- 1Dipartimento di Informatica, Università degli Studi di Milano, Milan, Italy
- 2Unit of Fetal Medicine and Prenatal Diagnosis, Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy
- 3Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Italy
- 4Department of Obstetrics and Gynecology and Center on Human Development and Disability (CHDD), School of Medicine, University of Washington, Seattle, WA, United States
During labor, uterine contractions trigger the response of the autonomic nervous system (ANS) of the fetus, producing sawtooth-like decelerations in the fetal heart rate (FHR) series. Under chronic hypoxia, ANS is known to regulate FHR differently with respect to healthy fetuses. In this study, we hypothesized that such different ANS regulation might also lead to a change in the FHR deceleration morphology. The hypothesis was tested in an animal model comprising nine normoxic and five chronically hypoxic fetuses that underwent a protocol of umbilical cord occlusions (UCOs). Deceleration morphologies in the fetal inter-beat time interval (FRR) series were modeled using a trapezoid with four parameters, i.e., baseline b, deceleration depth a, UCO response time τu and recovery time τr. Comparing normoxic and hypoxic sheep, we found a clear difference for τu (24.8±9.4 vs. 39.8±9.7 s; p < 0.05), a (268.1±109.5 vs. 373.0±46.0 ms; p < 0.1) and Δτ = τu − τr (13.2±6.9 vs. 23.9±7.5 s; p < 0.05). Therefore, the animal model supported the hypothesis that hypoxic fetuses have a longer response time τu and larger asymmetry Δτ as a response to UCOs. Assessing these morphological parameters during labor is challenging due to non-stationarity, phase desynchronization and noise. For this reason, in the second part of the study, we quantified whether acceleration capacity (AC), deceleration capacity (DC), and deceleration reserve (DR), computed through Phase-Rectified Signal Averaging (PRSA, known to be robust to noise), were correlated with the morphological parameters. DC, AC and DR were correlated with τu, τr and Δτ for a wide range of the PRSA parameter T (Pearson's correlation ρ > 0.8, p < 0.05). In conclusion, deceleration morphologies have been found to differ between normoxic and hypoxic sheep fetuses during UCOs. The same difference can be assessed through PRSA based parameters, further motivating future investigations on the translational potential of this methodology on human data.
1. Introduction
During labor, a fetus might suffer considerable stress due to uterine contractions, causing transient oxygen reduction and head compression, resulting in vagal and sympathetic stimulations. Nutrient deprivation, hypoxemia, hypoxia, acidemia and cardiovascular decompensation directly impact the autonomic nervous system (ANS) and thus affect the fetal heart rate variability (FHRV) (1, 2). The cardiotocography (CTG) remains the best available proxy of ANS' functional state through the analysis of fetal heart rate (FHR) and its variability. Considering that the standard processing of CTG series has been found poorly correlated to the relevant clinical outcomes, such as fetal brain injury or death, new FHR biomarkers are needed to better quantify the risk of fetal morbidity and mortality during labor (3, 4).
Phase-Rectified Signal Averaging (PRSA) analysis extracts quasi-periodic oscillations from HRV series and it is more resistant to non-stationarities, signal loss and artifacts (5) than conventional HRV analysis techniques, such as the well-known spectral analysis. It provides two measures that quantify the average cardiac acceleration (AC) and deceleration (DC) capacities from an inter-beat time interval series (RR). Practically, these measures quantify the average RR increase (or decrease) in milliseconds. When quantified on CTG signals or fetal RR series (FRR), AC and DC seem to perform better than the short term variation of FHR in identifying fetal growth restricted fetuses (6–8) and adverse outcome (9). In a study of fetal sheep exposed to repetitive umbilical cord occlusions (UCOs), a model of uterine contractions during labor, we found that there was a high correlation between AC and DC and acid/base balance (10); particularly, AC and DC progressively increased with the severity of the UCOs, suggesting an activation of ANS of healthy normoxic fetus exposed to acute hypoxemia.
In the same animal model, we recently observed that, at the beginning of each UCO, FRR adapted by a progressive increase (reduction in FHR) and quickly recovered when pressure was released. In order to quantify such adaptations, we modeled the FRR deceleration using a first-order exponential model, one of the possible models typically employed for system modeling tasks (11), for both response and recovery phases (10). These models were characterized by time constants, describing the speed of FRR adaptation (the larger the time constant, the slower the adaptation) and we found that healthy normoxic fetuses had longer UCO response times than the time necessary to return to the baseline level (10), suggesting the presence of asymmetric trends in the series during labor.
Motivated by this observation, we also proved that dissimilarities in AC and DC values arise when asymmetric increasing/decreasing trends appear in the series (12), which seem to occur during labor. We thus introduced the deceleration reserve (DR), a new PRSA-based metric for the quantification of such asymmetry (12). The DR is computed as the difference between DC and AC. Up to date, DR was tested on a near-term pregnant sheep model and human CTG recordings, obtaining promising results for distinguishing between normoxic and chronically hypoxic fetuses, and to detect fetal acidemia at birth. Even though PRSA processes the FRR series in its whole entirety, it is reasonable to hypothesize that AC, DC and DR are deeply linked with the FRR adaptation time due to uterine contractions during labor.
In this study, we hypothesized that the different ANS regulation under chronic hypoxia might also lead to a change in the FHR deceleration morphology, as a result of the uterine contraction, and that the adaptation times would be different from those of healthy fetuses. The hypothesis was tested in an animal model comprising nine normoxic and five chronically hypoxic fetuses that underwent a protocol of UCOs. Deceleration morphologies on the FRR series were modeled using a trapezoid with four parameters characterizing the adaptation times, baseline and deceleration depth. The parameters were compared between the two groups. We also quantified their correlation with biomarkers of acid/base balance. Assessing these morphological parameters during labor is challenging due to non-stationarity, phase desynchronization and noise. For this reason, in the second part of the study, we quantified whether AC, DC and DR were correlated with the morphological parameters. Given the fact that PRSA is more robust with respect to phase-desynchronization, a correlation might further support the opportunity of using AC, DC and DR in the clinical settings. In addition, given the link between the deceleration morphology and the area under the deceleration, i.e., the so-called “Deceleration Area” (DA), we quantified the relationship between PRSA-based parameters and DA.
