- 1Faculty of Medicine, Institute of Forensic Medicine “Milovan Milovanović,” University of Belgrade, Belgrade, Serbia
- 2Faculty of Medicine, Institute of Human Genetics, University of Belgrade, Belgrade, Serbia
- 3Faculty of Medicine, Institute of Histology and Embryology, University of Belgrade, Belgrade, Serbia
- 4Clinic for Infectious and Tropical Diseases, Clinical Centre of Serbia, Belgrade, Serbia
- 5Faculty of Medicine, University of Belgrade, Belgrade, Serbia
In Europe, the first case of coronavirus disease (COVID-19) and the first COVID-19-related death were reported in France on January 24th and February 15th, 2020, respectively. Officially, the first case of COVID-19 infection in the Republic of Serbia was registered on March 6th. Herein, we presented the first case of retrospective detection of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in the post-mortem-obtained vitreous humor (VH), which took place on February 5th, 2020. This is the first death in Europe proven to be caused by COVID-19 by means of post-mortem histopathological and molecular analyses. Based on this finding, it appears that SARS-CoV-2 has been spreading faster and started spreading much earlier than it had been considered and that COVID-19 was probably the cause of the much-reported pneumonia of unknown origin in January and February 2020.
Introduction
In December 2019 and January 2020, doctors around the world encountered an unusual situation—a significantly higher number of patients suffering from pneumonia of unknown origin compared to previous years (1, 2). In December 2019, it was obvious that Wuhan and other cities in China had faced a new public health challenge, recognized as coronavirus disease (COVID-19). The causative agent, identified from throat swab samples on January 7th, 2020, was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and quickly became a serious problem worldwide with a high mortality rate and the need for quarantine (3, 4). In Europe, the first case and the first death caused by COVID-19 were reported in France on January 24th and February 15th, 2020, respectively (5). Officially, the first case of COVID-19 in the Republic of Serbia was registered on March 6th (6). Herein, we presented the first case of retrospective detection of SARS-CoV-2 in the post-mortem-obtained vitreous humor (VH), more than a month before the first case was officially registered in the Balkan region (6)1. In addition, this is the first death caused by COVID-19 in Europe proven by post-mortem diagnostics.
Case Description
On February 5th 2020, a 56-year-old man presented to the hospital with fever, cough, and shortness of breath, which started 5 days before. The socio-epidemiological questionnaire showed1 that the patient worked in a construction company, was living in a Belgrade suburb, had not traveled abroad for a long time, had no chronic conditions, but was slightly overweight (BMI 25.2 kg/m2). On admission, the patient had a high fever (39.2°C) and hypoxia (PaO2 78%) and was hypotensive (blood pressure 100/50 mm/Hg). Chest radiography revealed massive bilateral pneumonia. The biochemical and blood gas analyses revealed the following (reference ranges are shown in parentheses): white blood cell 1.9 × 109/L (4.40–11.50), platelet 127 × 109/L (150–400), D-dimer 3.9 g/L (2–4), C-reactive protein 229 mg/dL (0–7), pO2 25 (80–100), pCO2 33 (35–45), and CHCO3 21 (22–28). The patient was intubated and connected to mechanical ventilation; unfortunately, he died within a few hours after admission to the hospital. The autopsy was performed the next day, according to the standard procedure. Gross autopsy findings revealed heavy, grossly firm, and rubbery lungs with severe bilateral edema. On the cut section, the lungs were dark red without purulent discharge. The hilar lymph nodes were slightly enlarged. The findings in other organs were unremarkable.
Initially, the death was attributed to pneumonia of unknown origin but after additional diagnostic procedures conducted 3 months later, it was proven that death was caused by COVID-19.
Histopathological Analysis
Histopathological analysis (HP) of the lungs revealed exudative and early organizing phases of diffuse alveolar damage (DAD) in all sections (Figure 1). The findings of intraalveolar protein-rich edema, capillary congestion, and formation of hyaline membranes corresponded to the exudative phase of DAD. DAD was focally associated with fibrinous pneumonia with a mixed inflammatory infiltrate in the lumen of the alveoli and interstitium, consisting of abundant monocytes/macrophages (CD68 staining), rare T-cells (CD3 staining), and scattered granulocytes. Megakaryocytes were observed in the microvasculature. The focal proliferation of type 2 pneumocytes, thickening of the alveolar septae, fibroblast proliferation, and loose interstitial fibrosis corresponded to the early organizing phase of DAD. Few hyaline microthrombi were present in sections. HP findings of other organs were unremarkable (Figure 1).
