
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Med., 04 February 2021
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.598037
Candida spp. is one of the most important components of human microecology. Among hospitalized patients, the isolation rate of Candida spp. by active screening is about 15%, while in critically ill patients, the rate can be as high as 25% (1). Although microbial colonization plays an important role in secondary infections, Candida pneumonia is seldom documented even in the intensive care unit (ICU). Thus, the common consensus is that anti-Candida therapy is rarely necessary in most cases and it should be considered as colonization in which Candida spp. are isolated from the respiratory tract (RT) (2).
The co-existence of bacteria and fungi has raised great concern in the last decade. It has been indicated by some studies that Candida colonization in the RT might be an independent risk factor that could promote ventilator-associated pneumonia (VAP) and even change the antibiotic resistance patterns of pathogenic bacteria by polymicrobial biofilm formation (3, 4). Therefore, the significance of Candida colonization in RT remains controversial, and many clinical problems need to be reinterpreted.
The rate of Candida spp. isolation in the RT is relatively high, especially in those with mechanical ventilation (MV) (3). However, whether VAP can be caused by Candida spp. remains controversial and the main reasons for this are listed as follows: (1) No matter what the pathogenic microorganism is, the diagnosis of VAP is still difficult due to the lack of pathological evidence. The clinical diagnostic criteria for suspected VAP are not specific, and it is difficult to distinguish between colonization and infection (5). (2) The understanding of the importance of bacterial and fungal co-existence is not deep enough. Some microbiological laboratories have not conducted further analysis when fast-growing Candida spp. are isolated from RT samples. What's more, only filamentous fungi isolation were reported in some institutions (6). (3) It is widely accepted that the cutoff value for the number of pathogenic bacteria for VAP diagnosis is 103 cfu/mL (protected specimen brush sample) or 104 cfu/mL (bronchoalveolar lavage fluid sample), but such a threshold has not yet been established for Candida (5). Therefore, Candida pneumonia must be diagnosed by histopathology.
Hence, it is generally thought that Candida pneumonia is quite rare in the ICU, and the guidelines for the management of Candida spp. of both the IDSA and ESCMID do not recommend antifungal treatment unless there is clear histological evidence of infection (2, 7).
Alveolar macrophages act as the first line of defense against Candida in critically ill patients. Toll-like receptor (TLR) induces a Th1 cytokine pattern to increase the levels of IFN-γ and TNF-α to facilitate the clearance of Candida spores from the alveoli. What is more, other researches have also indicated that IFN-γ favors the intracellular killing of the fungus after internalization in professional phagocytes (8). Thus, it can be inferred that Candida pneumonia may not exist in the ICU. An autopsy study with 135 patients who died of pneumonia showed that among them, 77 (57%) severely affected patients had Candida airway colonization during their hospital stay. However, none of these cases was pathologically confirmed as Candida pneumonia (9). Meanwhile, one controlled before-after study in a microbiology laboratory at Illinois University showed that limiting the identification of respiratory secretions (only filamentous fungi were reported) could reduce the prescription of antifungal drug treatment (21 vs. 39%) and shorten the length of hospital stay (10.1 vs. 12.1 days) compared with full identification (all rapidly growing yeasts were reported), p < 0.05 (6).
What should ICU physicians do when they receive a microbial culture report which indicates that Candida spp. are growing fast in airway secretions? The practice guidelines recommend that antifungal therapy should not be routinely used in those with Candida airway colonization (2, 7). However, should Candida colonization in the airway of critically ill patients simply be ignored? Some in vitro experiments on the co-existence of bacteria and fungi came to different conclusions.
