- 1Department of Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Critical Care Medicine, Zhongnan Hospital of Wuhan University, Wuhan, China
- 3Research Center for Translational Medicine, Wuhan Jinyin-Tan Hospital, Wuhan, China
- 4Department of Critical Care Medicine, Renmin Hospital, Wuhan University, Wuhan, China
- 5Department of Critical Care Medicine, Xiaogan Central Hospital, Xiaogan, China
- 6Department of Critical Care Medicine, Wuhan Pulmonary Hospital, Wuhan, China
- 7Department of Critical Care Medicine, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China
- 8Department of Critical Care, The Third People's Hospital of Yichang, Yichang, China
- 9Department of Critical Care Medicine, Xiangyang No.1 People's Hospital, Affiliated Hospital of Hubei University of Medicine, Xiangyang, China
- 10Department of Critical Care Medicine, Taihe Hospital Affiliated to Hubei University Medicine, Shiyan, China
- 11Department of Critical Care Medicine, Fifth Hospital of Wuhan, Wuhan, China
- 12Department of Critical Care Medicine, The Affiliated Hospital of Jianghan University, Wuhan, China
- 13Department of Critical Care, Wuhan No.1 Hospital, Wuhan, China
- 14Intensive Care Unit (ICU) Center of Xijing Hospital, Airforce Medical University, Xi'an, China
- 15Department of Critical Care Medicine, Huanggang Central Hospital, Huanggang, China
- 16Department of Intensive Care Unit (ICU), Huangshi Central Hospital, Affiliated Hospital of Hubei Polytechnic University, Edong Healthcare Group, Huangshi, China
- 17Department of Critical Care Medicine, Jingzhou Central Hospital, The Second Clinical Medical College, Yangtze University, Jingzhou, China
- 18Department of Critical Care Medicine, No.2 Hospital of Huangshi, Huangshi, China
- 19Department of Critical Care Medicine, The First People's Hospital of Jingmen, Jingmen, China
- 20Intensive Care Unit, Xiehe Wuhan Red Cross Hospital, Wuhan, China
- 21Department of Critical Care Medicine, Ezhou Central Hospital, Ezhou, China
- 22Department of Intensive Care Unit (ICU)/Emergency, Wuhan Third Hospital, Wuhan, China
- 23Department of Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: The outbreak of coronavirus disease 2019 (COVID-19) has led to a large and increasing number of patients requiring prolonged mechanical ventilation and tracheostomy. The indication and optimal timing of tracheostomy in COVID-19 patients are still unclear, and the outcomes about tracheostomy have not been extensively reported. We aimed to describe the clinical characteristics and outcomes of patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia who underwent elective tracheostomies.
Methods: The multi-center, retrospective, observational study investigated all the COVID-19 patients who underwent elective tracheostomies in intensive care units (ICUs) of 23 hospitals in Hubei province, China, from January 8, 2020 to March 25, 2020. Demographic information, clinical characteristics, treatment, details of the tracheostomy procedure, successful weaning after tracheostomy, and living status were collected and analyzed. Data were compared between early tracheostomy patients (tracheostomy performed within 14 days of intubation) and late tracheostomy patients (tracheostomy performed after 14 days).
Results: A total of 80 patients were included. The median duration from endotracheal intubation to tracheostomy was 17.5 [IQR 11.3–27.0] days. Most tracheotomies were performed by ICU physician [62 (77.5%)], and using percutaneous techniques [63 (78.8%)] at the ICU bedside [76 (95.0%)]. The most common complication was tracheostoma bleeding [14 (17.5%)], and major bleeding occurred in 4 (5.0%) patients. At 60 days after intubation, 31 (38.8%) patients experienced successful weaning from ventilator, 17 (21.2%) patients discharged from ICU, and 43 (53.8%) patients had died. Higher 60 day mortality [22 (73.3%) vs. 21 (42.0%)] were identified in patients who underwent early tracheostomy.
Conclusions: In patients with SARS-CoV-2 pneumonia, tracheostomies were feasible to conduct by ICU physician at bedside with few major complications. Compared with tracheostomies conducted after 14 days of intubation, tracheostomies within 14 days were associated with an increased mortality rate.
Introduction
The novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in a worldwide pandemic and a large and increasing number of patients who are critically ill and require endotracheal intubation and mechanical ventilation (1–3).
Tracheostomy is a common procedure for critically ill patients who require long-term mechanical ventilation (4). Compared with an orotracheal tube, a shorter tracheostomy tube that bypasses the mouth and pharynx can avoid oropharyngeal and laryngeal lesions, improve patient comfort and reduce sedative drug use (5). In addition, a tracheostomy tube can provide less airway dead space and thus less work of breathing, facilitate weaning from mechanical ventilation, make airway suctioning much easier, and potentially reduce the incidence of ventilator-associated pneumonia (6). In COVID-19 patients with requirements of prolonged ventilation, tracheostomy is one of the important clinical considerations for optimal management (7). However, in the current pandemic, there is significant uncertainty regarding the indication and timing of tracheostomy.