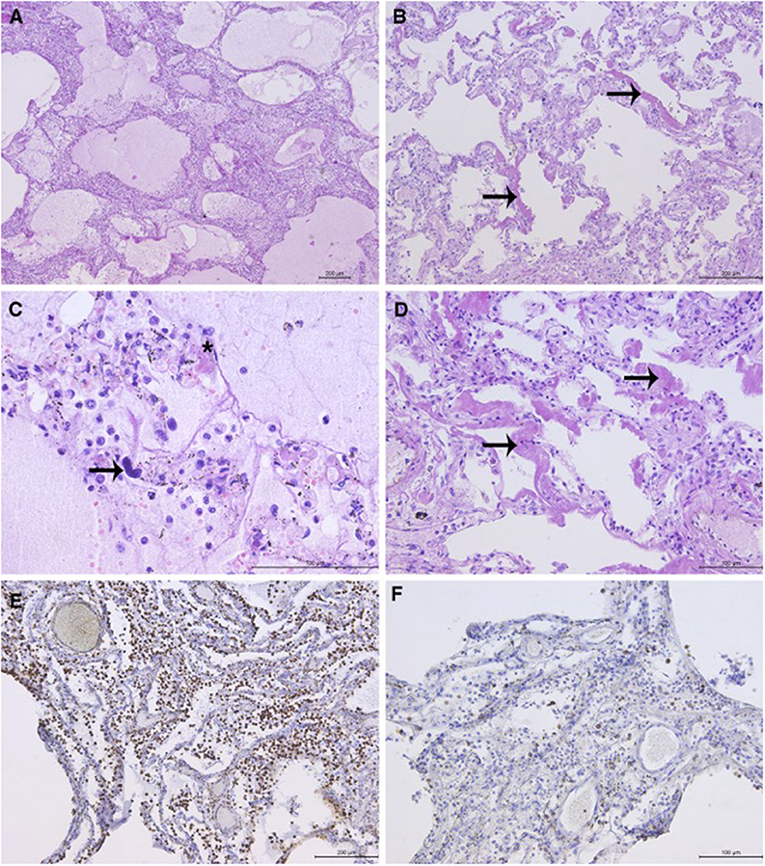
Figure 1. Representative lung sections showing diffuse alveolar damage (DAD): (A) DAD with associated pneumonia. Intraalveolar protein-rich edema and inflammatory cells in the alveolar lumen and interstitium. (B) Hyaline membranes (arrows) with widening of the alveolar septae. (C) Alveolar protein-rich edema. Mixed inflammatory infiltrate with predominance of macrophages. Scattered granulocytes can be observed. Megakaryocyte in the capillary (arrow). Hyaline microthrombus (asterisk). (D) Hyaline membranes (arrows), light interstitial fibrosis, and proliferation of type 2 pneumocytes. (E) Abundant macrophages in the interstitium and lumen of the alveoli (immunohistochemical staining with CD68 antibody) and (F) scattered T-lymphocytes (staining with CD3 antibody). *Microphotographs were taken using the Leica DM4000 B LED light microscope (Leica, Wetzlar, Germany) and Leica DFC295 digital camera (Leica, Heerbrugg, Switzerland).
