- 1Laboratory Analysis Unit, Azienda Ospedaliero Universitaria Policlinico “G. Rodolico-San Marco”, Catania, Italy
- 2Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy
- 3Ophthalmology Unit, Azienda di Rilievo Nazionale e di Alta Specializzazione Garibaldi, Catania, Italy
Fusarium is a filamentous fungus commonly found in the environment and is the major cause of fungal keratitis. We report a case of keratomycosis caused by Fusarium solani in a patient using disposable soft contact lenses. A delay in diagnosis led to the initiation of an empirical antifungal treatment with the subsequent deterioration of the patient's clinical condition. The use of the real-time quantitative PCR assay confirmed keratitis from F. solani providing a result in <48 h and therefore giving the possibility of quickly starting targeted antifungal therapy. The patient had an improvement in eye condition after the diagnosis of keratitis by F. solani and the rapid change to targeted antifungal treatment. For the rapid identification of corneal fungal pathogens, we believe that PCR may be added for the diagnosis of mycotic keratitis pending the isolation in culture that is necessary for in vitro susceptibility testing.
Introduction
Fungal keratitis (FK) is a potentially sight-threatening fungal infection of the cornea and has been reported to account for about half of all microbial keratitis cases requiring therapeutic penetrating keratoplasty (1, 2). FK develops rapidly and can lead to corneal ulcers and vision loss; therefore, early diagnosis and prompt treatment are essential to prevent long-term complications. It is typically caused by Aspergillus species, Candida species, and several species of the genus Fusarium, mostly Fusarium solani that is the most virulent, associated with the ability to generate resistance to many antifungal agents (3–7). Trauma to the cornea is the most common risk factor for FK, followed by topical corticosteroid use, ocular surface disease, contact lens use, and systemic immunosuppression (8–10). In all patients with a suspicion of keratomycosis, a timely diagnosis is among the most important factors because an early diagnosed episode of keratomycosis could favor a good prognosis (11).
Case Report
A 25-year-old female, who had been using disposable soft contact lens for several years, was assessed for a sensation of pain in her left eye. A sty was diagnosed, and medical therapy with tobramycin eye drops was prescribed.
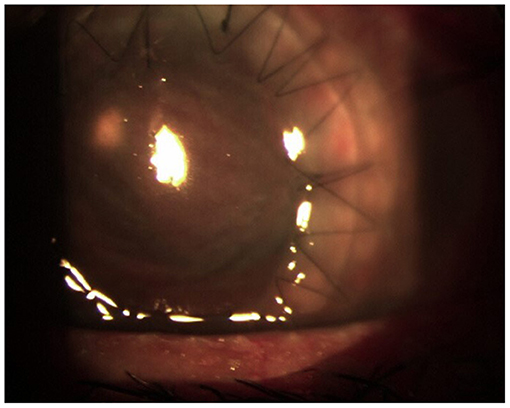
After 10 days, despite topical antibiotic therapy for the persistence of pain, the patient referred a reduction of visual acuity to 6/15, and because of the appearance of diffuse edema at the epithelial level, Acanthamoeba keratitis was suspected; a swab was taken and analyzed together with her contact lenses for a microbiological examination. Other than therapy with tobramycin drops every 2 h, Polihexanide 0.02% (PHMB) eye drops were also administered (2 drops × 6 v/die). The patient's condition subsequently deteriorated rapidly, and 3 days later, she was hospitalized. On local examination, corneal abscess with a collection of purulent inflammatory exudate of ~1 mm in size was observed (Figure 1). No view of the lens or fundus was possible, and vision was impaired. Routine blood tests were normal, and the search for Acanthamoeba from the swab and contact lenses was negative. Therapy with vancomycin intravenous 1 g daily and ceftazidime 1 g every 12 h daily was started. Atropine drops 3 per day, levofloxacin and tobramycin drops every 2 h, and PHMB (2 drops × 6 v/die) were also administered. Corneal scraping was performed 4 days later and sent to the Clinical Pathology Service that reported the presence of yeast cells on Gram stain, and that there was a fungal culture in progress. Considering the suspicion of probable Candida infection, vancomycin was changed to topical fluconazole (200 mg/100 ml) 1 drop every hour, and caspofungin intravenous (50 mg/die) was also administered, whereas the PHMB eye drops and tobramycin drops were stopped. After 6 days, the anterior chamber was washed with fluconazole (200 mg/100 ml), and fibrinous tissue was taken and reported as fibrin-leukocyte exudate. After a slight improvement in symptoms, the patient's condition worsened, and after a further anterior chamber washing with fluconazole, a corneal transplant was performed with removal of the membrane that covers the corneal iris angle and the crystalline lens, followed by intraocular lens (IOL) implantation. The patient was discharged from the hospital after 10 days. On the 26th day after the transplant, she was hospitalized again due to a worsening of her clinical condition (Figure 2).
