
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 18 February 2021
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.616511
This article is part of the Research TopicSpotlight on the Relationship between Sepsis and Infection: from Mechanisms to TherapyView all 26 articles
 Stefano Busani1
Stefano Busani1 Erika Roat1
Erika Roat1 Martina Tosi1
Martina Tosi1 Emanuela Biagioni1
Emanuela Biagioni1 Irene Coloretti1
Irene Coloretti1 Marianna Meschiari2
Marianna Meschiari2 Roberta Gelmini3
Roberta Gelmini3 Lucio Brugioni4
Lucio Brugioni4 Sara De Biasi5
Sara De Biasi5 Massimo Girardis1*
Massimo Girardis1*Septic shock still has a high mortality rate which has not hinted at decreasing in recent years. Unfortunately, randomized trials failed mainly because the septic patient was considered as a homogeneous entity. All this creates a sort of therapeutic impotence in everyday clinical practice in treating patients with septic shock. The need to customize therapy on each patient with sepsis has now become an established necessity. In this scenario, adjuvant therapies can help if interpreted as modulators of the immune system. Indeed, the host's immune response differs from patient to patient based on the virulence of the pathogen, comorbidity, infection site, and prolonged hospitalization. In this review, we summarize the rationale for using immunoglobulins as an adjunctive treatment. Furthermore, we would like to suggest a possible protocol to personalize treatment in the different clinical scenarios of the host's response to serious infectious events.
Septic shock is a complex syndrome occurring when sepsis is associated with circulatory, cellular, and metabolic abnormalities to such an extent that the risk of death is substantially increased compared to sepsis alone. The clinical criteria to define this condition have recently been modified to improve its identification (1).
Despite the progressive comprehension of its pathogenesis, mortality rates are high and did not significantly change in the last 10 years. Septic shock hospital mortality was described as around 40% in a recent meta-analysis analyzing data from 71 studies from Europe and North America (2).
Another worrying aspect of septic shock is randomized clinical trials (RCTs) designed in the last years to test additional therapies that gave discouraging results. Historically therapeutically strategies, some of them appearing promising in preclinical studies, have been developed based on septic shock pathogenesis.
One of the first targets identified and studied was endotoxin present in gram-negative bacteria, which has been blocked through different anti-lipid A antibodies without obtaining benefit in RTCs (3, 4). Similarly, the use of anti-TNF antibodies or anti-IL-1 antibodies was developed with the purpose to limit the innate immune hyperactivation responsible for tissue damage, but larger RTCs results were negative (5, 6). Endothelial dysfunction, frequently found in septic patients, was investigated trying to improve microcirculation and tissue oxygenation, but neither platelet-activating factor antagonist (7) nor activated protein C (8) reduced mortality.
Several different extracorporeal blood purification techniques have been developed in the last decades to remove inflammatory mediators. High volume hemofiltration was unable to reduce mortality in a recent meta-analysis (9), although was considered a safe technique. Hemoperfusion using filters coated with polymyxin B, aimed to remove endotoxins able to trigger the inflammatory response, displayed contrasting results in RCTs (10, 11). Concerning coupled plasma filtration and adsorption (CPFA) interesting results were obtained in the COMPACT 1 randomized study (12) but the COMPACT 2 trial was stopped earlier because of adverse events associated with CPFA. An urgent letter was sent to all CPFA users mentioning that CPFA is no longer indicated for the treatment of septic shock1. Among new membranes, Cytosorb a hemoperfusion cartridge able to remove broad-spectrum cytokines failed to find any decrease of IL-6 plasma levels over time (13); while a recent proof of concept pilot study demonstrated a significant effect on norepinephrine requirements (14). The new Oxyris membrane, a heparin-grafted membrane specifically designed for cytokine and endotoxin adsorption, tested on 16 patients seemed to effectively remove endotoxin and TNF-α, IL-6, IL-8, and IFNγ in patients with septic shock-associated acute renal failure (15).
Further, the unsuccessfully tested approach included immunomodulant and antioxidant therapies, aimed to reduce the overwhelming tissue damage caused by the excessive activation of the host's response. The very recent ACTS and ATESS RCTs failed to demonstrate improvement in organ function with a combination of vitamins and corticosteroids (16, 17). The promising results obtained by Meisel et al. about granulocyte-macrophage colony-stimulating factor in sepsis (18), unfortunately, have not yet found confirmation from other studies with adequate power.
As a consequence of this litany of negative studies, over the years the Recommendations provided by the Surviving Sepsis Campaign (SSC) Guidelines (19) for the management of septic shock have been progressively reduced, moreover being in most cases negative (i.e., prescribing not to do something), limiting the therapeutic space of maneuver for clinicians. Thus, although the role of the Guidelines is guided by the “not to harm” principle, stratification into subpopulations may be advisable in a heterogeneous setting.
Unfortunately, nowadays it is not easy to answer this question because sepsis is the expression of many different clinical scenarios. Patient characteristics, such as age, comorbidities, genetic factors, immune response, and previous antibiotics exposure are important factors involved in the evolution from sepsis to septic shock; thus, all these variables partly explain the difficulty of defining a unique treatment suitable for all patients.
