
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 16 February 2021
Sec. Rheumatology
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.622225
This article is part of the Research TopicDiagnostic and Therapeutic Challenges in Rare and Complex Autoimmune DiseasesView all 17 articles
Glucocorticoids therapy has greatly improved the outcome of lupus nephritis patients. Since their discovery, their adverse effects have counterbalanced their beneficial anti-inflammatory effects. Glucocorticoids exert their effects through both genomic and non-genomic pathways. Differential activation of these pathways is clinically relevant in terms of benefit and adverse effects. Ongoing aims in lupus nephritis treatment development focus on a better use of glucocorticoids combined with immunosuppressant drugs and biologics. Newer regimens aim to decrease the peak glucocorticoid dose, allow a rapid glucocorticoid tapering, and intend to control disease activity with a lower cumulative glucocorticoid exposure. In this review we discuss the mechanisms, adverse effects and recent strategies to limit glucocorticoid exposure without compromising treatment efficacy.
Cortisone (“compound E” or 17-hydroxy-11-dehydrocorticosterone) was identified in the 1930's by Edward Kendall and Tadeusz Reichstein, and later purified and synthesized in the 1940's. Compound E had strong anti-inflammatory effects but also potent mineralocorticoid effects which manifested as fluid retention, hypertension, and hypokalemia. Compound E was first applied for treatment in 1948 by Philip Hench. At that time, a young woman with severe rheumatoid arthritis would become the first patient treated with cortisone. The anti-inflammatory effects of the cortisone were remarkable but so were the adverse effects (1, 2).
Subsequently, other glucocorticoid (GC) preparations were developed for treating autoimmune diseases, including systemic lupus erythematosus (SLE) (3). The use of these anti-inflammatory steroids in lupus nephritis (LN) dramatically improved survival. For example, survival was 17% at 5 years in the pre-glucocorticoid era, but 55% at 5 years after introduction of glucocorticoids (4, 5). The addition of immunosuppressive drugs to GC, and later on, the development of biologic drugs, have transitioned LN management to one focused on improving kidney outcomes while minimizing adverse events. In this review, we discuss the use of GCs from mechanisms, adverse events to management of lupus nephritis, and current strategies to limit toxicity of these drugs.
Glucocorticoids are involved in regulatory processes throughout the body, such as energy and lipid metabolism, and adaptation to stress. Two of their most important effects are their strong anti-inflammatory and immunosuppressive effects, evident at concentrations above the physiological glucocorticoid levels (6).
Glucocorticoids and synthetic glucocorticoids have two mechanisms of action: the genomic and non-genomic mechanisms (Figure 1) (7). Genomic mechanisms are activated after GC, as lipophilic molecules, cross the cell membranes and bind to the multiprotein complex of chaperones (e.g., Hsp40, Hsp56, Hsp70, and Hsp90), immunophilins that act as co-chaperones (e.g., p23, FKBP51, FKBP52), and the intracellular cytoplasmic glucocorticoid receptor (cGR). After binding and subsequent dissociation from these proteins, the complex GC-cGR translocates to the nucleus and binds to DNA binding sites known as glucocorticoid response elements. The final result is a decreased transcription of genes encoding inflammatory cytokines (e.g., interleukin-6, interleukin-8, tumor necrosis factor-a), a process known as transrepression; and an increased transcription of anti-inflammatory genes (e.g., interleukin-10, IκB, annexin A1), known as transactivation (8).
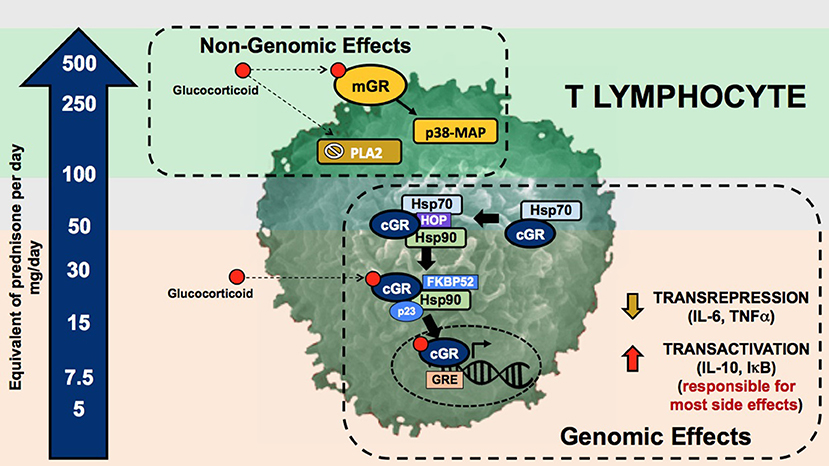
Figure 1. Genomic and non-genomic mechanisms of glucocorticoids. Glucocorticoid genomic pathway is mediated through the cytoplasmic glucocorticoid receptor (cGR) leading to the mechanisms of gene transactivation and transrepression. The non-genomic pathway is mediated through the membrane glucocorticoid receptor (mGR), inhibition of the phospholipase A2, and changes in cell membranes. The arrow in the left depicts the dose of prednisone required to activate these pathways. The upper and lower gray zones represent the doses were genomic (lower gray zone) and non-genomic (upper gray zone) are fully saturated without added benefit and with higher incidence of adverse effects. mGR, membrane glucocorticoid receptor; PLA2, phospholipase A2; cGR, cytoplasmic glucocorticoid receptor; GRE, glucocorticoid response element; Hsp70·HOP·Hsp90, multiprotein complex including chaperones such as heat shock proteins and the glucocorticoid receptor; Hsp90·FKBP52·p23, multiprotein complex including chaperones, co-chaperones, and the glucocorticoid receptor.
Genomic mechanisms are generally evident 30 min after GC administration. By contrast, a second type of non-genomic mechanisms produce effects within minutes after the administration. These non-genomic effects are mediated through changes in cellular membranes, inactivation of the phospholipase A2 enzyme, and interaction with membrane glucocorticoid receptors (mGR). Second messengers include kinases, such as the p38 MAP kinase. The final effect is decreased lymphocyte activity and proliferation (9).
Identification of genomic and non-genomic mechanisms is clinically important due to the differential adverse effect profile and differential activation exerted by currently used glucocorticoid dosages and preparations (Figure 1). Genomic effects are activated with low (<7.5 mg prednisone equivalent per day) to moderate (7.5–30 mg prednisone equivalent per day) GC doses, and cGRs are progressively saturated with high-doses above 30 to 50 mg per day (10). From this pharmacologic concept, prednisone doses above 50 mg per day approach the ceiling of cGR saturation, with limited additional anti-inflammatory benefit, yet increasing the risk for adverse effects. As will be further discussed, some adverse effects, such as avascular bone necrosis, are dependent on the peak GC dose and duration of high-dose exposure (tapering speed) (Figure 2).