Molecular Testing
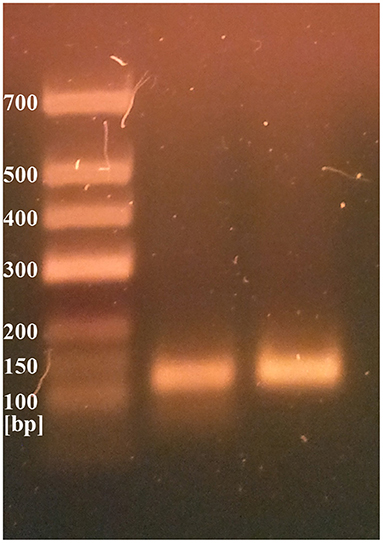
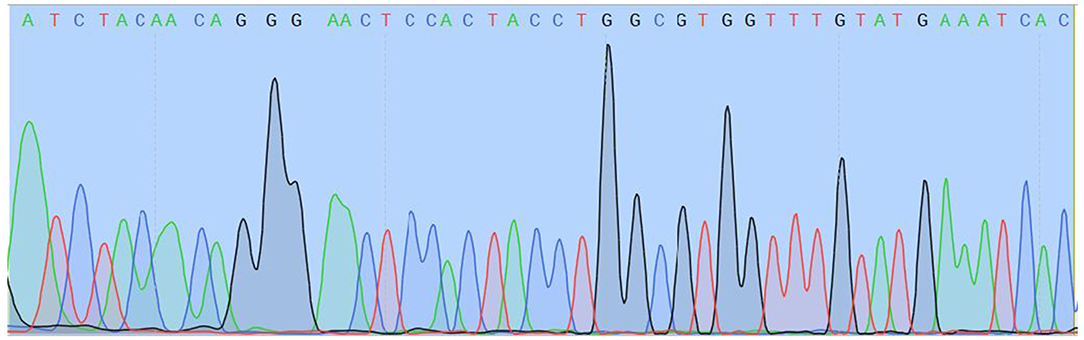
Two different genetic analyses of VH samples (RNA isolation/reverse transcription and Real-Time PCR/amplicon sequencing) were performed with the aim of confirming the validity of results and eliminating the possibility of false-positive results. Two regions of SARS-CoV-2 orf1ab were amplified using the Institute Pasteur primers (IP2 and IP4)1. To confirm the specificity, agarose gel extraction of IP4 amplicon and DNA sequencing were performed (Figure 2). The obtained sequence was aligned against reference genomes in the NCBI database using MEGABLAST. IP2 and IP4 regions were amplified with CT values 35 and 34, respectively. PCR products matched the expected lengths for IP2 and IP4 segments, with a minor non-specific band in the P2 lane. MEGABLAST results showed that the sequence without primer binding regions matched SARS-CoV-2 with an E value of 2e−15, as well as 97.92% of percentage identity with NC_045512.2 (Figure 3).
Confirmatory Assay
Since this was presumably the first confirmed case of COVID-19 in our country, we performed a second molecular analysis of different gene targets of SARS-CoV-2, as suggested by the Center for Disease Control and Prevention. Total RNA was separately isolated from an additional sample of 200 μl of VH. The sequences from the SARS-CoV-2-specific RdRp gene, as well as from the human RNase P gene as internal control, were detected.
Discussion
The case represents the very first fatal outcome of COVID-19 in Europe and the first post-mortem-confirmed COVID-19 case after retrospective VH analysis. The patient died on February 5th, after 5 days of intensive respiratory symptoms, indicating that he was probably infected in January. The fact that the patient had not traveled abroad and the fact that there were no known epidemiological links with other countries with an active COVID-19 epidemic at that moment indicate that SARS-CoV-2 had probably already been spreading among the population in the Balkans at the beginning of 2020. The study of Deslandes et al. (7) confirmed the suspicion that the virus had come to Europe earlier than it was officially registered. Based on the retrospective testing of respiratory swabs, this group of researchers detected one SARS-CoV-2-positive case in France in late December 2019 (7).
An important conclusion is that VH is a very useful post-mortem sample for SARS-CoV-2 preservation, as it allowed us to detect the virus 3 months after the death, especially in cases when the virus has not been isolated from the blood (8–11). We isolated the virus from VH, although VH underwent freezing at −20°C and subsequent thawing and the confirmatory analysis was done on that thawed sample a few weeks after the first one. This suggests that SARS-CoV-2 is more resistant to temperature changes. Recently published manuscripts showed different results regarding the presence of SARS-CoV-2 in the ocular structures (12–15). In the study of Sawant et al., vitreous swabs were positive for SARS-CoV-2 RNA in 2/10 patients with COVID-19 (14), while one other study did not find SARS-CoV-2 RNA in the vitreous fluid of COVID-19-positive patients (13). On the other hand, Casagrande et al. showed that the prevalence rate of SARS-CoV-2 RNA in the retinal biopsies of patients with COVID-19 is 21% (3/14) (15). Therefore, the possibility of infiltration of VH via retinal vasculature could not be eliminated. VH could be used as a valuable specimen for post-mortem virological analyses, as it stays stable and preserved for a long time. The sequencing of the viral RNA is undoubtedly confirmation that this was in fact SARS-CoV-2, while the possibility of a falsely positive result is excluded.