In light of this observation, some authors developed a clinical staging system called PIRO aimed to characterize septic patients through four components: Predisposition (P), Insult/Infection (I), Response (R), and finally Organ dysfunction (O) evaluating time and number of failing organs. PIRO model can stratify infected patients in 4 stages defined by a progressive increase in mortality trying to reduce the heterogeneity of patients and to help a more tailored therapy (20, 21). Despite this attempt, complexity, and variability have been so far ignored by clinical trials enrolling patients only based on a septic shock diagnosis without considering selecting a specific and more homogeneous subgroup. The lack of patients' selection may in part explain the negative results obtained in all the RCTs so far designed.
The growing comprehension of the essential role played by the immune response in septic shock pathophysiology led to develop and test of some adjunctive treatments based on the use of immunomodulant molecules. To enlighten this fundamental point, it is useful to remember that the immune response during septic shock can be very different among patients. Some patients develop an overwhelming immune response, with a massive production of pro-inflammatory mediators such as cytokines, that are responsible for tissue damage, organ dysfunction, and early death (22). On the other side, some patients develop a condition of immune paralysis, characterized by a reduced ability of the immune system to face pathogens, leading to secondary infections and long-term mortality (22, 23). Generally, mortality in septic shock follows a biphasic curve; only a part of these patients dies during the first 3 days due to an irrepressible immune response, whereas the majority have an unfavorable outcome often a few weeks later, showing a profound impairment of the immune response (24). To confirm this, severe apoptosis-related depletion of cells of both innate and adaptive immune systems along with an increase in regulatory T cells and myeloid-derived suppressor cells, able to inhibit effector immune cell's function, have been documented (23, 25).
Although these scenarios are extreme simplifications of the complex imbalance between activation and suppression of immune response characterizing sepsis and septic shock, it appears clear that agents affecting immune function could be a strategy for implementing sepsis' treatment and improve survival rates.
The evidence on Igs, here reported, was found through searches in the Medline (PubMed) and Scopus databases with the January 2000-September 2020 time limits. We narratively reviewed the efficacy and mechanisms of action of Igs, using the keywords “immunoglobulins,” “mechanism of action,” “efficacy” and “sepsis.”
Among immune-modulatory treatments, intravenous immunoglobulins (Igs) administration may be a promising approach. The rationale for Igs use is both related to their pleiotropic effects on immune response and alterations of Igs serum levels observed in sepsis and septic shock. The potential benefits of Igs administration are related to their immune-modulating functions acting both on pathogens and immune cells: IgG and IgM can scavenge and remove toxins, to bind pathogens promoting phagocytosis, through opsonization and bactericidal activity of CD8+ lymphocytes mediated by antibody-dependent cellular cytotoxicity (26) (Figure 1). These molecules also display direct anti-inflammatory effects mediated by Fc receptor consisting in the modulation of dendritic cell function and reduction in response to INF-γ (27), promote the clearance of apoptotic cells, exert an anti-apoptotic action on immune cells, reduce the activity of the classical complement pathway, due prevalently to IgM, stimulate anti-inflammatory cytokines production along with a reduction of the anti-inflammatory ones favoring a balance between activation and suppression of immune response typically defective during sepsis and septic shock. Igs have also an important synergic activity with antibiotics enhancing their antibacterial efficacy (Figure 1). In addition to the pathophysiological rationale, several studies found a correlation between the reduction of circulating Igs concentrations in septic shock patients and poor outcomes. Venet et al. measured IgG and IgM serum titers in septic shock patients during the first 4 days after diagnosis and found out that Igs deficiency is an important predictive factor for patients' mortality (28). Another study reported an important reduction in IgM levels when patients' conditions deteriorated from severe sepsis to septic shock, underling the importance of Igs kinetic to predict patient's evolution; moreover in vitro stimulation with phytohemagglutinin of lymphocytes isolated by septic shock patients displays a reduced ability to produce IgM compared to healthy subjects (29). Despite the relatively high number of studies evaluating this additional therapy (30–36), the scarce number of patients included and the heterogeneity in terms of the type of preparation, dosages, durations, the severity of enrolled subjects, and type of controls impaired the significance of results (Table 1). Nevertheless, two recent meta-analyses revealed a reduction in mortality using polyclonal Igs compared to the control arm and suggested that the highest total dose range is probably more effective (37, 38).