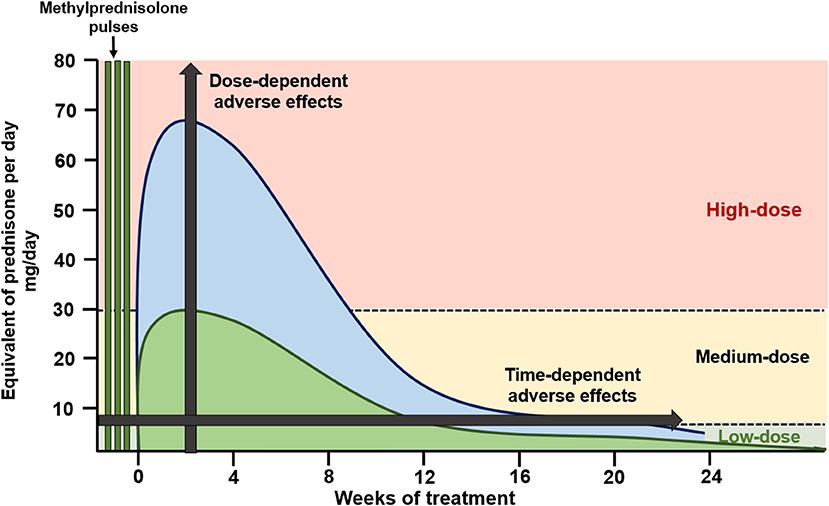
Figure 2. Glucocorticoid dosing in induction of remission schemes. High-dose oral glucocorticoid schemes (blue) apply starting doses of oral glucocorticoids at 0.8–1.0 mg/kg/day, with slow tapering, reaching low glucocorticoid doses by 24 weeks of therapy. Recent schemes (green) apply methylprednisolone pulses followed by medium starting doses of oral glucocorticoids (<0.5 mg/kg/day) with a faster tapering, reaching low glucocorticoid doses by 12 weeks of therapy.
Non-genomic mechanisms are activated with very-high GC dosages, such as those reached with methylprednisolone pulses. This activation starts at prednisone dosages of 100 mg, and reaching its maximum around 250 to 500 mg. In contrast to effects mediated by genomic mechanisms, non-genomic mechanisms are thought to be associated with less adverse effects, at least in part due to the short duration of administration (11).
The relative activation of these genomic and non-genomic pathways differs among different GC preparations. For example, dexamethasone and methylprednisolone activate the non-genomic pathway at a 3-fold greater rate than prednisone (12). Different GC preparations also differ in potency (expressed relative to hydrocortisone), mineralocorticoid effects, and duration of suppression of the hypothalamic-pituitary-adrenal axis (13). Other factors, such as time of administration (less suppression when administered in the morning) and their chronopharmacology, contribute to the degree of GC-axis suppression and in consequence to the severity of adverse effects, but are beyond the scope of this review (14).
Understanding these mechanisms is important to develop strategies to limit GC toxicity. As shown in Figure 2, GC administration strategies used in recent clinical trials have included intravenous methylprednisolone pulses, which activate non-genomic pathways, followed by lower peak oral GC dosages and a faster tapering of oral GCs. This strategy aims to maintain treatment efficacy while limiting GC-related adverse effects.
Both disease activity and glucocorticoid exposure have been associated with organ damage in SLE (15, 16). As patients with higher degree of disease activity are usually treated with higher GC doses, many of the reported studies suffer of confounding by indication (i.e., patients with more severe activity are administered higher GC doses). Also, as damage is frequently measured through indices that group several manifestations [e.g., the SLICC/ACR damage index (SDI)], it is difficult to distinguish organ damage caused by prednisone from that caused by disease activity or concomitant immunosuppressive medications (17). Finally, many studies also suffer from time bias, as the contribution of disease activity to damage is usually higher at earlier stages, while GC-related damage is greater at later stages (17).
Organ damage occurs in 50% of patients with SLE within 5-years of SLE diagnosis (18), with reported increased risk of 2.8% for each 1 mg prednisone per day (19). Organ damage has been reported to be minimized by achieving disease remission (15, 20), and by using maintenance doses of prednisone lower than 6.0 to 7.5 mg per day (21–23).
GC-related adverse effects have also been classified into those related to high dosing over a short period of time, and those related to cumulative GC doses. Table 1 summarizes the reported GC adverse effects according to the use of intravenous methylprednisolone pulses, the peak oral-GC dose, the duration of exposure to high-GC doses, and the GC cumulative dose.
Infections have been frequently associated with the peak dose of GC and the duration of exposure to high GC doses. Infections continue to be a major cause of hospitalization and mortality in SLE (24–26). An increased incidence of these infections occurs in patients with kidney disease (27). Although bacterial infections in lungs, skin, and urinary tract are far more frequent (25, 28, 29), the risk for both bacterial and opportunistic infections increases progressively with the use of medium- to high-dose of GC (30–33). The risk of infections associated with high-dose GC administration seems to be independent of the use of other immunosuppressive medications (21).
Studies of infections with administration of methylprednisolone pulses have also been confounded by indication, due to the traditional administration of this treatment in combination with other aggressive immunosuppressive regimens to sicker patients (32, 34). Some studies suggest that the risk of infection is lower with the use of methylprednisolone pulses of less than 1.5 g in total (35, 36). It has also been hypothesized that the shorter duration of pulse therapy (3–5 days) may limit the prolonged suppression of T-cell responses, which usually peaks after 21 days of GC administration (37). Therefore, methylprednisolone pulses of less than 1.5 g in total followed by reduced oral GC may potentially decrease the incidence of steroid induced infections. Additional preventive measures include vaccination and the use of prophylactic antibiotics and antivirals when indicated (38, 39).
Avascular bone necrosis occurs in 5–15% of patients with SLE. It is most commonly found in the femoral head, but may occur in other weight-bearing joints, and may occur bilaterally (40–42). The pathophysiology of avascular bone necrosis is not fully understood and suggested mechanisms are reviewed elsewhere (43). As for infections, avascular bone necrosis has been reported to occur more frequently in association with lupus nephritis (44, 45). Also, it has been associated with GC pulse therapy (46), the peak initial GC dose (47, 48), and the high cumulative GC doses in the first months of treatment (40, 49).
The prevalence of osteoporosis in SLE is 10 to 20%, with up to 20% of patients experiencing vertebral fractures (50). Glucocorticoids increase bone resorption and reduce bone formation. The former is more pronounced in the first months of steroid use while the latter becomes predominant with chronic GC use (51). Osteoporosis and vertebral fractures have been associated with higher GC doses, cumulative doses, and prolonged administration (52). The risk of osteoporotic fractures has been estimated to increase 4.2% for each 1 mg per day of prednisone (19). As bone loss develops over a long-time, many studies with short follow-up fail to assess the impact of GC therapy on bone density. Assessment of risk for fractures and of the need for concomitant preventive therapies including calcium, vitamin D, and bisphosphonates are recommended for all patients on GC therapy and are reviewed elsewhere (53, 54).
Long-term and high-dose GC therapy are associated with pro-atherogenic disturbances that characterize the metabolic syndrome (55). This syndrome occurs in 30 to 40% of patients with SLE and has been associated with higher disease activity, past or present history of LN, and higher oral doses of GCs. Its prevalence varies according to age and ethnicity as expected (56, 57).
Insulin resistance increases in patients with SLE on oral GC above 7.5 mg per day (58). Furthermore, the risk of diabetes increases 2- to 4-fold in non-diabetic patients with SLE, especially with increasing years of chronic GC use (59–61). In patients with pre-existing diabetes, exacerbation of the disease is particularly severe in patients with poor glycemic control at baseline (62, 63).
Hypertension is common in SLE and LN patients, with a prevalence up to 70% when assessed by 24-h blood pressure monitoring (64). Acute exacerbation of hypertension is frequent during pulse GC therapy. Although hypertension during an active LN is mediated by salt-sensitive mechanisms (65), the risk of hypertension has been also reported to be higher in patients exposed to GC, and has been associated with the duration of exposure and the daily dosage of GCs (61, 66, 67).
