTDM after kidney transplantation than BMI.
Posttransplant diabetes mellitus (PTDM), a metabolic complication after kidney transplantation, occurs in 10–40% of kidney recipients depending on the patient characteristics (1–4). Because PTDM correlates with adverse outcomes such as cardiovascular events and death, it is crucial to predict PTDM precisely and manage its occurrence in advance (4–6). Several risk factors for PTDM have been identified, such as obesity (7, 8), high blood pressure (9), immunosuppressive agents (10–12), infection with hepatitis C virus (4, 13), hyperuricemia (13), and hypertriglyceridemia (13). High values of body mass index (BMI), one of the crude measures for body fat, predict the risk of DM (14), but this relationship has not necessarily happened in PTDM (4, 15–18).
BMI is a simple and convenient measure for adiposity but does not reflect body shape and fat distribution, which leads to inevitable limitations in the precise estimation of visceral fat (VF) volumes (19). Furthermore, the relationship with worse outcomes may depend on the race as Asians have a higher proportion of body fat mass for a given BMI than Caucasians (20). A bioelectrical impedance analysis, dual-energy X-ray absorptiometry, and cross-sectional computed tomography (CT) have been used to substitute BMI (21–25). Analyzing body components in cross-sectional CT imaging is regarded as a reference standard. However, its clinical use remains limited because the analysis requires a considerable amount of time and effort of specialists.
The introduction of a deep-learning algorithm in medicine attempts to change the paradigm of the clinical process (26, 27), particularly of diagnostic imaging (28). Deep learning algorithms have shown potential in automatic fat quantification on CT images and thus can reduce the laborious work involved in fat segmentation (29). Herein, we addressed whether deep-learning-based volumetric fat quantification on CT images after segmenting body fat distribution predicted the risk of PTDM more precisely than BMI.
The study was approved by the institutional review board of the Seoul National University Hospital (no. H-1907-072-1047) and complied with the Declaration of Helsinki. Among 1,377 adults (aged ≥ 18 years) who consecutively underwent kidney transplantation at Seoul National University Hospital between 2003 and 2017, 983 patients who underwent abdominal CT scans within 1 year before transplantation were initially reviewed. Of these, 38 patients in whom the CT scan did not sufficiently cover the abdominal waist from the iliac crest to the lower margin of the ribs and 227 patients who had DM before transplantation were excluded. Accordingly, 718 patients were analyzed in the present study. Under the review board's approval, informed consent was waived.
Baseline information such as age, sex, weight, height, type of pretransplant dialysis, donor type (living or deceased), ABO incompatibility, positivity for hepatitis B surface antigen and anti-hepatitis C virus antibody, the number of human leukocyte antigen mismatches, and the immunosuppressive regimens for induction (e.g., basiliximab and anti-thymocyte globulin) and maintenance (e.g., steroid, calcineurin inhibitor, and mycophenolic acid) were collected. A combination therapy of steroids, tacrolimus, and mycophenolic acid was primarily used for maintenance in our center. BMI was calculated as weight (kg)/height (m2). Laboratory findings such as total cholesterol, high-density lipoprotein cholesterol, triglyceride, and uric acid were collected in the fasting state before kidney transplantation. Low-density lipoprotein cholesterol was calculated using the following formula: total cholesterol – high-density lipoprotein cholesterol – (triglyceride/5).
The primary outcome was PTDM. PTDM was diagnosed when recipients needed antidiabetic medications because of high blood glucose levels. The secondary outcomes were delayed graft function (i.e., the requirement of dialysis within 7 days after transplantation) and biopsy-proven acute rejection such as acute T-cell-mediated and antibody-mediated rejections.
All abdominal CT scans were performed using multidetector CT scanners without the intravenous administration of contrast media. The mean interval between CT scanning and transplantation was 91.1 ± 54.5 days. After uploading precontrast volumetric abdominal CT images to commercially available segmentation software (MEDIP Deep Catch v1.0.0.0, MEDICALIP Co. Ltd., Seoul, Korea), a 3D U-Net automatically generated a volumetric mask of 7 compartments in <1.5 min with the recommended specifications (30): skin, bone, muscle, VF, subcutaneous fat (SF), internal organs with vessels, and central nervous system. The network was developed using 39,286 labeled whole-body CT images and provided an average segmentation accuracy for VF and SF of 92.4–98.9% and 94.1–99.7%, respectively, in internal and external validation datasets of whole-body CT scans. After the volumetric segmentation of VF and SF, the range of the whole abdominal waist was automatically extracted between the iliac crest and the margin of the lowest rib, with subsequent calculation of the 3D volumes of VF and SF in the whole abdominal or waist area and 2D volumes at the midpoint of the abdominal waist (31). An experienced body radiologist (SH Yoon) identified whether the results of segmentation and the range of the abdominal waist were appropriate. VF and SF volumes were summed to calculate total fat (TF) volumes. All of the TF, VF, and SF volumes were normalized by the height squared (m2) (32).