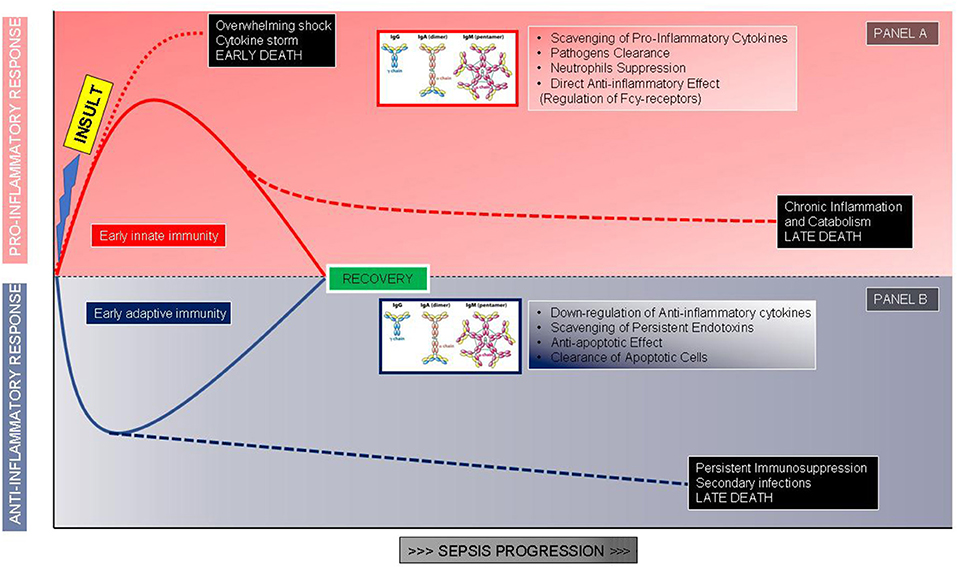
Figure 1. The proposed role of immunoglobulins correlated to the phases of the host response after an infectious insult. (A) Hyperinflammatory response and role of immunoglobulins in modulating the proinflammatory storm. (B) Immunosuppressive response and role of immunoglobulins as immune system adjuvants.
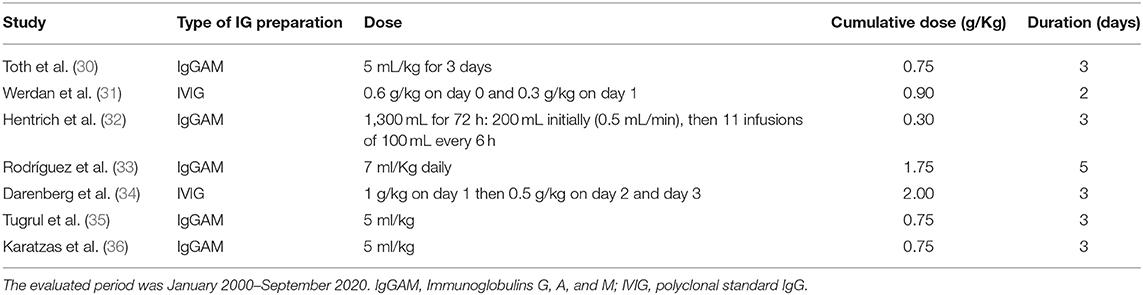
Table 1. Description of Igs preparation, dose prescribed, and duration of treatment in different randomized clinical trials in adults evaluating Igs efficacy as adjunctive therapy in sepsis and septic shock.
The SSC Guidelines (19) suggested against the use of intravenous Igs in patients with sepsis or septic shock. This conclusion was mainly guided by the results of the only RCT on the use of intravenous Igs judged satisfying in terms of numbers, design, and risk of bias. The study revealed no difference in survival at 28 days, but many limitations reduced its power: long duration from 1991 to 1995, publication only 12 years later, and lack of detail of patients' severity (31). To stress the deficiency of evidence and the lack of agreement among the experts it is important to remind that the last version of the Japanese guidelines for the management of sepsis mentions Igs as a possible therapeutic option (39). At present two different types of preparations obtained from plasma of healthy donors are available: polyclonal standard IgG (IVIG) and IgM-enriched formulation (IgGAM); IVIG contains at least 96% of polyclonal IgG whereas the composition of IgGAM, consisting in IgM 12%, IgA 12%, and IgG 76%, appears to trace more accurately the physiological antibodies' production in course of infection. Both preparations can induce pathogen clearance but the higher killing on gram-negative bacteria is obtained with IgM-enriched Igs (40). The presence of IgM seems to be particularly important because of their specific roles in immune response (29). The characteristics of IgM along with the better results obtained with IgGAM in terms of the odds ratio that is a reduction of mortality's relative risk compared to IVIG in the meta-analysis (41) suggest their preferential use in septic shock. Finally, a recent pilot trial evaluating the use of IgGAM in patients with a diagnosis of sepsis or septic shock observed an improvement in sublingual microcirculation in treated patients (42) suggesting a role in the restoration of endothelial cell function as shown in previous preclinical studies (43). The authors of a recent review evaluating the use of Igs as adjunctive therapies in severe infections in ICU suggest that so far insufficient evidence is available to support their use except for streptococcal toxic shock syndrome. However, they underline the importance to identify the proper Igs type of preparation together with dose, the timing of administration, and patients' characteristics (44). Considering the importance of timing to maximize the effect of immune-modulating therapies, Berlot et al. showed that early administration of IgGAM in septic shock patients was associated with a reduction in the risk of in-ICU mortality (45). A multicenter, double-blind, randomized, controlled trial using hyperimmune Igs to treat patients affected by severe H1N1 infection found that their early use within 5 days of symptom onset was associated with reduced viral load and reduced mortality (46). More recently Yun et al. observed that the administration within 48 h of admission in ICU of intravenous Igs as adjuvant therapy in patients suffering from SARS-CoV-2 pneumonia was associated not only with a reduction in the need for mechanic ventilation but also with a reduction of mortality and in-hospital length of stay (47). Summarizing, we can conclude that there is a pressing need for more precise use of Igs in terms of patients' selection, dosage, and timing rather than excluding their benefit in treating sepsis and septic shock.