Glucocorticoids contribute to weight gain by increasing the appetite for high caloric, high fat food intake (68, 69). The weight gain is characterized by central hypertrophy of adipose tissue with concomitant thinning of peripheral subcutaneous adiposity, providing a lipodystrophic appearance (Cushingoid phenotype) (70). Up to 60–70% of patients prescribed long-term GCs report weight gain (52), and this effect has been associated with doses of GC above 5 mg per day (52, 61).
It is known that the incidence of cardiovascular events is increased in SLE, particularly, in patients with lupus nephritis and chronic kidney disease (23). Although it is difficult to differentiate the effect of disease activity, traditional risk factors, and treatment-related factors; the use of medium- to high-dose GCs has been associated with increased cardiovascular events, subclinical atherosclerosis such as carotid intima-media thickening, severity of coronary calcifications, and severity of arterial stiffness (71, 72). The risk of cardiovascular events is estimated to increase 5-fold in SLE patients taking >20 mg per day of prednisone (23), and 3-fold in those who develop cushingoid features (73). Cardiovascular events may be reduced by administering lower peak GC doses, faster GC tapering, and by limiting cumulative dose. In fact, reductions in cumulative oral GC were associated with lower incidence of cardiovascular events in a reported cohort study (74).
The treatment of lupus nephritis has been traditionally divided into an induction phase of intense immunosuppression, aimed to quickly suppress inflammation, followed by a prolonged maintenance phase, directed to consolidate response and to prevent disease flares (75). For the induction phase, current guidelines recommend the use of medium to high-dose GCs, combined with an immunosuppressant such as mycophenolate mofetil, cyclophosphamide, and more recently, calcineurin inhibitors (38, 39). Next, we describe strategies aimed to reduce exposure while keeping treatment efficacy. These strategies have 3 main objectives: (1) reducing the peak GC dose, (2) reducing the duration of exposure to high-dose GC via a faster GC tapering, and (3) limiting the cumulative dose from prolonged administration.
Administration of methylprednisolone pulses may allow the use of lower initial oral GC doses (lower peak GC dose) with a faster tapering schedule (lower exposure to high GC doses). Several clinical studies in lupus nephritis have included methylprednisolone pulses, followed by moderate ( ≤0.5 mg/kg/day) doses of oral GCs (Figure 3) (76–78). The Euro Lupus Nephritis Trial (ELNT) scheme (77) included three 750 mg methylprednisolone pulses, followed by 0.5 mg/kg/day prednisone slowly tapered to 10 mg/day by 6 months. This trial reported renal response rates (complete and partial) around 20 and 50% at 6- and 12-months, respectively, and long-term preservation of kidney function (77, 79).
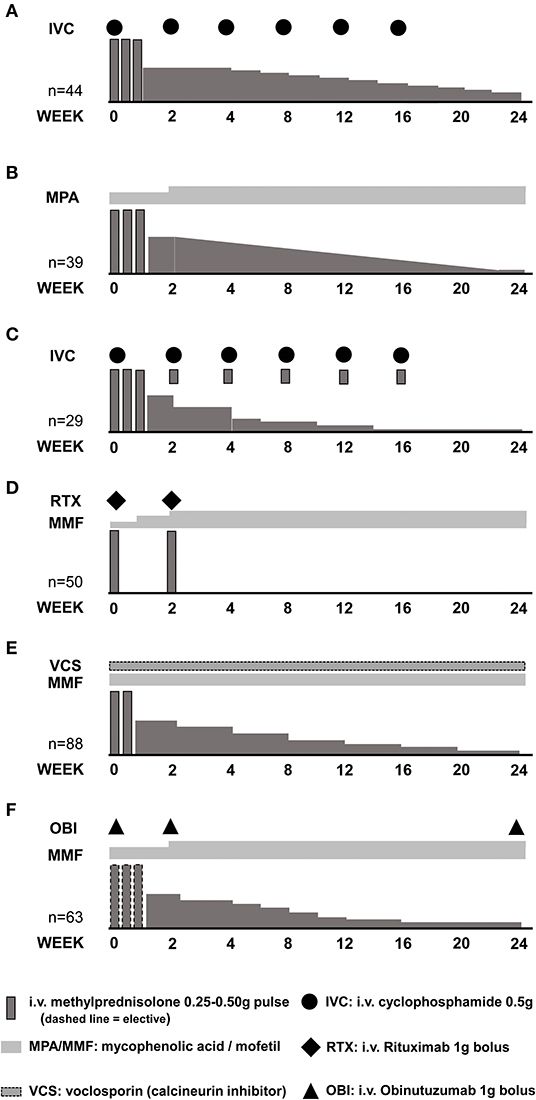
Figure 3. Induction of remission schemes in several studies. (A) Euro Lupus Nephritis Trial low-dose cyclophosphamide arm; (B) MYLUPUS reduced-dose glucocorticoid arm; (C) Lupus-Cruces protocol; (D) RITUXILUP protocol; (E) AURA-LV study voclosporin-treated arm; (F) NOBILITY study obinutuzumab-treated arm.
The MYLUPUS trial (80) is the only randomized clinical trial that compared the efficacy of medium-dose oral GC therapy to high-dose GC. In this trial, all patients received three 0.5 g methylprednisolone pulses plus extended-release mycophenolate acid. Subjects were randomized to either high-dose oral GC scheme (starting dose 1 mg/kg/day) or to a reduced-dose oral GC scheme (starting dose ≈0.5 mg/kg/day). Complete and total response rates were similar at 6 months, 19 vs. 21% and 67 vs. 56%, respectively, in both groups.
In a trial evaluating a combination of calcineurin inhibitor, mycophenolate mofetil and GCs vs. intravenous cyclophosphamide, all patients received three 0.5 g/day methylprednisolone pulses followed by 0.6 mg/kg oral prednisone slowly tapered to 10 mg/day by week 16. Response rates of 84 and 63% at 6-months, were documented in the multi-targeted therapy and cyclophosphamide groups, respectively, and of 78% in both groups by 2 years of therapy (81–83).
More recent clinical trials have used lower doses of methylprednisolone pulses combined with a lower and faster oral GC tapering. In the AURA-LV (84) and AURORA (NCT03021499) trials evaluating combination therapy of voclosporin (a novel calcineurin inhibitor) with mycophenolate mofetil and oral GC, patients were treated with two 0.25–0.5 g methylprednisolone pulses, followed by a fixed 20–25 mg/d starting oral prednisone rapidly tapered to 5 mg by 12 weeks. Among clinical trials in LN, these two trials used the lowest peak oral GC doses and the faster tapering (Table 2). At 12 months, complete and total renal response rates of 49 vs. 24%, and 67 vs. 48%, respectively, were observed in the multi-targeted treatment and control groups in the AURA-LV trial (84).