2. Materials and Methods
2.1. Animal Model and FHR Data
An established pregnant sheep model of labor was retrospectively analyzed. A comprehensive review on the pregnant sheep model and its translational significance for human physiology, in particular for studies of the ANS, can be found in Morrison et al. (13). The animal cohort comprised of nine normoxic and five spontaneously chronically hypoxic near-term pregnant sheep fetuses which underwent periodic UCOs mimicking uterine contractions during labor.
The animal and experimental models were described elsewhere (14). The animal study was reviewed and approved by University of Western Ontario Council on Animal Care/Canadian Council on Animal Care. Animal care followed the guidelines of the Canadian Council on Animal Care and was approved by the University of Western Ontario Council on Animal Care. Briefly, sheep fetuses were monitored over a 6 h period during which a mechanical stimulation was applied to the umbilical cord by using an inflatable silicon rubber cuff. A baseline period of approximately 1 h with no occlusion preceded the study. After that, UCOs were delivered every 2.5 min and lasted for 1 min. Three levels of occlusion strength, from partial to complete, were performed: mild (MILD, 60 min), moderate (MODERATE, 60 min) and complete (SEVERE, 60 min or until pH < 7.00 was reached). The stimulation protocol ended with a recovery period. During the stimulation protocol, pH, base deficit (BE) and lactate (hereafter referred to as “biomarkers”) were quantified by means of fetal arterial blood samples collected every 20 min.
Sheep fetuses were categorized as chronically hypoxic if O2Sat < 55%, as measured before the beginning of the UCO stimulation protocol. In this study, we refer to the two models as “normoxic” and “chronically hypoxic,” respectively. As per experimental protocol, both models showed a progressive worsening acidemia of the hypoxic status until pH < 7.00 was reached [see Figure 2 in (12)].
Fetal ECGs were collected by means of electrodes implanted into the left supra-scapular muscles, in the muscles of the right shoulder and in the cartilage of the sternum, and digitized at 1,000 Hz. FRR series were automatically extracted from the fetal ECG (15).
In this study, we only considered the SEVERE phase of UCOs since FHR mostly changed during this condition.
2.2. FHR Series Preprocessing
A preprocessing similar to the one proposed in Rivolta et al. (12) was adopted for both datasets. Briefly, FRR series were analyzed to determine whether they were suitable for further analysis in terms of noise level, by excluding those recordings with more than 10% gaps during the SEVERE phases. Two normoxic fetuses were excluded from the analysis because of the high amount of missing beats. Furthermore, FRR intervals greater than 1,500 ms (40 bpm) were labeled as artifacts and substituted with an equivalent number of beats (calculated dividing the length of each artifact by the median of the 20 nearby FRR samples). The reconstructed samples were used neither in the model fitting nor as anchor points in the PRSA analysis (in this latter case, however, they contributed to the selection of nearby anchor points).
2.3. Geometrical Model of Deceleration Morphology and Its Fitting to FHR Data
In this analysis, we quantified the average characteristics of the FHR response to UCOs in terms of baseline level, deceleration depth, time necessary to reach a steady condition of the FRR during both UCO stimulation and resting phase in the normoxic and hypoxic datasets. To do so, we used a simple model to describe the time evolution of FRR during each cycle of UCO and rest and extract the relevant information. The procedure took two steps. First, we time-aligned all the FRR segments of 150 s starting from the beginning of each UCO. Second, a piecewise linear model was fitted using a semi-automatic approach based on least squares. The model was as follows during UCO stimulation
and the following one for the resting phase
where t was the time (seconds), b (milliseconds), a (milliseconds), τr (seconds), and τu (seconds) were the morphological parameters to be estimated. In particular, b was the baseline FRR value in absence of UCOs, τu the time to reach the steady condition during UCO, a is the amplitude change of FRR, and τr the time to reach the baseline b after releasing the UCO. In addition, the difference Δτ = τu − τr was considered as measure of asymmetry to the response to UCO stimulation. Figure 1 shows an example of model fitting and a visual description of the morphological parameters.
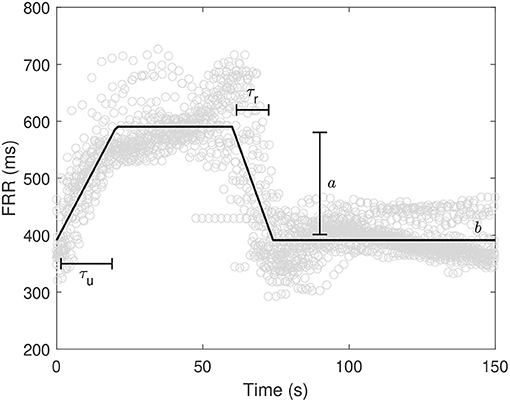
Figure 1. Example of model fitting along with variable definition. ° refers to FRR values from the beginning of the UCOs.
2.4. Correlation of Deceleration Morphology With Time and Biomarkers
In the second analysis, we determined whether the time intervals τu, τr and Δτ changed over time and were correlated with pH, base deficit and level of lactates, along the entire SEVERE phase. According to the stimulation protocol, blood samples were collected every 20 min up to the termination of the study. The same morphological parameters of Equations (1) and (2) were therefore estimated on all 20 min windows preceding each blood sample. Two correlation analyses were thus performed. First, we computed the correlation coefficients between τu, τr and Δτ, and blood sample time points. Second, we determined the correlation between τu, τr and Δτ with the biomarkers (pH, BE and lactate). To compensate for the fact that biomarkers' values changed over time according to the stimulation protocol, partial correlation coefficients were computed, by accounting for the progress of time.
2.5. PRSA, AC, DC, DR, and DA
A complete description of the PRSA algorithm can be found in Bauer et al. (5) and Rivolta et al. (12). The algorithm is divided into two steps. First, anchor points are identified on the time series x[k]. Each time index k that satisfies the condition
is inserted in the DC anchors' point list (for AC, the inequality sign must be flipped). The parameter T refers to the number of FRR intervals involved in the identification of anchor points. Second, all the windows of 2L elements centered on each anchor point are aligned (anchor points are located at the L + 1 sample) and then averaged. The parameter L defines the slowest FRR oscillation detectable by the algorithm (5). Such series of 2L averaged elements is the PRSA series.
