- 1The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2The Third Clinical Medical College of Yangtze University, Jingzhou Hospital of Traditional Chinese Medicine, Jingzhou, China
- 3Putuo People's Hospital, Department of Bioinformatics, Tongji University, Shanghai, China
- 4Department of Critical Care Medicine, Dongguan People's Hospital, Southern Medical University, Dongguan, China
- 5Department of Critical Care Medicine, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 6School of Medicine, Sun Yat-sen University, Shenzhen, China
Corticosteroid is commonly used to reduce damage from inflammatory reactions in coronavirus disease 2019 (COVID-19). We aim to determine the outcomes of corticosteroid use in critically ill COVID-19 patients. Ninety six critically ill patients, hospitalized in 14 hospitals outside Wuhan from January 16 to March 30, 2020 were enrolled in this study. Among 96 critical patients, 68 were treated with corticosteroid (CS group), while 28 were not treated with corticosteroids (non-CS group). Multivariable logistic regression were performed to determine the possible correlation between corticosteroid use and the treatment outcomes. Forty-six (68%) patients in the CS group died compared to six (21%) of the non-CS group. Corticosteroid use was also associated with the development of ARDS, exacerbation of pulmonary fibrosis, longer hospital stay and virus clearance time. On admission, no difference in laboratory findings between the CS and the non-CS group was observed. After corticosteroid treatment, patients treated with corticosteroids were associated with higher counts of white blood cells, neutrophils, neutrophil-to-lymphocyte ratio, alanine aminotransferase level and Sequential Organ Failure Assessment score. In conclusion, corticosteroid use in critically ill COVID-19 patients was associated with a much higher case fatality rate. Frequent incidence of liver injury and multi-organ failure in corticosteroid treated patients may have contributed to the adverse outcomes. The multi-organ failure is likely caused by more persistent SARS-CoV-2 infection and higher viral load, due to the inhibition of immune surveillance by corticosteroid.
Introduction
The pandemic of Coronavirus Disease 2019 (COVID-19) has caused many deaths, and no therapy with proven efficacy is available. The viral pathogen of COVID-19 is severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2), which, together with SARS-CoV, belongs to the species of SARSr-CoV (Severe acute respiratory syndrome-related coronavirus) (1). Both viruses are closely related to MERS-CoV, which causes Middle East respiratory syndrome (MERS).
Study with SARS patients indicated that immune-mediated mechanism rather than virus induced damage drives the clinical progression (2). Consistently, study with macaques indicated that severe symptoms of SARS infection were caused by elevated immune reactions rather than higher viral load, and the anti-inflammatory therapy with type I interferon reduced the respiratory symptoms (3). Similar to SARS and MERS (4), high levels of proinflammatory cytokines and chemokines, the cytokine storm, were observed in the peripheral blood of COVID-19 patients (5). Based on the pathological findings including significantly increased serum level of cytokines, and over-activation of T cells in severe COVID-19 patients, Xu et al. recommended to treat COVID-19 with corticosteroid to control immune reactions (6). Corticosteroid has been commonly administered to COVID-19 patients in (7, 8) and outside China (9).
However, the application of corticosteroids in coronavirus infection has consequences. The use of corticosteroids in SARS patients was associated with serious complications including avascular necrosis, diabetes and psychosis (10). Corticosteroid use also led to delayed viral RNA clearance in SARS (11) and MERS (12). In addition, suppression of the patients' immune system with corticosteroids leads to secondary infection, as observed in clinical trials of septic shock (13) and respiratory failure (14). To understand the beneficial and adverse effects of corticosteroid use in COVID-19, we reviewed the medical records of critical COVID-19 patients from 14 hospitals and found that the use of corticosteroid is associated with more severe respiratory symptoms and a much higher case fatality rate.