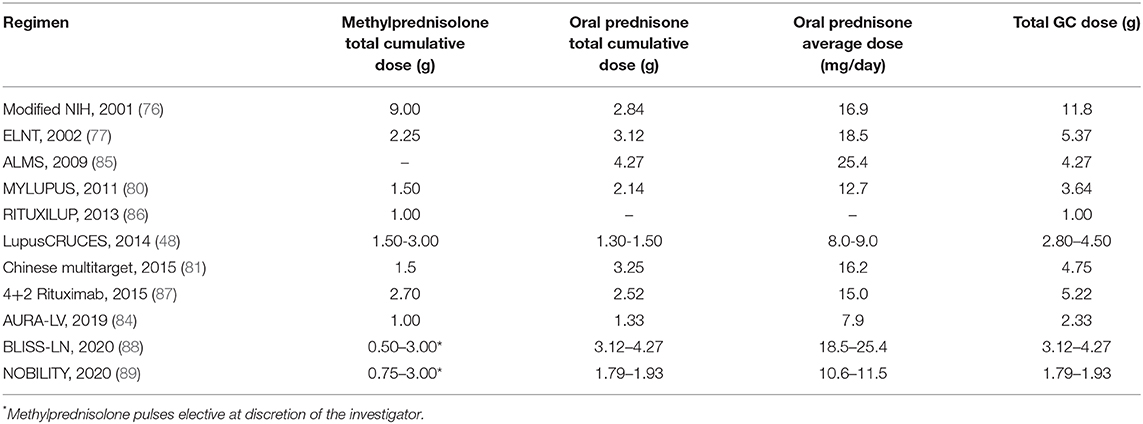
Table 2. Estimated cumulative glucocorticoid doses in a 24-week period for a 60 kg patient in different induction to remission schemes.
Uncontrolled single center experiences also suggest that treatment with methylprednisolone pulses allows a safe administration of lower starting oral GCs, and a faster tapering without compromising response and possibly reducing adverse effects. For example, the “Lupus Cruces” protocol for class III or IV LN includes the administration of three methylprednisolone pulses between 0.25–0.50 g, and an extra pulse of 0.1 g along with each cyclophosphamide bolus, following the ELNT scheme. The starting oral GC in this protocol was below 30 mg per day. In two reports, including 15 and 29 patients, response rates of 60 and 80%, and 86 and 87%, have been achieved at 6- and 12-months, respectively, with relapse rates below 15%. More importantly, the incidence of GC-related adverse effects was reduced to 7%, a significantly lower percentage when compared to that of historical or concurrent cohorts treated with higher doses of oral GC (48, 90).
Therefore, as evidenced in clinical trials and single-center experiences, the use of methylprednisolone pulses may allow reducing the starting oral GC doses and the duration of the exposure to high GC doses by allowing a faster tapering.
There are no specific reports evaluating the dose of methylprednisolone in lupus nephritis. Although the use of methylprednisolone pulses has been associated with higher risk of infection in some cohort studies (32), these studies do not control for methylprednisolone dose. A small clinical trial including 21 patients with SLE (6 of them with nephritis) suggested that clinical outcomes are similar when using three daily 100 mg vs. 1 g methylprednisolone pulses. However, this trial did not control for other important variables such as concomitant treatment (91). While quality evidence is still low to support lower doses of methylprednisolone pulses, pharmacologic studies suggest that pulse doses above 0.5 g provide little additional anti-inflammatory benefit, and as mentioned earlier, may be associated with a higher incidence of adverse effects.
Combination therapy of mycophenolate mofetil, calcineurin inhibitors, and glucocorticoids may facilitate the use of lower GC doses. As previously mentioned, the AURA-LV trial used a forced reduced steroid taper along with mycophenolate ± voclosporin. The multi-targeted group showed 67% response rate by 12 months of treatment (84).
A recent trial evaluated the combination therapy of obinutuzumab, a novel B-cell depleting therapy, with mycophenolic acid analogs. All patients received a starting oral prednisone dose of 0.5 mg/kg with a fast taper to 7.5 mg by week 12, and optional methylprednisolone pulses. This trial has reported 52-week CR rates of 35%, maintained at 40 and 41% by 76 and 104 weeks, respectively (89).
Other immunosuppressants such as Janus kinase inhibitors, spleen tyrosine kinase inhibitors and biologics such as anifrolumab and ustekinumab, are being tested in patients with LN. Their addition might also facilitate the use of lower dose glucocorticoids in the future.
After initial reports describing the potential use of rituximab without increasing GC dose in renal (92) and non-renal lupus (93), the UK group from the Imperial College in London reported their first 50 patient experience with the RITUXILUP scheme (86). This regimen consists of 2 doses of rituximab 1 g administered with 0.5 g methylprednisolone followed by mycophenolate mofetil and no oral glucocorticoids. The initial report, which included class III, IV, and V LN patients, showed 6-month complete and total response rates of 32 and 62%, respectively. During follow-up, kidney function was preserved in most patients, with 22% of patients experiencing nephrotic relapses. Importantly, unlike the LUNAR trial (94) that failed to demonstrate a benefit of added rituximab to the standard of care therapy, depletion of B-cells to <5 B lymphocytes/mL was achieved in 93% of patients. The importance of B-cell depletion is supported by a sub-analysis from the LUNAR trial showing that complete response was more frequent in those subjects with B cell depletion (95). Therefore, although not yet demonstrated in a clinical trial, the RITUXILUP scheme supports the concept that the use of biologic drugs may facilitate the administration of GC free regimen in some patients with LN.
Targeting the activated complement system with complement inhibitors may also promote GC-reduced or GC-free regimens. Although complement inhibition in lupus nephritis has been used in a few case reports (96, 97), particularly in the context of concomitant thrombotic microangiopathy, the CLEAR (98) and ADVOCATE (99) studies in ANCA-associated vasculitis suggest this may be an approach worth investigating in lupus nephritis. In these studies, administration of avacopan (an oral complement C5aR inhibitor) along with cyclophosphamide or rituximab, allowed the administration of a GC-free regimen with higher remission rates at 52 weeks of follow-up in patients with ANCA-associated vasculitis (99).
The use of antimalarial in all patients with SLE and lupus nephritis is recommended in recent guidelines (38, 39). Although unexplored in controlled trials, combination schemes with antimalarial may add to the use of lower doses of GC by an enhanced effect for remission (100–102). Other demonstrated benefits from antimalarial, as the protective effect for damage accrual (103, 104), infections (33), and mortality (105), may add to the potential benefit of GC-reduced regimens.
Maintenance therapy in lupus nephritis aims to consolidate the response obtained after the induction phase of therapy, and to prevent systemic and renal relapses. Current guidelines suggest tapering glucocorticoids to “the lowest possible dose” and to consider discontinuation after 12 months of complete remission (39).
Although there is no solid evidence in lupus nephritis, the CORTICOLUP trial (106) evaluated discontinuation of steroid in stable SLE patients (34–41% had history of LN). In this trial, patients receiving 5 mg of prednisone who have been stable for 1 year (the median quiescence duration was ≈5 years) were randomized to suspend or continue prednisone at the same dose. Disease flares were observed in 27% of patients who suspended prednisone vs. 7% in those who continued prednisone at 5 mg per day (RR 0.2, 0.01–0.7, p = 0.003). Only 3 patients had renal flares and the study was underpowered to evaluate the subgroup of patients with LN. Noteworthy, there were no differences in adverse events or damage accrual in both groups measured using the glucocorticoid toxicity index (107) and the SDI, respectively.
This study suggests that a low-dose of glucocorticoids at 5 mg per day may be safe and keeps patients free from disease flares. In other studies, the longer duration of the GC therapy before suspension has also been associated with less disease flares (108). A recent EULAR expert consensus suggested that at ≤ 5 mg/day, there is a low level of harm related to GC's main adverse effects (109), however, acknowledges that the actual risk of harm is patient-specific. Therefore, long-term glucocorticoid therapy must be balanced individually considering individual risk factors for flares (e.g., partial instead of complete response, persistently low C3), against individual risk factors for GC related adverse effects (e.g., age, cumulative GC dose, cardiovascular risk factors, presence of metabolic disease, etc.).