From the PRSA series, AC and DC are then derived with
DR is instead defined as the sum of DC and AC (note that AC is a negative quantity for RR series) (12).
We quantified AC, DC and DR for multiple values of T (s = T and L = 50). A correlation analysis was performed to assess which range of T mostly correlated with the time constants derived from the piecewise linear models. In particular, we computed the correlation between τu and DC, τr and AC, and Δτ and DR while varying the T value between 1 and 50.
Given the fact that: i) AC, DC and DR depend on the power of the series (12); ii) a difference in the power of FRR signals was previously observed between the normoxic and hypoxic fetuses in this dataset (12); and iii) a high variability in the deceleration depth a was observed (see section 3.1), we computed the partial correlation coefficients, while compensating for the amplitude a and baseline b. In this way, correlations were insensitive to linear relations of such quantities.
Finally, we derived the relationship between the PRSA-based parameters and DA. To do so, we first computed the DA by means of the area of the trapezoid (after converting the FRR into the FHR) for both normoxic and chronically hypoxic fetuses. Then, we compared the values obtained and discussed the relationship found with the PRSA-based parameters.
2.6. Statistical Analysis
Results are reported as mean ± standard deviation and quantities were compared between the normoxic and hypoxic fetuses using a student t-test. Correlations and partial correlations were computed using the Pearson's correlation coefficient. Considering the low sample size, hypothesis tests and correlation coefficients were considered statistically significant when p < 0.1 (we also specify when p < 0.05).
3. Results
3.1. Comparison of Morphological Parameters Between Normoxic and Hypoxic Sheep Fetuses
The morphological parameters obtained after model fitting were compared between the normoxic and hypoxic sheep fetuses. We obtained a model fitting achieving R2 values of 0.8±0.1. Figure 2 reports the scatter plots for all pairs of morphological parameters for both animal models. A clear difference was found for τu (normoxic vs. hypoxic; 24.8±9.4 vs. 39.8±9.7 s; p < 0.05), no difference for τr (11.6±4.8 vs. 16.0±3.9 s; p > 0.1), a difference in FRR change a (268.1±109.5 vs. 373.0±46.0 ms; p < 0.1), and no difference for the baseline b (357.0±34.1 vs. 372.6±23.6 ms; p > 0.1). Δτ was found different between normoxic and hypoxic fetuses (13.2±6.9 vs. 23.9±7.5 s; p < 0.05).
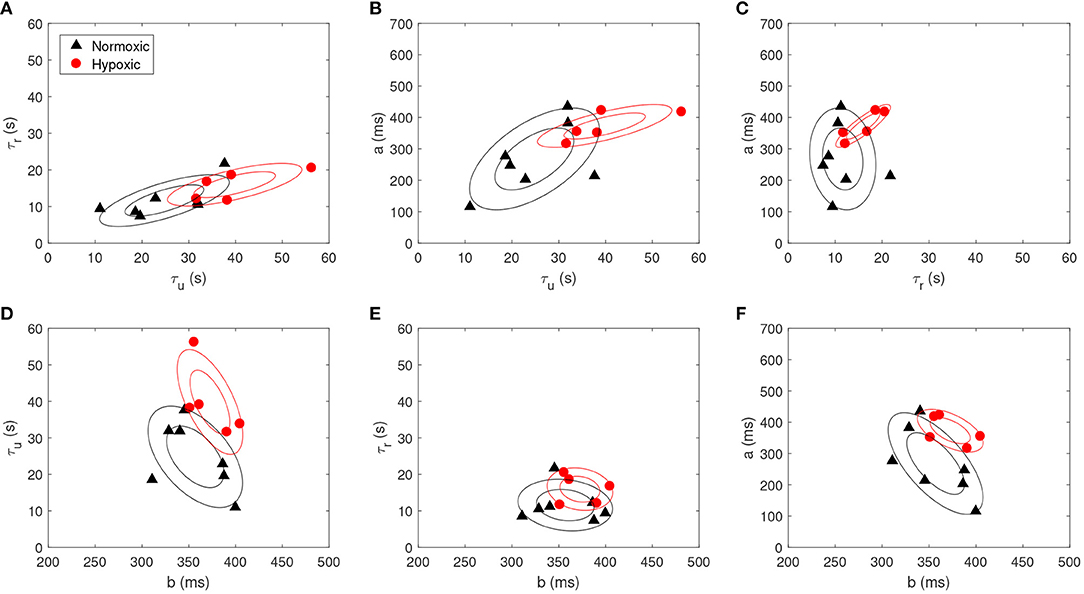
Figure 2. Scatter plot for each pair of the morphological parameters, i.e., (A) τu vs. τr, (B) τu vs. a, (C) τr vs. a, (D) b vs. τu, (E) b vs. τr and (F) b vs. a, for both normoxic (black triangle) and hypoxic fetuses (red circle). Contour plots are also reported (prepared with an assumption of Gaussianity, made for visualization purposes).
3.2. Time Progression of FHR Deceleration Morphology and Correlation With Biomarkers
A correlation analysis was performed to assess the time progression of the morphological parameters over the entire duration of the SEVERE phase. We found no correlation between the morphological parameters and time for both animal models when considered together (correlations between time vs. τu, τr, and Δτ were −0.2, −0.1, and −0.3, respectively; p > 0.1) or separated (normoxic fetuses: −0.1, 0.1, and −0.1 and hypoxic fetuses: 0.1, 0.3, and −0.1; p > 0.1). Non-significant correlations were likely due to the limited sample size. In fact, a similar analysis performed on the biomarkers resulted in a moderate (significant) correlation with time (pH vs. time: −0.5, p < 0.05; BE vs. time: −0.6, p < 0.05; lactate vs. time: 0.34, p < 0.1).
Consequently, when correlation was assessed between morphological parameters and biomarkers, partial correlation coefficients were calculated to account for this possible time variation. Partial correlations were found statistically significant for pH vs. τu (−0.5; p < 0.05) and pH vs. Δτ (−0.6; p < 0.05), and for BE vs. τu (−0.6; p < 0.05) and BE vs. Δτ (−0.7; p < 0.05), whereas no significant correlation was found for lactate. Such significant correlations were mostly due to the normoxic dataset (pH vs. τu: −0.5, pH vs. Δτ: −0.6, BE vs. τu: −0.6, BE vs. Δτ: −0.7; p < 0.05). Indeed, pH and BE were found correlated with the morphological parameters only for the normoxic data (p < 0.05). On the other hand, lactate was found correlated with only the morphological parameters of the hypoxic fetuses (coefficients for τu and Δτ were −0.8 and −0.8; p < 0.05).
