- 1Department of Epidemiology, School of Public Health, Nanjing Medical University, Nanjing, China
- 2Eastern Theater Command Centers for Disease Control and Prevention, Institute of Epidemiology and Microbiology, Nanjing, China
- 3Department of Epidemiology and Statistics, School of Public Health, Medical College of Soochow University, Suzhou, China
- 4Department of Critical Care Medicine, The Affiliated Yixing Hospital of Jiangsu University, Yixing, China
- 5Department of Infectious Diseases, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Direct-acting antiviral (DAA) treatment for 8 weeks has a sustained virological response rate in adults with chronic hepatitis C. We have conducted a systematic review and meta-analysis to compare the efficacy and safety of the 8-week vs. 12/24-week DAA treatment in adolescents and children with CHC. The PubMed, Web of Science, and Cochrane databases were searched for the relevant articles from January 1, 2017 to August 28, 2020 and further screened for literature reviews on April 1, 2021. Pool proportions with 95% CIs for SVR12 were summarized with fixed/random effects models using Freeman–Tukey double arcsine transformation. Subgroup analysis was used to explore the source of heterogeneity. Thirty-six relevant publications were identified. For adolescents aged 12–17 years old, the pooled SVR12 and AE rate were 99.4% (95% CI: 98.7–99.9) and 34.7% (95% CI: 31.9–37.6). No one discontinued treatment due to drug intolerance. In addition, the SVR12 adolescents treated for 12 and 8/24 weeks were 99.3% (95% CI: 98.4–99.9) and 100%, respectively. The pooled SVR12 rate, AEs, and SAEs for children younger than 12 years were 98.9% (95% CI: 97.3–99.8), 51.6% (95% CI: 47.0–56.2), and 1.1% (95% CI: 0.4–2.5), respectively. The most common AE was fatigue (28.4%). The SVR12 was 98.8% (95% CI: 97.1–99.8) and 100% for the pediatric patients treated for 12 weeks and 8/24 weeks, respectively. Taken together, DAAs are generally effective against CHC and well-tolerated by the adolescents and children. A treatment duration of 8 weeks is equally effective and safe as 12/24 weeks in this demographic group.
Introduction
Hepatitis C is caused by hepatitis C virus (HCV) infection and afflicted 71.7 million people or 1% of the global population in 2015 (1, 2), of which 13.2 (11.5–21.2) million were children and adolescents aged 1–15 years (3). Only 1.76 million (13%) of the patients received treatment, and 86% (1.51 million) were treated with direct-acting antivirals (DAAs) (1, 2).
Vertical HCV infection is cleared spontaneously without treatment in 20% of the pediatric patients, while the remaining 80% develop chronic infection in the first 4 years of life that usually persists into adulthood (4–6). Early diagnosis and treatment at younger age can reduce the prevalence of chronic infection in adulthood, and therefore reduce the global burden of HCV (7). However, although 5.5 million people with chronic HCV have been treated so far, most of these patients are adults that received the less effective interferon-based regimens (2).
The Food and Drug Administration (FDA) approved supplemental administration of sofosbuvir (SOF) and a combination of sofosbuvir and ledipasvir (SOF+LDV) in April 2017 to treat HCV in adolescents aged 12–17 years (8). In addition, several single-arm clinical trials conducted in the last 2 years have shown that DAAs are highly effective in pediatric CHC patients aged 6–12 years (9, 10). However, most of these studies have only analyzed the efficacy of DAAs on specific pediatric patient populations, such as those infected with HCV genotype 4 (GT) (11, 12), or the treatment experienced (TE) or treatment-naïve (TN) patients (13). The efficacy of short-duration (8 weeks) DAA treatment in adolescents and children with HCV infection has not been summarized so far.
The aim of this study was to comprehensively evaluate the efficacy and safety of 8-week vs. 12/24-week DAA regimens in adolescents and children with HCV infection using data from published studies. Our findings provide valuable information for medical professionals and researchers.
Materials and Methods
This systematic review and meta-analysis was conducted according to the preferred reporting items for systematic review and meta-analyses (PRISMA) statement (Supplementary Table 1) (14).
Literature Search
PubMed, Cochrane Library, and Web of Science databases were searched for the relevant articles from January 1, 2017 to August 28, 2020. Literature reviews were searched on April 1, 2021. There were no restrictions on the year of publication and language. To avoid missing any study, several keywords were replaced with their synonyms. The following search terms were applied: “hepatitis C virus” (e.g., “HCV”; “CHC”; “hepatitis c”); “direct-acting antiviral” (e.g., “DAA”; “Sofosbuvir”; “Dasabuvir”; “Daclatasvir”; “Ledipasvir”; “Ombitasvir”; “Elbasvir”; “Velpatasvir”; “Boceprevir”; “Telaprevir”; “Simeprevir”; “Asunaprevir”; “Paritaprevir”; “Grazoprevir”); “pediatric” (e.g., “paediatr”; “pediatr”); and “children” (e.g., “child”; “teenager”; “kid”; “adolescent”; “youngster”; “juvenile”) (Supplementary Table 2). All types of studies were collated initially. The procedure is outlined in Figure 1.
