- 1Department of Anesthesiology and Critical Care, University Hospital Würzburg, Julius-Maximilians-University Würzburg, Würzburg, Germany
- 2Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Goethe-University, Frankfurt, Germany
- 3Department of Anesthesiology and Critical Care, Klinikum Aschaffenburg-Alzenau, Aschaffenburg, Germany
- 4Department of Anesthesiology and Critical Care, Diakoneo Diak Klinikum Schwabisch Hall, Schwabisch-Hall, Germany
- 5Department of Critical Care, Emergency Medicine and Anesthesiology, ARDS/ECMO-Centre, Campus Kassel of the University of Southampton, Southampton, Germany
- 6Department of Anesthesiology and Critical Care, Sana-Klinikum Offenbach GmbH, Offenbach, Germany
- 7Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany
- 8Department of Internal Medicine II, University Hospital Würzburg, Würzburg, Germany
- 9Institute for Clinical Epidemiology and Biometry, Julius-Maximilians-University, Würzburg, Germany
- 10Clinical Trial Center, University Hospital Würzburg, Julius-Maximilians-University, Würzburg, Germany
Background: Proportions of patients dying from the coronavirus disease-19 (COVID-19) vary between different countries. We report the characteristics; clinical course and outcome of patients requiring intensive care due to COVID-19 induced acute respiratory distress syndrome (ARDS).
Methods: This is a retrospective, observational multicentre study in five German secondary or tertiary care hospitals. All patients consecutively admitted to the intensive care unit (ICU) in any of the participating hospitals between March 12 and May 4, 2020 with a COVID-19 induced ARDS were included.
Results: A total of 106 ICU patients were treated for COVID-19 induced ARDS, whereas severe ARDS was present in the majority of cases. Survival of ICU treatment was 65.0%. Median duration of ICU treatment was 11 days; median duration of mechanical ventilation was 9 days. The majority of ICU treated patients (75.5%) did not receive any antiviral or anti-inflammatory therapies. Venovenous (vv) ECMO was utilized in 16.3%. ICU triage with population-level decision making was not necessary at any time. Univariate analysis associated older age, diabetes mellitus or a higher SOFA score on admission with non-survival during ICU stay.
Conclusions: A high level of care adhering to standard ARDS treatments lead to a good outcome in critically ill COVID-19 patients.
Background
Following the first outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) in December 2019, the virus has spread worldwide. The coronavirus disease-19 (COVID-19) currently affects 188 countries and territories (1).
In Germany the first case of a SARS-CoV2 infection was diagnosed on February 27, 2020 (2). Although means of social distancing helped to contain virus transmission more than 175 000 people were infected (1). SARS-CoV2 was suggested to elicit a new ARDS-subphenotype, where hypoxemia often does not match lung compliance and ventilator responsiveness (3). The observed case-fatality ratios differ among countries, with the United States reporting 3.8% and Germany reporting 4.5%,respectively. This is lower compared to other European countries, for example, Italy (14.3%), United Kingdom (15.3%) or France (14.2%) (4). Understanding the specific characteristics of severe and fatal disease, as well as the therapeutic approaches to COVID-19 induced ARDS remains an urgent need to provide a basis for best practice models of standardized ARDS treatment.
In the current study, we report the epidemiologic features, clinical course, treatment patterns and outcome of patients requiring intensive care due to COVID-19 induced ARDS in five German centers.
Methods
This is a retrospective, observational multicenter study at the University Hospital Würzburg and University Hospital Frankfurt, as well as the municipal hospitals of Kassel, Offenbach and Aschaffenburg. Würzburg, Frankfurt, and Kassel are referral centers for adult extracorporeal membrane oxygenation (ECMO) and part of the German ARDS network. To guarantee an individual high level of ICU care all participating hospitals immediately improved ICU infrastructure by adding extra ICU nurses, physicians, medical students and other support workers to the COVID-19 ICUs.
The institutional ethic boards of the University of Würzburg and Frankfurt, as well as the medical association of Bavaria ethics board (Aschaffenburg) and Hessen (Offenbach, Kassel), respectively, approved the study. The need for informed consent from individual patients was waived due to the context of sole retrospective chart review within standard care.
Patient Selection
We included all patients consecutively admitted to the ICU in any of the participating hospitals due to an acute respiratory distress syndrome between March 12 and May 4, 2020. All patients submitted to the ICU had received the diagnosis of a SARS-CoV2 infection or were tested positive for COVID-19 during ICU treatment. SARS-CoV2 infection was detected with real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing based on the recommended World Health Organization standards. No patient tested positive for other respiratory viruses in primary diagnostics. All patients received venous thromboembolism (VTE) prophylaxis with pharmacologic anticoagulation according to the German guidelines on VTE (5). In case of contraindications against pharmacological anticoagulation, mechanical prophylaxis (intermittent pneumatic compression) was conducted. Follow-up ended with ICU discharge or death during ICU treatment, respectively.