Several recommendations and guidelines have discussed on when to perform a tracheostomy in COVID-19 patients, while the timing is varied across the literature. Recommendations from the UK and North America suggested that tracheostomy should be delayed until at least 14 days from endotracheal intubation to allow prognostic information to become clear and for viral load to sufficiently decline (1, 8–12). In contrast, recommendations from France proposed a more aggressive approach-favoring early tracheostomy so that patients can be weaned off intubation and transferred to a ventilatory weaning unit thus sparing ICU beds for new patients (13). These recommendations were based on expert opinion, and robust ICU outcome data are needed to give high level of evidence. At present, the outcomes about tracheostomy in COVID-19 patients have not been extensively reported.
In this study, we aimed to describe the clinical characteristics of patients with confirmed SARS-CoV-2 pneumonia who underwent elective tracheostomies and to explore the association between the timing of tracheostomy and the outcomes of these patients.
Materials and Methods
Study Design and Patients
This multicenter, retrospective, observational study was conducted in Hubei Province, China. Patients treated in intensive care units (ICUs) of 23 hospitals from January 8, 2020 to March 25, 2020 were screened. All patients who were diagnosed with COVID-19 and underwent elective tracheostomies were included. COVID-19 was diagnosed according to the World Health Organization interim guidance (14). The decision of tracheostomy was made by treating clinicians. This study was approved by the Ethics Committee of Union Hospital, and written informed consent was waived.
Data Collection
Medical records of patients were reviewed, and data were collected by investigators at each ICU by using a standardized case-report form. Sociodemographic and clinical data were collected for all patients, including age, sex, chronic medical histories, vital signs, laboratory tests, acute physiology and chronic health evaluation II (APACHE II) scores and sequential organ failure