The cell wall of Candida spp. is combined with polysaccharides and proteins. Among them, Beta-glucan (BG) is a proinflammatory factor that can cause dysfunction of macrophages and neutrophils in alveoli as well as reduce the production of reactive oxygen species (10). It is also reported that there is a strong interaction among Candida, Gram-positive and Gram-negative bacteria through quorum sensing (QS) molecules, and the extensive interaction of metabolic processes and intercellular communication among them are the basis of synergistic and antagonistic interactions (11). Through an observational study of rats injected with active Candida albicans, it was found that the increased production of cellular inflammatory factors, including interleukin-6, interferon-γ and tumor necrosis factor-α, inhibited phagocytosis by alveolar macrophages. This phenomenon led to changes in airway microecology, and an increase in the airway colonization rate of Pseudomonas aeruginosa was found (12). Moreover, this effect was not unique to Pseudomonas aeruginosa. Another study showed that Candida colonization was also beneficial for the colonization of Staphylococcus aureus and Enterobacteriaceae, which led to an increase in bacterial pneumonia (13).
Candida biofilms show a reticular structure composed of Candida spores and hyphae and are easily found on the surfaces of artificial materials (such as endotracheal tubes). The biofilm matrix contains polysaccharides, proteins and other unknown components, which show strong adhesion and are difficult to remove (14) (Figure 1). Biofilms not only have a protective effect on Candida but also have a strong adsorption effect on co-existing bacteria. Animal experiments and electron microscopic studies show that bacteria and fungi can produce small molecules to interact with each other and change their morphology, function and growth environment, resulting in bacteria that are firmly adsorbed between Candida spores or biofilms. Such structures are difficult to remove. Even though the spore activity of some Candida spp. is decreased, the adsorption phenomenon is still observed (4, 15).
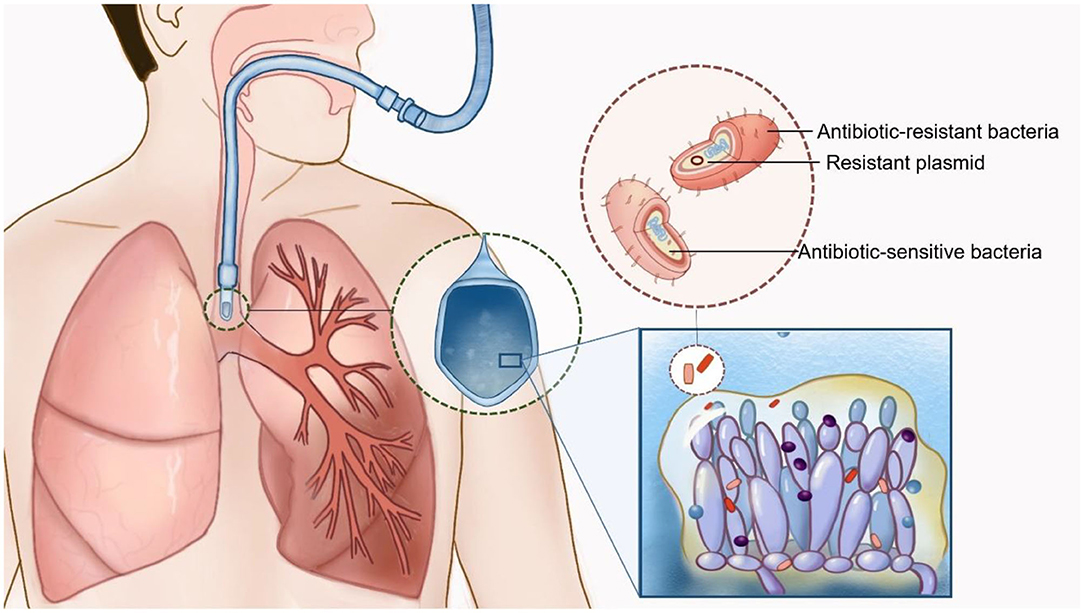
Figure 1. Interaction of Candida spp. and bacteria in patients with mechanical ventilation. Candida biofilms are easily found on the respiratory tract or the surfaces of endotracheal tubes. Biofilms not only have a protective effect on Candida but also have a strong adsorption effect on co-existing bacteria. Multidrug-resistant bacteria could be isolated by the transmission of drug-resistant plasmid transmission and polymicrobial biofilm formation (Drawn by Chunhui Xu).