Data Collection
Specific treatment protocols were not defined. Routine clinical data were continuously recorded using patient data management systems (PDMS) (University of Würzburg: COPRA6 RM1.0, COPRA System GmbH, Berlin, Germany; University of Frankfurt: Metavision 5.0, imd soft, Dusseldorf, Germany) or assessed via handwritten records (Aschaffenburg, Offenbach, Kassel). The data were retrieved according to the diagnostic standards of the individual centers. Demographic data, pre-existing medical conditions and medications were gathered from prior written records or discharge letters, questionnaires at the time of hospital admission, as well as personal communication with family members. Lung edema on chest radiographs was evaluated via the Radiographic Assessment of Lung Edema (RALE) score (6) in all patients admitted to the ICU in Würzburg. Severity of ARDS was categorized in line with the Berlin definition (mild: 200 mm Hg < PaO2/FIO2 ≤ 300 mm Hg; moderate: 100 mm Hg < PaO2/FIO2 < 200 mm Hg and severe PaO2/FIO2 < 100 mm Hg) (7). Since treatment and data acquisition were conducted according to the standard procedures of the respective hospital, diagnostics and reported parameters varied to some degree between the centers. Hence, if applicable the nominators and denominators are reported for each parameter separately, since not all parameters could be retrieved in the whole cohort of patients. All participating hospitals reported their data via a unified sheet (Microsoft® Excel 2019, Version 16.41, Microsoft® Corporation, Redmond, WA).
Statistical Analysis
Median and interquartile range (25–75%) were reported for continuous data, absolute and relative frequencies for categorical variables. Percentages are based on the total number of patients with complete information in the respective category. Continuous variables were tested for normality using histogram and QQ-plot. To compare differences between survivors and non-survivors in continuous variables the Mann-Whitney rank-sum test or the Wilcoxon matched-pairs signed rank test, respectively, was used as appropriate, as most of the variables were not normally distributed. The Chi2-Test or Fisher exact test was used to assess the association of dichotomous variables and the outcome. Age-adjusted logistic regression analyses were performed to identify factors associated with death during ICU treatment. Wilson score method was used to estimate 95%-confidence intervals for the crude proportion of survival during ICU stay; Kaplan-Meier estimates were used for estimating survival probability. All tests were two-tailed, a p-value <0.05 was considered as statistically significant. The univariate p-values were based on Mann-Whitney U Test, Chir2-Test or Fisher's exact Test as appropriate. The adjusted p-values are based on a logistic regression adjusted for age.