There are no absolute contraindications to the use of Igs. Each product containing Igs is different so a patient with a life-threatening reaction to one product may have no reactions with other preparations. Thus, the contraindications are related to the particular component of the Igs product (48).
Even if in most cases Igs infusions are well-tolerated, several adverse effects have been reported having a wide range of incidence (49); these side effects are more frequently transient but rarely are serious and can lead to long term disability. Two types of risk factors for adverse effects have been identified: one related to Igs preparation and another to the patient's characteristics. Considering Igs formulation a higher concentration of IgA, anti-Rh and anti-RhD increase the prevalence of adverse reactions (50), on the other side some authors reported a greater risk of side effects in patients with primary antibody defects particularly in those with low IgA levels (51), attention should be paid also in patients with a previous history of allergies and thrombotic events. Immediate adverse effects are the majority and consist of flu-like symptoms (which are the most frequent), dermatologic reactions, arrhythmia, hypotension, and transfusion-related acute lung injury (52). On the contrary delayed adverse reactions, although affecting <1% of treated patients, can be severe and in rare cases lethal; these include renal impairment, hematological and neurological disorders, electrolytes alterations, transfusion-related infections, and thrombotic events (52). The incidence of the adverse effects is strictly related to the rate of Igs infusion, therefore during the first administration, it is recommended to start slowly in the first 30 min increasing subsequently the rate (53).
Based on the need to select the correct phenotype for Igs treatment we decided to use “case examples.” The use of these “case examples” could be beneficial to reduce complexity and define some categories of patients uniform in terms of the immune response.
The first “example” is represented by a previously healthy young adult who develops meningococcemia or severe pneumonia sustained by Streptococcus pneumonia or toxic shock syndrome; in this patient, we could expect an overwhelming pro-inflammatory response, not balanced by an adequate anti-inflammatory response, able to eradicate bacteria but leading to tissue damages and multiorgan failure in the early phase of shock (Figure 1A). In this case, we could take advantage of the Igs ability in pathogen clearance, toxin, and mediators scavenging along with the anti-inflammatory effects; the administration should be early and at high dosages to block as soon as possible the hyperactivation of the efficient immune system and limit organ damages (26, 54, 55). Swedish surveillance data on Igs administered in toxic shock syndrome reported a significantly improved survival in treated patients with an odds ratio of 5.6 for Igs use (56). While a very recent prospective multicenter Scandinavian study identified as a risk factor associated with mortality the non-administration of Igs in patients with necrotizing soft-tissue infections (57).
The second “example” is a patient with persisting or breakthrough infection after a first sepsis episode: i.e., candidemia after abdominal surgery or multi-drug resistant (MDR) pathogen infection after a previous infection. The context, in this case, is a reduced pro-inflammatory response combined with a pronounced or sustained anti-inflammatory state with persisting or secondary infections. The patient may manage to limit the pathogen growth, thanks mainly to antibiotic therapy, although a complete clearance of bacteria is not reached, with the persistence of infection leading at a later time to the patient's death (Figure 1B). Another typical example is the appearance of breakthrough infections sustained by opportunistic relatively avirulent pathogens such as Acinetobacter or Stenotrophomonas species. In this condition Igs will be useful thanks to the anti-apoptotic effect on immune cells and the ability to clear apoptotic cells, rather than for pathogen phagocytosis; the time window of administration, in this case, could be broader. Infections caused by opportunistic pathogens, often MDR, are a clear marker of an immune-suppressed status and some evidence suggests the use of IgGAM in this specific condition (26, 55). In our experience on septic shock patients sustained by MDR, preexisting cancer, by itself a condition of immune response impairment, and Acinetobacter baumanii infection were independently correlated with an increased risk of 30-days mortality whereas only IgGAM administration appeared to be beneficial (58); similar results were obtained in Greece, a country where the MDR prevalence is extremely high, by Giamarellos-Bourboulis et al. showing, in severe infections by MDR gram-negative bacteria, a protective effect of IgGAM administration on 28-days mortality (59).
To categorize patients and progress toward a more personalized medicine we developed a protocol for IgGAM use in septic shock (Figure 2) aimed at applying this adjunctive therapy to appropriate patients at the right time and dosage.