3.3. Correlation Analysis Between PRSA and Morphological Parameters, and DA Computation
Partial correlation coefficients were calculated between the PRSA and morphological parameters on both animal models. Partial correlation coefficients were computed to compensate for the a and b values. An example of FRR after this compensation, for both animal models, is shown in Figure 3. A slower FHR adaptation, as response to UCO, becomes clearly visible for the hypoxic fetus. A wide range of statistically significant correlations was found for DC vs. τu, AC vs. τu and DR vs. Δτ, reaching values larger than −0.9 (p < 0.1) for T < 20. In particular, the normoxic fetuses showed correlations between T = 6 and T = 18 (Figure 4A), whereas the hypoxic ones between T = 1 and T = 13 (Figure 4B).
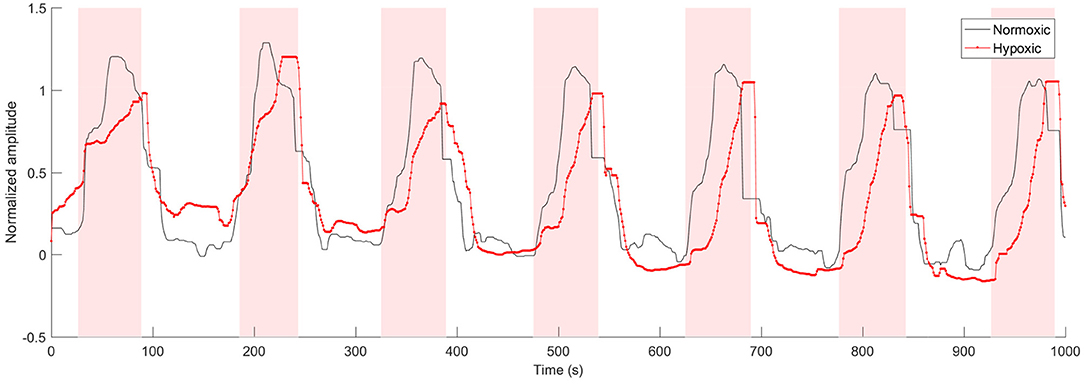
Figure 3. Example of normalized FRR signals from one normoxic and one hypoxic fetus (a median filter was applied to the signals to enhance the trend). The shaded area corresponds to UCOs.
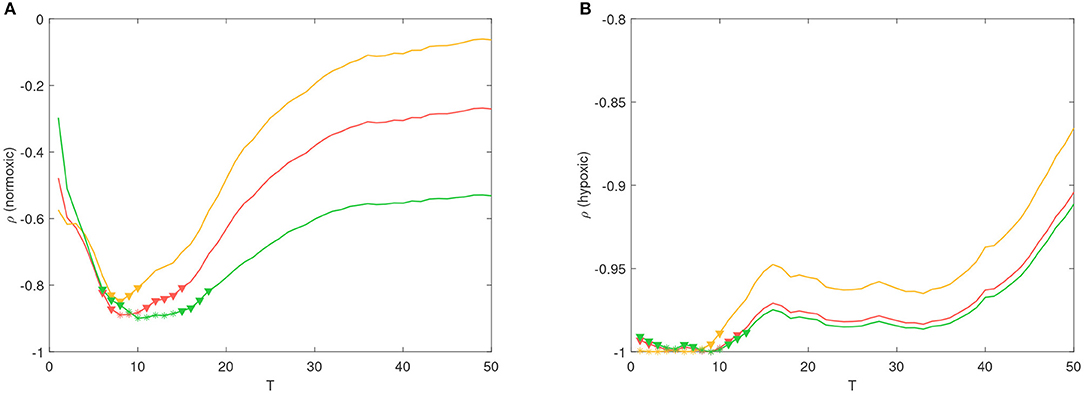
Figure 4. Partial correlation coefficient ρ between τu vs. DC (red line), τr vs. AC (yellow line) and Δτ vs. DR (green line), for different values of T. Coefficients were computed from the (A) normoxic and (B) hypoxic fetuses. It is worth noting that the number of data points available for the computation were 7 and 5 at each T-value. * (p < 0.05) and ▽ (p < 0.1) refer to statistically significant correlations.
For both animal models, DA was quantified and a comparison between the DA of the two populations was performed. DA was not found significantly different between normoxic and hypoxic fetuses in our data (62.9±19.5 vs. 64.4±6.6 beats; p > 0.1).
4. Discussion
4.1. Morphological Differences in FHR Decelerations
When comparing the morphological differences of FHR decelerations, the significant differences in τu, Δτ and a between normoxic and chronically hypoxic fetuses suggest a different FHR response to the ANS stimulation caused by UCOs in hypoxic fetus. These findings are in line with other reports of different response to external hypoxic stimulation, as in our case by UCOs, in normoxic vs. already hypoxic fetuses (16). The reduction of FHR in the presence of hypoxic stimuli in a normoxic fetus represents a protective mechanism while it reduces the oxygen consumption via reduced myocardial work (17). In the presence of already established acidemia, the ANS modulation changes (18, 19), and some of the adaptive mechanisms, such as chemoreceptor-mediated circulatory adaptation, might be altered due to progressive tissue damage, including brain damage. In this view, the fact that the correlation between biomarkers (pH and BE) and time was driven mainly by the normoxic fetuses should not be surprising. Both pH and BE are strong stimulators of chemoreceptors in normoxic fetus (20). Thus, we speculate that the absence of the correlation between pH and BE in hypoxic fetus might represent a sign of already altered ANS and chemoreceptor function which are no longer able to respond and contribute to maintaining the balance of the aerobic metabolism. Moreover, large observational studies shows that the risk of neonatal morbidity only increases with very low pH values (< 7.00) (21, 22). Certainly, it has to be acknowledged that the number of available measurements was different for normoxic and hypoxic fetuses (21 vs. 9), resulting in a much shorter SEVERE phase for the hypoxic ones. On the other hand, we found that the lactate correlated only with the morphological parameters in the hypoxic fetuses. A possible explanation could be due to the fact that the lactate represents the end product of anaerobic glucose metabolism, reflecting, thus, the metabolic acidosis (23). Indeed, it has been suggested that lactate concentrations, on fetal scalp and umbilical artery at birth, might be a better predictor of poor neonatal outcome than pH (24) and even if its use has not been universally adopted, lactate monitoring is recommended in several national and international guidelines (23).