Data were analyzed using SAS® Software, Version 9.4. Copyright SAS Institute Inc. Cary, NC, USA, R, R Version 3.6.2., Prism 5 for Mac OS X (GraphPad Software, San Diego, CA), Stata version 14.2 (Stata Corp, College Station, TX) or SigmaPlot®, version 10.0 (Systat Software, Erkrath, Germany).
Results
A total of 106 ICU patients were treated for COVID-19 induced ARDS. None of these patients remained in ICU care at the end of the study period. Three patients were transferred from Italy to the ICU in Würzburg. Two of these patients were excluded from the analysis due to an advanced clinical course at the time of their transfer, as well as incomplete records and short-term ICU stay.
Epidemiologic Characteristics and Outcome
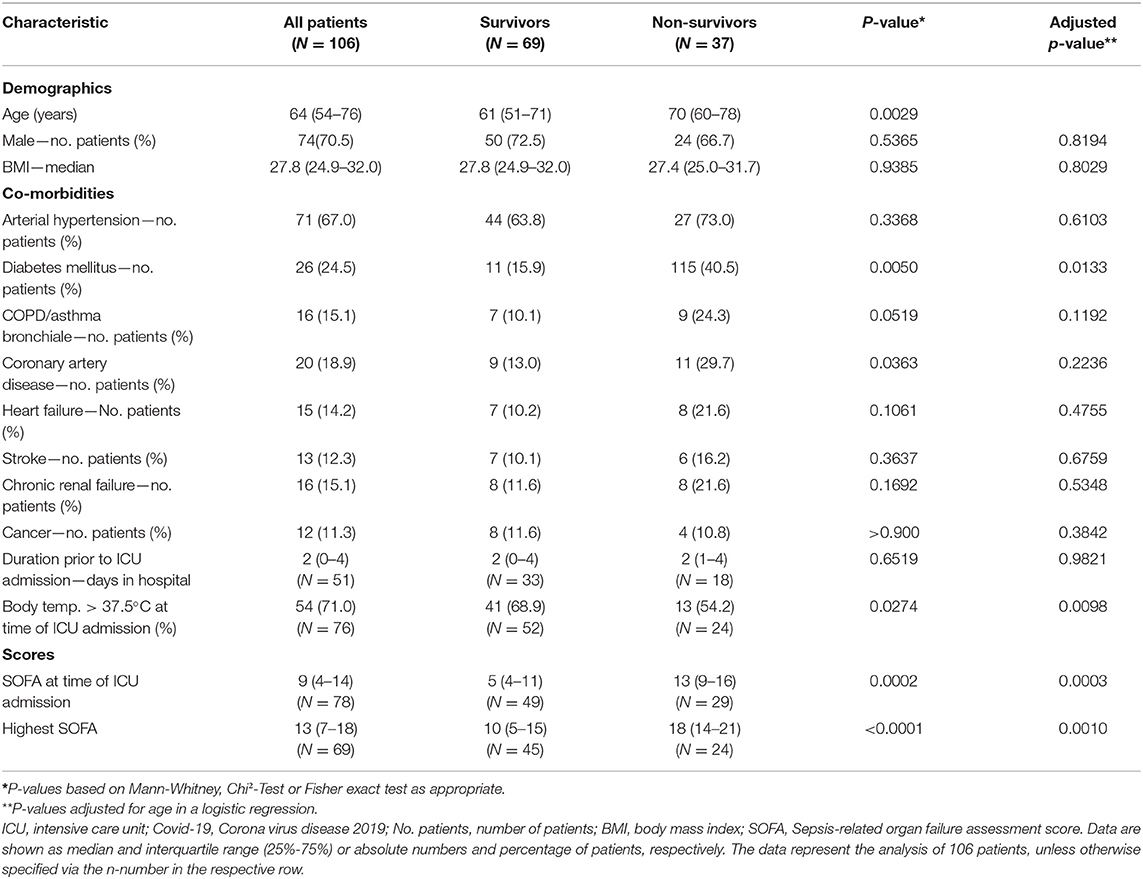
Median age of the patients was 64 (IQR 54–76) years, 70.5% were males. Median time from hospital to ICU admission was 2 (IQR 1–4) days. Overall, 37 patients died during ICU stay, constituting an overall survival of 65.0% (95% CI 55.6–73.5) (Figure 1). Considering only severe ARDS (PaO2/FiO2 < 100) (7), survival in critical care was 59.7% (CI 46.7–71.4) (Supplementary Table 1). Median duration of ICU treatment was 11 (IQR 7–19) days. Reported comorbidities were present in 79.3% of the cases, with arterial hypertension as most common comorbidity followed by diabetes mellitus (Table 1). Patients surviving ICU treatment were significantly younger. Although the majority of patients were male, a gender difference with respect to survival was not observed. Diabetes mellitus [age-adjusted Odds Ratio (OR) 3.4; 95-CI 1.3–8.7] and a higher SOFA score on admission (age-adjusted OR 1.2; 95%-CI 1.1–1.4) were associated with non-survival in univariate and age-adjusted analyses.
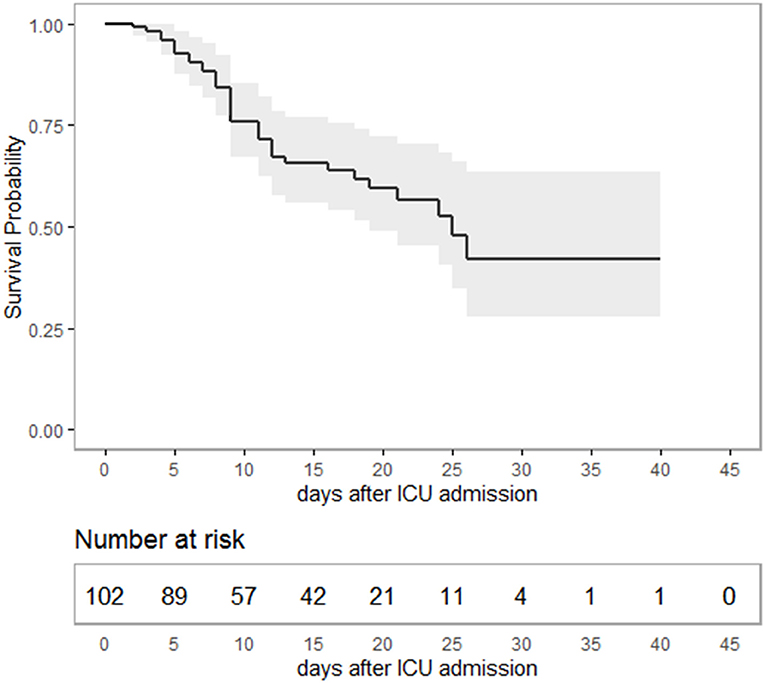
Figure 1. Kaplan-Meier-plot showing survival probability as a function of time in intensive care. Overall, 65% (95%-CI 55.6–73.5) of the patients survived ICU treatment with a median duration of 11 (IQR: 7–19) days. The study period ended with ICU discharge or death, respectively. Hence, survival data are terminally censored resulting in a horizontal line on the far right.