Methods
Study Design and Participants
We reviewed 410 patient charts of suspected COVID-19. Excluding 56 records for negative SARS-CoV-2 nucleic acid test, 24 for duplicated records, and 128 for the lack of relevant data, we identified 202 SARS-CoV-2 RNA positive patients hospitalized at 14 hospitals (Figure 1) in Jingzhou, China, from January 16 to March 30, 2020. Our COVID-19 patients were admitted to the hospitals because of fever, cough, dyspnea and chest computed tomography (CT) findings indicating SARS-CoV-2 pneumonia. Diagnosis of COVID-19 was based on positive SARS-CoV-2 nucleic acid test. Ninety-six critically ill patients were enrolled in this study. Critically ill patients were defined as those admitted to the ICU, requiring mechanical ventilation, or had a fraction of inspired oxygen (FiO2) of at least 60% (15, 16).
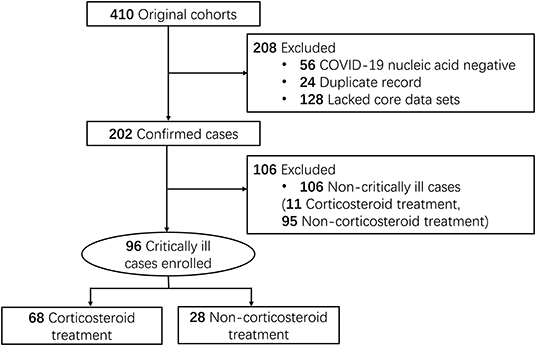
Figure 1. Study flow diagram. Medical records of COVID-19 patients (106 non-critically ill and 96 critically ill) included for the study of the corticosteroid effects were accessed from Jingzhou Hospital of Traditional Chinese Medicine (61 cases), Jianli Hospital of Traditional Chinese Medicine (44), Jingzhou First Hospital (9 cases), Jingzhou Central Hospital (29 cases), Jingzhou First People's Hospital (11 cases), Jingzhou Chest Hospital (9 cases), Gongan People's Hospital (8 cases), Honghu People's Hospital (10 cases), Honghu Hospital for the Control of Schistosomiasis (3 cases), Honghu Central Hospital (2 cases), Jianli People's Hospital (3 cases), Jiangling People's Hospital (2 cases), Shishou Hospital of Traditional Chinese Medicine (5 cases), and Songzi People's Hospital (6 cases).
Treatment of the infection followed the fifth edition of the Diagnosis and Treatment Guideline for COVID-19, National Health Commission of the People's Republic of China. Besides oxygen supplementation and respiratory support, patients were routinely given antibiotics, usually Moxifloxacin, and antivirus drugs, usually Lopinavir and Ritonavir. Mechanical ventilation was conducted when hypoxemia and dyspnea persisted despite non-invasive oxygen supplementation.
The use of corticosteroid in COVID-19 patients is controversial, and this is reflected in the fifth edition of the Diagnosis and Treatment Guideline for COVID-19, National Health Commission of the People's Republic of China, which recommended optional use of corticosteroid when evidence presented for deteriorating (or persistent) dyspnea or chest CT results. Corticosteroid [at a dose not to exceed the equivalent of 1~2 mg methylprednisolone /kg/day, and should be used for a short period of time (3 to 5 days)] was prescribed according to physicians' preference. Sixty-eight of the critically ill patients were treated with corticosteroids. We compared the demographics, symptoms, treatments and outcomes between critical COVID-19 patients treated with corticosteroids (CS group) and those not treated with corticosteroids (non-CS group).
This study was approved by the Institutional Review Boards of Sun Yat-sen University, and participating hospitals. Informed consent was waived for this retrospective chart review.