Antimalarials have been associated with lower incidence of disease flares in several observational cohort studies (110, 111). Moreover, reports of successful withdrawal of therapy in SLE patients have repeatedly found antimalarial treatment and duration of remission as the main factors associated with decreased odds of flares (112, 113). Also, as previously mentioned, antimalarials may reduce long-term damage from the disease activity (114).
Although evidence is still scarce, there is growing data suggesting that the use of certain biologics during the maintenance phase may aid in achieving sustained remission. For patients already on glucocorticoids, the RITUXIRESCUE regimen includes the administration of rituximab and methylprednisolone without increasing oral GC dose. This regimen showed a response rate of 78% in LN relapses, furthermore it allowed reduction or discontinuation of oral GC in more than 50% of patients during follow up (92).
An Italian strategy consisting of four 375 g/m2 rituximab doses reinforced by two additional doses at 1 and 2 months after (the 4+2 rituximab scheme), showed no flares during follow-up without the need for additional maintenance therapy beyond 5 mg of prednisone per day (87). Other small reports have highlighted the potential role of rituximab as a maintenance drug allowing glucocorticoid suspension (115). Therefore, although rituximab has not been tested for maintenance in a clinical trial, its use may aid in preventing flares during GC withdrawal.
In the BLISS-LN trial, the addition of belimumab to standard of care therapy (MMF or cyclophosphamide plus GC) showed a better response and a stable glomerular filtration rate beyond the induction phase, for up to 2 years of follow up (88). Furthermore, there have been small reports (116–118) suggesting that belimumab therapy may allow reduction or suspension of maintenance GCs, but this remains to be further studied.
Likewise, the NOBILITY trial has reported that the addition of obinutuzumab to standard of care therapy favored a sustained response, better glomerular filtration rate, and better serological profile at 76 weeks and onwards (89). This suggests that B cell targeted therapy may potentially facilitate GC withdrawal or at least a safe reduction to <5 mg/d of prednisone.
Although recent advances in drug development in lupus nephritis promote the use of lower glucocorticoid doses, we must acknowledge that “one size does not fit all” patients. For example, patients with severe lupus nephritis presenting with a glomerular filtration rate below 30 mL/min/1.73 m2 have been excluded from most clinical trials, and there are no data to support the effectiveness of reduced glucocorticoid doses in this group of patients. Moreover, many of the published studies are single-center and observational reports subject to bias. Therefore, caution and case-by-case evaluation is recommended in selecting an appropriate glucocorticoid therapy.
Future studies in lupus nephritis will likely aim at using the lowest effective dose of glucocorticoids or glucocorticoid-free regimens. Studying the safety and efficacy of calcineurin inhibitors, biologic drugs or perhaps complement inhibitors in combination with standard of care therapy might lead successfully to this aim.
The anti-inflammatory properties of GC have always been counterbalanced by their side effects. Adverse effects may be associated with peak doses, time under high doses, or cumulative doses. An objective for current and future management of lupus nephritis is to develop strategies that increase response to therapy with the least glucocorticoid exposure.
JM-V and IA designed the concept, planned, and performed this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Hench PS, Kendall EC, Slocumb CH, Polley HF. Adrenocortical hormone in arthritis : preliminary report. Ann Rheum Dis. (1949) 8:97–104. doi: 10.1136/ard.8.2.97
2. Burns CM. The history of cortisone discovery and development. Rheum Dis Clin North Am. (2016) 42:1–14. doi: 10.1016/j.rdc.2015.08.001
3. Cameron JS. A Comparison of cortisone and prednisone in treatment of rheumatoid arthritis. Br Med J. (1957) 2:199–202. doi: 10.1136/bmj.2.5038.199
4. Cameron JS. Lupus nephritis: an historical perspective 1968-1998. J Nephrol. (1999) 12(Suppl. 2):S29–41.
5. Pollak VE, Pirani CL, Schwartz FD. The natural history of the renal manifestations of systemic lupus erythematosus. 1964. J Am Soc Nephrol. (1997) 8:1189–98.
6. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids — New mechanisms for old drugs. N Engl J Med. (2005) 353:1711–23. doi: 10.1056/NEJMra050541
7. Buttgereit F, Wehling M, Burmester G-R. A new hypothesis of modular glucocorticoid actions: steroid treatment of rheumatic diseases revisited. Arthritis Rheum. (1998) 41:761–73. doi: 10.1002/1529-0131(199805)41:5<761::AID-ART2>3.0.CO;2-M
8. Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. (2008) 4:525–33 doi: 10.1038/ncprheum0898
9. Strehl C, Buttgereit F. Unraveling the functions of the membrane-bound glucocorticoid receptors: first clues on origin and functional activity. Ann N Y Acad Sci. (2014) 1318:1–6. doi: 10.1111/nyas.12364
10. Buttgereit F. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. (2002) 61:718–22. doi: 10.1136/ard.61.8.718
11. Buttgereit F, Straub RH, Wehling M, Burmester GR. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum. (2004) 50:3408–17. doi: 10.1002/art.20583
12. Schmid D, Burmester GR, Tripmacher R, Kuhnke A, Buttgereit F. Bioenergetics of human peripheral blood mononuclear cell metabolism in quiescent, activated, and glucocorticoid-treated states. Biosci Rep. (2000) 20:289–302. doi: 10.1023/A:1026445108136
13. Williams DM. Clinical pharmacology of corticosteroids. Respir Care. (2018) 63:655–70. doi: 10.4187/respcare.06314
14. Scherholz ML, Schlesinger N, Androulakis IP. Chronopharmacology of glucocorticoids. Adv Drug Deliv Rev. (2019) 151–2:245–261. doi: 10.1016/j.addr.2019.02.004
15. Zen M, Iaccarino L, Gatto M, Bettio S, Nalotto L, Ghirardello A, et al. Prolonged remission in Caucasian patients with SLE: prevalence and outcomes. Ann Rheum Dis. (2015) 74:2117–22. doi: 10.1136/annrheumdis-2015-207347
16. Nossent J, Kiss E, Rozman B, Pokorny G, Vlachoyiannopoulos P, Olesinska M, et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus. (2010) 19:949–56. doi: 10.1177/0961203310366572