Laboratory Findings
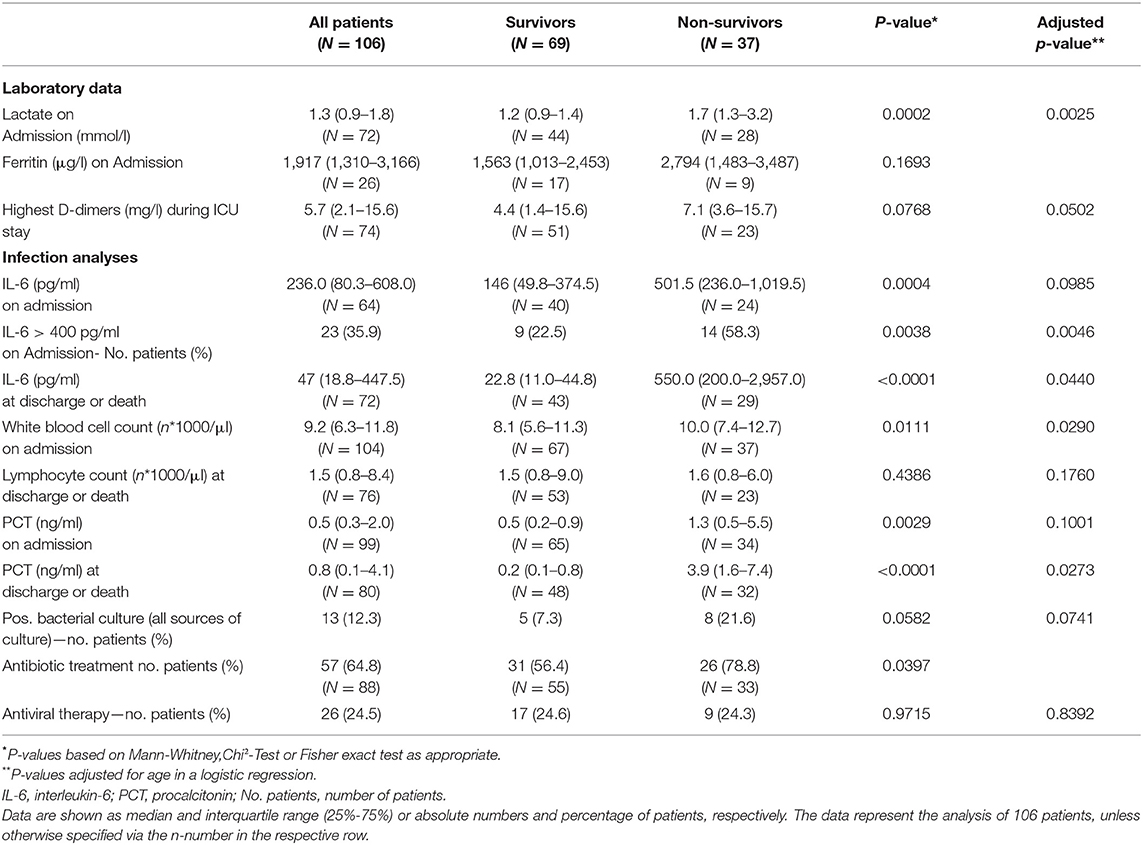
Laboratory findings are presented in Table 2. Patients who survived ICU treatment had lower levels of inflammatory markers on admission and during the course of therapy. A near three-fold difference in interleukin-6 (IL-6) was present between survivors and non-survivors at the time of ICU admission. 58.3% percent of the non-survivors had IL-6 levels >400 pg/ml. Bacterial specimens were found in 12.3% of the patients with no significantly differences between survivors or non-survivors. Nevertheless, a high percentage was already treated with antibiotics prior to ICU admission.