4.2. Correlation Between Morphological Parameters and AC, DC and DR
Morphological parameters τu, τr and Δτ were found to correlate with DC, AC and DR for T values less than 20. In our previous study on the same animal model, we found that DR achieved the highest discriminatory power in distinguishing between normoxic and hypoxic sheep fetuses during the SEVERE phase of the protocol, with T ranging between 5 and 9 (12). Such high discriminatory power was likely related to the larger Δτ measured on hypoxic fetuses in this study. In other words, we found that the ANS regulation of already hypoxic fetuses during labor affects the deceleration morphology (particularly Δτ), thus further highlighting that the presence of asymmetric trends in the series is relevant for risk stratification.
Correlations were not statistically significant for the entire range of T values considered. This was an expected result because the T value acts as frequency selector, specifically as a band-pass filter (25), whose frequency band shrinks when T increases. Although there is no clear evidence about the optimal T value for the detection of already hypoxic fetuses, previous studies employed effectively, for the detection of intra-uterine growth restriction (IUGR) during antepartum fetal monitoring, values of T corresponding to the range 2.5 s to 10 s (6, 9, 26). On the other hand, fetal acidemia occurring during labor seems better detected at lower time scales between 0.5 and 1.25 s (12, 27), thus suggesting a different mechanism for healthy fetuses during acute stress.
The PRSA series is also amplitude-dependent. In our previous study, we found a perfect linear relationship between the standard deviation of the series and the PRSA parameters (12) for Gaussian processes during stationary condition (e.g., a situation likely occurring during antepartum fetal monitoring). A similar relation is expected for other indices of variability, such as the short-term variation (STV). In fact, considering results obtained during fetal monitoring of IUGR fetuses, Huhn et al. (26) and Graatsma et al. (28) found a correlation of about 0.7 between STV and AC for IUGR fetuses antepartum. On the other hand, given the non-stationary nature of FHR series during labor and the fact that the PRSA algorithm is applied to the entire recording, the relationship between STV and PRSA may break, as supported by the study of Georgieva et al. (27) who reported a significant correlation of about 0.3 during labor. It sounds therefore reasonable that the long-term variability of FHR series may better correlate with PRSA parameters during labor. In fact, the deceleration depth a affects the variability of the entire FHR series (the standard deviation of the FHR series is proportional to a), and in turn, it affects the values of AC, DC and DR. However, while a was found larger in the hypoxic dataset, it is still unclear whether such morphological parameter would turn out to be important for risk stratification or needs to be considered as a confounding factor.
Assessing whether AC, DC and DR reflect changes in the acid/base balance is challenging. On one hand, for the normoxic fetuses, we found that i) AC/DC varied across the phases of the stimulation protocol and were correlated with changes of the biomarkers (10), ii) morphological parameters τu and Δτ were correlated to pH and BE in the SEVERE phase, and iii) correlations between morphological parameters τu vs. DC, τr vs. AC, and Δτ vs. DR were observed in the SEVERE phase. In this normoxic condition, the acid/base balance of the healthy fetus is continuously maintained by the placenta and its gas exchanges. The establishment of hypoxia, and a subsequent metabolic acidosis, depends both on the extent, duration and repetitiveness of the events responsible for a reduced supply of fetal oxygen, and on the metabolic reserves of the fetus at the onset of labor (29). These findings suggest that the advancing labor, along with the increased intensity and frequency of uterine contractions, causes fetal ANS adaptation that directly affects the acid/base balance and produces modifications of the deceleration morphology. The latter is thus captured by the PRSA algorithm. Our speculation is corroborated by the superior performance of PRSA-based parameters to detect acidemia at birth with respect to STV (27). On the other hand, for chronically hypoxic fetuses, we found that only lactate correlated with τu and Δτ during the SEVERE phase. It is difficult to speculate about additional relationships between PRSA-based parameters and the acid/base balance during labor for these fetuses. In fact, to the best of our knowledge, no studies quantified the correlation between PRSA-based parameters and the biomarkers in animal models of chronic hypoxia. However, several are the clinical studies suggesting that the PRSA-based parameters may differentiate between healthy and IUGR fetuses (whose our chronically hypoxic sheep are a model of) at different gestational age (6, 8) or detecting the short-term outcome for these compromised fetuses (9). Thus, confirming that the PRSA algorithm detects a deterioration of the acid/base balance of chronically hypoxic fetuses, rather than other factors, is at this point difficult to claim. We leave the quantification of the correlation between biomarkers and PRSA-based parameters on our chronically hypoxic sheep model for future works.
4.3. Comparison With Deceleration Area
The results reported so far are in line with other attempts of “capturing” the deceleration morphology for risk stratification. For example, the well-known DA quantifies the severity of the deceleration taking into account both its depth and duration (the number of “missed” beats due to the deceleration). In the work of Cahill et al. (30), DA was quantified by approximating the deceleration by a triangle having a base as long as its duration and height corresponding to the depth, and then computing its area. DA was found to perform well for detecting fetal acidemia (AUC = 0.76). Using the morphological parameters, which are depicted in Figure 1, DA is then given by
where dUCO = 60 s is the duration of the UCO, and 1, 000 is the conversion factor from milliseconds to seconds. In our case, given the severity of the stimulation protocol, UCOs caused FHR responses which were better approximated by a trapezoidal model than a triangle. Thus, it was possible to calculate DA using also the following formula
From these formulas, the relationship between DA and the morphological parameters, which we studied in this paper, is evident. The second formula also points out a link between DA and the asymmetry value Δτ = τu − τr, which is not captured in the triangular approximation usually employed. The relationship with Δτ hints the importance of looking at asymmetric trends in the series present during labor. We leave this point to future investigations.