assessment (SOFA) scores. We also collected details of the tracheostomy procedure, including timing, type (percutaneous or surgical), location, the clinicians performing the procedure, and complications. Whether successful weaning was achieved was also recorded, and successful weaning was defined as no need for mechanical ventilation for more than 48 h at any time after tracheostomy. Treatment was recorded for the duration of hospitalization. The living status at 60 days after intubation was also recorded.
Statistical Analysis
Normally distributed and non-normally distributed continuous variables are presented as the mean (SD) and median [IQR], respectively. Categorical variables are presented as numbers (%). Early tracheostomy was defined as tracheostomy within 14 days of intubation, and late tracheostomy was defined as tracheostomy after 14 days. The comparison between the two groups was conducted using Student's t-test, Mann-Whitney U test or Fisher's exact test when appropriate. The Kaplan-Meier method was used to depict survival curves, and the log-rank test was used to compare the survival rates between the early tracheostomy group and the late tracheostomy group. Cox proportional hazards regression analysis was used to explore the hazard ratio (HR) of variables with a p < 0.05 in univariate analysis. No imputation was made for missing data. A 2-tailed p < 0.05 was regarded as statistically significant. All statistical analyses were performed using the SPSS software system (vision 20.0, SPSS, Inc., Chicago, IL) and GraphPad Prism 5 software.
Results
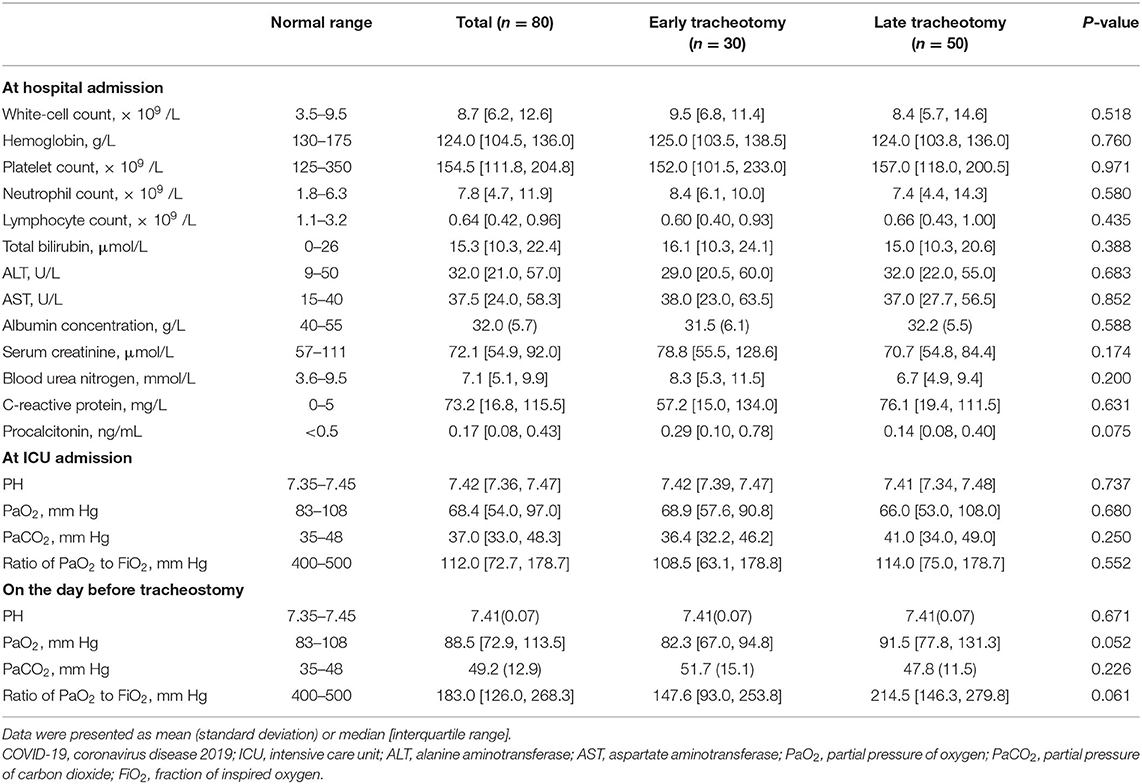
From January 8 to March 25, 2020, a total of 80 patients from 23 hospitals (2 [IQR 1–4] patients per center) in Hubei Province, China, were included in our study. Their mean (SD) age was 63.9 (14.0) years, and 61 (70.1%) were male. The median duration from intubation to tracheostomy was 17.5 [IQR 11.3–27.0] days. Sixty (69.0%) patients had chronic medical illnesses, and the most common illnesses were hypertension (40.0%), coronary heart disease (21.1%), diabetes (17.5%), and cerebrovascular disease (10.0%) (Table 