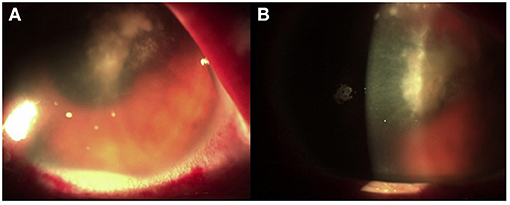
Figure 1. (A) Corneal abscess with fibrinous branches. (B) Collection of purulent inflammatory exudate.
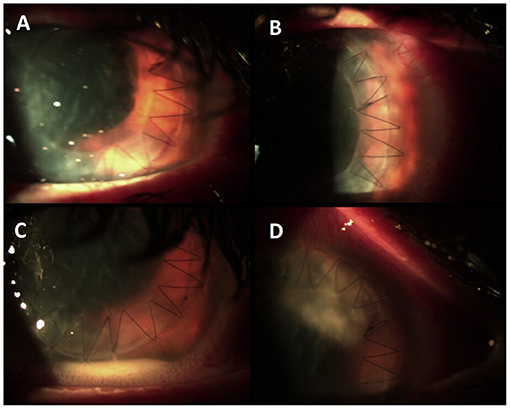
Figure 2. (A,B) Condition after corneal transplant at the time of discharge. (C) Appearance of hypopyon and abscess (D) at the time of the second hospitalization.
After 2 days, a new corneal transplant was performed. The explanted corneal flap and the purulent material of the anterior chamber were sent to the Mycology Laboratory of the University Hospital of Catania, Sicily, Italy. For the mycological examination, conventional and molecular diagnostic methods by microscopic, fungal culture in Sabouraud Dextrose Agar (SDA) medium supplemented with 1% chloramphenicol and gentamycin and real-time quantitative PCR (qPCR) assay for the detection of Fusarium and Aspergillus were carried out.
The qPCR assay was used for the detection of Fusarium spp. using the primers and probes as described by Koo et al. (12), whereas the AsperGenius® multiplex PCR (PathoNostics, Maastricht, Netherlands) was used to detect the most clinically relevant Aspergillus species. DNA was extracted by using the GenoXtract instrument (Hain Lifescience, Nehren, Germany) following the manufacturer's instructions. qPCR was performed adding 5 μl of DNA extract to the PCR mix, and a Rotor-Gene Q (Qiagen) was used for amplification and melting curve analysis. A positive control (Fusarium falciforme ATCC® MYA3636™) and a negative template control (NTC) were included in each PCR run. The AsperGenius® multiplex PCR was performed according to the manufacturer's instructions.
The direct microscopic examination with 15% potassium hydroxide (KOH) showed several hyaline septate hyphae. Fusarium spp. was detected by qPCR, whereas Aspergillus DNA was negative. After 4 days of incubation at 30°C, the colonies were clearly visible on SDA medium. The colonies were wooly, cottony, flat, and white. Identification of the isolate was performed by standard phenotypic methods based on the macroscopic and microscopic morphological studies. In particular, the microscopic morphological study showed long monophialidic conidiogenous cells, moderately curved macroconidia and cylindrical to oval microconidia with thick walls, and single-celled to two-celled. The pathogen was identified as F. solani complex. The results of the mycological examinations are shown in Figure 3.
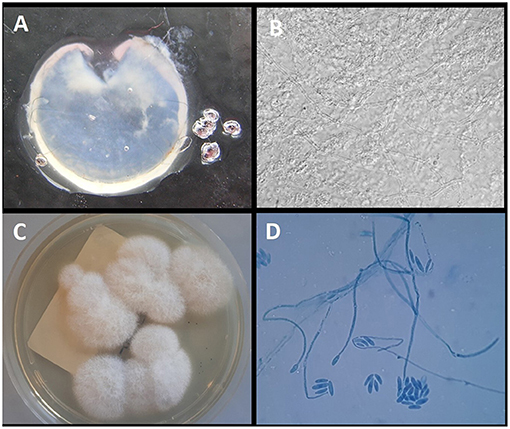
Figure 3. (A) Explanted corneal flap. (B) Direct examination of the corneal flap with 15% KOH (original magnification ×40); (C) growth on Sabouraud Dextrose Agar with gentamycin and chloramphenicol after 7 days of incubation at 32°C; (D) Microscopic structure of the colony showing long monophialidic conidiogenous cells and numerous microconidia of F. solani.
Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS) on a Microflex LT (Bruker Daltonics, Bremen, Germany) platform after ethanol–formic acid extraction identified the isolate as F. solani (score: 1.646). Susceptibility to fluconazole, itraconazole, voriconazole, posaconazole, caspofungin, anidulafungin, micafungin, flucytosine, and amphotericin B was evaluated. Minimum inhibitory concentration (MIC) values of >256 μg/ml for fluconazole, >16 μg/ml for itraconazole, 0.256 μg/ml for voriconazole, >8 μg/ml for posaconazole, >8 μg/ml for caspofungin, >8 μg/ml for anidulafungin, >8 μg/ml for micafungin, >64 μg/ml for flucytosine, and 1 μg/ml for amphotericin B were obtained. The patient was put on topical voriconazole eye drops every h and voriconazole 200 mg i.v. twice a day, and there was an almost immediate improvement of her eye condition (Figure 4). The systemic therapy with voriconazole 200 mg per os twice a day was continued for at least 3 months postoperatively.
At 3 months, despite the control of her fungal infection, the cornea is opaque due to a fibrinous reaction that also affects the IOL. After controlling the infection, the visual function can probably be restored with a new cornea transplant and replacement of the IOL.
Discussion
We report a case of keratomycosis caused by F. solani in a patient who had been wearing disposable soft contact lens for several years, often also used overnight. Outbreaks of Fusarium keratitis associated with the use of specific contact lens solutions as well as the ability of Fusarium to penetrate soft contact lenses forming biofilm have been described (13). Keratomycosis caused by Fusarium is important among the clinical conditions responsible because of ocular morbidity and blindness. It is a clinical challenge due to its slow pathological process, characteristics similar to other microbial keratitis, and possible complications. Therefore, a timely diagnosis and adequate therapy are the most important factors that favor a good prognosis. In our case, an Acanthamoeba keratitis was initially suspected but was excluded after the negative results of the tests carried out on the swab and contact lenses. Later, a probable Candida keratitis was suspected based on the presence of yeast cells on Gram stain after corneal scraping.
This determined the start of an empirical treatment with topical fluconazole and intravenous caspofungin; however, there was a subsequent deterioration of her clinical condition. Considering the worsening clinical condition of the patient, notwithstanding antifungal therapy, we excluded Candida as a possible etiological agent of keratitis because hypha formation is an essential step in the pathogenesis of Candida albicans in keratitis (14) and also in light of it not even being isolated in culture. Probably, in this initial phase, had the corneal scraping been sent to a specialized laboratory equipped for molecular and conventional mycologic diagnoses, a correct diagnosis could have been made, and a correct antifungal treatment could have been started without the necessity of a second transplant.
In fact, only at the second transplant, on the explanted corneal flap, was FK due to F. solani confirmed and treatment with topical and intravenous voriconazole started that resulted in an improvement of the condition of her eye.
The gold standard of laboratory diagnosis of FK includes the microscopic examination by histopathology or the KOH preparation of corneal scraping and a fungal culture. Generally, the sensitivity of the KOH examination may be negatively affected by the insufficient amount of scraping material, the small size of the corneal ulcer, and the lack of experience of the microscope observer. Moreover, the Gram stain, commonly used in clinical laboratories, has a significantly lower sensitivity than the KOH examination (15). The cultures, which are widely used in clinical laboratories, remain negative in several positive microscopy cases, as well as being associated with a long turnaround time.
In our case, FK was confirmed by microscopic examination in KOH, isolation in culture, and PCR even if the use of the qPCR assay identified Fusarium sp. keratitis in <48 h. PCR, as in other clinical settings (16), proved to be an effective, rapid method for the diagnosis of FK and was more sensitive than microscopy and culture methods; therefore, we believe that PCR should be added as a screening diagnosis test when an early mycotic keratitis is suspected (17).
However, there are problems related to the diagnosis and the management of Fusarium keratitis; considering that Fusarium species exhibit broad resistance to the spectrum of antifungals currently available, the isolation in culture is important to carry out an in vitro susceptibility. Data on their in vitro susceptibility to various antifungal agents indicate variable susceptibility to amphotericin B and extended-spectrum triazoles, such as itraconazole, voriconazole, isavuconazole, and posaconazole (18). The fungal sample that was isolated in our case showed susceptibility to amphotericin B and voriconazole with MIC values of 1 and 0.256 μg/ml, respectively, whereas resistance was demonstrated against all the other tested antifungals.
In conclusion, for the rapid identification of corneal fungal pathogens, we believe that PCR should be added for the diagnosis of mycotic keratitis pending the isolation in culture necessary for in vitro susceptibility tests. However, the diagnostic challenge of FK lies in the use of laboratories with expertise in medical mycology so as to assure an appropriate diagnostic course correlated to the presentation of the clinical picture.
Data Availability Statement
The original contributions generated for the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LT and SO performed the conventional and molecular diagnoses and wrote the manuscript. AM and GP performed the image acquisition and contributed the clinical details. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank the Scientific Bureau of the University of Catania for language support.