Data Collection
Charts were reviewed for demographic, clinical, laboratory, treatment and outcome data. Demographic data included age, gender, and comorbidities including hypertension, diabetes, cardiovascular disease, chronic obstructive pulmonary disease, chronic liver disease and malignancy. Clinical data recorded included vital signs such as temperature, respiratory rate, blood pressure, and oxygen saturation. In addition to fever, cough and dyspnea, other clinical characteristics recorded included sputum production, diarrhea (three or more loose or liquid stools per day), bloody stool (stool positive for occult blood test or white blood cell test), myalgia/muscle fatigue and haemoptysis. Laboratory data included blood cell counts (white blood cell, lymphocyte, neutrophil, monocyte, and platelet), markers for coagulation function (activated partial thromboplastin time (APTT), d-dimer and fibrinogen), infection-related biomarkers (C-reactive protein (CRP), Erythrocyte sedimentation rate (ESR), neutrophil-to-lymphocyte ratio (NLR) and procalcitonin), and other blood biochemistry measurements (aspartate transaminase (AST), alanine aminotransferase (ALT), creatine kinase (CK), creatinine, blood urea nitrogen (BUN), lactate dehydrogenase (LDH), total bilirubin and Sequential Organ Failure Assessment (SOFA) score). Cytokines were not measured for most patients.
The hospital course was reviewed for treatments and severity of disease. The need for supplemental oxygen, respiratory support and admission to the intensive care unit (ICU) were recorded.
Statistics
SPSS (Statistical Package for the Social Sciences) version 22.0 software (SPSS Inc.) was used for Student t test, Mann-Whitney U test, Chi-Square test, Fisher's exact test, multivariable Cox regression analysis, univariable and multivariable logistic regressions. Significance of the differences between two study groups were tested by Student t test or Mann-Whitney U test for numerical data, when appropriate. For multiple comparisons, p-values were adjusted by FDR method in R. Comparisons of categorical data were performed by Chi-Square test or Fisher's exact test, as appropriate. Age was transformed to categorical variable at the threshold of 60. Laboratory findings before discharge were transformed to categorical variables based on reference values. Multivariable logistic regressions were performed to identify clinical features and treatments associated with case fatality and corticosteroid treatment, adjusted for multiple potential confounders identified from univariable regressions. All statistical tests were two sided, with p-values of < 0.05 considered to be statistically significant.
Results
Demographics, Clinical Characteristics and the Treatments of the Critical COVID-19 Patients
A large proportion (68 of 96, 71%) of the critically ill COVID-19 patients were treated with corticosteroids, compared to 10% (11 of 106) of the non-critical patients (Figure 1). Therefore, this study only concerned the critical patients, among which 68 patients were treated with corticosteroids (CS group) while 28 patients were not treated with corticosteroid (non-CS group). Fifty-three patients (78%) in the CS group were 60 or older compared to 14 (50%) in the non-CS group (Table 1). No significant difference in gender ratio was found between the two groups.
On admission, the most common symptoms for both groups were fever (87% in the CS group), cough (74%, CS), and dyspnea (46%, CS), while less common symptoms included sputum production (32%, CS), myalgia (26%, CS), diarrhea (18%, CS), cephalalgia (15%, CS), and hemoptysis (1%, CS). No difference in the incidences of these symptoms was observed between the CS group and the non-CS group (Table 1).
The most frequently recorded comorbidities for both groups of patients included hypertension (47%, CS), diabetes (16%, CS), and cardiovascular disease (10%, CS). No difference in the incidence of any comorbidity between the CS group and the non-CS group was observed (Table 1).
Following the diagnosis and treatment guideline of the National Health Commission (China), the most common treatments included supplemental oxygen (100%, CS), antiviral treatments (90%, CS), and antibiotics (87%, CS). No difference was observed for these treatments between the CS and the non-CS groups (Table 1). The two groups were also similarly treated with thymosin α1, mechanical ventilation and ECMO. It is noteworthy that some patients in the CS group were treated with interferon (25%), immunoglobulin (29%) and albumin (7%), which were not used for patients in the non-CS group (Table 1).