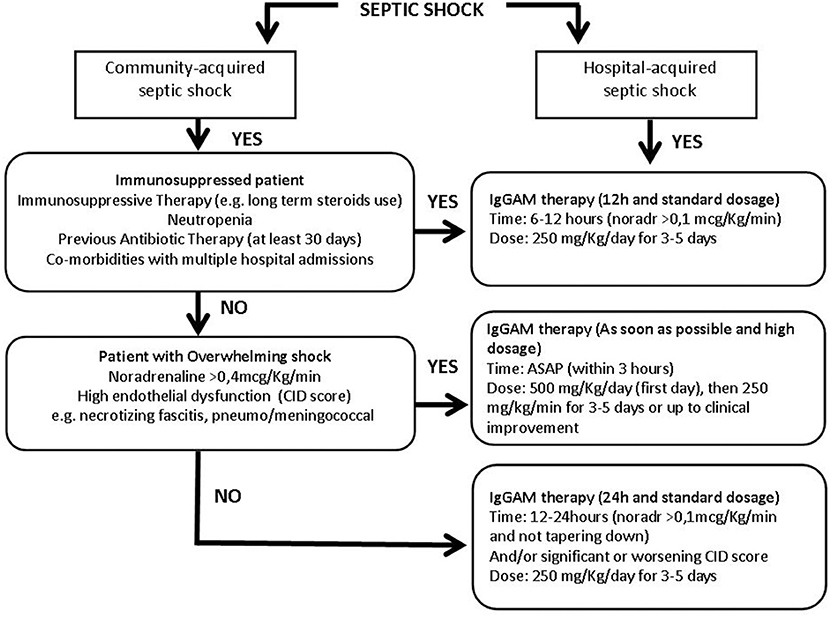
Figure 2. Flow chart of the immunoglobulins' treatment protocol in patients with different septic shock phenotypes. More details in the text. IgGAM, Immunoglobulins G, A, and M; ASAP, as soon as possible; Noradr, Noradrenaline.
Community and hospital-acquired septic shocks were first of all distinguished. In the context of the community-acquired infection, the cornerstone is the immediate identification of an overwhelming shock condition, such as necrotizing fasciitis or meningococcemia. Community-acquired septic shocks, developed in non-immunocompromised patients and characterized by the need for high noradrenaline dosages (>0.4 mcg/kg/min) and deep endothelial dysfunction must be promptly identified as overwhelming shock conditions. Endothelial dysfunction may be defined through the CID score, a simple score based on laboratory data: platelet count, fibrinogen, and fibrin markers (i.e., D-dimer or fibrin split products) plasma concentration and prothrombin time, used to identify endothelial alteration and risk of disseminated intravascular coagulation (60). In these conditions, IgGAM therapy should be administered as soon as possible, in any case within 3 h, starting the first day with a higher dose of 500 mg/kg/day (doubling the standard dose) then continuing with 250 mg/kg/day for 3–5 days or up to clinical improvement (Figure 2). The aim is to use IgGAM to scavenge toxins and to improve the clearance of bacteria boosting antibiotic effect and promoting phagocytosis.
If an overwhelming shock is not present, patients coming from the community should be evaluated to identify a possible immune-suppressed status: a previous antibiotic exposure in the last 30 days, neutropenia, defined by a neutrophil count <1,000/mm3, or on immunosuppressive therapy, including long term use of corticosteroids are even individually sufficient to define a condition of immune-depression. In this case, Igs administration should be started within 6–12 h from shock occurrence, when noradrenaline dosages are higher than 0.1 mcg/Kg/min. IgGAM in that instance should be used at a standard dose of 250 mg/Kg/day for 3–5 days (Figure 2); we don't need a high dose to control an excessive cytokine release or to reduce the bacterial burden, but we take advantage of the immune support given by the treatment, being usually endogenous Igs reduced. In this case, the goal is to favor the balance between pro and anti-inflammatory response thanks to the Igs' role in modulation of pattern recognition receptors (inflammasomes), signaling pathways (NF-kB), and effector molecules (cytokines) and their direct anti-apoptotic effect on immune cells.
If a septic shock patient coming from the community does not show any signs of overwhelming shock nor of immune suppression the administration of a standard dose of 250 mg/Kg/day for 3–5 days should be considered after 12–24 h (Figure 2), when noradrenaline administration >0.1 mcg/Kg/min is persistently necessary to maintain target pressure and/or in case of a significant worsening of the CID score (60) indicating a non-positive evolution of the patient's status.
Hospitalized patients often have an impaired immune function due to multiple predisposing factors including multiple comorbidities, frequent exposition to antibiotics, high risk of MDR pathogens colonization/infection and, therefore, these patients should be a priori considered as immune-compromised and should be treated in the same way of immuno-suppressed patients coming from the community: within 6–12 h from shock occurrence, when noradrenaline dosages are higher than 0,1 mcg/Kg/min, at the standard dose of 250 mg/Kg/day for 3–5 days (Figure 2).
This simple protocol could help to roughly divide patients based on pre-existent conditions and shock characteristics waiting for further studies aimed to better clarify the efficacy of Igs administrations in this setting.
Alongside this suggested clinical protocol, we are running a multicenter RCT “Efficacy and Safety of Adjunctive IgM-enriched Immunoglobulin Therapy with a Personalized Dose Based on Serum IgM-titers vs. Standard Dose in Patients with Septic Shock,” which aims to compare whether a personalized dosage of IgGAM, to achieve and maintain serum titers above 100 mg/dl, could show a different impact on 28-day mortality than a standard dose (250 mg/kg for 3 days) (NCT04182737).