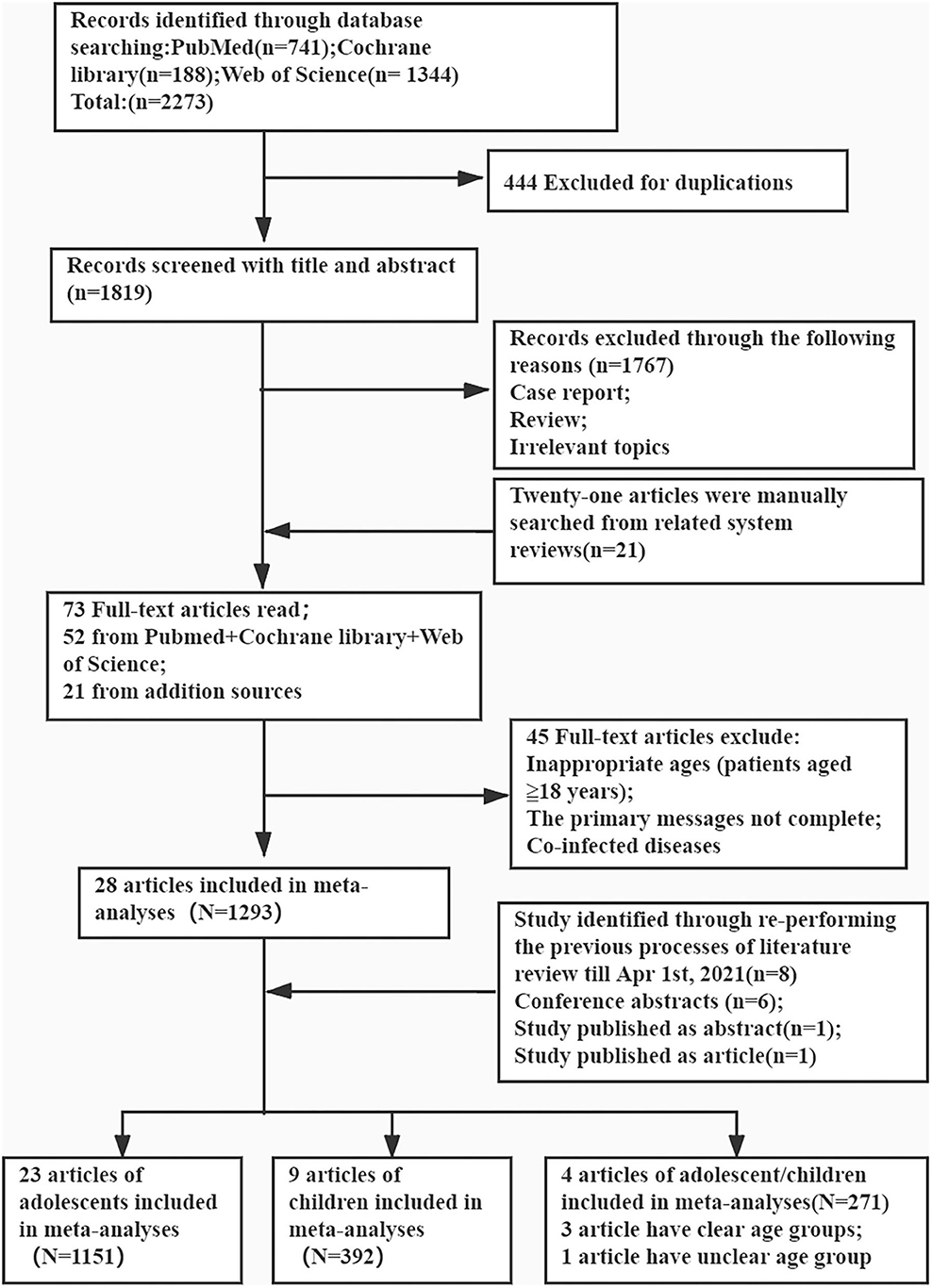
Figure 1. Preferred reporting items for the review flow diagram for identification of relevant studies.
Inclusion and Exclusion Criteria
Studies that met the following criteria were included: (i) HCV infection (HCV RNA positive in blood) (8), (ii) adolescents (12–17 years) or pediatric (<12 years of age) patients, (iii) DAA treatment regimen, (iv) all HVC genotypes, (v) definite outcome variables (SVR12), (vi) TN or TE patients, and (vii) informed consent.
The exclusion criteria of the studies were as follows: (i) co-infection with HBV or HIV, (ii) evidence of HCC or other malignancy, (iii) history of solid organ or bone marrow transplantation, (iv) decompensated liver disease or chronic liver disease of a non-HCV etiology, (v) review, case report, or articles with >10 subjects, and (vi) not treated with any DAA-containing regimens.
Study Selection
The duplicate studies were first eliminated using Endnote software, and the unrelated studies were excluded by browsing through the titles and abstracts. Studies with only adult subjects or lacking DAAs in the treatment regimens were excluded, and those reporting on the efficacy or safety of DAA treatment in children were retained. The bibliographies of the most recent relevant literature reviews were manually inspected to obtain additional articles. To avoid selection bias caused by one person, two reviewers (Mr. Fu and Miss Yue) evaluated all abstracts and selected the relevant studies for full-text reading. Any disagreement was resolved by consensus among all authors.
Research Outcomes
The primary outcome was the efficacy of DAA regimens in adolescents and children, which was defined as the percentage of patients with SVR12 [HCV RNA < the lower limit of quantitation (LLOQ) at 12 weeks after cessation of therapy]. The SVR12 in this meta-analysis was the intention-to-treat (ITT) SVR12. The second outcome was the percentage of patients with adverse events (AEs) and serious AEs (SAEs). The AEs were defined as any unfavorable medical event reported by patients or any aberrations observed by the clinicians from the baseline laboratory indices after administration of the first dose until 30 days after the last dose. Common AEs included fatigue, nausea, and so on. The SAEs were defined as any event causing disability, congenital malformation, or death (8, 15–17). The worsening of laboratory test values from baseline was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (18). The safety of DAA regimen for HCV-infected patients was evaluated by the rate of drug-related AEs, SAEs, discontinuation, and laboratory abnormalities (19).
Data Extraction and Quality Evaluation
All relevant data including SVR12 (the primary endpoints of interest), side effects, study characteristics (e.g., study author, publication date, study type, and study sites), patient characteristics at baseline (e.g., age, sex rate, genotype, and treatment regimen/duration), and possible factors that affect the outcomes of treatment were extracted from the articles. The study subjects were divided into the adolescents (12–17 years) and children (<12 years of age) groups.
All studies were assessed for methodological quality using the tool of Review Manager 5.2. The items of evaluation refer to a National Institutes of Health quality assessment tool: the tool for “Before-After (Pre-Post) Studies With No Control Group” (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) (Supplementary Table 3). Each criterion was graded as “yes,” “no,” or “unclear,” which corresponded to “low bias risk,” “high bias risk,” and “unclear risk of bias,” respectively.
Statistical Analyses
R x64-3.6.1 software (The R Foundation for Statistical Computing) was used for the meta-analysis. Pool proportions with 95% CIs for SVR were summarized with fixed effects models using Freeman–Tukey double arcsine transformation (20). Fixed/random effects models were used in all analyses, and statistical heterogeneity was calculated with the I2 method and subgroup differences using the Q-test. I2 was calculated as follows: I2 (%) = 100 × (Q − df)/Q, where Q is Cochrane's heterogeneity statistic and df indicates the degree of freedom. Negative values for I2 were set to zero, and an I2 ≥ 50% was considered to have substantial heterogeneity. Publication bias was analyzed by Funnel plots. P < 0.05 was considered statistically significant.
Results
A total of 741, 1,344, and 188 studies were initially identified in the PubMed, Web of Science, and Cochrane Library databases, respectively, of which 444 duplicate articles were excluded. After screening the titles and abstracts, 1,767 articles were further excluded. After including 21 additional articles from manual search of the reference lists, a total of 73 papers were eligible for full-text screening, of which 45 were excluded for incomplete data and/or inappropriate age groups (patients aged ≥18 years) and 7 for patients with co-morbidities. Another eight articles were included after the later literature search. Finally, 36 articles were included for further review, except for one that included both children and adolescents (Figure 1).