1). Thirty (37.5%) patients received tracheostomies within 14 days after intubation, and their median duration between intubation and tracheostomy was significantly shorter than that of the late tracheostomy group (9.5 [IQR 5.0–13.0] days vs. 24.5 [IQR 18.8–32.0] days, p < 0.001). Compared with patients in the early tracheostomy group, the patients in the late tracheostomy group had lower SOFA scores (5 [IQR 4–7] vs. 6 [IQR 4–9], p = 0.014) and APACHE II scores (11 [IQR 9–17] vs. 15 [IQR 11–21], p = 0.034) at ICU admission and lower APACHE II scores [13 (SD 4) vs. 17 (SD 6), p = 0.010] before tracheostomy. Among all 80 patients, lymphocytopenia and hypoalbuminemia at hospital admission and hypoxemia at ICU admission were prominent (Table 2). However, no differences were identified between the two groups.
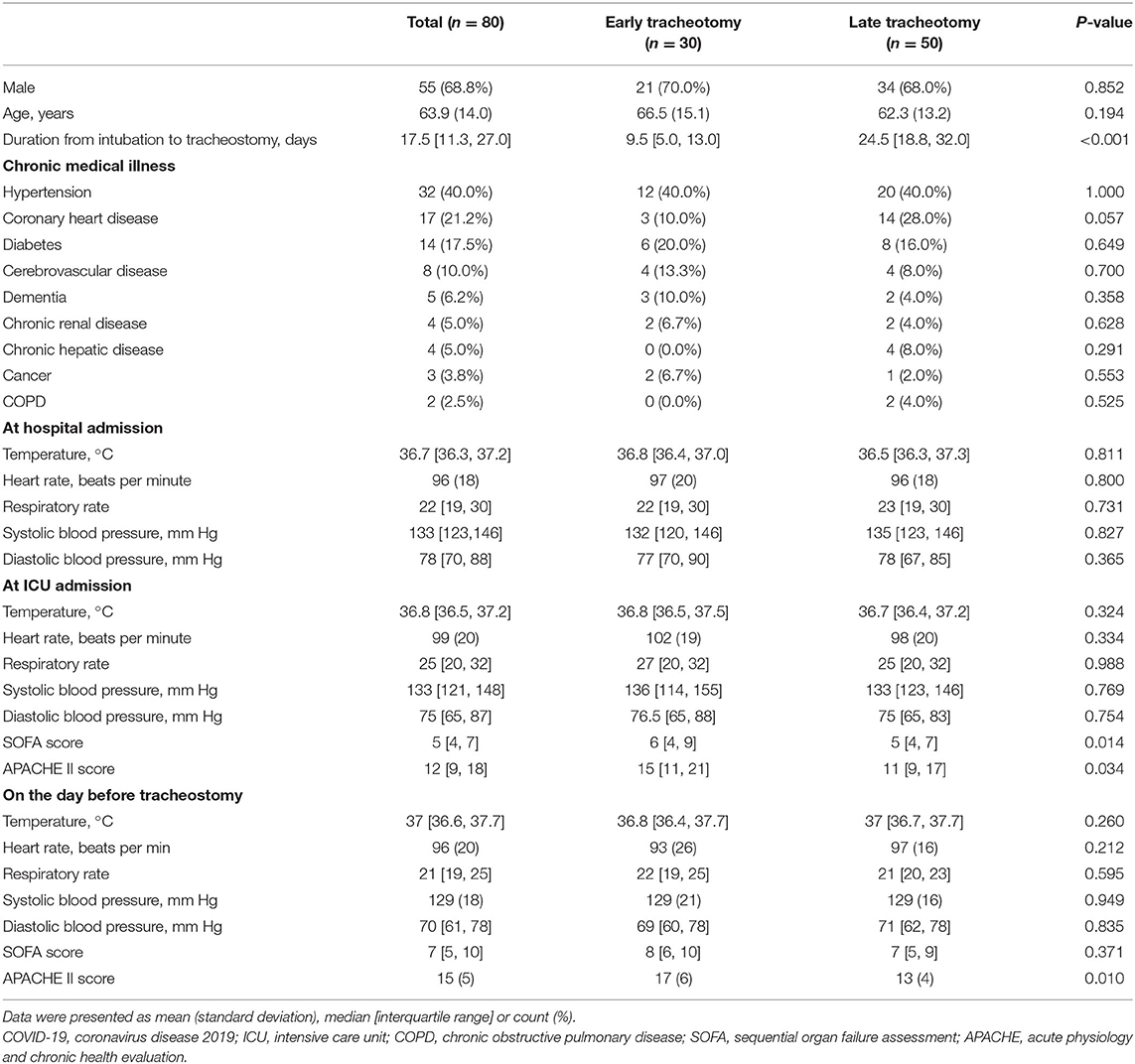
Table 1. Demographic data and vital signs in 80 COVID-19 patients receiving early and late tracheostomies.
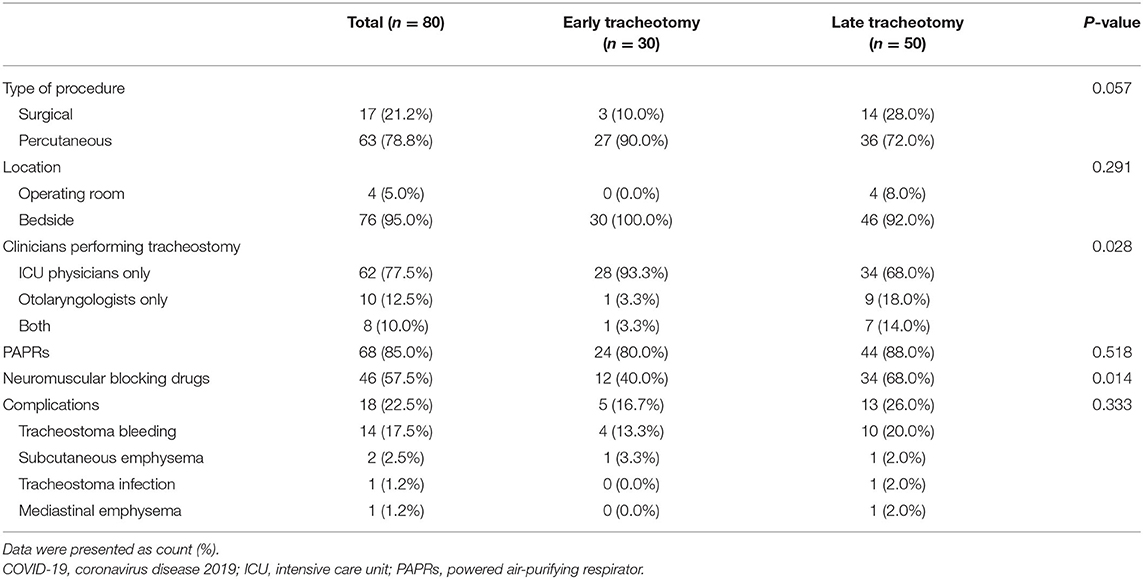
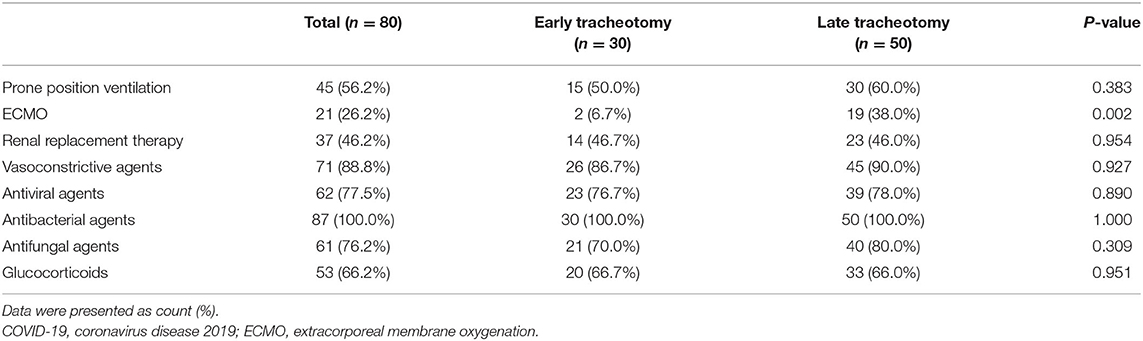
Most tracheotomies were performed by ICU physicians [62 (77.5%)] and using percutaneous techniques [63 (78.8%)] at the ICU bedside [76 (95.0%)]. Powered air-purifying respirators (PAPRs) were used by operating teams in 68 (85.0%) tracheostomies (Table 3). Furthermore, neuromuscular blocking drugs were applied in 46 (57.5%) patients, which may help avoid coughing-induced viral aerosolization. The most common complication was tracheostoma bleeding, which occurred in 14 (17.5%) patients. Major bleeding occurred in 4 (5.0%) patients, who received transfusion of red blood cells. Other complications included subcutaneous emphysema (2.5%), tracheostoma infection (1.2%), and mediastinal emphysema (1.2%) (Table 3). No differences were identified between the early and late tracheostomy groups in terms of complications. For treatments, no differences were identified between the two groups, except extracorporeal membrane oxygenation (ECMO). Compared with early tracheostomy patients, more patients who underwent late tracheostomy received ECMO [19 (8.0%) vs. 2 (6.7%), p = 0.002] (Table 4).