All statistical analyses were performed with the STATA (version 15.1; StataCorp, College Station, TX, USA) and R (version 3.5.0; R Core Team) software. Continuous variables are presented as the mean and standard deviation or median and interquartile ranges and compared by Student's t-test or the Wilcoxon rank-sum test, respectively. Categorical variables are presented as percentages and compared by the chi-squared test. Ordinary least-squares linear regression and fractional polynomial regression with continuous variables were used to determine a nonlinear relationship. Univariate and multivariable Cox regression models were applied to estimate the hazard ratio of the risks of outcomes. The Stata function mkspline was used to create a restricted cubic spline function to describe the hazard ratio of outcomes according to the fat parameters. The areas under the receiver operating characteristic curves (AUROCs) for predicting the risk of PTDM were compared between fat parameters using permutation tests (33, 34). The AUROCs for cumulative predictive probability depending on the follow-up duration were drawn using the survivalROC package in R. For the risk of delayed graft function, a multivariate logistic regression model was applied. A P-value of <0.05 was considered significant.
The mean age was 45.2 ± 12.6 years old, and 431 patients (60.0%) were male. A total of 81.8% of patients were treated with anti-hypertensive agents. A total of 65.5% of patients received transplants from living donors. The mean preoperative BMI was 22.5 ± 3.4 kg/m2. Other baseline characteristics of kidney recipients are shown in Table 1.
Figure 1 shows the schematic diagram to measure 2D waist, 3D waist, and 3D abdominal fat volumes using the deep neural network algorithm on 3D-reconstructed CT images. The mean values of 2D waist, 3D waist, and 3D abdominal TF volumes were 0.66 ± 0.41, 0.41 ± 0.36, and 2.08 ± 1.42 m3/m2, respectively. Although all the 2D and 3D fat volumes correlated with BMI (Table 2), their coefficients of determination (r2) in linear regression models were <0.6. When the nonlinear relationship was subsequently applied, a J-shaped relationship, but not a linear one, was shown between them (Figure 2).
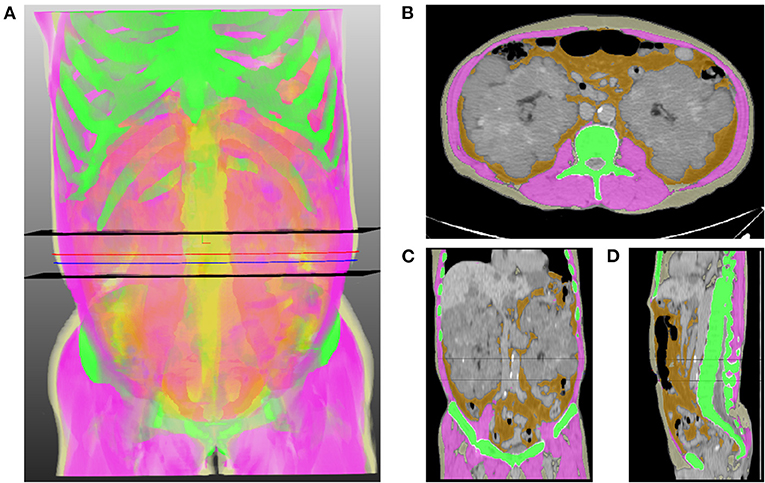
Figure 1. Representative image of the volumetric extraction of body compositions. (A) 3D translucent image shows a volumetric segmentation of muscle (pink), subcutaneous fat (light yellow), abdominal visceral fat (orange), and bone (green) using a deep neural network. Two black horizontal planes indicate the range of the abdominal waist between the lowest end of the rib cage and the uppermost end of the iliac crest. Blue and red lines indicate the levels of the umbilicus and the middle of the abdominal waist, respectively. Axial (B), coronal (C), and sagittal (D) images show the results of segmentation, which are overlaid on orthogonal cross-sectional images.