Studies and Patients' Characteristics
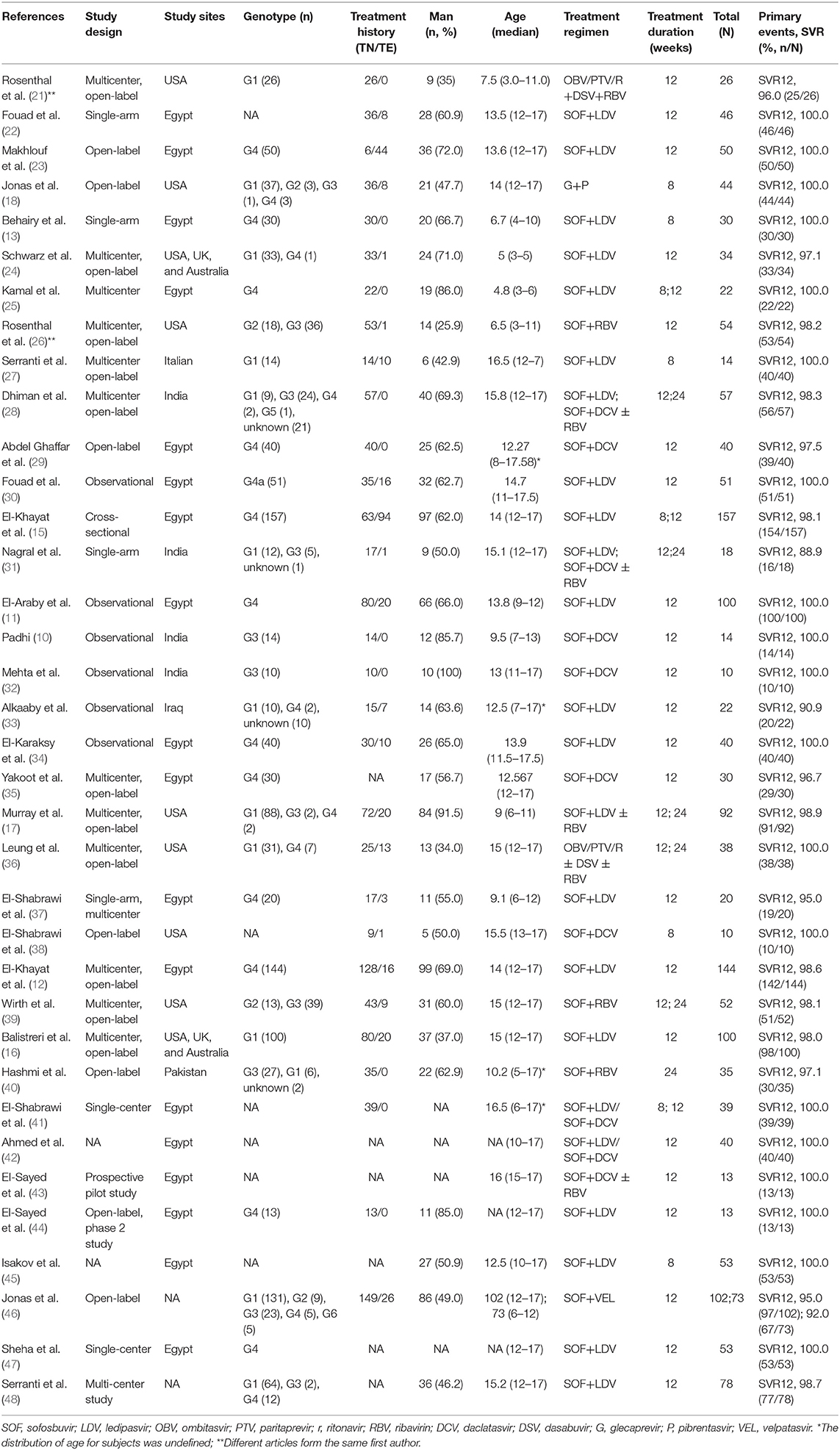
The main characteristics of the patients and studies are summarized in Table 1. A total of 28 studies were included, of which 18 were from Egypt, 7 from the United States, 4 from India, and 5 from multiple or other countries. All studies were observational, and 14 were multi-center studies. Except for three studies that did not specify the age groups, a total of 1,718 patients (1,253 adolescents and 465 children) were included in the studies, of which 792 were infected with HCV GT4, 545 with HCV GT1, 156 with GT3, 43 with HCV GT2, 1 with HCV GT5, and 213 with unknown GTs. Apart form 267 patients with unavailable treatment history, 1,216 were TN and 272 were TE. The majority of the patients (59%, 951/1,612) were males.
The methodological quality of each study is shown in Supplementary Figures 1, 2. The quality assessment criteria according to the National Institute of Health quality assessment tools are listed in Supplementary Table 3 (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). As shown in Supplementary Figure 2, most items had good level of research quality except for Q5, which was the result of patient specificity.
Efficacy Analysis of DAAs in Adolescents With CHC
A total of 24 studies including 1,253 adolescents patients were included for evaluating SVR12. The fixed-effect model showed that the pooled SVR12 rate was 99.4% (837/1,253, 95% CI: 98.7–99.9) (12, 15, 16, 18, 22, 23, 27–32, 34–36, 38, 39, 42–48). There was no significant heterogeneity (I2 = 0%, P = 0.83) (Figure 2A) or publication bias (t = 0.22, P = 0.828) (Supplementary Figure 3A) among these studies. There were three different treatment cycles of 8, 12, and 24 weeks. As shown in Table 2, the SVR12 rate was 100% (193/194, 95% CI: 98.7–100) for patients treated for 8 weeks, 99.3% (998/1,015, 95% CI: 98.4–99.9) for those treated for 12 weeks, and 100% (43/44, 95% CI: 98.9–100) for those treated for 24 weeks. The pooled SVR12 rate was 100% (95% CI: 100.0–100.0) (Supplementary Figure 4A), with litter heterogeneity among the three groups (P = 0.398). In addition, there were no significant differences in the pooled SVR12 rates when analyzed for the genotype, treatment history, and treatment regimen subgroups (Supplementary Figures 6A–8A).