Outcomes of the Patients Treated With or Without Corticosteroids
Forty-six of the 68 (68%) patients in the CS group died compared to six of the 28 (21%) in the non-CS group (Table 2). After adjusting for potential confounding factors including age, gender, hypertension, interferon, immunoglobulin, and thymosin α1, corticosteroids treatment was identified as a risk factor for case fatality in critically ill COVID-19 with an adjusted OR of 4.05 (95%CI: 1.37–11.98, p = 0.012, R2 = 0.413) (Table 3). We used the threshold of age> =60 in the multivariable logistic regression model because of the dichotomized distribution of the age of deceased patients (Supplementary Figure 1): out of the 52 deceased patients, 40 were 60 years old or older. This pattern is consistent with the reports that age >60 was a risk factor for critical illness in COVID-19 (17, 18). Similar result was obtained with survival analysis: after adjusting for the effects of age and other confounding factors, mutivariable analysis with the Cox proportional-hazards model indicated a lower survival rate in the CS group (HR 4.52 [95%CI: 1.79–11.41], p = 0.001, Supplementary Table 1). In line with higher case fatality rate, more of the patients treated with corticosteroids presented ARDS (CS vs. non-CS: 63 vs. 36%) and exacerbation of pulmonary fibrosis (CS vs. non-CS: 56 vs. 21%) than patients not treated with corticosteroids (Table 2). A higher proportion of the CS group required mechanical ventilation than the non-CS group, but statistical significance was not achieved. Among the surviving patients, the length of hospital stay was higher in the CS group [24 (IQR: 18.3–38.8) days] than in the non-CS group [17 (13.8–25.3) days]. The length of virus clearance time was also longer in the CS group [21 (16.8–26.3) days] than in the non-CS group [13.5 (12–16) days].
Table 3. Logistic univariate and multivariate regression analyses on the association between the case fatality rate and the clinical features.
Laboratory Findings
For better understanding of the elevated case fatality in the CS group, we reviewed the laboratory findings for blood cell counts, markers for coagulation function, inflammatory biomarkers and the markers for cellular, tissue and organ damage.
On admission, no difference in laboratory findings was observed between the CS and the non-CS groups (Table 4). Decreased lymphocyte counts in both groups of critical patients reflected the inflammatory characteristics of these patients. Other outstanding observations included the elevated CRP, ESR, and LDH in both groups (Table 4), reflecting viral infections, inflammatory reactions and tissue damage.
After corticosteroid treatments, patients treated with corticosteroids were associated with higher counts of white blood cells, neutrophils, and neutrophil-to-lymphocyte ratio (Table 5). The differences in the counts of white blood cells remained significant after adjustment for confounding factors (Table 6).
Table 6. Logistic univariate and multivariate regression analyses on the association between the corticosteroid treatment and the clinical features.
No difference was observed for coagulation function markers APTT, fibrinogen or d-dimer (Table 5). Serum markers for inflammation CRP and ESR were elevated in both the CS and the non-CS groups, but not different between the study groups.
Regarding markers for tissue and organ damage, multivariate logistic regression revealed higher incidence of elevated ALT and SOFA score > 10 in the CS group (Table 6). Levels of LDH, BUN, AST, and total bilirubin were elevated in both the CS and the non-CS group, but not different between the groups (Table 5).
Discussion
Our data showed that critical COVID-19 patients treated with corticosteroid had a much higher case fatality rate than those not treated with corticosteroid. This is in line with our observations that increased incidences of ARDS and exacerbation of pulmonary fibrosis, longer hospital stay and virus clearance time were associated with the use of corticosteroid in critical COVID-19 patients. Further, laboratory results provided evidence for increased incidence of tissue damage in corticosteroid users.