In the presented case, clinical, radiological, and biochemical findings are in accordance with the severe form of COVID-19. Macroscopically, it is typical of COVID-19 pneumonia to be presented with large and heavy lungs due to retained fluid, as in the presented case. Edler et al. noticed that the lung surface sometimes showed signs of pleurisy and mosaic-like pattern of pale fields and slightly protruding dark purple sections with prominent capillary drawing (16). Also, cut sections of the lungs revealed either ubiquitously dark red or, alternately, faded appearance. In some COVID-19 deaths, purulent respiratory tract infection with abscessed bronchopneumonia was observed (16–18), which is macroscopically different from our findings. Here, the main HP finding was DAD in exudative and early organizing phases, in accordance with the recently published studies (19, 20). Capillary congestion and multifocal microthrombotic disease in capillaries and small vessels were noticed and described in some papers (20). In comparison to other published data, we only detected scattered hyaline microthrombi in the lung capillaries (21, 22). In addition, an increased number of megakaryocytes in the lungs (and other organs) is described in COVID patients, along with some COVID-19-unrelated conditions, like intravascular coagulation, acute infection, shock, and fever (21, 23).
Based on this case, it appears that SARS-CoV-2 has been spreading much earlier than it was considered and that COVID-19 was probably the cause of much-reported pneumonia of unknown origin in early 2020. Important insights into this novel disease and its course can be obtained by post-mortem HP and molecular analyses of the VH, as an excellent sample for SARS-CoV-2 detection, in patients with pneumonia of unknown origin who died in the last one year.
Data Availability Statement
The datasets generated for this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/nuccore/MW471658.1/.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MB, IS, TA, and AB: drafted manuscript. IS, OS, VP, SS, ZM, and BR: autopsy and pathohistological and molecular analyses. TA, ID, and SD: review of literature. All authors: revised and prepared together the final version of manuscript.
Funding
This research was supported by the Science Fund of the Republic of Serbia, FORACOVID project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.612758/full#supplementary-material
Supplementary Figure 1. SYBR green RT PCR amplification using Pasteur primers. Linear scale representation. Order of Ct values: 34 for IP2, 33 for IP4, 40 for internal (human) control.
Supplementary Figure 2. SYBR green RT PCR amplification using Pasteur primers. Log scale representation. Order of Ct values: 34 for IP2, 33 for IP4, 40 for internal (human) control.
Supplementary Figure 3. Result of RT-PCR assay.
Footnotes
1. ^https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf (accessed on September 10, 2020).
References
1. European Centre for Disease Prevention and Control. Threats and Outbreaks: COVID-19 Secondary Threats and Outbreaks: COVID-19. (2020). Available online at: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/
2. Böhmer MM, Buchholz U, Corman VM, Hoch M, Katz K, Marosevic DV, et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. (2020) 20:920–8. doi: 10.1016/S1473-3099(20)30314-5
3. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
4. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. (2020) 109:102433. doi: 10.1016/j.jaut.2020.102433
5. Bernard Stoecklin S, Rolland P, Silue Y, Mailles A, Campese C, Simondon A, et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. (2020) 25:2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094
6. Puca E, Civljak R, Arapović J, Popescu C, Christova I, Raka L, et al. Short epidemiological overview of the current situation on COVID-19 pandemic in Southeast European (SEE) countries. J Infect Dev Ctries. (2020) 14:433–7. doi: 10.3855/jidc.12814