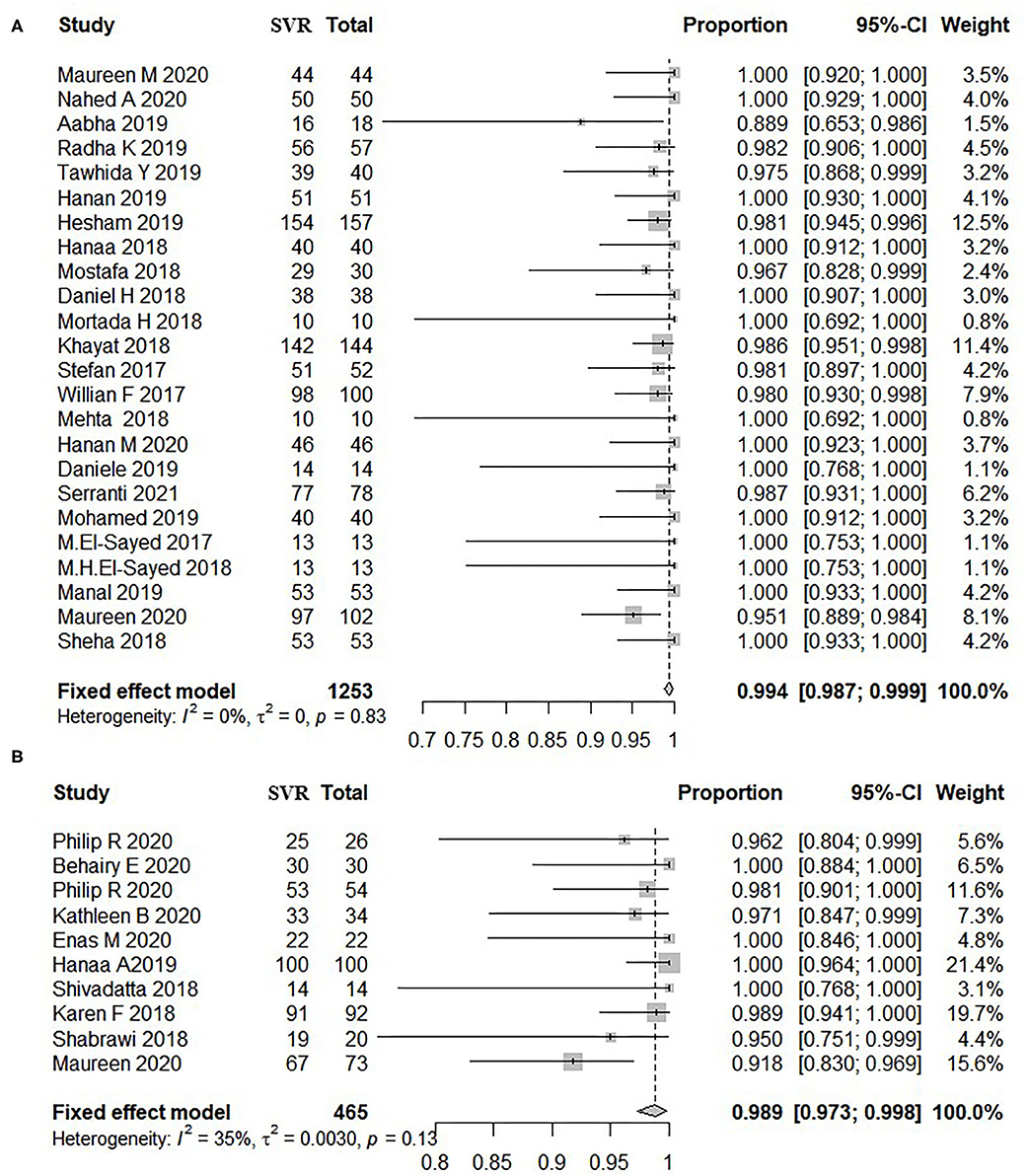
Figure 2. Overall rate of SVR12 in patients treated by DAAs. (A) Patients aged 12–17 years old. (B) Patients below 12 years old. The size of the square represents the weight of the study in the meta-analysis; the line width represents the 95% confidence interval of the study; the vertical line represents the “no effect line”; the diamond-shaped block represents the combined effect estimate of each study (fixed effect model or random effects model). (A) Patients aged 12–17 years old. (B) Patients below 12 years old. SVR, sustained virological response; DAAs, direct-acting antivirals; CI, confidence interval; Total, sum of patients treated by DAA.
Safety Analysis of DAAs in Adolescents With CHC
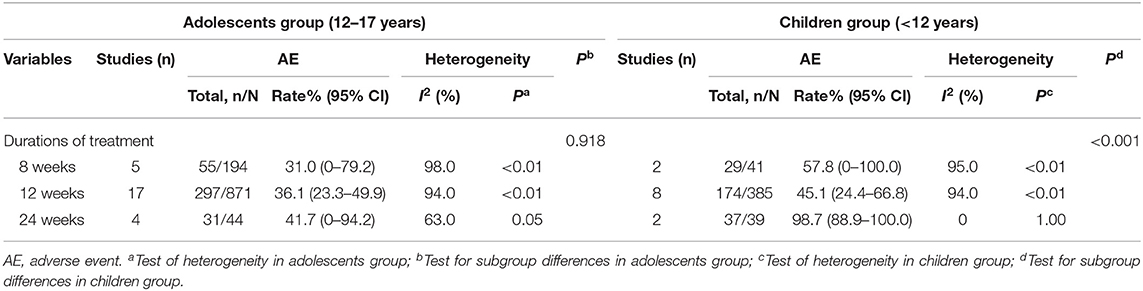
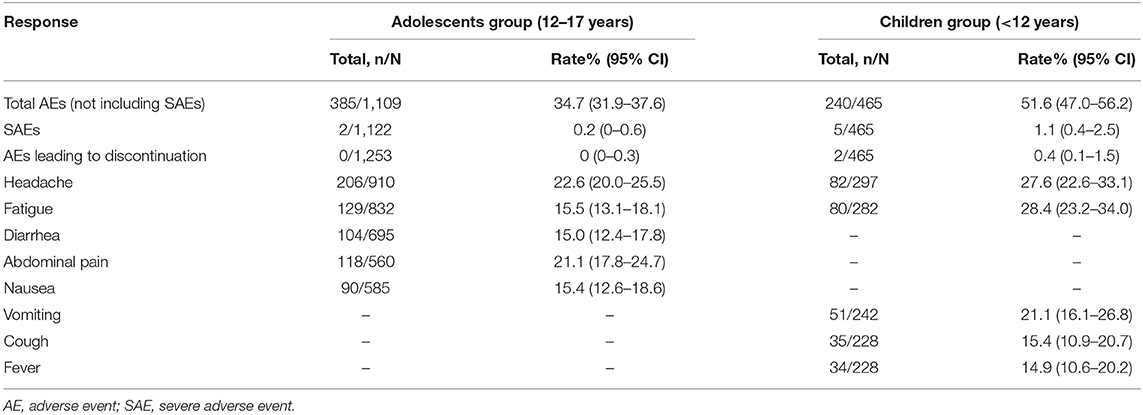
As shown in Table 3 and Supplementary Figure 5A, the AE rate was 31, 36.1, and 41.7% among adolescents treated with DAAs for 8, 12, and 24 weeks, respectively. No significant heterogeneity was observed among three groups (31.0 vs. 36.1 vs. 41.7%, P = 0.918). Furthermore, the pooled AE rate for the adolescents aged 12–17 years was 34.7% (385/1,109, 95% CI: 31.9–37.6) and the SAE rate was 0.2% (95% CI: 0–0.6). The top AEs in adolescents were headache (22.6%, 206/910), abdominal pain (21.1%, 118/560), fatigue (15.5%, 129/832), nausea (15.4%, 90/585), and diarrhea (15.0%, 104/695).