Respiratory Support
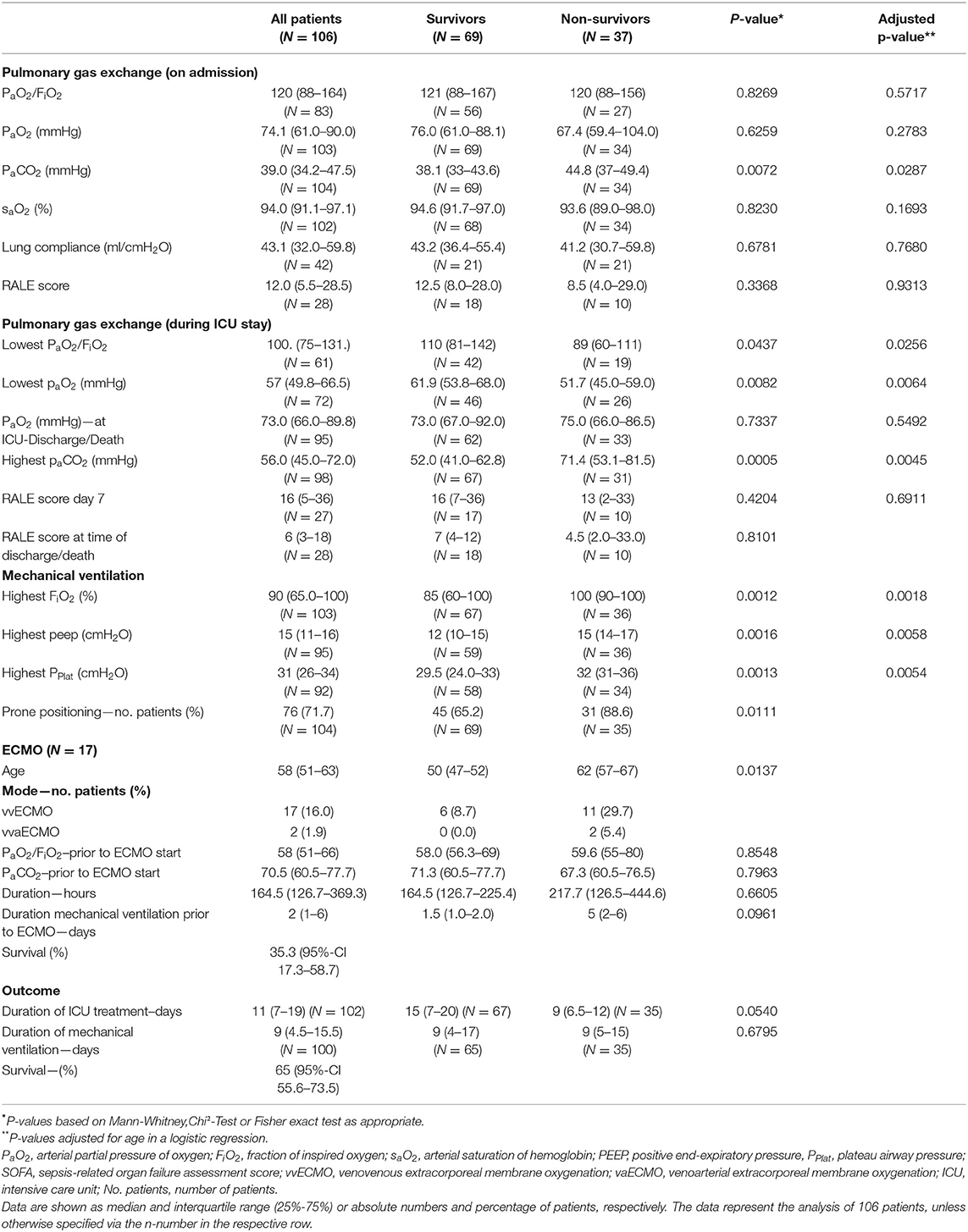
The median arterial oxygenation index (PaO2/FiO2) at the time of admission was 120 (IQR 88–164), indicating moderate to severe ARDS in the majority of patients. Overall, 55.6% had a moderate ARDS at admission; 35.8% of all patients and 63.8% of the non-survivors already suffered from a severe ARDS (PaO2/FiO2 < 100) at the time of ICU admission. Pulmonary gas exchange worsened in both populations. Prone positioning was performed in 78.9% of the cases. However, comparing the PaO2 at the time of ICU discharge or death, respectively, there was no significant difference. Median duration of mechanical ventilation was 9 (IQR 5.5–15.5) days and not significantly different between survivors and non-survivors. The same applies to lung mechanics or radiographic findings (Table 3). Chest X-ray pathologies were relatively minor compared to the degree of hypoxemia at admission. While deteriorating during the course of therapy, RALE scores were never significantly different between survivors and non-survivors. Moreover, RALE scores recovered in both groups toward the end of therapy.
Extracorporeal Membrane Oxygenation (ECMO)
Venovenous (vv) ECMO was utilized in 16.3% (n = 17) of the patients with a median age of 58 (IQR 51–63) years. Two patients received venoveno-arterial (vva) support due to acute cor pulmonale. ECMO patients had been on mechanical ventilation for a median of two (IQR 1–6) days. In three quarters of all cases, the use of ECMO was indicated due to refractory hypoxemia. Median PaO2/FiO2 at the time of ECMO commencement was 58 (IQR 51–66). Six patients (35.3%; 95%-CI 17.3–58.7) survived until ICU discharge.