In our study, however, DA was not found significantly different between normoxic and hypoxic fetuses in our data (62.9±19.5 vs. 64.4±6.6 beats; p > 0.1). A possible explanation could be the fact that Δτ and a were higher for the hypoxic fetuses, and such quantities correlated with DA in opposite directions, thus making DA values indistinguishable due to balancing effects and (likely) the limited sample size. Another possible reason is that chronic hypoxia (in this study) and acidemia at birth (30) might trigger different regulatory ANS responses.
4.4. Clinical Implications
Over the past 50 years, the cardiotocography has become the most frequently used method for intrapartum surveillance of fetal wellbeing. Intrapartum FHR monitoring, in particular as part of the CTG and computerized systems such as those developed by Dawes and Redman, has reduced perinatal mortality (31). In 1991, Dawes et al. (32) reported that low short-term variation was associated with adverse perinatal outcome, however its application required antepartum monitoring to identify at-risk fetuses which to-date has not become the standard of care. Recent developments in the remote transabdominal ECG technology are enabling the antepartum monitoring, so more progress is to be expected in incorporating various computerized FHR algorithms into the standard of care in the coming years (33). In the meantime, the intrapartum CTG as currently practiced in over 90% of the delivering hospitals remains to be characterized by high intra and inter-observer interpretative variability, and by low specificity in identifying fetal acidosis and fetal acidosis at birth. Its poor performance is compounded by the fact that fetal acidosis itself is a poor predictor of perinatal brain injury (34). Thus, CTG does not reduce perinatal morbidity and mortality, but it does increase the rate of operative deliveries (35, 36). Indeed, there is still debate regarding CTG application during labor (37). Randomized controlled trials failed to demonstrate improved outcomes with the use of CTG during labor (38, 39). Moreover, recent evidence suggests that the type of deceleration [defined by NICHD FHR parameters criteria (40)] does not predict low pH at birth (30), and that the deceleration area is the most discriminative in the identification of fetal acidemia (41). Thus, the authors concluded that there is no need to determine the type of deceleration at all, which is prone to high interobserver variation. However, this statement is not shared by other authors (42), arguing that the success of CTG depends on team effort by the obstetricians with in-depth practical knowledge.
Since singular parameters generally have been shown to have a poor predictive value, a multiparametric approach to FHR patterns is also emphasized. Recently, Eden et al. (43) developed a new promising multiparametric metric, the fetal reserve index, that showed a reduction in emergency operative deliveries and in adverse fetal/neonatal outcomes (44, 45). Building upon the notion of multiparametric approaches and the focus on fetal outcomes instead of poorly correlated outcome metrics such as fetal acidemia and leveraging deep learning, recent advances have been made in detecting preventable fetal distress and brain injury from multiple CTG patterns (34). Overall, there is evidence to suggest the new metrics and types of analysis of FHR might improve what, at the moment, represents the standard clinical practice. Based on our result, AC, DC and DR, computed through PRSA, are linked to the deceleration morphology itself. Thus, they may lead to a better categorization of the deceleration type. Moreover, the deceleration morphologies have been found to differ between normoxic and hypoxic sheep fetuses during UCO, thus, motivating future investigation on the translational potential of this methodology. Clinical cohorts are needed to validate these findings and evaluate the clinical performance of these new metrics in identifying compromised fetuses during labor.
4.5. Limitations of the Study
First, the sample size is small, dictated by the complexity of the animal model. Second, UCOs implemented in the experiments do not necessarily generalize to human labor, where the contractions are not equally regular nor are they all producing a complete occlusion of the umbilical cord. Third, sheep were analyzed during complete UCOs. However, changes in ANS activity in response to UCOs also occur earlier in time, when the UCOs are less severe or the recovery time between the UCOs is longer, and may reflect differences in the chronically hypoxic fetuses compared to the normoxic ones. Identifying these potentially earlier differences will be the subject of future studies. Fourth, all sheep fetuses displayed an individual pattern of pathological hypotensive responses to UCOs with regard to the timing of its emergence, with hypotensive responses to FHR decelerations showing well ahead of the severe UCOs in some instances (46, 47). As our present study focused on the differences between the hypoxic and normoxic fetuses in the severe stage of UCOs, it did not investigate the relationship between the PRSA-based metrics and the timing of the onset of pathological hypotension. We leave this to future work.
4.6. Conclusions
Our study motivates further investigations on PRSA-related quantities to determine their potential advantage for risk stratification. It might also open interesting scenarios for interpreting PRSA-based results and improving FHR monitoring. The evaluation of the performance of these new metrics in identifying compromised fetuses during labor is still underway.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The dataset can be made available upon request. Requests to access these datasets should be directed to Martin G. Frasch, bWZyYXNjaEB1dy5lZHU=.
Ethics Statement
The animal study was reviewed and approved by University of Western Ontario Council on Animal Care.
Author Contributions
MR designed and implemented the analyses and drafted the manuscript. MF collected the data. MR and RS optimized the proposed mathematical framework. MB, TS, and MF were involved in the clinical interpretation of the results. All authors read, revised, and approved the final manuscript.
Conflict of Interest
MF has a patent pending on abdominal ECG signal separation for FHR monitoring (WO2018160890).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge Dr. Bryan Richardson and his Perinatal Research Lab at the University of Western Ontario for the original design of the animal experiments that enabled the acquisition of the dataset underlying the present study. The preprint version of this manuscript is available on biorxiv.org (48).