Efficacy Analysis of DAAs in Children With CHC
A total of nine studies including 465 pediatric patients were included for SVR12 evaluation, and the fixed-effect model showed that the pooled SVR12 rate was 98.9% (454/465, 95% CI: 97.3–99.8) (10, 11, 13, 17, 21, 24–26, 37, 46). There was little heterogeneity among these studies (I2 = 35%, P = 0.13) (Figure 2B), and no significant publication bias was observed as per the funnel plot (t = −0.68, P = 0.519) (Supplementary Figure 3B). Eight of these studies reported the efficacy of 12-week treatment, two studies reported the efficacy of 8-week treatment, and only one study observed the outcomes of 24-week treatment. The SVR12 rates were 100% (41/41, 95% CI: 95.9–100), 98.8% (410/421, 95% CI: 97.1–99.8), and 100% (3/3, 95% CI: 50.0–100.0) for patients treated for 8, 12, and 24 weeks, respectively (Supplementary Figure 4B). No distinct heterogeneity was observed among these groups (P = 0.676). Moreover, the pooled SVR12 rate for children with CHC was independent of HCV genotypes, treatment history, and treatment regimens (Supplementary Figures 6B–8B).
Safety Analysis of DAAs in Children With CHC
As shown in Table 3, the AE rates in pediatric patients treated with DAAs for 8, 12, and 24 weeks were 57.8, 45.1, and 98.7%, respectively (Supplementary Figure 5B). Thus, the AE rate increased significantly when the treatment was continued for 24 weeks (98.7 vs. 57.8/45.1%, P < 0.001). The pooled AE rate was 51.6% (240/465, 95% CI: 47.0–56.2) and the SAE rate was 1.1% (5/465, 95% CI: 0.4–2.5) for children (<12 years of age), and the most common AEs were fatigue (28.4%, 80/282), headache (27.6%, 82/297), vomiting (21.1%, 51/242), cough (15.4%, 35/228), and fever (14.9%, 34/228) (Table 4).
Discussion
Compared to adult patients, there are significant gaps regarding the data of adolescents and children with HCV infection. Although several DAAs are effective and safe in adolescents with hepatitis C (8, 49), it is unclear whether a shorter 8-week treatment cycle would achieve similar outcomes as the 12-week or even 24-week cycles. To this end, we systematically analyzed the studies published so far on the therapeutic efficacy of DAA-containing regimens in children and adolescents with HCV infection.
Prior to the regulatory approval of DAAs for pediatric patient, the standard treatment for adolescents and children infected with HCV was 24 weeks of pegIFN and RBV for GT 2 and 3, and 48 weeks for GT 1 and 4 (50–58). This combination resulted in an SVR of around 52% in patients infected with HCV GT 1 and 4, and 89% in those infected with HCV GT 2 and 3, but was associated with significant side effects (54–56, 58). Compared to IFN-based regimens, DAAs not only are more efficient but also have fewer side effects (10–12, 15–17, 22, 24–26, 28, 29, 31, 34–39). We found that the overall SVR12 rate for the adolescents and children treated with DAAs was 99.4 and 98.9%, respectively, although the frequency of AEs was substantial (34.7 and 51.6%). Nevertheless, SAEs were rare (0.2 and 1.1%) and no adolescent patients discontinued treatment due to the AEs since most were tolerable, such as headaches (22.6%), abdominal pain (21.1%), and fatigue (15.5%). Moreover, children were more likely to experience side effects compared to teenagers (51.6 vs. 34.7%). The most common AE among children was fatigue (28.4%), most likely due to “abnormal drug taste” (24, 26). Thus, DAAs are relatively well-tolerated by both children and adolescents.
Apart from efficacy and safety, cost-effectiveness is also an important issue for any drug regimen (59). Kohli et al. (60), Latt et al. (61), and Kattakuzhy et al. (62) analyzed the outcomes of HCV treatment shorter than 12 weeks and reported ambiguous results. A recent review has shown that 8 weeks of glecaprevir/pibrentasvir (G/P) is equally effective in treatment-naive non-cirrhotic adults (63). We did not detect any significant differences between the various treatment durations in terms of efficacy in adolescents or children (Pb = 0.398, Pd = 0.716). Hesham et al. also found that 8 weeks of treatment with the SOF/LDV combination was as effective and safe as the 12-week regimen in adolescent GT4 patients (15). Similar results were reported by Mortada et al. (38). As for the treatment cycle of 24 weeks, most just appeared in RBV-based regimen in children, because SOF+RBV was also a suboptimal regimen for persons with GT 3 infection, especially if they have liver cirrhosis (8). We found that both 8-week and 12/24-week treatment courses were well-tolerated in adolescents (31 vs. 36.1%/41.7%, P = 0.918), whereas the AE rate at 24 weeks was greater than that at 8/12 weeks (98.7 vs. 57.8%/45.1%, P < 0.001) in children with CHC. This can be attributed to RBV intolerance, as well as the fact that a longer treatment duration would also increase the chances of detecting AEs that manifest late. The correlation between treatment duration and AEs needs to be studied further.
Given the underdeveloped immune system of children and the limited time for which DAAs have been administered to this group, our findings should be interpreted with caution. In addition, we only evaluated the efficacy of DAAs in terms of SVR12, and some subgroups did not have a corresponding control due to ethical reasons. Secondly, stratified analysis of SVR showed that the heterogeneity within the three treatment cycles was somewhat large, but the inter-group heterogeneity was not statistically significant. Lastly, only the FDA-approved DAAs were analyzed in the review. Therefore, the treatment outcomes of novel DAAs will have to be continuously monitored in children.