17. Gladman DD, Urowitz MB, Rahman P, Ibañez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. (2003) 30:1955–9.
18. Karlson EW, Daltroy LH, Lew RA, Wright EA, Partridge AJ, Fossel AH, et al. The relationship of socioeconomic status, race, and modifiable risk factors to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum. (1997) 40:47–56. doi: 10.1002/art.1780400108
19. Al Sawah S, Zhang X, Zhu B, Magder LS, Foster SA, Iikuni N, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus–the Hopkins Lupus Cohort. Lupus Sci Med. (2015) 2:e000066. doi: 10.1136/lupus-2014-000066
20. Zen M, Iaccarino L, Gatto M, Bettio S, Saccon F, Ghirardello A, et al. The effect of different durations of remission on damage accrual: results from a prospective monocentric cohort of Caucasian patients. Ann Rheum Dis. (2017) 76:562–5. doi: 10.1136/annrheumdis-2016-210154
21. Singh JA, Hossain A, Kotb A, Wells G. Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis. BMC Med. (2016) 14:137. doi: 10.1186/s12916-016-0673-8
22. Thamer M, Hernan MA, Zhang Y, Cotter D, Petri M. Prednisone, lupus activity, and permanent organ damage. J Rheumatol. (2009) 36:560–4. doi: 10.3899/jrheum.080828
23. Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. (2012) 176:708–19. doi: 10.1093/aje/kws130
24. Ward MM, Pyun E, Studenski S. Causes of death in systemic lupus erythematosus, long-term follow up of an inception cohort. Arthritis Rheum. (1995) 38:1492–9. doi: 10.1002/art.1780381016
25. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period. Medicine. (2003) 82:299–308. doi: 10.1097/01.md.0000091181.93122.55
26. Yap DYH, Tang CSO, Ma MKM, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. (2012) 27:3248–54. doi: 10.1093/ndt/gfs073
27. Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, et al. Serious infections among adult medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. (2015) 67:1577–85. doi: 10.1002/art.39070
28. Gladman DD, Hussain F, Iban D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus. (2002) 11:234–9. doi: 10.1191/0961203302lu170oa
29. Noel V. Risk factors and prognostic influence of infection in a single cohort of 87 adults with systemic lupus erythematosus. Ann Rheum Dis. (2001) 60:1141–4. doi: 10.1136/ard.60.12.1141
30. Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, Martinez-Berriotxoa A, Egurbide M-V, Aguirre C. Predictors of major infections in systemic lupus erythematosus. Arthritis Res Ther. (2009) 11:R109. doi: 10.1186/ar2764
31. Rúa-Figueroa I, López-Longo FJ, Del Campo V, Galindo-Izquierdo M, Uriarte E, Torre-Cisneros J, et al. Bacteremia in systemic lupus erythematosus in patients from a Spanish Registry: risk factors, clinical and microbiological characteristics, and outcomes. J Rheumatol. (2020) 47:234–40. doi: 10.3899/jrheum.180882
32. Pimentel-Quiroz VR, Ugarte-Gil MF, Harvey G, Wojdyla D, Pons-Estel GJ, Quintana R, et al. Factors predictive of serious infections over time in systemic lupus erythematosus patients: data from a multi-ethnic, multi-national, Latin American lupus cohort. Lupus. (2019) 28:1101–10. doi: 10.1177/0961203319860579
33. González-Echavarri C, Capdevila O, Espinosa G, Suárez S, Marín-Ballvé A, González-León R, et al. Infections in newly diagnosed Spanish patients with systemic lupus erythematosus: data from the RELES cohort. Lupus. (2018) 27:2253–61. doi: 10.1177/0961203318811598
34. Teh CL, Wan SA, Ling GR. Severe infections in systemic lupus erythematosus: disease pattern and predictors of infection-related mortality. Clin Rheumatol. (2018) 37:2081–6. doi: 10.1007/s10067-018-4102-6
35. Badsha H, Kong KO, Lian TY, Chan SP, Edwards CJ, Chng HH. Low-dose pulse methylprednisolone for systemic lupus erythematosus flares is efficacious and has a decreased risk of infectious complications. Lupus. (2002) 11:508–13. doi: 10.1191/0961203302lu243oa
36. Badsha H, Edwards CJ. Intravenous pulses of methylprednisolone for systemic lupus erythematosus. Semin Arthritis Rheum. (2003) 32:370–7. doi: 10.1053/sarh.2002.50003
37. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev. (2008) 8:153–5. doi: 10.1016/j.autrev.2008.07.010
38. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 Update of the joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. (2020) 79:713–23. doi: 10.1136/annrheumdis-2020-eular.3936
39. KDIGO: Kidney Disease Improving Global Outcomes Clinical Practice Guideline for Glomerulonephritis. Kidney Int. (2021). Available online at: kdigo.org/guidelines/
40. Petri M. Musculoskeletal complications of systemic lupus erythematosus in the Hopkins lupus cohort: an update. Arthritis Care Res. (1995) 8:137–45. doi: 10.1002/art.1790080305
41. Gladman DD, Dhillon N, Su J, Urowitz MB. Osteonecrosis in SLE: prevalence, patterns, outcomes and predictors. Lupus. (2018) 27:76–81. doi: 10.1177/0961203317711012
42. Sayarlioglu M, Yuzbasioglu N, Inanc M, Kamali S, Cefle A, Karaman O, et al. Risk factors for avascular bone necrosis in patients with systemic lupus erythematosus. Rheumatol Int. (2012) 32:177–82. doi: 10.1007/s00296-010-1597-9
43. Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskelet Med. (2015) 8:201–9. doi: 10.1007/s12178-015-9277-8
44. Hussein S, Suitner M, Béland-Bonenfant S, Baril-Dionne A, Vandermeer B, Santesso N, et al. Monitoring of osteonecrosis in systemic lupus erythematosus: a systematic review and metaanalysis. J Rheumatol. (2018) 45:1462–76. doi: 10.3899/jrheum.170837
45. Mok CC, Lau CS, Wong RWS. Risk factors for avascular bone necrosis in systemic lupus erythematosus. Rheumatology. (1998) 37:895–900. doi: 10.1093/rheumatology/37.8.895
46. Mosca M, Tani C, Carli L, Bombardieri S. Glucocorticoids in systemic lupus erythematosus. Clin Exp Rheumatol. (2011) 29:S126–9.
47. Sciascia S, Mompean E, Radin M, Roccatello D, Cuadrado MJ. Rate of adverse effects of medium- to high-dose glucocorticoid therapy in systemic lupus erythematosus: a systematic review of randomized control trials. Clin Drug Investig. (2017) 37:519–24. doi: 10.1007/s40261-017-0518-z
48. Ruiz-Irastorza G, Danza A, Perales I, Villar I, Garcia M, Delgado S, et al. Prednisone in lupus nephritis: how much is enough? Autoimmun Rev. (2014) 13:206–14. doi: 10.1016/j.autrev.2013.10.013
49. Chen HL, Shen LJ, Hsu PN, Shen CY, Hall SA, Hsiao FY. Cumulative burden of glucocorticoid-related adverse events in patients with systemic lupus erythematosus: findings from a 12-year longitudinal study. J Rheumatol. (2018) 45:83–9. doi: 10.3899/jrheum.160214
50. Bultink IEM, Lems WF, Kostense PJ, Dijkmans BAC, Voskuyl AE. Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum. (2005) 52:2044–50. doi: 10.1002/art.21110
51. Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos Int. (2019) 30:1145–56. doi: 10.1007/s00198-019-04906-x
52. Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. (2006) 55:420–6. doi: 10.1002/art.21984
53. Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. (2017) 69:1095–110. doi: 10.1002/acr.23279
54. Adams J, Wilson N, Hurkmans E, Bakkers M, Balážová P, Baxter M, et al. 2019 EULAR points to consider for non-physician health professionals to prevent and manage fragility fractures in adults 50 years or older. Ann Rheum Dis. (2021) 80:57–64. doi: 10.1136/annrheumdis-2020-216931
55. Parker B, Bruce IN. The metabolic syndrome in systemic lupus erythematosus. Rheum Dis Clin North Am. (2010) 36:81–97. doi: 10.1016/j.rdc.2009.12.004
56. Parker B, Urowitz MB, Gladman DD, Lunt M, Bae SC, Sanchez-Guerrero J, et al. Clinical associations of the metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis. (2013) 72:1308–14. doi: 10.1136/annrheumdis-2012-202106
57. Parker B, Urowitz MB, Gladman DD, Lunt M, Donn R, Bae SC, et al. Impact of early disease factors on metabolic syndrome in systemic lupus erythematosus: data from an international inception cohort. Ann Rheum Dis. (2015) 74:1530–6. doi: 10.1136/annrheumdis-2013-203933
58. Sabio JM, Vargas-Hitos JA, Navarrete N, Hidalgo-Tenorio C, Jiménez-Alonso J, Grupo Lupus Virgen de las Nieves. Effects of low or medium-dose of prednisone on insulin resistance in patients with systemic lupus erythematosus. Clin Exp Rheumatol. (2010) 28:483–9.
59. Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care. (2006) 29:2728–9. doi: 10.2337/dc06-1499
60. Burt MG, Willenberg VM, Petersons CJ, Smith MD, Ahem MJ, Stranks SN. Screening for diabetes in patients with inflammatory rheumatological disease administered long-term prednisolone: a cross-sectional study. Rheumatology. (2012) 51:1112–9. doi: 10.1093/rheumatology/kes003
61. Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. (2009) 68:1119–24. doi: 10.1136/ard.2008.092163
62. Reynolds RM, Labad J, Sears AV, Williamson RM, Strachan MW, Deary IJ, et al. Glucocorticoid treatment and impaired mood, memory and metabolism in people with diabetes: the Edinburgh Type 2 Diabetes Study. Eur J Endocrinol. (2012) 166:861–8. doi: 10.1530/EJE-12-0041
63. Feldman-Billard S, Lissak B, Kassaei R, Benrabah R, Héron E. Short-term tolerance of pulse methylprednisolone therapy in patients with diabetes mellitus. Ophthalmology. (2005) 112:511–5. doi: 10.1016/j.ophtha.2004.10.032
64. Mejia-Vilet JM, López-Hernández YJ, Trujeque-Matos M, Santander-Velez JI, Cano-Verduzco ML, Cruz C, et al. High frequency of nocturnal hypertension in lupus nephritis: should ABPM be implemented in usual practice? Clin Rheumatol. (2020) 39:1147–55. doi: 10.1007/s10067-019-04830-9
65. Mathis KW, Venegas-Pont M, Masterson CW, Wasson KL, Ryan MJ. Blood pressure in a hypertensive mouse model of SLE is not salt-sensitive. Am J Phys Regul Integr Comp Phys. (2011) 301:R1281–5. doi: 10.1152/ajpregu.00386.2011
66. Conn HO, Poynard T. Corticosteroids and peptic ulcer: meta-analysis of adverse events during steroid therapy. J Intern Med. (1994) 236:619–32. doi: 10.1111/j.1365-2796.1994.tb00855.x
67. Panoulas VF, Douglas KKMJ, Stavropoulos-Kalinoglou A, Metsios GS, Nightingale P, Kita MD, et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology. (2008) 47:72–5. doi: 10.1093/rheumatology/kem311
68. Strack AM, Sebastian RJ, Schwartz MW, Dalllman MF. Glucocorticoids and insulin: reciprocal signals for energy balance. Am J Physiol. (1995) 268:R142–9 doi: 10.1152/ajpregu.1995.268.1.R142
69. Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H, Akana SF. Minireview: glucocorticoids- food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology. (2004) 145:2633–8. doi: 10.1210/en.2004-0037
70. Fardet L, Cabane J, Lebbé C, Morel P, Flahault A. Incidence and risk factors for corticosteroid-induced lipodystrophy: a prospective study. J Am Acad Dermatol. (2007) 57:604–9. doi: 10.1016/j.jaad.2007.04.018
71. Tselios K, Sheane BJ, Gladman DD, Urowitz MB. Optimal monitoring for coronary heart disease risk in patients with systemic lupus erythematosus: a systematic review. J Rheumatol. (2016) 43:54–65. doi: 10.3899/jrheum.150460
72. Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, et al. Subclinical atherosclerosis in patients with systemic lupus erythematosus: a systemic review and meta-analysis. Autoimmun Rev. (2015) 15:22–37. doi: 10.1016/j.autrev.2015.10.002
73. Fardet L, Petersen I, Nazareth I. Risk of cardiovascular events in people prescribed glucocorticoids with iatrogenic Cushing's syndrome: cohort study. BMJ. (2012) 345:e4928. doi: 10.1136/bmj.e4928
74. Ruiz-Arruza I, Lozano J, Cabezas-Rodriguez I, Medina JA, Ugarte A, Erdozain JG, et al. Restrictive use of oral glucocorticoids in systemic lupus erythematosus and prevention of damage without worsening long-term disease control: an observational study. Arthritis Care Res. (2018) 70:582–91. doi: 10.1002/acr.23322
75. Mejia-Vilet JM, Rovin BH. Chapter 59. Epidemiology and Management of Lupus Nephritis. In: Wallace Daniel J and Hahn Bevra H, editors. Dubois Systemic Lupus Erythematosus. Philadelphia, PA: Elsevier. (2019). p. 727–44. doi: 10.1016/B978-0-323-47927-1.00059-1
76. Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarboro CH, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. (2001) 135:248. doi: 10.7326/0003-4819-135-4-200108210-00009
77. Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, Garrido E de R, Danieli MG, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. (2002) 46:2121–31. doi: 10.1002/art.10461
78. Yee CS. EULAR randomised controlled trial of pulse cyclophosphamide and methylprednisolone versus continuous cyclophosphamide and prednisolone followed by azathioprine and prednisolone in lupus nephritis. Ann Rheum Dis. (2004) 63:525–9. doi: 10.1136/ard.2002.003574
79. Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. (2010) 69:61–4. doi: 10.1136/ard.2008.102533
80. Zeher M, Doria A, Lan J, Aroca G, Jayne D, Boletis I, et al. Efficacy and safety of enteric-coated mycophenolate sodium in combination with two glucocorticoid regimens for the treatment of active lupus nephritis. Lupus. (2011) 20:1484–93. doi: 10.1177/0961203311418269
81. Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, et al. Multitarget therapy for induction treatment of lupus nephritis. Ann Intern Med. (2015) 162:18. doi: 10.7326/M14-1030
82. Zhang H, Liu Z, Zhou M, Liu Z, Chen J, Xing C, et al. Multitarget therapy for maintenance treatment of lupus nephritis. J Am Soc Nephrol. (2017) 28:3671–8. doi: 10.1681/ASN.2017030263
83. Ayoub I, Rovin BH. Calcineurin inhibitors in the treatment of lupus nephritis: a hare versus turtle story? J Am Soc Nephrol. (2017) 28:3435–7. doi: 10.1681/ASN.2017080830
84. Rovin BH, Solomons N, Pendergraft WF, Dooley MA, Tumlin J, Romero-Diaz J, et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. (2019) 95:219–31. doi: 10.1016/j.kint.2018.08.025
85. Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. (2009) 20:1103–12. doi: 10.1681/ASN.2008101028
86. Condon MB, Ashby D, Pepper RJ, Cook HT, Levy JB, Griffith M, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. (2013) 72:1280–6. doi: 10.1136/annrheumdis-2012-202844
87. Roccatello D, Sciascia S, Baldovino S, Rossi D, Alpa M, Naretto C, et al. A 4-year observation in lupus nephritis patients treated with an intensified B-lymphocyte depletion without immunosuppressive maintenance treatment—Clinical response compared to literature and immunological re-assessment. Autoimmun Rev. (2015) 14:1123–30. doi: 10.1016/j.autrev.2015.07.017
88. Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. (2020) 383:1117–28. doi: 10.1056/NEJMoa2001180
89. Rovin BH, Aroca-Martinez G, Alvarez A, Fragoso-Loyo HE, Zuta AE, Furie R, et al. Two-year results from a randomized, controlled study of obinutuzumab for proliferative lupus nephritis. J Am Soc Nephrol. (2020) 31:53.