References
1. Shukla PK, Kumar M, Keshava GB. Mycotic keratitis: an overview of diagnosis and therapy. Mycoses. (2008) 51:183–99. doi: 10.1111/j.1439-0507.2007.01480.x
2. Chen WL, Wu CY, Hu FR, Wang IJ. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 1987 to 2001. Am J Ophthalmol. (2004) 137:736–43. doi: 10.1016/j.ajo.2003.11.010
3. Trovato L, Rapisarda MF, Greco AM, Galata F, Oliveri S. In vitro susceptibility of nondermatophyte molds isolated from onycomycosis to antifungal drugs. J Chemother. (2009) 21:403–7. doi: 10.1179/joc.2009.21.4.403
4. Ibrahim MM, Vanini R, Ibrahim FM, Fioriti LS, Furlan EM, Provinzano LM, et al. Epidemiologic aspects and clinical outcome of fungal keratitis in southeastern Brazil. Eur J Ophthalmol. (2009) 19:355–61. doi: 10.1177/112067210901900305
5. Acharya Y, Acharya B, Karki P. Fungal keratitis: study of increasing trend and common determinants. Nepal J Epidemiol. (2017) 7:685–93. doi: 10.3126/nje.v7i2.17975
6. Anaissie EJ, Kuchar RT, Rex JH, Francesconi A, Kasai M, Müller FM, et al. Fusariosis associated with pathogenic fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin Infect Dis. (2001) 33:1871–8. doi: 10.1086/324501
7. Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. (1994) 7:479–504. doi: 10.1128/CMR.7.4.479
8. Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. (2004) 15:321–7. doi: 10.1097/00055735-200408000-00008
9. Jurkunas U, Behlau I, Colby K. Fungal keratitis: changing pathogens and risk factors. Cornea. (2009) 28:638–43. doi: 10.1097/ICO.0b013e318191695b
10. Cho CH, Lee SB. Clinical analysis of microbiologically proven fungal keratitis according to prior topical steroid use: a retrospective study in South Korea. BMC Ophthalmol. (2019) 19:207. doi: 10.1186/s12886-019-1212-0
11. Mahmoudi S, Masoomi A, Ahmadikia K, Tabatabaei SA, Soleimani M, Rezaie S, et al. Fungal keratitis: an overview of clinical and laboratory aspects. Mycoses. (2018) 61:916–30. doi: 10.1111/myc.12822
12. Koo SH, Teoh YL, Koh WL, Ochi H, Tan SK, Sim D, et al. Development and validation of a real-time multiplex PCR assay for the detection of dermatophytes and Fusarium spp. J Med Microbiol. (2019) 68:1641–8. doi: 10.1099/jmm.0.001082
13. Chang DC, Grant GB, O'Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. (2006) 296:953–63. doi: 10.1001/jama.296.8.953
14. Jackson BE, Wilhelmus KR, Mitchell BM. Genetically regulated filamentation contributes to Candida albicans virulence during corneal infection. Microb Pathog. (2007) 42:88–93. doi: 10.1016/j.micpath.2006.11.005
15. Bharathi MJ, Ramakrishnan R, Vasu S, Meenakshi R, Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol. (2003) 51:315–21.
16. Trovato L, Oliveri S, Domina M, Patamia I, Scalia G, De Pasquale R. Molecular diagnosis of kerion celsi caused by Trichophyton tonsurans in a Italian child. Med Mycol Case Rep. (2019) 24:72–4. doi: 10.1016/j.mmcr.2019.04.010
17. Ferrer C, Alió JL. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. J Ophthalmic Inflamm Infect. (2011) 1:15–22. doi: 10.1007/s12348-011-0019-9
Keywords: Fungal keratitis, Fusarium solani, contact lenses, diagnosis, PCR
Citation: Trovato L, Marino A, Pizzo G and Oliveri S (2021) Case Report: Molecular Diagnosis of Fungal Keratitis Associated With Contact Lenses Caused by Fusarium solani. Front. Med. 8:579516. doi: 10.3389/fmed.2021.579516
Received: 02 July 2020; Accepted: 17 February 2021;
Published: 24 March 2021.
Edited by:
Ana Afonso, University of São Paulo, BrazilReviewed by:
Anupma Kindo, Sri Ramachandra Institute of Higher Education and Research, IndiaClaudy Oliveira Dos Santos, Radboud University Nijmegen Medical Centre, Netherlands
Copyright © 2021 Trovato, Marino, Pizzo and Oliveri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Trovato, bHRyb3ZhdG9AdW5pY3QuaXQ=
 Laura Trovato
Laura Trovato Antonio Marino3
Antonio Marino3 Salvatore Oliveri
Salvatore Oliveri