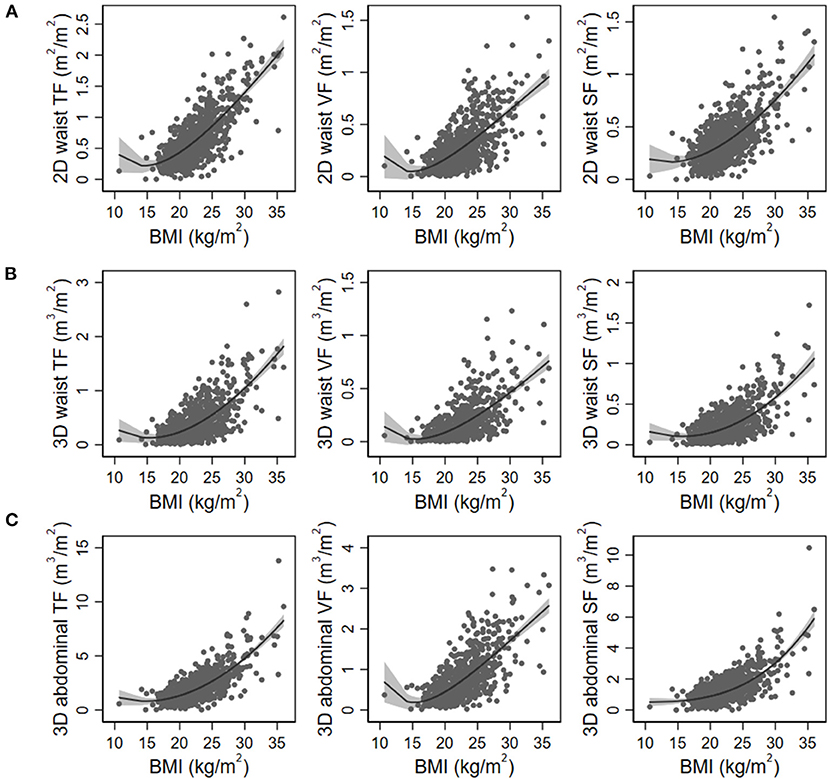
Figure 2. Nonlinear regression models of body mass index (BMI) with 2D waist (A), 3D waist (B), and 3D abdominal fat volumes (C). The gray area indicates 95% confidence intervals. TF, total fat; VF, visceral fat; SF, subcutaneous fat.
During the median follow-up duration of 5 years (2.5–8.6 years), PTDM occurred in 179 patients (24.9%). The prevalence of PTDM was 13.2 and 18.1% at 1 year and 3 years after transplantation, respectively. Among the baseline clinical variables, age, ABO incompatibility, induction regimens, and serum levels of high-density lipoprotein cholesterol were associated with the risk of PTDM (Supplementary Table 1). All 2D and 3D fat parameters and BMI were associated with the risk of PTDM irrespective of adjustment for multiple variables (Table 3). When a nonlinear relationship was applied, the relationship with the PTDM risk seemed to be more prominent in the VF and TF volumes compared with the SF volumes and BMI (Figure 3).