Septic shock requires further studies on the use of adjuvant therapies. To date, a process of selecting potentially profitable patients in terms of personalized medicine is preferable. To this purpose, we believe that a protocolized use of Igs therapy may be an option in the treatment of septic shock, however any type of protocol must be validated by a RCT before extensive clinical use.
SB, ER, MT, EB, and IC have written the initial draft. MM, SD, LB, RG, and MG reviewed the final version. ER, MT, EB, and IC also helped to draw the figures. All authors have written and reviewed the manuscript.
MG has consulted for Biotest-Germany.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. ^Available online at: http://www.salute.gov.it/imgs/C_17_AvvisiSicurezza_8135_azione_itemAzione0_files_itemFiles2_fileAzione.pdf
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. (2019) 23:196. doi: 10.1186/s13054-019-2478-6
3. The National Committee for the Evaluation of Centoxin. The French national registry of HA-1A (Centoxin) in septic shock: a cohort study of 600 patients. Arch Intern Med. (1994) 154:2484–91. doi: 10.1001/archinte.154.21.2484
4. McCloskey RV, Straube RC, Sanders C, Smith SM, Smith CR. Treatment of septic shock with human monoclonal antibody HA-1A. A randomized, double-blind, placebo-controlled trial. CHESS trial study group. Ann Intern Med. (1994) 121:11–21. doi: 10.7326/0003-4819-121-1-199407010-00001
5. Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II study group. Lancet. (1998) 351:929–33. doi: 10.1016/S0140-6736(05)60602-2
6. Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The interleukin-1 receptor antagonist sepsis investigator group. Crit Care Med. (1997) 25:1115–24. doi: 10.1097/00003246-199707000-00010
7. Opal S, Laterre PF, Abraham E, Francois B, Wittebole X, Lowry S, et al. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit Care Med. (2004) 32:332–41. doi: 10.1097/01.CCM.0000108867.87890.6D
8. Annane D, Timsit JF, Megarbane B, Martin C, Misset B, Mourvillier B, et al. Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am J Respir Crit Care Med. (2013) 187:1091–7. doi: 10.1164/rccm.201211-2020OC
9. Borthwick EM, Hill CJ, Rabindranath KS, Maxwell AP, McAuley DF, Blackwood B. High-volume haemofiltration for sepsis in adults. Cochrane Database Syst Rev. (2017) 1:CD008075. doi: 10.1002/14651858.CD008075.pub3
10. Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. (2015) 41:975–84. doi: 10.1007/s00134-015-3751-z
11. Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, et al. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. (2018) 320:1455–63. doi: 10.1001/jama.2018.14618
12. Livigni S, Bertolini G, Rossi C, Ferrari F, Giardino M, Pozzato M, et al. Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open. (2014). 4:e003536. doi: 10.1136/bmjopen-2013-003536
13. Schädler D, Pausch C, Heise D, Meier-Hellmann A, Brederlau J, Weiler N, et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE. (2017) 12:e0187015. doi: 10.1371/journal.pone.0187015
14. Hawchar F, László I, Öveges N, Trásy D, Ondrik Z, Molnar Z. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J Crit Care. (2019) 49:172–8. doi: 10.1016/j.jcrc.2018.11.003
15. Broman ME, Hansson F, Vincent JL, Bodelsson M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: a randomized crossover double-blind study. PLoS ONE. (2019) 14:e0220444. doi: 10.1371/journal.pone.0220444
16. Moskowitz A, Huang DT, Hou PC, Gong J, Doshi PB, Grossestreuer AV, et al. Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. JAMA. (2020) 324:642–50. doi: 10.1001/jama.2020.11946
17. Hwang SY, Ryoo SM, Park JE, Jo YH, Jang DH, Suh GJ, et al. Combination therapy of vitamin C and thiamine for septic shock: a multi-centre, double-blinded randomized, controlled study. Intensive Care Med. (2020) 46:2015–25. doi: 10.1007/s00134-020-06191-3
18. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. (2009) 180:640–8. doi: 10.1164/rccm.200903-0363OC
19. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. (2017) 43:304–77. doi: 10.1007/s00134-017-4683-6
20. Marshall JC. The PIRO (predisposition, insult, response, organ dysfunction) model: toward a staging system for acute illness. Virulence. (2014) 5:27–35. doi: 10.4161/viru.26908
21. Cardoso T, Teixeira-Pinto A, Rodrigues PP, Aragão I, Costa-Pereira A, Sarmento AE. Predisposition, insult/infection, response and organ dysfunction (PIRO): a pilot clinical staging system for hospital mortality in patients with infection. PLoS ONE. (2013) 8:e70806. doi: 10.1371/journal.pone.0070806
22. Rubio I, Osuchowski MF, Shankar-Hari M, Skirecki T, Winkler MS, Lachmann G, et al. Current gaps in sepsis immunology: new opportunities for translational research. Lancet Infect Dis. (2019) 19:e422–36. doi: 10.1016/S1473-3099(19)30567-5
23. Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. (2019) 10:782. doi: 10.1038/s41419-019-2015-1
24. Daviaud F, Grimaldi D, Dechartres A, Charpentier J, Geri G, Marin N, et al. Timing and causes of death in septic shock. Ann Intensive Care. (2015) 5:16. doi: 10.1186/s13613-015-0058-8
25. Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. (2013) 13:260–8. doi: 10.1016/S1473-3099(13)70001-X
26. Jarczak D, Kluge S, Nierhaus A. Use of intravenous immunoglobulins in sepsis therapy-a clinical view. Int J Mol Sci. (2020) 21:5543. doi: 10.3390/ijms21155543
27. Nagelkerke SQ, Kuijpers TW. Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol. (2014) 5:674. doi: 10.3389/fimmu.2014.00674
28. Venet F, Gebeile R, Bancel J, Guignant C, Poitevin-Later F, Malcus C, et al. Assessment of plasmatic immunoglobulin G, A and M levels in septic shock patients. Int Immunopharmacol. (2011) 11:2086–90. doi: 10.1016/j.intimp.2011.08.024
29. Giamarellos-Bourboulis EJ, Apostolidou E, Lada M, Perdios I, Gatselis NK, Tsangaris I, et al. Kinetics of circulating immunoglobulin M in sepsis: relationship with final outcome. Crit Care. (2013). 17:R247. doi: 10.1186/cc13073
30. Toth I, Mikor A, Leiner T, Molnar Z, Bogar L, Szakmany T. Effects of IgM-enriched immunoglobulin therapy in septic-shock-induced multiple organ failure: pilot study. J Anesth. (2013) 27:618–22. doi: 10.1007/s00540-012-1553-9
31. Werdan K, Pilz G, Bujdoso O, Fraunberger P, Neeser G, Schmieder RE, et al. Score-based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med. (2007) 35:2693–701. doi: 10.1097/00003246-200712000-00003
32. Hentrich M, Fehnle K, Ostermann H, Kienast J, Cornely O, Salat C, et al. IgMA-enriched immunoglobulin in neutropenic patients with sepsis syndrome and septic shock: a randomized, controlled, multiple-center trial. Crit Care Med. (2006) 34:1319–25. doi: 10.1097/01.CCM.0000215452.84291.C6
33. Rodríguez A, Rello J, Neira J, Maskin B, Ceraso D, Vasta L, et al. Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock. (2005) 23:298–304. doi: 10.1097/01.shk.0000157302.69125.f8
34. Darenberg J, Ihendyane N, Sjölin J, Aufwerber E, Haidl S, Follin P, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis. (2003) 37:333–40. doi: 10.1086/376630
35. Tugrul S, Ozcan PE, Akinci O, Seyhun Y, Cagatay A, Cakar N, et al. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis [ISRCTN28863830]. Crit Care. (2002) 6:357–62. doi: 10.1186/cc1523
36. Karatzas S, Boutzouka E, Venetsanou K, Myrianthefs P, Fildisis G, Baltopoulos G. The effects of IgM-enriched immunoglobulin preparations in patients with severe sepsis: another point of view. Crit Care. (2002) 6:543–4. doi: 10.1186/cc1837
37. Busani S, Damiani E, Cavazzuti I, Donati A, Girardis M. Intravenous immunoglobulin in septic shock: review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol. (2016) 82:559–72. Available online at: https://www.minervamedica.it
38. Yang Y, Yu X, Zhang F, Xia Y. Evaluation of the effect of intravenous immunoglobulin dosing on mortality in patients with sepsis: a network meta-analysis. Clin Ther. (2019) 41:1823–38.e4. doi: 10.1016/j.clinthera.2019.06.010
39. Nishida O, Ogura H, Egi M, Fujishima S, Hayashi Y, Iba T, et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2016 (J-SSCG 2016). J Intensive Care. (2018) 6:7. doi: 10.1186/s40560-017-0270-8
40. Rossmann FS, Kropec A, Laverde D, Saaverda FR, Wobser D, Huebner J. In vitro and in vivo activity of hyperimmune globulin preparations against multiresistant nosocomial pathogens. Infection. (2015) 43:169–75. doi: 10.1007/s15010-014-0706-1
41. Soares MO, Welton NJ, Harrison DA, Peura P, Shankar-Hari M, Harvey SE, et al. An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): incorporating a systematic review, meta-analysis and value of information analysis. Health Technol Assess. (2012) 16:1–186. doi: 10.3310/hta16070
42. Domizi R, Adrario E, Damiani E, Scorcella C, Carsetti A, Giaccaglia P, et al. IgM-enriched immunoglobulins (pentaglobin) may improve the microcirculation in sepsis: a pilot randomized trial. Ann Intensive Care. (2019) 9:135. doi: 10.1186/s13613-019-0609-5
43. Hoffman JN, Fertmann JM, Vollmar B, Laschke MW, Jauch KW, Menger MD. Immunoglobulin M-enriched human intravenous immunoglobulins reduce leukocyte-endothelial cell interactions and attenuate microvascular perfusion failure in normotensive endotoxemia. Shock. (2008) 29:133–9. doi: 10.1097/SHK.0b013e318123e5a6
44. Aubron C, Berteau F, Sparrow R L. Intravenous immunoglobulin for adjunctive treatment of severe infections in ICUs. Curr Opin Crit Care. (2019) 25:417–22. doi: 10.1097/MCC.0000000000000639
45. Berlot G, Vassallo MC, Busetto N, Nieto Yabar M, Istrati T, Baronio S, et al. Effects of the timing of administration of IgM- and IgA-enriched intravenous polyclonal immunoglobulins on the outcome of septic shock patients. Ann Intensive Care. (2018) 8:122. doi: 10.1186/s13613-018-0466-7
46. Hung IFN, To KKW, Lee CK, Lee KL, Yan WW, Chan K, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. (2013) 144:464–73. doi: 10.1378/chest.12-2907
47. Xie Y, Cao S, Dong H, Li Q, Chen E, Zhang W, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. (2020) 81:318–56. doi: 10.1016/j.jinf.2020.03.044
48. Arumugham VB, Rayi A. Intravenous immunoglobulin (IVIG). In: StatPearls. Treasure Island, FL: StatPearls Publishing (2020).