References
1. Divon MY, Muskat Y, Platt LD, Paldi E. Increased beat-to-beat variability during uterine contractions: a common association in uncomplicated labor. Am J Obstet Gynecol. (1984) 149:893–6. doi: 10.1016/0002-9378(84)90611-2
2. Bennet L, Gunn AJ. The fetal heart rate response to hypoxia: insights from animal models. Clin Perinatol. (2009) 36:655–72. doi: 10.1016/j.clp.2009.06.009
3. Jonsson M, Agren J, Nordén-Lindeberg S, Ohlin A, Hanson U. Neonatal encephalopathy and the association to asphyxia in labor. Am J Obstet Gynecol. (2014) 211:667.e1-8. doi: 10.1016/j.ajog.2014.06.027
4. Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. (2017) 2:CD006066. doi: 10.1002/14651858.CD006066.pub3
5. Bauer A, Kantelhardt JW, Bunde A, Barthel P, Schneider R, Malik M, et al. Phase-rectified signal averaging detects quasi-periodicities in non-stationary data. J Phys A. (2006) 364:423–34. doi: 10.1016/j.physa.2005.08.080
6. Stampalija T, Casati D, Montico M, Sassi R, Rivolta MW, Maggi V, et al. Parameters influence on acceleration and deceleration capacity based on trans-abdominal ECG in early fetal growth restriction at different gestational age epochs. Eur J Obstet Gynecol Reprod Biol. (2015) 188:104–12. doi: 10.1016/j.ejogrb.2015.03.003
7. Stampalija T, Casati D, Monasta L, Sassi R, Rivolta MW, Muggiasca ML, et al. Brain sparing effect in growth-restricted fetuses is associated with decreased cardiac acceleration and deceleration capacities: a case-control study. BJOG. (2016) 123:1947–54. doi: 10.1111/1471-0528.13607
8. Tagliaferri S, Fanelli A, Esposito G, Esposito FG, Magenes G, Signorini MG, et al. Evaluation of the acceleration and deceleration phase-rectified slope to detect and improve IUGR clinical management. Comput Math Methods Med. (2015) 2015:1–9. doi: 10.1155/2015/236896
9. Lobmaier SM, van Charante NM, Ferrazzi E, et al. Phase-rectified signal averaging method to predict perinatal outcome in infants with very preterm fetal growth restriction- a secondary analysis of TRUFFLE-trial. Am J Obstet Gynecol. (2016) 215:630.e1–630.e7. doi: 10.1016/j.ajog.2016.06.024
10. Rivolta MW, Stampalija T, Casati D, Richardson BS, Ross MG, Frasch MG, et al. Acceleration and deceleration capacity of fetal heart rate in an in-vivo sheep model. PLoS ONE. (2014) 9:e104193. doi: 10.1371/journal.pone.0104193
11. Warrick PA, Hamilton EF, Precup D, Kearney RE. Classification of normal and hypoxic fetuses from systems modeling of intrapartum cardiotocography. IEEE Trans Biomed Eng. (2010) 57:771–9. doi: 10.1109/TBME.2009.2035818
12. Rivolta MW, Stampalija T, Frasch MG, Sassi R. Theoretical value of deceleration capacity points to deceleration reserve of fetal heart rate. IEEE Trans Biomed Eng. (2020) 67:1176–85. doi: 10.1109/TBME.2019.2932808
13. Morrison JL, Berry MJ, Botting KJ, Darby JRT, Frasch MG, Gatford KL, et al. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol Regul Integr Comp Physiol. (2018) 315:R1123–53. doi: 10.1152/ajpregu.00391.2017
14. Xu A, Durosier LD, Ross MG, Hammond R, Richardson BS, Frasch MG. Adaptive brain shut-down counteracts neuroinflammation in the near-term ovine fetus. Front Neurol. (2014) 5:110. doi: 10.3389/fneur.2014.00110
15. Durosier LD, Green G, Batkin I, Seely AJ, Ross MG, Richardson BS, et al. Sampling rate of heart rate variability impacts the ability to detect acidemia in ovine fetuses near-term. Front Pediatr. (2014) 2:38. doi: 10.3389/fped.2014.00038
16. Parer J, Dijkstra H, Harris J, Krueger T, Reuss M. Increased fetal heart rate variability with acute hypoxia in chronically instrumented sheep. Eur J Obstet Gynaecol Reprod Biol. (1980) 10:393–9. doi: 10.1016/0028-2243(80)90025-8
17. Fletcher AJW, Gardner DS, Edwards CMB, Fowden AL, Giussani DA. Development of the ovine fetal cardiovascular defense to hypoxemia towards full term. Am J Physiol Heart Circ Physiol. (2006) 291:H3023–34. doi: 10.1152/ajpheart.00504.2006
18. Murotsuki J, Bocking AD, Gagnon R. Fetal heart rate patterns in growth restricted fetal sheep induced by chronic fetal placental embolization. Am J Obstet Gynecol. (1997) 176:282–90. doi: 10.1016/S0002-9378(97)70486-1
19. Siira S, Ojala T, Vahlberg T, Rosén K, Ekholm E. Do spectral bands of fetal heart rate variability associate with concomitant fetal scalp pH? Early Hum Dev. (2013) 89:739–42. doi: 10.1016/j.earlhumdev.2013.05.007
20. Itskovitz J, Rudolph AM. Denervation of arterial chemoreceptors and baroreceptors in fetal lambs in utero. Am J Physiol Heart Circ Physiol. (1982) 242:H916–20. doi: 10.1152/ajpheart.1982.242.5.H916
21. Yeh P, Emary K, Impey L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: analysis of 51,519 consecutive validated samples. BJOG. (2012) 119:824–31. doi: 10.1111/j.1471-0528.2012.03335.x
22. Mittendorf R, Won SY, Gianopoulos JG, Pryde PG, Roizen N. Relationships between umbilical cord arterial blood pH levels at delivery and bayley psychomotor development index scores in early childhood. Perinat Med. (2008) 36:335–40. doi: 10.1515/JPM.2008.043
23. Cummins G, Kremer J, Bernassau A, Brown A, Bridle H, Schulze H, et al. Sensors for fetal hypoxia and metabolic acidosis: a review. Sensors. (2018) 18:2648. doi: 10.3390/s18082648
24. Kruger K, Hallberg B, Blennow M, Kublickas M, Westgren M. Predictive value of fetal scalp blood lactate concentration and pH as markers of neurologic disability. Am J Obstet Gynecol. (1999) 181:1072–8. doi: 10.1016/S0002-9378(99)70083-9
25. Sassi R, Stampalija T, Casati D, Ferrazzi E, Bauer A, Rivolta MW. A methodological assessment of phase-rectified signal averaging through simulated beat-to-beat interval time series. Comput Cardiol. (2014) 41:601–4. Available online at: https://www.cinc.org/archives/2014/pdf/0601.pdf