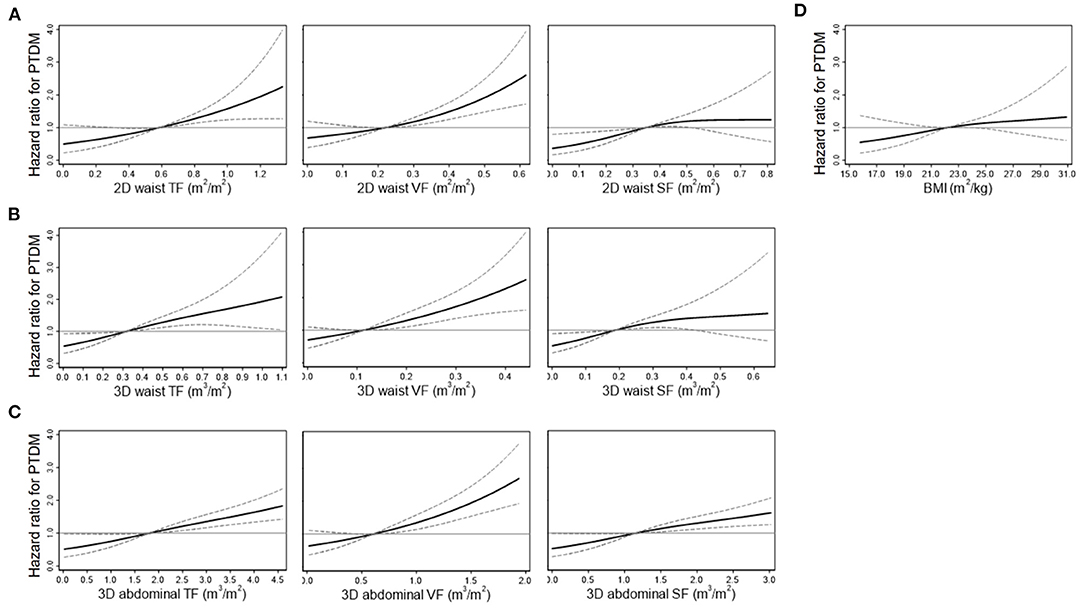
Figure 3. Restricted cubic spline curves for the risk of posttransplant diabetes mellitus (PTDM) according to the 2D waist (A), 3D waist (B), 3D abdominal fat volumes (C), and body mass index (BMI) (D). Curves were adjusted by multiple variables, such as age, sex, ABO incompatibility, anti-hepatitis C virus antibody, the induction agents, and serum levels of high-density lipoprotein cholesterol. Solid and dashed lines indicate hazard ratios and 95% confidence intervals, respectively. TF, total fat; VF, visceral fat; SF, subcutaneous fat.
The AUROCs for predicting the 3-year risk of PTDM were higher in VF and TF volumes from 2D and 3D quantification than in BMI (Table 4). The highest value was identified in 3D abdominal VF volumes. The corresponding curves support these results (Supplementary Figure 1). When the cumulative AUROCs were evaluated, VF volumes had higher values than BMI irrespective of the follow-up period (Supplementary Figure 2). We evaluated whether the addition of fat parameters to the risk model with other clinical factors, which had P < 0.05 in Supplementary Table 1, increased the overall predictability for the 3-year PTDM. The 3D abdominal VF volumes elevated the predictability of the model when added (P = 0.015), but BMI did not (P = 0.206). The corresponding ROC curves support these results (Figure 4).
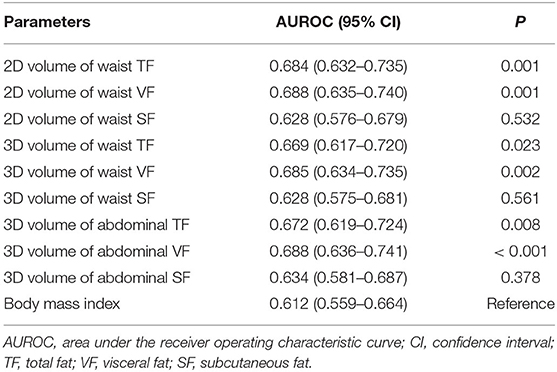
Table 4. Area under the receiver operating characteristic curves of fat parameters in predicting 3-year posttransplant diabetes mellitus.
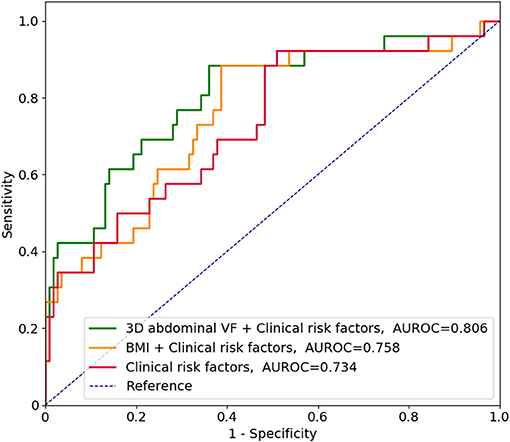
Figure 4. Receiver operating characteristic curves in predicting the 3-year risk of posttransplant diabetes mellitus along with clinical risk factors. According to Supplementary Table 1, clinical risk factors included age, ABO incompatibility, induction regimens, and serum levels of high-density lipoprotein cholesterol. VF, visceral fat; AUROC, area under the receiver operating characteristic curve; BMI, body mass index.
Because high fat volumes may confer the risk of rejection and delayed graft function according to previous studies (35, 36), other risks such as delayed graft function and rejections were assessed. For delayed graft function, the 3D volumes of abdominal VF and TF were predictors with odds ratios of 2.08 (1.12–3.87) and 1.33 (1.01–1.75) per 1-unit increase, respectively, but other fat parameters, including BMI, were not (Supplementary Table 2). None of the fat parameters were associated with the risk of rejections in the present cohort (Supplementary Table 3).