In conclusion, DAAs are overall effective and well-tolerated in adolescents and children with chronic hepatitis C. The 8-week treatment course is as effective as 12/24 weeks in both adolescents and children.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
ZF and MY: study design and protocol, searches, title, abstract, full-text screening, data abstraction, statistical analyses, interpretation of the data, and drafting the article. ZG and CW: data verification and statistical analyses. CS and YW: statistical analyses and interpretation of the data. JL and CZ: interpretation of the data. PH, CD, and YZ: study design and protocol, interpretation of the data, and drafting the article. ZF, CD, PH, and MY: manuscript revision and question answer. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81703273 and 81773499), the Natural Science Foundation of Jiangsu Province of China (BK20171054), the Science Foundation for Distinguished Young Scholars of Jiangsu Province (BK20190106), Jiangsu Program for Young Medical Talents (QNRC2016616), and the Key Project of Yunnan Province Applied Basic Research Program (2019FA005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.608760/full#supplementary-material
References
1. Spearman CW, Dusheiko GM, Hellard M, Sonderup M. Hepatitis C. Lancet. (2019) 394:1451–66. doi: 10.1016/S0140-6736(19)32320-7
3. El-Sayed MH, Razavi H. Global estimate of HCV infection in the pediatric and adolescent population. J Hepatol. (2015) 62:S831–2. doi: 10.1016/S0168-8278(15)31458-6
4. Bortolotti F, Verucchi G, Camma C, Cabibbo G, Zancan L, Indolfi G, et al. Long-term course of chronic hepatitis C in children: from viral clearance to end-stage liver disease. Gastroenterology. (2008) 134:1900–7. doi: 10.1053/j.gastro.2008.02.082
5. Network. The European Paediatric Hepatitis C Virus Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. (2005) 41:45–51. doi: 10.1086/430601
6. Resti M, Jara P, Hierro L, Azzari C, Giacchino R, Zuin G, et al. Clinical features and progression of perinatally acquired hepatitis C virus infection. J Med Virol. (2003) 70:373–7. doi: 10.1002/jmv.10405
7. Modin L, Arshad A, Wilkes B, Benselin J, Lloyd C, Irving WL, et al. Epidemiology and natural history of hepatitis C virus infection among children and young people. J Hepatol. (2019) 70:371–8. doi: 10.1016/j.jhep.2018.11.013
8. World Health Organisation. Guidelines for the Care and Treatment of Persons Diagnosed With Chronic Hepatitis C Virus Infection. Geneva: World Health Organisation (2018).
9. Thorne C, Indolfi G, Turkova A, Giaquinto C, Nastouli E. Treating hepatitis C virus in children: time for a new paradigm. J Virus Erad. (2015) 1:203–5. doi: 10.1016/S2055-6640(20)30500-8
10. Padhi S, Maharshi S, Gupta GK, Garg KS. Nijhawan efficacy and safety of direct acting antiviral therapy for chronic hepatitis C in thalassemic children. J Pediatr Hematol Oncol. (2018) 40:511–4. doi: 10.1097/MPH.0000000000001217
11. El-Araby HA, Behairy BE, El-Guindi MA, Adawy NM, Allam AA, Sira AM, et al. Generic sofosbuvir/ledipasvir for the treatment of genotype 4 chronic hepatitis C in Egyptian children (9-12 years) and adolescents. Hepatol Int. (2019) 13:706–14. doi: 10.1007/s12072-019-09985-w
12. El-Khayat HR, Kamal EM, El-Sayed MH, El-Shabrawi M, Ayoub H, Riz KA, et al. The effectiveness and safety of ledipasvir plus sofosbuvir in adolescents with chronic hepatitis C virus genotype 4 infection: a real-world experience. Aliment Pharmacol Ther. (2018) 47:838–44. doi: 10.1111/apt.14502
13. Behairy BE, El-Araby HA, El-Guindi MA, Basiouny HM, Fouad OA, Ayoub BA, et al. Safety and EFFICACY of 8 weeks Ledipasvir/Sofosbuvir for chronic hepatitis C genotype 4 in children aged 4-10 years. J Pediatr. (2020) 219:106–10. doi: 10.1016/j.jpeds.2019.12.034
14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
15. El-Khayat H, Kamal EM, Yakoot M, Gawad MA, Kamal N, El Shabrawi M, et al. Effectiveness of 8-week sofosbuvir/ledipasvir in the adolescent chronic hepatitis C-infected patients. Eur J Gastroenterol Hepatol. (2019) 31:1004–9. doi: 10.1097/MEG.0000000000001360
16. Balistreri WF, Murray KF, Rosenthal P, Bansal S, Lin CH, Kersey K, et al. The safety and effectiveness of ledipasvir-sofosbuvir in adolescents 12-17 years old with hepatitis C virus genotype 1 infection. Hepatology. (2017) 66:371–8. doi: 10.1002/hep.28995
17. Murray KF, Balistreri WF, Bansal S, Whitworth S, Evans HM, Gonzalez-Peralta RP, et al. Safety and efficacy of Ledipasvir-Sofosbuvir with or without ribavirin for chronic hepatitis C in children ages 6-11. Hepatology. (2018) 68:2158–66. doi: 10.1002/hep.30123
18. Jonas MM, Squires RH, Rhee SM, Lin CW, Bessho K, Feiterna-Sperling C, et al. Pharmacokinetics safety, and efficacy of Glecaprevir/Pibrentasvir in adolescents with chronic hepatitis C virus: part 1 of the DORA study. Hepatology. (2020) 71:456–62. doi: 10.1002/hep.30840
19. Wang X, Fan X, Deng H, Zhang X, Zhang K, Li N, et al. Efficacy and safety of glecaprevir/pibrentasvir for chronic hepatitis C virus genotypes 1-6 infection: a systematic review and meta-analysis. Int J Antimicrob Agents. (2019) 54:780–9. doi: 10.1016/j.ijantimicag.2019.07.005