In the 80 COVID-19 patients who underwent elective tracheostomies, 43 (53.8%) patients had died at 60 days. Higher 60 day mortality [22 (73.3%) vs. 21 (42.0%), p = 0.007] was identified in patients who underwent early tracheostomy (Figure 1). At 60 days after intubation, 31 (38.8%) patients experienced successful weaning from the ventilator, and 17 (21.2%) patients were discharged from the ICU. Because collinearity existed between the SOFA and APACHE II scores at ICU admission, only the SOFA score was incorporated into the Cox proportional hazards regression analysis. After adjusting for SOFA [HR 1.00 (95% CI, 0.91–1.11)] and ECMO [HR 1.06 (95% CI, 0.49–2.28)], late tracheostomy was identified with a decreased risk of death [HR 0.34 (95% CI, 0.17–0.70)] (Table 5).
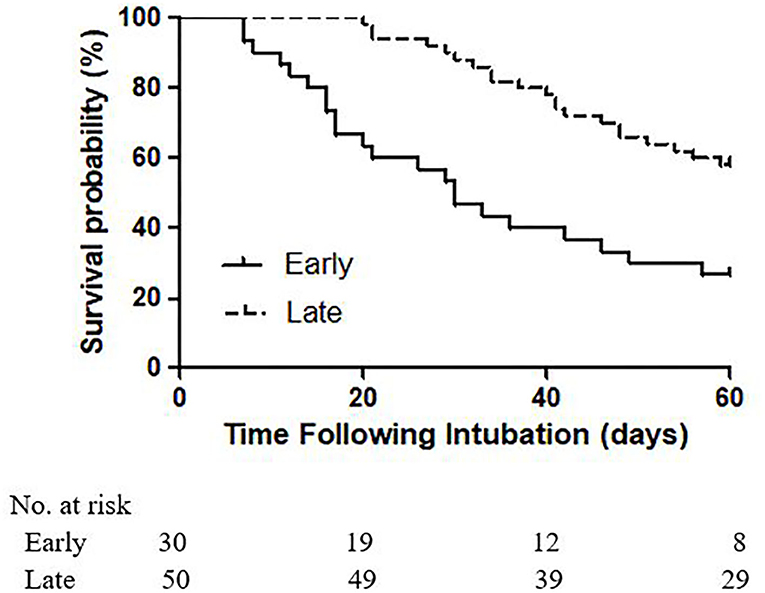
Figure 1. Kaplan-Meier analysis of survival in patients receiving early and late tracheostomies for 60 days (p log−ranktest = 0.0003).
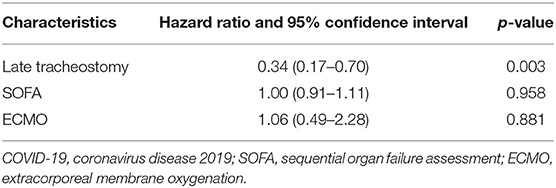
Table 5. Cox proportional hazards regression analysis in 80 COVID-19 patients receiving tracheostomy.
Discussion
As the number of patients infected by SARS-CoV-2 around the world is increasing, the demand for endotracheal intubation and invasive mechanical ventilatory support secondary to acute respiratory failure is increasing accordingly (15, 16). In our study, most procedures were performed by ICU physicians using percutaneous techniques at bedside, which avoided the unnecessary transport of ventilated patients and repeated connection and disconnection of ventilatory circuits during transfer. Regarding the type of tracheostomy performance, one of the concerns is complications of bleeding and stomal infections. Long et al. (17) compared percutaneous with surgical tracheotomy in patients with COVID-19, and they found there were no significant differences in complication rates between the two methods. Another concern is the potential risk of viral transmission. Some argued against percutaneous tracheostomy performed in COVID-19 patients because it usually involves opening the ventilator circuit more frequently than surgical tracheostomy, and serial dilations during the procedure may put surgeons in face of the airway from the beginning (18). However, there is currently no evidence across the literature to advise which approach is less aerosol generating (19).