The present study used the deep learning algorithm to quantify the 2D and 3D fat volumes in pretransplant CT images and identified that their relationship with BMI was not linear. Although all the fat parameters were associated with the risk of PTDM, the predictability was greater in VF volumes than in BMI. The addition of 3D abdominal VF volume to the model with clinical risk factors increased the predictability of PTDM, but BMI did not. The present results indicate that precise quantification of fat volumes by deep learning algorithm may help to alert clinicians of the risk of PTDM.
Precise measurement of fat components is a critical issue in classifying risky patients based on obesity-related outcomes. BMI, which is based on weight and height, is a commonly used method to measure fat mass, but it does not take into account other body compositions such as muscle and bone. BMI seems to be an insufficient marker to assess PTDM based on inconsistent research results (16–18). VF components have been revealed as a risk factor for metabolic and cardiovascular diseases in the general population, independent of BMI (37). VF was related to glucose intolerance in kidney recipients (38). Based on both the previous and present results, the estimation of VF volumes is needed to predict the risk of PTDM more precisely than BMI.
Abdominal imaging methods, including CT, have been used to assess the volumes of fat components using computer calculator more than before (9, 39–41). This method has been validated in several studies, but optimization is needed to reduce bias and the time consumed by the task (42–44). The present study applied a deep learning algorithm to automatically segment the VF and SF components and exclude muscle and bone, which eventually detected the fat volumes quickly and unbiasedly for a number of images. Because kidney transplant recipients undergo abdominal CT scans for routine preoperative work-up, our approach using readily available software is implementable for more accurate prediction of PTDM than BMI, which may help in designing a plan to prevent PTDM occurrence.
Despite the valuable findings of our study, there are some limitations that need to be addressed. Waist circumference, a useful method for fat volume, was not evaluated. Follow-up CT images may be helpful to predict the risk of PTDM, but the present study could not obtain these data. Other unidentified factors, such as diet and exercise information, could have an interacting effect on the relationships observed in the study. Only Korean patients were analyzed, and no other populations were analyzed. Nevertheless, the primary purpose of the study was to address the application of the deep-learning-algorithm-based quantification of 2D and 3D fat volumes in kidney recipients, not to build a final model. A prospective application and adjustment of our algorithm to other cohorts is warranted in future studies.
Quantification of VF components with a deep learning algorithm successfully predicts PTDM, which is better than the measurement of BMI. Deep-learning-based approaches are increasingly used in many clinical aspects, and the present results will be a basis for application in the transplant field.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Seoul National University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JK: data analysis and interpretation and manuscript drafting. SP: technical support. YCK, S-IM, and JH: data collection. YSK: technical support and supervision. SY and SH: project development, data interpretation, supervision, and manuscript editing. All authors contributed to the article and approved the submitted version.
SP is the CEO of MEDICALIP Co. Ltd., Seoul, Korea.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.632097/full#supplementary-material
1. Baron PW, Infante S, Peters R, Tilahun J, Weissman J, Delgado L, et al. Post-transplant diabetes mellitus after kidney transplant in hispanics and caucasians treated with tacrolimus-based immunosuppression. Ann Transplant. (2017) 22:309–14. doi: 10.12659/AOT.903079
2. Terjimanian MN, Underwood PW, Cron DC, Augustine JJ, Noon KA, Cote DR, et al. Morphometric age and survival following kidney transplantation. Clin Transplant. (2017) 31:e13066. doi: 10.1111/ctr.13066
3. David-Neto E, Lemos FC, Fadel LM, Agena F, Sato MY, Coccuza C, et al. The dynamics of glucose metabolism under calcineurin inhibitors in the first year after renal transplantation in nonobese patients. Transplantation. (2007) 84:50–5. doi: 10.1097/01.tp.0000267647.03550.22
4. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. (2003) 3:178–85. doi: 10.1034/j.1600-6143.2003.00010.x
5. Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. (2006) 69:588–95. doi: 10.1038/sj.ki.5000116
6. Ducloux D, Kazory A, Chalopin JM. Posttransplant diabetes mellitus and atherosclerotic events in renal transplant recipients: a prospective study. Transplantation. (2005) 79:438–43. doi: 10.1097/01.TP.0000151799.98612.EB
7. Chang S, Jiang J. Association of body mass index and the risk of new-onset diabetes after kidney transplantation: a meta-analysis. Transplant Proc. (2018) 50:1316–25. doi: 10.1016/j.transproceed.2018.02.075
8. Parikh CR, Klem P, Wong C, Yalavarthy R, Chan L. Obesity as an independent predictor of posttransplant diabetes mellitus. Transplant Proc. (2003) 35:2922–6. doi: 10.1016/j.transproceed.2003.10.074
9. Cron DC, Noon KA, Cote DR, Terjimanian MN, Augustine JJ, Wang SC, et al. Using analytic morphomics to describe body composition associated with post-kidney transplantation diabetes mellitus. Clin Transplant. (2017) 31:e13040. doi: 10.1111/ctr.13040
10. Ciancio G, Burke GW, Gaynor JJ, Ruiz P, Roth D, Kupin W, et al. A randomized long-term trial of tacrolimus/sirolimus versus tacrolimums/mycophenolate versus cyclosporine/sirolimus in renal transplantation: three-year analysis. Transplantation. (2006) 81:845–52. doi: 10.1097/01.tp.0000203894.53714.27
11. Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the symphony study. Nephrol Dial Transplant. (2010) 25:2004–10. doi: 10.1093/ndt/gfp778
12. Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States multicenter FK506 kidney transplant group. Transplantation. (1996) 62:900–5. doi: 10.1097/00007890-199610150-00005
13. Sharif A, Cohney S. Post-transplantation diabetes-state of the art. Lancet Diabetes Endocrinol. (2016) 4:337–49. doi: 10.1016/S2213-8587(15)00387-3
14. Hanson RL, Narayan KM, McCance DR, Pettitt DJ, Jacobsson LT, Bennett PH, et al. Rate of weight gain, weight fluctuation, and incidence of NIDDM. Diabetes. (1995) 44:261–6. doi: 10.2337/diab.44.3.261
15. Numakura K, Satoh S, Tsuchiya N, Horikawa Y, Inoue T, Kakinuma H, et al. Clinical and genetic risk factors for posttransplant diabetes mellitus in adult renal transplant recipients treated with tacrolimus. Transplantation. (2005) 80:1419–24. doi: 10.1097/01.tp.0000181142.82649.e3
16. Sumrani NB, Delaney V, Ding ZK, Davis R, Daskalakis P, Friedman EA, et al. Diabetes mellitus after renal transplantation in the cyclosporine era–an analysis of risk factors. Transplantation. (1991) 51:343–7. doi: 10.1097/00007890-199102000-00014
17. Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. (2002) 25:583–92. doi: 10.2337/diacare.25.3.583
18. Gourishankar S, Jhangri GS, Tonelli M, Wales LH, Cockfield SM. Development of diabetes mellitus following kidney transplantation: a Canadian experience. Am J Transplant. (2004) 4:1876–82. doi: 10.1111/j.1600-6143.2004.00591.x
19. Zafon C. Oscillations in total body fat content through life: an evolutionary perspective. Obes Rev. (2007) 8:525–30. doi: 10.1111/j.1467-789X.2007.00377.x
20. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. (2002) 3:141–6. doi: 10.1046/j.1467-789X.2002.00065.x
21. Browning LM, Mugridge O, Chatfield MD, Dixon AK, Aitken SW, Joubert I, et al. Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obesity. (2010) 18:2385–91. doi: 10.1038/oby.2010.71
22. Ryo M, Maeda K, Onda T, Katashima M, Okumiya A, Nishida M, et al. A new simple method for the measurement of visceral fat accumulation by bioelectrical impedance. Diabetes Care. (2005) 28:451–3. doi: 10.2337/diacare.28.2.451
23. Kim SK, Kim HJ, Hur KY, Choi SH, Ahn CW, Lim SK, et al. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. (2004) 79:593–9. doi: 10.1093/ajcn/79.4.593
24. Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol. (2012) 85:e826–30. doi: 10.1259/bjr/57987644
25. Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. (1982) 36:172–7. doi: 10.1093/ajcn/36.1.172
26. Patel VL, Shortliffe EH, Stefanelli M, Szolovits P, Berthold MR, Bellazzi R, et al. The coming of age of artificial intelligence in medicine. Artif Intell Med. (2009) 46:5–17. doi: 10.1016/j.artmed.2008.07.017
27. Darcy AM, Louie AK, Roberts LW. Machine learning and the profession of medicine. JAMA. (2016) 315:551–2. doi: 10.1001/jama.2015.18421
28. Jha S, Topol EJ. Adapting to artificial intelligence: radiologists and pathologists as information specialists. JAMA. (2016) 316:2353–4. doi: 10.1001/jama.2016.17438
29. Weston AD, Korfiatis P, Kline TL, Philbrick KA, Kostandy P, Sakinis T, et al. Automated abdominal segmentation of CT scans for body composition analysis using deep learning. Radiology. (2019) 290:669–79. doi: 10.1148/radiol.2018181432