One immediate question is which came first, more severe symptoms or the use of corticosteroids? Use of corticosteroids was likely the lead because patients in the two study groups were both critically ill, with similar symptoms and laboratory results on admission. One possible argument is that patients in the CS group were perceived sicker by the physicians, because these patients were significantly older, and many patients in the CS group were treated with interferon, immunoglobulin and albumin, which were not prescribed for the patients in the non-CS group. To address this concern, the influences of age, possible more severe symptoms represented by the use of interferon and immunoglobulin, as well as other confounding factors, were adjusted in the multivariate logistic regression, resulted in an OR of 4.05 (1.37–11.98) for case fatality, indicating that corticosteroid use caused a much higher case fatality in critical patients.
In our hospitals, corticosteroids were usually used in the second week of disease onset when severe symptoms presented. In theory, this is a good timing for the suppression of inflammatory reactions that cause damage in ARDS and viral pneumonia (19). However, the beneficial outcomes seen with SARS patients and other types of pneumonia did not occur with our COVID-19 patients. Instead, we observed higher case fatality rate and other adverse events in critical patients treated with corticosteroids. Although pulmonary dysfunction was common in our patients treated with corticosteroids, elevated SOFA scores of these patients indicated that multi-organ failure often contributed to corticosteroid related death. In support of this, blood biochemistry revealed frequent incidence of liver injury in corticosteroid treated patients. The multi-organ failure was likely caused by more persistent viral infection and higher viral load, which could be a consequence of the inhibition of the body's immune surveillance in corticosteroid treated patients. Our retrospective study is very limited on relevant data for the evaluation of the corticosteroid effects on patient immunity and subsequent infection risk (viral load, cytokine levels, etc). Available data related to immunity and subsequent infection risk include blood cell counts, inflammation markers and viral clearance time. Before discharge, white blood cell count, lymphocyte count, and NLR were higher in the corticosteroid group, a reflection of being recovered from viral infection. As it is well-established that corticosteroid reduces inflammatory activities in viral pneumonia (19), our data support that, patients' immunity was suppressed by short-term corticosteroid treatment, which may lead to increased viral replication, extended viral clearance, and secondary infections, and therefore subsequent inflammatory reactions in multiple organs. In addition, corticosteroid treatment may directly affect SARS-CoV-2 replication, similarly as it increases viral replication of rhinovirus and influenza A virus through reduced expression of innate anti-viral genes (20). Although data is not available in our patients, known adverse effects of corticosteroids include pancreatitis (21), heart failure, ischemic heart disease (22), and myopathy (23), which may contribute to the elevated SOFA score in corticosteroid treated patients.
Our observations are different from some of the published studies. Zha et al. examined the outcome of 11 mild COVID-19 patients treated with corticosteroid (24). Compared with patients not treated with corticosteroid, the treated patients exhibited no significant difference in every outcome measures, likely due to the small sample size. However, it is noteworthy that the corticosteroid treated patients consistently exhibited a larger median for the virus clearance time, for the length of hospital stay, and for the duration of symptoms.
Recently, in contrast to our findings, two randomized open-label trials reported beneficial effects of corticosteroid therapy on severe COVID-19. The RECOVERY study reported reduced case fatality in patients treated with corticosteroid (25), while the similarly designed REMAP-CAP study observed an increased odds of improvement in organ support-free days within 21 days in the corticosteroid treatment group (26). Inconsistently, data from the REMAP-CAP study also indicated a trend of increased length of ICU stay and hospital stay for the corticosteroid treated patients. One of the limitations in both REMAP-CAP and RECOVERY studies, as reported by the authors, was that 15% of the no corticosteroid group received systemic corticosteroids. Another important limitation in both studies was that they enrolled suspected COVID-19 patients. Without positive viral RNA diagnosis, these studies provide little support for beneficial effects of corticosteroid therapy in COVID-19.
It is worth noting that one earlier review summarizing all data on corticosteroid use with COVID-19 before July 2020 concluded that corticosteroid therapy is associated with the improvement in symptoms and oxygenation for individuals with severe COVID-19, but case fatality rate in the corticosteroid group was significantly higher than that in the non-corticosteroid group (27). This conflict between improved symptoms and increased case fatality rate could be a consequence of the heterogeneous nature of the different studies being reviewed. In contrast, we observed, consistently, higher case fatality rate and higher odds for other adverse outcomes after corticosteroid treatment, which may due to the strict enforcement of our inclusion criteria.