Tracheotomy for patients with COVID-19 is considered a highly-risk procedure, and appropriate personal protective equipment (PPE) is critical to reduce infection rates among health care workers (20). In our study, standard PPE was systematically used in all of the procedures, including N95 mask, gowns, caps, boots, double gloves and face shield/eye protection. Additionally, the PAPR, which was advised by several recommendations (1, 12, 21), was used in more than half of the procedures in our study. Other principles, including limiting the number of personnel present, ensuring complete paralysis, adequate sedation, and minimizing suction during the procedure, also help to improve protection for health care workers from SARS-CoV-2 (22).
Indications for tracheostomy in patients with COVID-19 remain unclear. Mattioli et al. suggested that tracheostomy has the potential to facilitate ventilator weaning and promote early discharge of COVID-19 patients from ICU to lower intensity care wards and thus free up resources (23). However, Shiba et al. argued that tracheostomy does not provide any benefit on the outcome in patients with COVID-19 due to rapid evolution of the disease, and they did not believe that tracheostomy had widespread indication (24). Above all, before consideration of tracheostomy, ICU physicians and surgery teams should fully assess the prognosis and associated benefit from the procedure. Tracheostomy is preferably be offered to patients with an expectation of recovery or a long-term need of an artificial airway.
Timing for elective tracheostomy performance is always controversial. Outside the context of the COVID-19 pandemic, a systematic review suggested that early tracheostomy (within 7 days) was associated with a reduced duration of mechanical ventilation, less mortality rate and shorter length of ICU stay (25). Furthermore, A Cochrane review found lower mortality rates and a higher probability of discharge from the ICU at day 28 among patients with early tracheotomy (26). In contrast, meta-analyses published by Griffiths et al. (27) and Siempos et al. (28) suggested that early tracheostomy is not associated with lower mortality than late tracheostomy. Moreover, a TracMan randomized trial (29), comparing 455 patients undergoing early tracheostomy (within 4 days) and 454 patients undergoing late tracheostomy (after 10 days), found that there were no differences in 30 day mortality and 1 and 2 year survival or length of ICU stay between them. During the pandemic of COVID-19, the focus has changed dramatically. Tracheostomy is an aerosol generating procedure which theoretically increases the risk of viral transmission, and the viral load may be high in the early course of the disease (7, 21). The timing should balance the benefits of tracheostomy for mechanically ventilated patients and the risk of viral transmission to the team involved in the procedure. Both the US and Canadian recommendations strongly advised that test for COVID-19 should be negative before performing an elective tracheostomy (8, 30).
Our study suggested that, compared with tracheostomies conducted after 14 days of intubation, tracheostomies within 14 days were associated with an increased mortality rate. Univariate analysis showed that patients who underwent early tracheostomies had higher SOFA scores and APACHE II scores, and less of these patients received ECMO. However, after adjusting SOFA and ECMO, the timing of tracheostomy was the only variable significantly associated with mortality. A prospective cohort study assessed 50 patients with confirmed COVID-19 reported that early tracheotomy (≤ 10 days) was associated with shorter mechanical ventilation duration and hospital stay, and no differences were found in mortality rate (31). The overall mortality in our study was as high as 53.8%, which was consistent with other studies reported > 50% mortality rate for patients who are placed on the ventilator (2, 32, 33). Given the high mortality rate, lack of proven benefit, and concern for viral exposure, it is reasonable to consider tracheostomy no sooner than 14 days of endotracheal intubation, and preferably at least tests of specimens from the respiratory tract for SARS-CoV-2 RNA are negative.
This study has several limitations. First, the sample size of our study was relatively small, which might cause bias and limit the reliability or generalizability of our results. Second, some patients were still hospitalized at the end of this study, so some clinical outcomes, such as length of ICU stay and hospital stay, were unavailable at the time of analysis. Third, due to its retrospective design, the lack of randomization for patients who underwent early and late tracheostomy may increase the possibility of confounding in the subsequent comparison. Forth, results of SARS-CoV-2 tests from clinicians involved in tracheostomies were not available. Even if they were test positive for SARS-CoV-2-RNA, we were unable to ascertain whether the clinicians contracted it during the procedures. In future research, rigorous prospective randomized trials with large samples are needed to elucidate any potential benefit from tracheostomy in COVID-19 patients and determine the optimal timing of this procedure.