20. Miller John J. The inverse of the freeman – Tukey double arcsine transformation. Am Statist. (1978) 32:138. doi: 10.1080/00031305.1978.10479283
21. Rosenthal P, Narkewicz MR, Yao BB, Jolley CD, Lobritto SJ, Wen J, et al. Ombitasvir, paritaprevir, ritonavir, and dasabuvir mini-tabs plus ribavirin for children aged 3-11 years with hepatitis C genotype 1a. Adv Ther. (2020) 37:3299–310. doi: 10.1007/s12325-020-01389-9
22. Fouad HM, Ahmed Mohamed A, Sabry M, Abdel Aziz H, Eysa B, Rabea M. The effectiveness of Ledipasvir/Sofosbuvir in youth with genotype 4 hepatitis C virus: a single egyptian center study. Pediatr Infect Dis J. (2019) 38:22–5. doi: 10.1097/INF.0000000000002189
23. Makhlouf NA, Abdelmalek MO, Ibrahim ME, Abu-Faddan NH, Kheila AE, Mahmoud AA. Ledipasvir/Sofosbuvir in adolescents with chronic hepatitis C genotype with and without hematological disorders: virological efficacy and impact on liver stiffness. J Pediatric Infect Dis Soc. (2020) 4:7–13. doi: 10.1093/jpids/piaa006
24. Schwarz KB, Rosenthal P, Murray KF, Honegger JR, Hardikar W, Hague R, et al. Ledipasvir-Sofosbuvir for 12 weeks in children 3 to <6 years old with chronic hepatitis C. Hepatology. (2019) 71:422–30. doi: 10.1002/hep.30830
25. Kamal EM, El-Shabrawi M, El-Khayat H, Yakoot M, Sameh Y, Fouad Y, et al. Effects of sofosbuvir/ledipasvir therapy on chronic hepatitis C virus genotype 4, infected children of 3-6 years of age. Liver Int. (2019) 40:319–23. doi: 10.2139/ssrn.3429908
26. Rosenthal P, Schwarz KB, Gonzalez-Peralta RP, Lin CH, Kelly DA, Nightingale S, et al. Ribavirin therapy for children aged 3 to <12 years with hepatitis C virus genotype 2 or 3 infection. Hepatology. (2019) 71:31–43. doi: 10.1002/hep.30821
27. Serranti D, Dodi I, Nicastro E, Cangelosi AM, Riva S, Ricci S, et al. Shortened 8-week course of Sofosbuvir/Ledipasvir therapy in adolescents with chronic hepatitis C infection. J Pediat Gastroenterol Nutr. (2019) 69:595–8. doi: 10.1097/MPG.0000000000002449
28. Dhiman RK, Grover GS, Premkumar M, Taneja S, Duseja A, Rathi S, et al. Direct-acting antiviral therapy is safe and effective in pediatric chronic hepatitis C: the public health perspective. J Pediatr Gastroenterol Nutr. (2019) 68:74–80. doi: 10.1097/MPG.0000000000002139
29. Abdel Ghaffar TY, El Naghi S, Abdel Gawad M, Helmy S, Abdel Ghaffar A, Yousef M. Safety and efficacy of combined sofosbuvir/daclatasvir treatment of children and adolescents with chronic hepatitis C Genotype 4. J Viral Hepat. (2019) 26:263–70. doi: 10.1111/jvh.13032
30. Fouad HM, Sabry MA, Ahmed A, Hassany M, Al Soda MF, Abdel Aziz H. Generic Ledipasvir-Sofosbuvir treatment for adolescents with chronic hepatitis C virus infection. J Pediatric Infect Dis Soc. (2020) 9:386–9. doi: 10.1093/jpids/piz041
31. Nagral A, Jhaveri A, Sawant S, Parikh NS, Nagral N, Merchant R, et al. Treatment of chronic hepatitis C infection with direct acting antivirals in adolescents with thalassemia major. Ind J Pediatr. (2019) 86:148–53. doi: 10.1007/s12098-018-2752-7
32. Mehta R, Kabrawala M, Nandwani S, Desai P, Bhayani V, Patel S, et al. Safety and efficacy of sofosbuvir and daclatasvir for hepatitis C virus infection in patients with beta-thalassemia major. J Clin Exp Hepatol. (2018) 8:3–6. doi: 10.1016/j.jceh.2017.06.002
33. Alkaaby BA, Al-Ethawi AE. The effectiveness of oral antiviral (Sofosbuvir/Ledipasvir) in treating children with HCV infection. Pak J Med Sci. (2018) 34:1353–6. doi: 10.12669/pjms.346.15722
34. El-Karaksy H, Mogahed EA, Abdullatif H, Ghobrial C, El-Raziky MS, El-Koofy N, et al. Sustained viral response in genotype 4 chronic hepatitis C virus-infected children and adolescents treated with Sofosbuvir/Ledipasvir. J Pediatr Gastroenterol Nutr. (2018) 67:626–30. doi: 10.1097/MPG.0000000000002101
35. Yakoot M, El-Shabrawi MH, AbdElgawad MM, Mahfouz AA, Helmy S, Abdo AM, et al. Dual Sofosbuvir/Daclatasvir therapy in adolescent patients with chronic hepatitis C infection. J Pediatr Gastroenterol Nutr. (2018) 67:86–9. doi: 10.1097/MPG.0000000000001968
36. Leung DH, Wirth S, Yao BB, Viani RM, Gonzalez-Peralta RP, Jonas MM, et al. Ombitasvir/Paritaprevir/Ritonavir with or without dasabuvir and with or without ribavirin for adolescents with HCV genotype 1 or 4. Hepatol Commun. (2018) 2:1311–9. doi: 10.1002/hep4.1250
37. El-Shabrawi MHF, Kamal NM, El-Khayat HR, Kamal EM, AbdElgawad M. A pilot single arm observational study of sofosbuvir/ledipasvir (200 + 45 mg) in 6- to 12- year old children. Aliment Pharmacol Ther. (2018) 47:1699–704. doi: 10.1111/apt.14677
38. El-Shabrawi MH, Abdo AM, El-Khayat HR, Yakoot M. Shortened 8 weeks course of dual Sofosbuvir/Daclatasvir therapy in adolescent patients, with chronic hepatitis C infection. J Pediatr Gastroenterol Nutr. (2018) 66:425–7. doi: 10.1097/MPG.0000000000001838
39. Wirth S, Rosenthal P, Gonzalez-Peralta RP, Jonas MM, Balistreri WF, Lin CH, et al. Sofosbuvir and ribavirin in adolescents 12-17 years old with hepatitis C virus genotype 2 or 3 infection. Hepatology. (2017) 66:1102–10. doi: 10.1002/hep.29278
40. Hashmi M A., Cheema H. A. Effectiveness and Safety of Sofosbuvir in Treatment-NäiveChildren with Hepatitis C Infection. J Coll Physicians Surg Pak. (2017) 27:423–6.