Conflicting results have also been reported on the use of corticosteroids in ARDS and infectious pneumonia. Studies from Meduri' group (28–31), including a randomized, double-blind, placebo-controlled trial (28), and other groups (32–35) reported beneficial effects of methylprednisolone on ARDS. However, in a prospective, randomized, double-blind, placebo-controlled trial in 99 patients with ARDS, high dose of methylprednisolone did not affect case fatality, the reversal of ARDS, or the incidence of secondary bacterial infection (36). Similarly, in a prospective, randomized, double-blind, placebo-controlled trial of treating severe sepsis and septic shock with high-dose methylprednisolone, no significant difference were found in the prevention or reversal of shock, or overall case fatality of the patients (37). Adverse outcomes from corticosteroid treatment in sepsis were also frequently reported and summarized in a meta-analysis, which concluded that corticosteroid treatment increased case fatality rate and caused a trend of increased rate of secondary infection (38).
In a retrospective cohort study similarly designed as our study, Auyeung et al. examined the effect of corticosteroid in the treatment of SARS patients (39). They reported a 20.7-fold increase in risk of either ICU admission or case fatality, after adjusting for the effects of age and disease severity. Extrapulmonary injury may have contributed to the adverse outcomes, as their corticosteroid treated patients exhibited a trend of elevated lactate dehydrogenase. These results suggest that, although damage caused by inflammation in SARS and COVID-19 pneumonia can be lethal, turning off the immune surveillance with corticosteroids may cause additional adverse outcomes. Both SARS-CoV-2 and SARS-CoV are known to infect lung, intestines and other organs (40, 41), and the use of corticosteroid may exacerbate the viral infections leading to multi-organ failure.
Other limitations of our study include small sample size and imbalanced age between groups. The sample size did not allow sufficient power for many of the comparisons between the study groups. We are lucky that, an R2 value of 0.413 was achieved in the multivariable logistic regression of the case fatality rate, indicating a decent power for the analysis of the primary outcome. The age of the CS group was apparently older and the difference was statistically significant before adjustment for multiple comparisons. Considering the potential impact of the age on the clinical outcomes, age was included in the multivariate regressions. Our results indicated that adverse outcomes remained significantly associated with corticosteroid use in critical COVID-19 after adjusting for the influence of age and other confounding factors.
In conclusion, corticosteroid use in critically ill COVID-19 patients was associated with a much higher case fatality rate. Frequent incidence of liver injury and multi-organ failure in corticosteroid treated patients may have contributed to the adverse outcomes. The multi-organ failure is likely caused by more persistent SARS-CoV-2 infection and higher viral load, due to the inhibition of immune surveillance by corticosteroid.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of the Six Affiliated Hospital of Sun Yat-sen University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
GY, LZ, and RZ conceived and designed this study. YL, JL, YZ, LZ, SC, and YH collected data. YL, JK, NJ, JL, LZ, GY, and RZ analyzed data. YL, JK, NJ, and LZ prepared the first draft. All authors critically revised the manuscript and approved the final version.