90. Ruiz-Irastorza G, Ugarte A, Saint-Pastou Terrier C, Lazaro E, Iza A, Couzi L, et al. Repeated pulses of methyl-prednisolone with reduced doses of prednisone improve the outcome of class III, IV and V lupus nephritis: an observational comparative study of the Lupus-Cruces and lupus-Bordeaux cohorts. Autoimmun Rev. (2017) 16:826–32. doi: 10.1016/j.autrev.2017.05.017
91. Edwards JCW, Snaith ML, Isenberg DA. A double blind controlled trial of methylprednisolone infusions in systemic lupus erythematosus using individualised outcome assessment. Ann Rheum Dis. (1987) 46:773–6. doi: 10.1136/ard.46.10.773
92. Pepper R, Griffith M, Kirwan C, Levy J, Taube D, Pusey C, et al. Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Nephrol Dial Transplant. (2009) 24:3717–23. doi: 10.1093/ndt/gfp336
93. Ezeonyeji AN, Isenberg DA. Early treatment with rituximab in newly diagnosed systemic lupus erythematosus patients: a steroid-sparing regimen. Rheumatology. (2012) 51:476–81. doi: 10.1093/rheumatology/ker337
94. Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. (2012) 64:1215–26. doi: 10.1002/art.34359
95. Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P, Dall'Era M, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol. (2018) 13:1502–9. doi: 10.2215/CJN.01070118
96. Sciascia S, Radin M, Yazdany J, Tektonidou M, Cecchi I, Roccatello D, et al. Expanding the therapeutic options for renal involvement in lupus: eculizumab, available evidence. Rheumatol Int. (2017) 37:1249–55. doi: 10.1007/s00296-017-3686-5
97. Wright RD, Bannerman F, Beresford MW, Oni L. A systematic review of the role of eculizumab in systemic lupus erythematosus-associated thrombotic microangiopathy. BMC Nephrol. (2020) 21:245 doi: 10.1186/s12882-020-01888-5
98. Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. (2017) 28:2756–67. doi: 10.1681/ASN.2016111179
99. Jayne DR, Merkel PA, Yue H, Kelleher CL, Schall TJ, Bekker P. Complement C5a receptor inhibitor avacopan improves renal function in ANCA vasculitis. J Am Soc Nephrol. (2020) 31:53.
100. Kasitanon N, Fine DM, Haas M, Magder LS, Petri M. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus. (2006) 15:366–70. doi: 10.1191/0961203306lu2313oa
101. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. (2010) 69:20–8. doi: 10.1136/ard.2008.101766
102. Mejia-Vilet JM, Cordova-Sanchez BM, Uribe-Uribe NO, Correa-Rotter R. Immunosuppressive treatment for pure membranous lupus nephropathy in a Hispanic population. Clin Rheumatol. (2016) 35:2219–27. doi: 10.1007/s10067-016-3366-y
103. Fessler BJ, Alarcón GS, McGwin G, Roseman J, Bastian HM, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. (2005) 52:1473–80. doi: 10.1002/art.21039
104. Pons-Estel GJ, Alarcón GS, McGwin G, Danila MI, Zhang J, Bastian HM, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. (2009) 61:830–9. doi: 10.1002/art.24538
105. Shinjo SK, Bonfá E, Wojdyla D, Borba EF, Ramirez LA, Scherbarth HR, et al. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum. (2010) 62:855–62. doi: 10.1002/art.27300
106. Mathian A, Pha M, Haroche J, Cohen-Aubart F, Hié M, Pineton de Chambrun M, et al. Withdrawal of low-dose prednisone in SLE patients with a clinically quiescent disease for more than 1 year: a randomised clinical trial. Ann Rheum Dis. (2020) 79:339–46. doi: 10.1136/annrheumdis-2019-216303
107. Miloslavsky EM, Naden RP, Bijlsma JWJ, Brogan PA, Brown ES, Brunetta P, et al. Development of a Glucocorticoid Toxicity Index (GTI) using multicriteria decision analysis. Ann Rheum Dis. (2017) 76:543–6. doi: 10.1136/annrheumdis-2016-210002
108. Goswami RP, Sit H, Ghosh P, Sircar G, Ghosh A. Steroid-free remission in lupus: myth or reality; an observational study from a tertiary referral centre. Clin Rheumatol. (2019) 38:1089–97. doi: 10.1007/s10067-018-4377-7
109. Strehl C, Bijlsma JWJ, de Wit M, Boers M, Caeyers N, Cutolo M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis. (2016) 75:952–7. doi: 10.1136/annrheumdis-2015-208916
110. Costedoat-Chalumeau N, Amoura Z, Hulot JS, Hammoud HA, Aymard G, Cacoub P, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum. (2006) 54:3284–90. doi: 10.1002/art.22156
111. Costedoat-Chalumeau N, Galicier L, Aumaître O, Francès C, Guern V Le, Lioté F, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis. (2013) 72:1786–92. doi: 10.1136/annrheumdis-2012-202322
112. Zen M, Saccon F, Gatto M, Montesso G, Larosa M, Benvenuti F, et al. Prevalence and predictors of flare after immunosuppressant discontinuation in patients with systemic lupus erythematosus in remission. Rheumatology. (2020) 59:1591–8. doi: 10.1093/rheumatology/kez422
113. Moroni G, Gallelli B, Quaglini S, Banfi G, Rivolta E, Messa P, et al. Withdrawal of therapy in patients with proliferative lupus nephritis: long-term follow-up. Nephrol Dial Transplant. (2006) 21:1541–8. doi: 10.1093/ndt/gfk073
114. Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum. (2012) 64:4021–8. doi: 10.1002/art.34672
115. Camous L, Melander C, Vallet M, Squalli T, Knebelmann B, Noël LH, et al. Complete remission of lupus nephritis with rituximab and steroids for induction and rituximab alone for maintenance therapy. Am J Kidney Dis. (2008) 52:346–52. doi: 10.1053/j.ajkd.2008.03.036
116. Sciascia S, Radin M, Yazdany J, Levy R, Roccatello D, Dall'Era M, et al. Efficacy of belimumab on renal outcomes in patients with systemic lupus erythematosus: a systematic review. Autoimmun Rev. (2017) 16:287–93. doi: 10.1016/j.autrev.2017.01.010
117. Binda V, Trezzi B, Del Papa N, Beretta L, Frontini G, Porata G, et al. Belimumab may decrease flare rate and allow glucocorticoid withdrawal in lupus nephritis (including dialysis and transplanted patient). J Nephrol. (2020) 33:1019–25. doi: 10.1007/s40620-020-00706-3
Keywords: glucocorticoids, lupus nephritis, systemic lupus erythematosus, prednisone, methylpredisolone, steroids, adverse effect
Citation: Mejía-Vilet JM and Ayoub I (2021) The Use of Glucocorticoids in Lupus Nephritis: New Pathways for an Old Drug. Front. Med. 8:622225. doi: 10.3389/fmed.2021.622225
Received: 28 October 2020; Accepted: 20 January 2021;
Published: 16 February 2021.
Edited by:
Savino Sciascia, University of Turin, ItalyReviewed by:
Claudio Ponticelli, Retired, ItalyCopyright © 2021 Mejía-Vilet and Ayoub. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabelle Ayoub, SXNhYmVsbGUuQXlvdWJAb3N1bWMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.