Antiviral Therapies
The majority of patients (75.5%) did not receive any antiviral or anti-inflammatory therapy, while 24.5% received adjunct therapies including oseltamvir (n = 10), remdesivir (n = 1), chloroquins (n = 10) or tocilizumab (n = 3). However, as the choice and duration of therapy was purely at the discretion of the attending physicians, a large number of heterogeneous substances and protocols were used. Hence, no further analyses were performed due to the small sample sizes.
Discussion
The current study focused on the characteristics and outcome of COVID-19 patients admitted to the ICU in five German centers. Our study population mainly consisted of high-risk patients, where ARDS mortality rates of 40 to 46% can be expected (8). Half of our patients suffered from severe ARDS. Major findings include the identification of age, diabetes mellitus and higher SOFA scores on admission as factors associated with non-survival during ICU treatment. Furthermore, our observations indicate that standard ARDS treatment resolves acute hypoxemia in the majority of cases.
The proportion of patients surviving ICU care was 65.0% with a corresponding 95% CI of 55.6–73.5. Survival rates of ICU patients varied substantially between previous studies and different countries, for example, between 22 to 84% in China (9–12), 50% in Seattle (13), 33% in Washington State (14) and 61% in New York (15). In a retrospective cohort study from Italy only 46.6% of the patients requiring hospital admission survived (16). The ICNARC currently reports a survival of 60% in intensive care from the United Kingdom (17). A recent analysis of COVID-19 patients via the claims of the German Local Health Care Funds revealed an overall mortality of 22% and a mortality of 53% in patients requiring invasive ventilation. However, ARDS subtypes were not classified and risk factors of non-survival were not identified (18). Differences between countries may be due to variations in patient characteristics, as well as ICU admission criteria, criteria for ECMO, or availability of ICU capacities. All of the participating hospitals had sufficient resources to provide the best available standard care at any time. The workforce on the ICU of the participating hospitals was actually increased to counteract the big challenges associated with COVID-19, including a high number of patients requiring prone positioning, as well as time and effort associated with the use of personal protective equipment.
Advanced age has been uniformly reported as a risk factor for severe disease (12, 19) and was also associated with a worse outcome in our study. Diabetes mellitus was also reported as a factor associated with death from COVID-19 in critically ill in New York City and Lombardy (15, 16). It was associated with an approximately three-fold increased risk of death in our study. Arterial hypertension on the other hand was the most frequent comorbidity. Nevertheless its presence was not associated with a worse outcome and likely only represents the overall disease frequency (20). Although previously reported as a predictor of sepsis mortality (21), lymphocytopenia was not a distinctive feature in our ICU population. We did observe differences in SOFA scores and IL-6. IL-6 is perceived to be the central mediator of a cytokine release syndrome (22) and survivors had significantly lower IL-6 levels at the time of ICU admission. In this regard, preliminary data indicate that the administration of dexamethasone could improve survival in patients receiving respiratory support (23). Nevertheless, in our study treatment protocols for the use of glucocorticoids were not defined and dexamethasone was not utilized in any of the patients. Moreover, due to the small sample size, no multivariable prediction model to identify potential predictors of survival could be build.
The standards of ARDS treatment consist of prone positioning and protective mechanical ventilation with higher PEEP levels. All centers adhered to these guideline recommended therapies (24), although PPlat values indicate difficulties in maintaining lung protective ventilation at all times. Both survivors and non-survivors had worsening lung injury during the course of treatment with a high percentage of prone positioning. Patients dying during ICU treatment suffered from a worse pulmonary function at time of ICU admission, however, interestingly the duration of mechanical ventilation was not significantly different to patients surviving ICU care. Furthermore, paO2 values do not indicate hypoxemia at the time of death. The same applies to the RALE score or lung compliance, emphasizing that radiographic findings and lung mechanics often do not match the severity of disease (3). Antiviral or anti-inflammatory treatments were only utilized in a minority of the patients. The use of remdesivir was recently associated with faster COVID-19 recovery times, whereupon beneficial effects could not be shown in patients receiving mechanical ventilation or ECMO (25). In our cohort, approximately one fourth received antiviral treatment, whereas no significant difference in survival was observed.
Seventeen patients (16.3%) received vvECMO therapy. The overall rate of vvECMO treatment was higher compared to what has been reported from China (11, 12), the United States (15) and Italy (26). German Local Health Care Fund data recorded ECMO treatment in 7% of all ventilated patients in 920 German hospitals (18). The high ECMO rate in our study population emphasizes the severity of disease and that mainly specialized centers participated in the study. Nevertheless, the survival rate was lower in these patients and worse compared to other causes of ARDS.