30. MEDICAL IP. (2018). Available online at: http://www.medicalip.com/sub.php?p=deepcatch (accessed June 18, 2020).
31. Lean ME, Han TS, Deurenberg P. Predicting body composition by densitometry from simple anthropometric measurements. Am J Clin Nutr. (1996) 63:4–14. doi: 10.1093/ajcn/63.1.4
32. Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in caucasians aged 18-98 y. Int J Obes Relat Metab Disord. (2002) 26:953–60. doi: 10.1038/sj.ijo.0802037
33. Bandos AI, Rockette HE, Gur D. A permutation test sensitive to differences in areas for comparing ROC curves from a paired design. Stat Med. (2005) 24:2873–93. doi: 10.1002/sim.2149
34. Bandos AI, Rockette HE, Gur D. A permutation test for comparing ROC curves in multireader studies a multi-reader ROC, permutation test. Acad Radiol. (2006) 13:414–20. doi: 10.1016/j.acra.2005.12.012
35. Kwan JM, Hajjiri Z, Metwally A, Finn PW, Perkins DL. Effect of the obesity epidemic on kidney transplantation: obesity is independent of diabetes as a risk factor for adverse renal transplant outcomes. PLoS ONE. (2016) 11:e0165712. doi: 10.1371/journal.pone.0165712
36. Molnar MZ, Kovesdy CP, Mucsi I, Bunnapradist S, Streja E, Krishnan M, et al. Higher recipient body mass index is associated with post-transplant delayed kidney graft function. Kidney Int. (2011) 80:218–24. doi: 10.1038/ki.2011.114
37. Zhang M, Hu T, Zhang S, Zhou L. Associations of different adipose tissue depots with insulin resistance: a systematic review and meta-analysis of observational studies. Sci Rep. (2015) 5:18495. doi: 10.1038/srep18495
38. von During ME, Jenssen T, Bollerslev J, Asberg A, Godang K, Eide IA, et al. Visceral fat is better related to impaired glucose metabolism than body mass index after kidney transplantation. Transpl Int. (2015) 28:1162–71. doi: 10.1111/tri.12606
39. Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. (2012) 256:255–61. doi: 10.1097/SLA.0b013e31826028b1
40. Krishnamurthy V, Zhang P, Ethiraj S, Enchakalody B, Waljee AK, Wang L, et al. Use of analytic morphomics of liver, spleen, and body composition to identify patients at risk for cirrhosis. Clin Gastroenterol Hepatol. (2015) 13:360–8 e5. doi: 10.1016/j.cgh.2014.07.042
41. Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. (2001) 50:425–35. doi: 10.1053/meta.2001.21693
42. Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. (1986) 250(6 Pt 1):E736–45. doi: 10.1152/ajpendo.1986.250.6.E736
43. Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes. (2007) 31:500–6. doi: 10.1038/sj.ijo.0803454
Keywords: artificial intelligence, body mass index, fat, deep learning, kidney transplantation, post-transplant diabetes mellitus
Citation: Kim JE, Park SJ, Kim YC, Min S-I, Ha J, Kim YS, Yoon SH and Han SS (2021) Deep Learning-Based Quantification of Visceral Fat Volumes Predicts Posttransplant Diabetes Mellitus in Kidney Transplant Recipients. Front. Med. 8:632097. doi: 10.3389/fmed.2021.632097
Received: 22 November 2020; Accepted: 30 April 2021;
Published: 25 May 2021.
Edited by:
Marian Klinger, Opole University, PolandReviewed by:
Hoon Young Choi, Yonsei University, South KoreaCopyright © 2021 Kim, Park, Kim, Min, Ha, Kim, Yoon and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung Seok Han, aGFuc3dheTgwQGdtYWlsLmNvbQ==; Soon Ho Yoon, eXNob2thQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.