26. Huhn EA, Lobmaier S, Fischer T, Schneider R, Bauer A, Schneider KT, et al. New computerized fetal heart rate analysis for surveillance of intrauterine growth restriction. Prenat Diagn. (2011) 31:509–14. doi: 10.1002/pd.2728
27. Georgieva A, Papageorghiou AT, Payne SJ, Moulden M, Redman CW. Phase-rectified signal averaging for intrapartum electronic fetal heart rate monitoring is related to acidaemia at birth. BJOG. (2014) 121:889–94. doi: 10.1111/1471-0528.12568
28. Graatsma EM, Mulder EJH, Vasak B, Lobmaier SM, Pildner von Steinburg S, Schneider KTM, et al. Average acceleration and deceleration capacity of fetal heart rate in normal pregnancy and in pregnancies complicated by fetal growth restriction. J Matern Fetal Neonatal Med. (2012) 25:2517–22. doi: 10.3109/14767058.2012.704446
29. Yli BM, Kjellmer I. Pathophysiology of foetal oxygenation and cell damage during labour. Best Pract Res Clin Obstet Gynaecol. (2016) 30:9–21. doi: 10.1016/j.bpobgyn.2015.05.004
30. Cahill AG, Tuuli MG, Stout MJ, López JD, Macones GA. A prospective cohort study of fetal heart rate monitoring: deceleration area is predictive of fetal acidemia. Am J Obstet Gynecol. (2018) 218:e1–523.e12. doi: 10.1016/j.ajog.2018.01.026
31. Redman C. 45 years of fetal heart rate monitoring in BJOG. BJOG. (2015) 122:536. doi: 10.1111/1471-0528.13101
32. Dawes GS, Moulden M, Redman CW. The advantages of computerized fetal heart rate analysis. J Perinat Med. (1991) 19:39–45. doi: 10.1515/jpme.1991.19.1-2.39
33. Mhajna M, Schwartz N, Levit-Rosen L, Warsof S, Lipschuetz M, Jakobs M, et al. Wireless, remote solution for home fetal and maternal heart rate monitoring. Am J Obstet Gynecol MFM. (2020) 2:100101. doi: 10.1016/j.ajogmf.2020.100101
34. Frasch MG, Strong S, Nilosek D, Leaverton J, Schiffrin BS. Detection of preventable fetal distress during labor from scanned cardiotocogram tracings using deep learning. Front Pediatr. (2021).
35. Clark SL, Hamilton EF, Garite TJ, Timmins A, Warrick PA, Smith S. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. (2017) 216:163.e1–163.e6. doi: 10.1016/j.ajog.2016.10.009
36. Johnson GJ, Salmanian B, Denning SG, Belfort MA, Sundgren NC, Clark SL. Relationship between umbilical cord gas values and neonatal outcomes: implications for electronic fetal heart rate monitoring. Obstet Gynecol. (2021) 138:366–73. doi: 10.1097/AOG.0000000000004515
37. Frasch MG, Boylan GB, Wu HT, Devane D. Commentary: computerised interpretation of fetal heart rate during labour (INFANT): a randomised controlled trial. Front Physiol. (2017) 8:721. doi: 10.3389/fphys.2017.00721
38. Vintzileos AM, Antsaklis A, Varvarigos I, Papas C, Sofatzis I, Montgomery JT. A randomized trial of intrapartum electronic fetal heart rate monitoring versus intermittent auscultation. Obstet Gynecol. (1993) 81:899–907.
39. The-INFANT-Collaborative-Group. Computerised interpretation of the fetal heart rate during labour: a randomised controlled trial (INFANT). Lancet. (2017) 389:1719–29. doi: 10.1016/S0140-6736(17)30568-8
40. ACOG. ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. (2009) 114:192–202. doi: 10.1097/AOG.0b013e3181aef106
41. Furukawa A, Neilson D, Hamilton E. Cumulative deceleration area: a simplified predictor of metabolic acidemia. J Matern Fetal Neonatal Med. (2019) 30:1–8. doi: 10.1080/14767058.2019.1678130
42. Sholapurkar SL. The present and future of intrapartum computerized cardiotocography: role of pattern recognition incorporating single vs. multiple parameters. J Matern Fetal Neonatal Med. (2021) 17:1–7. doi: 10.1080/14767058.2021.1949453
43. Eden RD, Evans MI, Evans SM, Schifrin BS. The “Fetal reserve Index”: re-engineering the interpretation and responses to fetal heart rate patterns. Fetal Diagn Ther. (2018) 43:90–104. doi: 10.1159/000475927
44. Eden RD, Evans MI, Britt DW, Evans SM SB. Safely lowering the emergency cesarean and operative vaginal delivery rates using the fetal reserve index. J Matern Fetal Neonatal Med. (2020) 33:1473–9. doi: 10.1080/14767058.2018.1519799
45. Evans MI, Eden RD, Britt DW, Evans SM SB. Re-engineering the interpretation of electronic fetal monitoring to identify reversible risk for cerebral palsy: a case control series. J Matern Fetal Neonatal Med. (2019) 32:2561–9. doi: 10.1080/14767058.2018.1441283
46. Gold N, Frasch MG, Herry CL, Richardson BS, Wang X. A doubly stochastic change point detection algorithm for noisy biological signals. Front Physiol. (2018) 8:1112. doi: 10.3389/fphys.2017.01112
47. Gold N, Herry CL, Wang X, Frasch MG. Fetal cardiovascular decompensation during labor predicted from the individual heart rate: a prospective study in fetal sheep near term and the impact of low sampling rate. arXiv e-prints. (2019) arXiv:1911.01304.
Keywords: phase-rectified signal averaging (PRSA), animal model, fetal heart rate (FHR), electronic fetal monitoring (EFM), heart rate variability (HRV), fetal hypoxia, labor
Citation: Rivolta MW, Barbieri M, Stampalija T, Sassi R and Frasch MG (2021) Relationship Between Deceleration Morphology and Phase Rectified Signal Averaging-Based Parameters During Labor. Front. Med. 8:626450. doi: 10.3389/fmed.2021.626450
Received: 05 November 2020; Accepted: 31 October 2021;
Published: 25 November 2021.
Edited by:
Mark I. Evans, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Serena Xodo, University of Udine, ItalyTanja Premru-Sršen, University Medical Centre Ljubljana, Slovenia
Copyright © 2021 Rivolta, Barbieri, Stampalija, Sassi and Frasch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo W. Rivolta, bWFzc2ltby5yaXZvbHRhQHVuaW1pLml0
 Massimo W. Rivolta
Massimo W. Rivolta Moira Barbieri
Moira Barbieri Tamara Stampalija
Tamara Stampalija Roberto Sassi
Roberto Sassi Martin G. Frasch
Martin G. Frasch