Candida colonization can also change the virulence and/or host immune function of colonized bacteria. A series of animal experiments have shown that after the mixed inoculation of Candida and bacteria in the airway of mice, even if the number of inoculated Candida is very small, the bacterial load still occupies a high percentage of the alveoli. It has been suggested that the presence of Candida albicans protects the bacteria from clearance by normal alveolar macrophages (16). Acinetobacter baumannii can affect the morphology of Candida albicans through the QS molecule N-acyl homoserine lactone, whereas farnesol is the main QS molecule of Candida albicans (11). This can affect the movement ability and virulence factor expression of Acinetobacter baumannii. An animal experiment has also found that the degree of alveolar invasiveness of Acinetobacter baumannii in mice with Candida colonization during pneumonia is much higher than that of Acinetobacter baumannii during pulmonary infection (17).
The existence of biofilms can also increase the resistance of bacteria to antibiotics. It is showed that Staphylococcus aureus could form a single biofilm (monoculture biofilm) in serum, but its integrity was poor, and it was easy to dissociate. If there is co-growth with Candida albicans, Staphylococcus aureus can form microcolonies on the fungal biofilm, which is closely connected to the bottom hyphae “scaffold,” to form a multi-bacterial biofilm (polymicrobial biofilm) (Supplementary Figure 1). Staphylococcus aureus matrix staining showed different phenotypes of multi-bacterial biofilms and single cell membranes (18), indicating that Staphylococcus aureus may be encapsulated in the matrix secreted by Candida albicans, resulting in an increase in its resistance to vancomycin.
Further studies showed that in the environment of multi-bacterial biofilm formation, 27 Staphylococcus aureus-specific proteins were identified by gel electrophoresis, some of which could upregulate the expression of L-lactate dehydrogenase I, confer the ability to resist host-derived oxidative stress to bacteria and enhance resistance to antibiotics, while other proteins could downregulate the expression of the virulence factor CodY (19). These findings suggest that the occurrence of VAP caused by MRSA in patients with Candida albicans airway colonization is not only the result of the expression of QS molecules but can also be attributed to the differential regulation of specific drug resistance genes and virulence factors. Similar results have been obtained in other studies of Gram-negative bacteria (20, 21). In vitro studies suggest that there is mutual induction of the process of the co-existence of bacteria and fungi, so it is necessary to further describe and study the complex interactions between pathogens at the molecular level. The transition from basic research to clinical research may help to design new treatment or prevention and control strategies for bacterial and fungal superinfection.
Clinical studies have pointed out that the isolation rate of Candida from the RT of ICU patients with MV could be as high as 50%, which prolonged the median hospital stay (59.9 vs. 38.6 days, p = 0.006) or even increased the hospital mortality (34.2 vs. 21.0%, p = 0.003) (22). Moreover, it might be associated with persistent immunosuppression and inflammation (23). Candida airway colonization and its concomitant secretion of inflammatory factors may affect host cellular immune function, especially in immunosuppressed hosts with severe monocyte and lymphocyte dysfunction, which results in a decrease in the effective clearance of bacteria and fungi and an increase in the incidence of VAP (24).
However, the effect of Candida RT colonization on bacterial colonization and antibacterial resistance patterns has always been controversial in clinical research. It is still unclear whether Candida airway colonization could increase the incidence of VAP and whether patients with Candida airway colonization can benefit from antifungal therapy (Supplementary Table 1).
One early prospective cohort study reported that Candida RT colonization could increase the incidence of VAP caused by Pseudomonas aeruginosa (9 vs. 4.8%, p = 0.048), and Candida RT colonization was proven to be an independent risk factor (18). Similarly, another single-center retrospective case-control study indicated that antifungal therapy in those with Candida albicans airway colonization could prevent the occurrence of Pseudomonas aeruginosa VAP (25). Some studies have also pointed out that Candida airway colonization is associated with the pathogenesis of Acinetobacter baumannii VAP. In addition, another cohort study showed that aerosol inhalation of amphotericin B in patients with MV significantly reduced the Candida load in the airway but did not change the morbidity due to VAP or mortality during the ICU stay (26, 27).