49. Seidling V, Hoffmann JH, Enk AH, Hadaschik EN. Analysis of high-dose intravenous immunoglobulin therapy in 16 patients with refractory autoimmune blistering skin disease: high efficacy and no serious adverse events. Acta Derm Venereol. (2013) 93:346–9. doi: 10.2340/00015555-1471
50. Manlhiot C, Tyrrell PN, Liang L, Atkinson AR, Lau W, Feldman BM. Safety of intravenous immunoglobulin in the treatment of juvenile dermatomyositis: adverse reactions are associated with immunoglobulin A content. Pediatrics. (2008) 121:e626–30. doi: 10.1542/peds.2007-1218
51. Dashti-Khavidaki S, Aghamohammadi A, Farshadi F, Movahedi M, Parvaneh N, Pouladi N, et al. Adverse reactions of prophylactic intravenous immunoglobulin; a 13-year experience with 3004 infusions in Iranian patients with primary immunodeficiency diseases. J Investig Allergol Clin Immunol. (2009) 19:139–45. Available online at: http://www.jiaci.org
52. Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. (2018) 9:1299. doi: 10.3389/fimmu.2018.01299
53. Thornby KA, Henneman A, Brown DA. Evidence-based strategies to reduce intravenous immunoglobulin-induced headaches. Ann Pharmacother. (2015) 49:715–26. doi: 10.1177/1060028015576362
54. Peetermans M, Wan RYY, Camporota L, Barrett NA, Retter A. Use of intravenous immunoglobulins in patients with suspected toxin-mediated shock requiring extracorporeal membrane oxygenation. Shock. (2020) 54:209–12. doi: 10.1097/SHK.0000000000001519
55. Nierhaus A, Berlot G, Kindgen-Milles D, Müller E, Girardis M. Best-practice IgM-and IgA-enriched immunoglobulin use in patients with sepsis. Ann Intensive Care. (2020) 10:132. doi: 10.1186/s13613-020-00740-1
56. Linnér A, Darenberg J, Sjölin J, Henriques-Normark B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis. (2014) 59:851–7. doi: 10.1093/cid/ciu449
57. Bruun T, Rath E, Bruun Madsen M, Oppegaard O, Nekludov M, Arnell P, et al. Risk factors and predictors of mortality in streptococcal necrotizing soft-tissue infections: a multicenter prospective study. Clin Infect Dis. (2020) 10:ciaa027. doi: 10.1093/cid/ciaa027
58. Busani S, Serafini G, Mantovani E, Venturelli C, Giannella M, Viale P, et al. Mortality in patients with septic shock by multidrug resistant bacteria: risk factors and impact of sepsis treatments. J Intensive Care Med. (2019) 34:48–54. doi: 10.1177/0885066616688165
59. Giamarellos-Bourboulis EJ, Tziolos N, Routsi C, Katsenos C, Tsangaris I, Pneumatikos I, et al. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin Microbiol Infect. (2016) 22:499–506. doi: 10.1016/j.cmi.2016.01.021
60. Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. (2001). 86:1327–30. doi: 10.1055/s-0037-1616068
Keywords: immunoglobulins, septic shock, adjunctive treatment, sepsis, protocol
Citation: Busani S, Roat E, Tosi M, Biagioni E, Coloretti I, Meschiari M, Gelmini R, Brugioni L, De Biasi S and Girardis M (2021) Adjunctive Immunotherapy With Polyclonal Ig-M Enriched Immunoglobulins for Septic Shock: From Bench to Bedside. The Rationale for a Personalized Treatment Protocol. Front. Med. 8:616511. doi: 10.3389/fmed.2021.616511
Received: 12 October 2020; Accepted: 27 January 2021;
Published: 18 February 2021.
Edited by:
Alessandro Russo, University of Pisa, ItalyReviewed by:
Daniele Roberto Giacobbe, Azienda Ospedaliera Universitaria San Martino (IRCCS), ItalyCopyright © 2021 Busani, Roat, Tosi, Biagioni, Coloretti, Meschiari, Gelmini, Brugioni, De Biasi and Girardis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Girardis, bWFzc2ltby5naXJhcmRpc0B1bmltb3JlLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.