Conclusion
In patients with severe SARS-CoV-2 pneumonia, tracheostomies were feasible to conduct by ICU physicians at bedside with few major complications. Compared with tracheostomies conducted after 14 days of intubation, tracheostomies within 14 days were associated with an increased mortality rate. Despite the results, further research and data from other institutions are warranted to more accurately verify these findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Union Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YT, YW, FZ, XY, CH, GH, WX, MH, LZ, AC, ZX, BL, SH, GZ, XF, XZh, YY, HF, LY, BW, ZL, YP, ZS, SF, YO, JX, and XZo collected the epidemiological and clinical data. YT, YW, and FZ summarized all the data. YT, YW, FZ, XY, CH, and GH drafted the manuscript. MF, ZY, BH, and YS revised the final manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the patients and their families involved in the study. This manuscript has been released as a pre-print at [Research Square] (34).
Abbreviations
COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ICU, Intensive care unit; COPD, chronic obstructive pulmonary disease; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation; ECMO, extracorporeal membrane oxygenation; PPE, Personal Protective Equipment; PAPRs, Powered air-purifying respirators.
References
1. Miles BA, Schiff B, Ganly I, Ow T, Cohen E, Genden E, et al. Tracheostomy during SARS-CoV-2 pandemic: recommendations from the New York Head and Neck Society. Head Neck. (2020) 42:1282–90. doi: 10.1002/hed.26166
2. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
3. Yu Y, Xu D, Fu S, Zhang J, Yang X, Xu L, et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. (2020) 24:219. doi: 10.1186/s13054-020-02939-x
4. Scales DC, Ferguson ND. Tracheostomy: it's time to move from art to science. Crit Care Med. (2006) 34:3039–40. doi: 10.1097/01.CCM.0000242924.24342.9D
5. Bosel J, Schiller P, Hook Y, Andes M, Neumann JO, Poli S, et al. Stroke-related early tracheostomy versus prolonged orotracheal intubation in neurocritical care trial (SETPOINT): a randomized pilot trial. Stroke. (2013) 44:21–8. doi: 10.1161/STROKEAHA.112.669895
6. Robba C, Galimberti S, Graziano F, Wiegers E, Lingsma HF, Iaquaniello C, et al. Tracheostomy practice and timing in traumatic brain-injured patients: a CENTER-TBI study. Intensive Care Med. (2020) 46:983–94. doi: 10.1007/s00134-020-05935-5
7. Mcgrath BA, Brenner MJ, Warrillow SJ, Pandian V, Arora A, Cameron TS, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. (2020) 8:717–25. doi: 10.1016/S2213-2600(20)30230-7
8. American Academy of Otolaryngology and Head and Neck Surgery. AAO Position Statement: Tracheotomy Recommendations During the COVID-19 Pandemic. (2020). Available online at: https://www.entnet.org/content/aao-position-statement-tracheotomy-recommendations-during-covid-19-pandemic (accessed November 12, 2020).
9. Chao TN, Braslow BM, Martin ND, Chalian AA, Atkins J, Haas AR, et al. Tracheotomy in ventilated patients with COVID-19. Ann Surg. (2020) 272:e30–2. doi: 10.1097/SLA.0000000000003956
10. David AP, Russell MD, El-Sayed IH, Russell MS. Tracheostomy guidelines developed at a large academic medical center during the COVID-19 pandemic. Head Neck. (2020) 42:1291–6. doi: 10.1002/hed.26191
11. Givi B, Schiff BA, Chinn SB, Clayburgh D, Iyer NG, Jalisi S, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg. (2020) 146:579–84. doi: 10.1001/jamaoto.2020.0780
12. Takhar A, Walker A, Tricklebank S, Wyncoll D, Hart N, Jacob T, et al. Recommendation of a practical guideline for safe tracheostomy during the COVID-19 pandemic. Eur Arch Otorhinolaryngol. (2020) 277:2173–84. doi: 10.1007/s00405-020-05993-x
13. Schultz MJ, Pattnaik R, Dondorp AM. Walking the line between benefit and harm from tracheostomy in COVID-19. Lancet Respir Med. (2020) 8:656–7. doi: 10.1016/S2213-2600(20)30231-9
14. World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance. Available online at: https://apps.who.int/iris/handle/10665/330893 (accessed November 12, 2020).
15. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. (2020) 55:105924. doi: 10.1016/j.ijantimicag.2020.105924
16. Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. (2020) 18:1469–72. doi: 10.1111/jth.14848
17. Long SM, Chern A, Feit NZ, Chung S, Ramaswamy AT, Li C, et al. Percutaneous and open tracheostomy in patients with COVID-19: comparison and outcomes of an institutional series in New York City. Ann Surg. (2020) doi: 10.1097/SLA.0000000000004428
18. Tay JK, Khoo ML, Loh WS. Surgical considerations for tracheostomy during the COVID-19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg. (2020) 146:517–8. doi: 10.1001/jamaoto.2020.0764
19. Chiesa-Estomba CM, Lechien JR, Calvo-Henriquez C, Fakhry N, Karkos PD, Peer S, et al. Systematic review of international guidelines for tracheostomy in COVID-19 patients. Oral Oncol. (2020) 108:104844. doi: 10.1016/j.oraloncology.2020.104844
20. Loeb M, Mcgeer A, Henry B, Ofner M, Rose D, Hlywka T, et al. SARS among critical care nurses, Toronto. Emerg Infect Dis. (2004) 10:251–5. doi: 10.3201/eid1002.030838
21. Sommer DD, Engels PT, Weitzel EK, Khalili S, Corsten M, Tewfik MA, et al. Recommendations from the CSO-HNS taskforce on performance of tracheotomy during the COVID-19 pandemic. J Otolaryngol Head Neck Surg. (2020) 49:23. doi: 10.1186/s40463-020-00414-9
22. Thal AG, Schiff BA, Ahmed Y, Cao A, Mo A, Mehta V, et al. Tracheotomy in a high-volume center during the COVID-19 pandemic: evaluating the surgeon's risk. Otolaryngol Head Neck Surg. (2020) doi: 10.1177/0194599820955174
23. Mattioli F, Fermi M, Ghirelli M, Molteni G, Sgarbi N, Bertellini E, et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol. (2020) 277:2133–5. doi: 10.1007/s00405-020-05982-0
24. Shiba T, Ghazizadeh S, Chhetri D, St JM, Long J. Tracheostomy considerations during the COVID-19 pandemic. OTO Open. (2020) 4:2473974X20922528X. doi: 10.1177/2473974X20922528
25. Adly A, Youssef TA, El-Begermy MM, Younis HM. Timing of tracheostomy in patients with prolonged endotracheal intubation: a systematic review. Eur Arch Otorhinolaryngol. (2018) 275:679–90. doi: 10.1007/s00405-017-4838-7
26. Andriolo BN, Andriolo RB, Saconato H, Atallah ÁN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. (2015) 1: CD7271. doi: 10.1002/14651858.CD007271.pub3
27. Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. (2005) 330:1243. doi: 10.1136/bmj.38467.485671.E0
28. Siempos II, Ntaidou TK, Filippidis FT, Choi A. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. (2015) 3:150–8. doi: 10.1016/S2213-2600(15)00007-7
29. Young D, Harrison DA, Cuthbertson BH, Rowan K. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. (2013) 309:2121–9. doi: 10.1001/jama.2013.5154
30. Canadian Society of Otolaryngology-Head and Neck Surgery. Recommendations From the CSO-HNS Taskforce on Performance of Tracheotomy During the COVID-19 Pandemic. (2020). Available online at: https://www.entcanada.org/wp-content/uploads/COVID-19-Guidelines-CSOHNS-Task-Force-Mar-23-2020.pdf (accessed November 20, 2020).
31. Avilés-Jurado FX, Prieto-Alhambra D, González-Sánchez N, de Ossó J, Arancibia C, Rojas-Lechuga MJ, et al. Timing, complications, and safety of tracheotomy in critically ill patients with COVID-19. JAMA Otolaryngol Head Neck Surg. (2020) doi: 10.1001/jamaoto.2020.3641
32. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934–43. doi: 10.1001/jamainternmed.2020.0994
33. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
Keywords: COVID-19, tracheostomy, mechanical ventilation, intensive care unit, critically ill patients
Citation: Tang Y, Wu Y, Zhu F, Yang X, Huang C, Hou G, Xu W, Hu M, Zhang L, Cheng A, Xu Z, Liu B, Hu S, Zhu G, Fan X, Zhang X, Yang Y, Feng H, Yu L, Wang B, Li Z, Peng Y, Shen Z, Fu S, Ouyang Y, Xu J, Zou X, Fang M, Yu Z, Hu B and Shang Y (2020) Tracheostomy in 80 COVID-19 Patients: A Multicenter, Retrospective, Observational Study. Front. Med. 7:615845. doi: 10.3389/fmed.2020.615845
Received: 10 October 2020; Accepted: 27 November 2020;
Published: 17 December 2020.
Edited by:
Jiapeng Huang, University of Louisville, United StatesReviewed by:
Julian Bösel, Heidelberg University, GermanyCassiano Teixeira, UFCSPA Medical School, Brazil
Copyright © 2020 Tang, Wu, Zhu, Yang, Huang, Hou, Xu, Hu, Zhang, Cheng, Xu, Liu, Hu, Zhu, Fan, Zhang, Yang, Feng, Yu, Wang, Li, Peng, Shen, Fu, Ouyang, Xu, Zou, Fang, Yu, Hu and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You Shang, eW91X3NoYW5naHVzdEAxNjMuY29t; Bo Hu, aG9iYmllcjE5NzlAMTYzLmNvbQ==; Zhui Yu, eXV6aHVpQHdodS5lZHUuY24=; Minghao Fang, ZmFuZ21oQHRqaC50am11LmVkdS5jbg==
†These authors have contributed equally to this work
 Yun Tang
Yun Tang Yongran Wu1†
Yongran Wu1† Fangfang Zhu
Fangfang Zhu Xiaobo Yang
Xiaobo Yang You Shang
You Shang