Taken together, standard ARDS treatment according to published guidelines resolved acute hypoxemia in the majority of cases. Advanced age and diabetes mellitus increased the risk of non-survival. ICU triage with population-level decision making was not necessary and sufficient ICU equipment and personnel resources were available at any time. If the number of COVID-19 ICU patients re-increases, standard ARDS treatment provides a strong basis to ensure a good outcome in critically ill COVID-19 ARDS patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Würzburg and Frankfurt, as well as the medical association of Bavaria ethics board (Aschaffenburg) and Hessen (Offenbach, Kassel). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JH, EA, KZ, and CL contributed substantially to the conception and design of the study, the acquisition, analysis, interpretation of the data, and drafted the article. PM, QN, PHel, MS, PU-P, AS, IT, CaR, ChR, MK, JS, and DG-S contributed substantially to the acquisition and analysis of the data. PK, AB, ToS, BS, DW, HK, TW, SF, GE, RM, HM, and YZ contributed substantially to the acquisition of data, the interpretation of the data and provided critical revision of the article. ThS contributed substantially to the critical revision of the article. PHeu contributed substantially to the analysis, interpretation of the data and provided critical revision of the article. VR contributed substantially to the analysis of the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
PHeu reports grants from German Ministry of Research and Education, German Research Foundation, European Union, Charité—Universitätsmedizin Berlin, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert Koch Institute, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovationfond, University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), Charité—Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), outside the submitted work. SF reports grants from DFG, BMBF, grants and personal fees from Abiomed, Amgen, Akzea, AstraZeneca, Bayer, Berlin-Chemie, Braun, Bristol-Myers Squibb, Boehringer, Daiichi Sankyo, MSD, Novartis, Pfizer, Sanofi-Aventis, Servier, Siemens, Zoll, outside the submitted work. GE reports grants and personal fees from Bayer, grants and personal fees from Novartis, grants and personal fees from Vifor Pharma Deutschland GmbH, outside the submitted work; PK reports other from FreseniusKabi, personal fees from BBraun, grants, personal fees and other from TEVARatiopharm, other from CSL Behring, other from Pajunk, other from APEPTICO Forschung und Entwicklung GmbH, outside the submitted work; KZ reports personal fees from Aesculap Akademie GmbH, personal fees from Affinites Sante, grants from Ashai Kasai Pharma, grants and personal fees from B. Braun AG, grants and personal fees from B. Braun Avitum AG, personal fees from Bayer AG, grants from Biotest AG, personal fees from Christian Doppler Stiftung, grants and personal fees from CSL Behring GmbH, personal fees from Cyto Sorbents GmbH, personal fees from Edward Lifescience Corporation, personal fees from Executive Insight AG, personal fees from Fresenius Kabi GmbH, personal fees from Fresenius Medical Care, personal fees from Haemonetics Corporation, personal fees from Hartmannbund Landesverband, personal fees from Health Adcances GmbH, personal fees from Heinen + Löwenstein GmbH, personal fees from Hexal AG, grants from INC Research, personal fees from Johnson and Johnson, personal fees from Josef Gassner, personal fees from Maquet GmbH, personal fees from Markus Lücke Kongress Organization, personal fees from Masimo International, personal fees from med Update GmbH, personal fees from Medizin and Markt Gesundheitswerk, personal fees from MSD Sharp and Dohme GmbH, personal fees from Nordic Group, personal fees from Nordic Pharma, grants from Novo Nordisc Pharma GmbH, grants from Pfizer Pharma GmbH, personal fees from Pharmacosmos, personal fees from Ratiopharm GmbH, personal fees from Salvia Medical GmbH, personal fees from Schering Stiftung, personal fees from Schöchl Medical Österreich, personal fees from Serumwerke, personal fees from Verlag für Printmedien und PR, Forum Sanitas, grants and personal fees from Vifor Pharma GmbH, personal fees from Wellington, personal fees from Werfen, outside the submitted work; HK served as a speaker and/or an Advisory Board Member for AbbVie, BMS, Gilead, Hexal, Janssen, MSD, Pfizer, ViiV and has received research funding from AbbVie, Arrowhaed, BMS, Gilead, Janssen, MSD, Novartis, German Liver Foundation, Hector Foundation, Virtual University of Bavaria, Federal Ministry of Education and Research, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All investigators would very much like to thank all nurses, physicians, and supporting staff in Würzburg, Aschaffenburg, Frankfurt, Offenbach, and Kassel for their dedication and relentless work in order to achieve the best possible outcome for their patients.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.599533/full#supplementary-material
Abbreviations
COVID-19, coronavirus disease-19; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; vvECMO, venovenous extracorporeal membrane oxygenation; RT-PCR, real-time reverse transcriptase polymerase chain reaction; SOFA, sepsis-related organ failure assessment; FiO2, fraction of inspired oxygen; paO2/FiO2, arterial partial pressure of oxygen/fraction of inspired oxygen oxygenation index; PEEP, positive end-expiratory pressure; PPlat, maximum airway plateau pressure; Pmean, mean airway pressure; RALE, radiographic assessment of lung edema; IQR, interquartile range (25–75%); PCT, procalcitonin; IL-6, interleukin 6; VTE, venous thromboembolism.