41. El-Shabrawi M, Baroudy S, Hassanin F, Behairy AS, Yakoot M, Ahmed A. Follow-up of chronic paediatric hepatitis C virus in a low-/middle-income country. Acta Paediatr. (2020) 109:2699–705. doi: 10.1111/apa.1533310.1111/apa.15333
42. Ahmed M, Hanno A, Hamouda S, Abdelgawad M, Abouelkhier H. THU-113-comparison between safety and efficacy of two treatment regimens for pediatric patients with chronic hepatitis C virus: Sofosbuvir/ledipasvir versus sofosbuvir/daclatasvir regimen. J Hepatol. (2019) 70(Suppl. 1):e208. doi: 10.1016/S0618-8278(19)30388-3
43. El-Sayed M, Hassany M, Asem N. THU-412 - A pilot study for safety and efficacy of 12 weeks sofosbuvir plus daclatasvir with or without ribavirin in egyptian adolescents with chronic hepatitis C virus Infection. J Hepatol. (2017) 66:S178. doi: 10.1016/S0168-8278(17)30642-6
44. El-Sayed MH, Ebeid FSES, Zekri AR, Massetto B, Kersey K, Osinusi A, et al. Ledipasvir/Sofosbuvir for 12 weeks is safe and effective in adolescents with chronic hepatitis C virus infection and hematological malignancies undergoing chemotherapy. J Hepatol. (2018) 68:S515. doi: 10.1016/S0168-8278(18)31278-9
45. Isakov V, Gankina N, Morozov V, Kersey K, Lu S, Osinusi A, et al. THU-136-Ledipasvir/sofosbuvir for 8 weeks cures genotype 4 chronic hepatitis C in non-cirrhotic children and adolescents. J Hepatol. (2019) 70:e221. doi: 10.1016/S0618-8278(19)30411-6
46. Jonas M, Romero R, Sokal E, Rosenthal P, Verucchi G, Lin H, et al. IDDF2020-ABS-0059 Safety and efficacy of sofosbuvir/velpatasvir (SOF/VEL) in pediatric patients 6 to <18 years old with chronic hepatitis C (CHC) infection. Gut. (2020) 69:1832–40. doi: 10.1136/gutjnl-2020-IDDF.142
47. Sheha G, Elsayed R, Elbasiony M, Mikhail N. Ledipasvir 90 mg/sofosbuvir 400 mg for treatment of children with CHC genotype 4: single centre experience. J Hepatol. (2018) 68:S267. doi: 10.1016/S0168-8278(18)30748-7
48. Serranti D, Nebbia G, Cananzi M, Nicastro E, Di Dato F, Nuti F, et al. Efficacy of Sofosbuvir/Ledipasvir in adolescents with chronic hepatitis C genotypes 1, 3, and 4: a real-world study. J Pediatr Gastroenterol Nutr. (2021) 72:95–100. doi: 10.1097/MPG.0000000000002900
49. Indolfi G, Giometto S, Serranti D, Bettiol A, Bigagli E, De Masi S, et al. Systematic review with meta-analysis: the efficacy and safety of direct-acting antivirals in children and adolescents with chronic hepatitis C virus infection. Aliment Pharmacol Ther. (2020) 52:1125–33. doi: 10.1111/apt.16037
50. Baker RD, Dee D, Baker SS. Response to pegylated interferon alpha-2b and ribavirin in children with chronic hepatitis C. J Clin Gastroenterol. (2007) 41:111–4. doi: 10.1097/MCG.0b013e31802dd2f6
51. Jara P, Hierro L, de la Vega A, Diaz C, Camarena C, Frauca E, et al. Efficacy, safety of peginterferon-alpha2b ribavirin combination therapy in children with chronic hepatitis C infection. Pediatr Infect Dis J. (2008) 27:142–8. doi: 10.1097/INF.0b013e318159836c
52. Tajiri H, Inui A, Kiyohara Y, Suzuki M, Kagimoto S, Etani Y, et al. Peginterferon alpha-2b and ribavirin for the treatment of chronic hepatitis C in Japanese pediatric and young adult patients: a survey of the Japan society of pediatric hepatology. Eur J Gastroenterol Hepatol. (2009) 21:1256–60. doi: 10.1097/MEG.0b013e32832a4e97
53. Sokal EM, Bourgois A, Stephenne X, Silveira T, Porta G, Gardovska D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in children and adolescents. J Hepatol. (2010) 52:827–31. doi: 10.1016/j.jhep.2010.01.028
54. Wirth S, Ribes-Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, et al. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol. (2010) 52:501–7. doi: 10.1016/j.jhep.2010.01.016
55. Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, et al. NASPGHAN practice guidelines: diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr. (2012) 54:838–55. doi: 10.1097/MPG.0b013e318258328d
56. Druyts E, Thorlund K, Wu P, Kanters S, Yaya S, Cooper CL, et al. Efficacy and safety of pegylated interferon alfa-2a or alfa-2b plus ribavirin for the treatment of chronic hepatitis C in children and adolescents: a systematic review and meta-analysis. Clin Infect Dis. (2013) 56:961–7. doi: 10.1093/cid/cis1031
57. Indolfi G, Nebbia G, Cananzi M, Maccabruni A, Zaramella M, D'Antiga L, et al. Kinetic of virologic response to pegylated interferon ribavirin in children with chronic hepatitis C predicts the effect of treatment. Pediatr Infect Dis J. (2016) 35:1300–3. doi: 10.1097/INF.0000000000001325
58. Wirth S, Pieper-Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology. (2005) 41:1013–8. doi: 10.1002/hep.20661
59. Rosenthal ES, Graham CS. Price and affordability of direct-acting antiviral regimens for hepatitis C virus in the United States. Infect Agent Cancer. (2016) 11:24. doi: 10.1186/s13027-016-0071-z
60. Kohli A, Kattakuzhy S, Sidharthan S, Nelson A, McLaughlin M, Seamon C, et al. Four-week direct-acting antiviral regimens in noncirrhotic patients with hepatitis C virus genotype 1 infection: an open-label, nonrandomized trial. Ann Intern Med. (2015) 163:899–907. doi: 10.7326/M15-0642
61. Latt NL, Yanny BT, Gharibian D, Gevorkyan R, Sahota AK. Eight-week ledipasvir/sofosbuvir in non-cirrhotic, treatment-naïve hepatitis C genotype-1 patients with hepatitis C virus-RNA <6 million: single center, real world effectiveness and safety. World J Gastroenterol. (2017) 23:4759–66. doi: 10.3748/wjg.v23.i26.4759
62. Kattakuzhy S, Wilson E, Sidharthan S, Sims Z, McLaughlin M, Price A, et al. Moderate sustained virologic response rates with 6-week combination directly acting anti-hepatitis C virus therapy in patients with advanced liver disease. Clin Infect Dis. (2016) 62:440–7. doi: 10.1093/cid/civ897
Keywords: hepatitis C virus, direct-acting antivirals regimens, adolescents and children, sustained virological response, treatment duration
Citation: Fu Z, Dong C, Ge Z, Wang C, Zhang Y, Shen C, Li J, Zhu C, Wang Y, Huang P and Yue M (2021) High SVR12 With 8-Week Course of Direct-Acting Antivirals in Adolescents and Children With Chronic Hepatitis C: A Comprehensive Analysis. Front. Med. 8:608760. doi: 10.3389/fmed.2021.608760
Received: 21 September 2020; Accepted: 30 April 2021;
Published: 08 June 2021.
Edited by:
Jianpeng Sheng, Nanyang Technological University, SingaporeCopyright © 2021 Fu, Dong, Ge, Wang, Zhang, Shen, Li, Zhu, Wang, Huang and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Huang, aHVhbmdwZW5nQG5qbXUuZWR1LmNu; Ming Yue, eXVlbWluZ0Buam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Zuqiang Fu
Zuqiang Fu Chen Dong3†
Chen Dong3† Yan Wang
Yan Wang Peng Huang
Peng Huang Ming Yue
Ming Yue