Funding
This study was partially supported by National Natural Science Foundation of China 81770571 (to LZ), 81774152 (to RZ), Guangdong Province Pearl River Talent Plan Innovation and Entrepreneurship Team Project 2019ZT08Y464 and the National Key Clinical Discipline of China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Yingfang Liu (Sun Yat-sen University School of Medicine) for critical review of the manuscript before submission.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.604263/full#supplementary-material
References
1. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
2. Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. (2003) 361:1767–72. doi: 10.1016/s0140-6736(03)13412-5
3. Smits SL, de Lang A, van den Brand JMA, Leijten LM, van IJcken WF, et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. (2010) 6:e1000756. doi: 10.1371/journal.ppat.1000756
4. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. (2017) 39:529–39. doi: 10.1007/s00281-017-0629-x
5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
6. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
7. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
8. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934–43. doi: 10.1001/jamainternmed.2020.0994
9. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
10. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. (2020) 395:473–5. doi: 10.1016/S0140-6736(20)30317-2
11. Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. (2004) 31:304–9. doi: 10.1016/j.jcv.2004.07.006
12. Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, et al. Saudi critical care trial: corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. (2018) 197:757–67. doi: 10.1164/rccm.201706-1172OC
13. Sprung CL, Caralis PV, Marcial EH, Pierce M, Gelbard MA, Long WM, et al. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. (1984) 311:1137–43. doi: 10.1056/NEJM198411013111801
14. Weigelt JA, Norcross JF, Borman KR, Snyder WH 3rd. Early steroid therapy for respiratory failure. Arch Surg. (1985) 120:536–40. doi: 10.1001/archsurg.1985.01390290018003
15. Fowler RA, Lapinsky SE, Hallett D, Detsky AS, Sibbald WJ, Slutsky AS, et al. Toronto: critically ill patients with severe acute respiratory syndrome. JAMA. (2003) 290:367–73. doi: 10.1001/jama.290.3.367
16. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
17. Cheng S, Wu D, Li J, Zou Y, Wan Y, Shen L, et al. Risk factors for the critical illness in SARS-CoV-2 infection: a multicenter retrospective cohort study. Respir Res. (2020) 21:277. doi: 10.1186/s12931-020-01492-z
18. Liu D, Cui P, Zeng S, Wang S, Feng X, Xu S, et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. EClinicalMedicine. (2020) 25:100471. doi: 10.1016/j.eclinm.2020.100471
19. Gomersall CD. Pro/con clinical debate: steroids are a key component in the treatment of SARS. Crit Care. (2004) 8:105–7. doi: 10.1186/cc2452
20. Thomas BJ, Porritt RA, Hertzog PJ, Bardin PG, Tate MD. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep. (2014) 4:7176. doi: 10.1038/srep07176
21. Sadr-Azodi O, Mattsson F, Bexlius TS, Lindblad M, Lagergren J, Ljung R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: a population-based nested case-control study. JAMA Intern Med. (2013) 173:444–9. doi: 10.1001/jamainternmed.2013.2737
22. Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts AC, Leufkens HG, et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart. (2004) 90:859–65. doi: 10.1136/hrt.2003.020180
23. Bowyer SL, LaMothe MP, Hollister JR. Steroid myopathy: incidence and detection in a population with asthma. J Allergy Clin Immunol. (1985) 76(2 Pt 1):234–42. doi: 10.1016/0091-6749(85)90708-0
24. Zha L, Li S, Pan L, Tefsen B, Li Y, French N, et al. Villanueva: corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust. (2020) 212:416–20. doi: 10.5694/mja2.50577
25. Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. (2020). doi: 10.1056/NEJMoa2021436
26. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane AW, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. (2020) 324:1317–29. doi: 10.1001/jama.2020.17022
27. Yang JW, Yang L, Luo RG, Xu JF. Corticosteroid administration for viral pneumonia: COVID-19 and beyond. Clin Microbiol Infect. (2020) 26:1171–7. doi: 10.1016/j.cmi.2020.06.020
28. Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. (1998) 280:159–65. doi: 10.1001/jama.280.2.159
29. Meduri GU, Belenchia JM, Estes RJ, Wunderink RG, el Torky M, Leeper KV, et al. Fibroproliferative phase of ARDS. Clinical findings and effects of corticosteroids. Chest. (1991) 100:943–52. doi: 10.1378/chest.100.4.943
30. Meduri GU, Chinn AJ, Leeper KV, Wunderink RG, Tolley E, Winer-Muram HT, et al. Corticosteroid rescue treatment of progressive fibroproliferation in late ARDS. Patterns of response and predictors of outcome. Chest. (1994) 105:1516–27. doi: 10.1378/chest.105.5.1516
31. Meduri GU, Headley S, Tolley E, Shelby M, Stentz F, Postlethwaite A. Plasma and BAL cytokine response to corticosteroid rescue treatment in late ARDS. Chest. (1995) 108:1315–25. doi: 10.1378/chest.108.5.1315
32. Hooper RG, Kearl RA. Established ARDS treated with a sustained course of adrenocortical steroids. Chest. (1990) 97:138–43. doi: 10.1378/chest.97.1.138
33. Hooper RG, Kearl RA. Established adult respiratory distress syndrome successfully treated with corticosteroids. South Med J. (1996) 89:359–64. doi: 10.1097/00007611-199604000-00001
34. Ashbaugh DG, Maier RV. Idiopathic pulmonary fibrosis in adult respiratory distress syndrome. Diagnosis and treatment. Arch Surg. (1985) 120:530–5. doi: 10.1001/archsurg.1985.01390290012002
35. Biffl WL, Moore FA, Moore EE, Haenel JB, McIntyre RC Jr, Burch JM. Are corticosteroids salvage therapy for refractory acute respiratory distress syndrome? Am J Surg. (1995) 170:591–5; discussion 595–6. doi: 10.1016/s0002-9610(99)80022-1
36. Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. (1987) 317:1565–70. doi: 10.1056/NEJM198712173172504
37. Bone RC, Fisher CJ Jr, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. (1987) 317:653–8. doi: 10.1056/NEJM198709103171101
38. Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. (1995) 23:1430–9. doi: 10.1097/00003246-199508000-00019
39. Auyeung TW, Lee JS, Lai WK, Choi CH, Lee HK, Lee JS, et al. The use of corticosteroid as treatment in SARS was associated with adverse outcomes: a retrospective cohort study. J Infect. (2005) 51:98–102. doi: 10.1016/j.jinf.2004.09.008
40. Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. (2003) 200:282–9. doi: 10.1002/path.1440
Keywords: SARS-CoV-2, mortality, critical, gluocorticoid, steroid, COVID-19, ARDS, cytokine storm
Citation: Li Y, Li J, Ke J, Jiao N, Zhu L, Shen L, Chen L, Jiang Z, Cheng S, Huang Y, Zou Y, Zhu R and Yan G (2021) Adverse Outcomes Associated With Corticosteroid Use in Critical COVID-19: A Retrospective Multicenter Cohort Study. Front. Med. 8:604263. doi: 10.3389/fmed.2021.604263
Received: 09 September 2020; Accepted: 21 January 2021;
Published: 10 February 2021.
Edited by:
Fabrizio Ricci, University of Studies G. d'Annunzio Chieti and Pescara, ItalyReviewed by:
Zuo-jiong Gong, Renmin Hospital of Wuhan University, ChinaMinxue Shen, Central South University, China
Qiang Deng, Fudan University, China
Youhua Xie, Shanghai Medical College of Fudan University, China
Copyright © 2021 Li, Li, Ke, Jiao, Zhu, Shen, Chen, Jiang, Cheng, Huang, Zou, Zhu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Zhu, emh1bHg2QG1haWwuc3lzdS5lZHUuY24=; Ruixin Zhu, cnh6aHVAdG9uZ2ppLmVkdS5jbg==; Guangjun Yan, MTQxNTk2MTg4OUBxcS5jb20=
†These authors have contributed equally to this work
 Yichen Li
Yichen Li Jie Li2†
Jie Li2† Na Jiao
Na Jiao Lixin Zhu
Lixin Zhu Lihan Shen
Lihan Shen Sijing Cheng
Sijing Cheng Yibo Huang
Yibo Huang Ruixin Zhu
Ruixin Zhu