References
1. Available online at: https://coronavirus.jhu.edu/map.html (accessed on July 20, 2020).
2. Available online at: https://www.sueddeutsche.de/bayern/coronavirus-bayern-erster-ausbruch-rueckblick-1.4794769 (accessed on April 27, 2020).
3. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. (2020) 201:1299–300. doi: 10.1164/rccm.202003-0817LE
4. Available online at: https://coronavirus.jhu.edu/data/mortality (accessed on July 20, 2020).
5. Haas S, Encke A, Kopp I. [German S3 practice guidelines on prevention of venous thromboembolism–New and established evidence]. Dtsch Med Wochenschr. (2016) 141:453–6. doi: 10.1055/s-0042-100484
6. Warren MA, Zhao Z, Koyama T, Bastarache JA, Shaver CM, Semler MW, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. (2018) 73:840–6. doi: 10.1136/thoraxjnl-2017-211280
7. Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
8. Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. (2016) 315:788–800. doi: 10.1001/jama.2016.0291
9. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
10. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 33:1061–9. doi: 10.1001/jama.2020.1585
11. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
12. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
13. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. COVID-19 in critically ill patients in the Seattle region—case series. (2020). N Engl J Med. (2020). 382:2012–22. doi: 10.1056/NEJMoa2004500
14. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. (2020) 323:1612–4. doi: 10.1001/jama.2020.4326
15. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. (2020) 395:1763–70. doi: 10.1016/S0140-6736(20)31189-2
16. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. (2020) 180:1345–55. doi: 10.1001/jamainternmed.2020.3539
18. Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. (2020) 8:853–62. doi: 10.1016/S2213-2600(20)30316-7
19. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:934–43. doi: 10.1001/jamainternmed.2020.0994
20. Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. (2003) 289:2363–9. doi: 10.1001/jama.289.18.2363
21. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. (2014) 42:383–91. doi: 10.1097/SHK.0000000000000234
22. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. (2014) 124:188–95. doi: 10.1182/blood-2014-05-552729
23. Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with covid-19—preliminary report. N Engl J Med. (2020). doi: 10.1056/NEJMoa2021436. [Epub ahead of print].
24. Fichtner F, Moerer O, Weber-Carstens S, Nothacker M, Kaisers U, Laudi S. Clinical guideline for treating acute respiratory insufficiency with invasive ventilation and extracorporeal membrane oxygenation: evidence-based recommendations for choosing modes and setting parameters of mechanical ventilation. Respiration. (2019) 98:357–72. doi: 10.1159/000502157
25. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. (2020) 383:1813–26. doi: 10.1056/NEJMc2022236
Keywords: COVID-19, ARDS (acute respiratory distress syndrome), intensive care medicine, pandemia, Germany
Citation: Herrmann J, Adam EH, Notz Q, Helmer P, Sonntagbauer M, Ungemach-Papenberg P, Sanns A, Zausig Y, Steinfeldt T, Torje I, Schmid B, Schlesinger T, Rolfes C, Reyher C, Kredel M, Stumpner J, Brack A, Wurmb T, Gill-Schuster D, Kranke P, Weismann D, Klinker H, Heuschmann P, Rücker V, Frantz S, Ertl G, Muellenbach RM, Mutlak H, Meybohm P, Zacharowski K and Lotz C (2020) COVID-19 Induced Acute Respiratory Distress Syndrome—A Multicenter Observational Study. Front. Med. 7:599533. doi: 10.3389/fmed.2020.599533
Received: 27 August 2020; Accepted: 27 November 2020;
Published: 18 December 2020.
Edited by:
Jiapeng Huang, University of Louisville, United StatesReviewed by:
Jesus Rico-Feijoo, Hospital Universitario Río Hortega, SpainTommaso Tonetti, University of Bologna, Italy
Copyright © 2020 Herrmann, Adam, Notz, Helmer, Sonntagbauer, Ungemach-Papenberg, Sanns, Zausig, Steinfeldt, Torje, Schmid, Schlesinger, Rolfes, Reyher, Kredel, Stumpner, Brack, Wurmb, Gill-Schuster, Kranke, Weismann, Klinker, Heuschmann, Rücker, Frantz, Ertl, Muellenbach, Mutlak, Meybohm, Zacharowski and Lotz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Lotz, bG90el9jQHVrdy5kZQ==
†These authors share first authorship
‡These authors share senior authorship
 Johannes Herrmann1†
Johannes Herrmann1† Elisabeth Hannah Adam
Elisabeth Hannah Adam Quirin Notz
Quirin Notz Kai Zacharowski
Kai Zacharowski Christopher Lotz
Christopher Lotz