7. Deslandes A, Berti V, Tandjaoui-Lambotte Y, Alloui C, Carbonnelle E, Zahar JR, et al. SARS-CoV-2 was already spreading in France in late December 2019. Int J Antimicrob Agents. (2020) 55:106006. doi: 10.1016/j.ijantimicag.2020.106006
8. Salducci M, La Torre G. COVID-19 emergency in the cruise's ship: a case report of conjunctivitis. Clin Ter. (2020) 171:e189–91. doi: 10.7417/CT.2020.2212
9. Garland J, Tse R, Cala AD. Neisseria meningitidis isolated in postmortem vitreous humor in a death due to meningococcal sepsis. Am J Forensic Med Pathol. (2016) 37:233–5. doi: 10.1097/PAF.0000000000000269
10. Ridpath AD, Halse TA, Musser KA, Wroblewski D, Paddock CD, Shieh WJ, et al. Postmortem diagnosis of invasive meningococcal disease. Emerg Infect Dis. (2014) 20:453–5. doi: 10.3201/eid2003.131245
11. Henwood AF. Coronavirus disinfection in histopathology. J Histotechnol. (2020) 16:1–3. doi: 10.1080/01478885.2020.1734718
12. Lauermann P, Storch M, Weig M, Tampe B, Winkler M, Hoerauf H, et al. There is no intraocular affection on a SARS-CoV-2—Infected ocular surface. Am J Ophthalmol Case Rep. (2020) 20:100884. doi: 10.1016/j.ajoc.2020.100884
13. List W, Regitnig P, Kashofer K, Gorkiewicz G, Zacharias M, Wedrich A, et al. Occurrence of SARS-CoV-2 in the intraocular milieu. Exp Eye Res. (2020) 201:108273. doi: 10.1016/j.exer.2020.108273
14. Sawant OB, Singh S, Wright RE III, Jones KM, Titus MS, Dennis E, et al. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul Surf . (2020) 19:332–9. doi: 10.1101/2020.10.05.20201574
15. Casagrande M, Fitzek A, Püschel K, Aleshcheva G, Schultheiss HP, Berneking L, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. (2020) 28:721–5. doi: 10.1080/09273948.2020.1770301
16. Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, et al. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. (2020) 134:1275–84. doi: 10.1007/s00414-020-02317-w
17. Aguiar D, Lobrinus JA, Schibler M, Fracasso T, Lardi C. Inside the lungs of COVID-19 disease. Int J Legal Med. (2020) 134:1271–4. doi: 10.1007/s00414-020-02318-9
18. Suess C, Hausmann R. Gross and histopathological pulmonary findings in a COVID-19 associated death during self-isolation. Int J Legal Med. (2020) 134:1285–90. doi: 10.1007/s00414-020-02319-8
19. Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. (2020) 396:320–32. doi: 10.1016/S0140-6736(20)31305-2
20. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. (2020) 20:1135–40. doi: 10.1016/S1473-3099(20)30434-5
21. Repkiewicz AV, Mai X, Carson SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. E Clinical Medicine. (2020) 24:100434. doi: 10.1016/j.eclinm.2020.100434
22. Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. (2020) 77:198–209. doi: 10.1111/his.14134
Keywords: COVID-19, SARS-CoV-2, vitreous humor, autopsy, Europe (central)
Citation: Bogdanović M, Skadrić I, Atanasijević T, Stojković O, Popović V, Savić S, Mihailović Z, Radnić B, Aćimović T, Damjanjuk I, Despotović S and Barać A (2021) Case Report: Post-mortem Histopathological and Molecular Analyses of the Very First Documented COVID-19-Related Death in Europe. Front. Med. 8:612758. doi: 10.3389/fmed.2021.612758
Received: 30 September 2020; Accepted: 19 January 2021;
Published: 19 February 2021.
Edited by:
John Hay, University at Buffalo, United StatesReviewed by:
Salvatore Rubino, University of Sassari, ItalyAlberto Enrico Maraolo, University of Naples Federico II, Italy
Copyright © 2021 Bogdanović, Skadrić, Atanasijević, Stojković, Popović, Savić, Mihailović, Radnić, Aćimović, Damjanjuk, Despotović and Barać. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandra Barać, YWxla3NhbmRyYWJhcmFjODVAZ21haWwuY29t
 Milenko Bogdanović
Milenko Bogdanović Ivan Skadrić
Ivan Skadrić Tatjana Atanasijević
Tatjana Atanasijević Oliver Stojković
Oliver Stojković Vesna Popović
Vesna Popović Slobodan Savić1
Slobodan Savić1 Bojana Radnić
Bojana Radnić Sanja Despotović
Sanja Despotović Aleksandra Barać
Aleksandra Barać