The EMPIRICUS study is a randomized trial to evaluate the efficacy of micafungin for the treatment of patients with Candida colonization in multiple sites and sepsis with organ failure (28). The study noted that the incidence of VAP and the 28-days mortality during the ICU stay did not decrease in the micafungin group compared with those in the placebo group (32 vs. 39.8%, p > 0.05). Therefore, the above studies led to a change in the understanding of the co-existence of bacteria and fungi and their effects on immune function in clinical studies. FUNGIBACT, as a prospective cohort study, included 146 patients with MV for more than 96 h. After adjusting for the immune index mHLA-DR, it was concluded that there was no correlation between airway Candida colonization and the incidence of VAP [HR: 0.98; 95% CI (0.59–1.65), p = 0.95] (29). Another retrospective study reviewed 269 systemic lupus erythematosus patients with hospital-acquired pneumonia. Among them, 186 (69.1%) were found to have airway Candida colonization. Compared with that in the non-colonized group, the detection rate of multidrug-resistant bacteria was higher (58.6 vs. 36.1%, p < 0.001), and the secreted IgA and IL-17 levels returned to normal range faster after anti-fungal treatment, but this had no effect on 28-days mortality (14.5 vs. 10.8, p > 0.05) (30).
One meta-analysis about the influence of Candida spp. airway colonization on clinical outcomes in patients with VAP included four prospective studies, three retrospective studies, and one cross-sectional study (31). It revealed that those with airway Candida colonization had longer durations of MV. The most noteworthy feature of the meta-analysis is that patients with Candida colonization had higher 28-days mortality (RR: 1.64; 95% CI: 1.27–2.12) and ICU mortality (RR: 1.57; 95% CI: 1.26–1.94) than those without Candida colonization. Although it has included almost all the clinical research about airway Candida colonization with high quality, limitations still exist. First, attributable mortality rate could hardly find in these studies duo to the effects of confounding factors and the insufficient sample size. Second, a highly heterogeneity could be recognized in the baseline of the enrolled patients.
Reasons for MV, severity of VAP, antibiotic exposures before the diagnosis of VAP and the immune state was probably diverse among studies.
Although “Candida pneumonia” is rarely confirmed in critically ill patients, Candida airway colonization may affect bacterial colonization and antibacterial resistance patterns, playing an important role in the development of bacterial pneumonia. However, the conclusions of current clinical studies are not consistent. Future clinical studies are needed to re-evaluate the potential benefits of pre-emptive antifungal therapy for preventing VAP.
Y-TY and JL: conception and design. D-CC: administrative support. C-HX: provision of study materials or patients. Y-TY and C-HX: data analysis and interpretation. All authors: collection and assembly of data, manuscript writing, and final approval of manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.598037/full#supplementary-material
1. Epelbaum O, Chasan R. Candidemia in the intensive care unit. Clin Chest Med. (2017) 38:493–509. doi: 10.1016/j.ccm.2017.04.010
2. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of Candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. (2016) 62:e1–50. doi: 10.1093/cid/civ933
3. Hamet M, Pavon A, Dalle F, Pechinot A, Prin S, Quenot JP, et al. Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med. (2012) 38:1272–9. doi: 10.1007/s00134-012-2584-2
4. Gabrilska RA, Rumbaugh KP. Biofilm models of polymicrobial infection. Future Microbiol. (2015) 10:1997–2015. doi: 10.2217/fmb.15.109
5. Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. (2016) 63:e61–111. doi: 10.1093/cid/ciw353
6. Barenfanger J, Arakere P, Cruz RD, Imran A, Drake C, Lawhorn J, et al. Improved outcomes associated with limiting identification of Candida spp. in respiratory secretions. J Clin Microbiol. (2003) 41:5645–9. doi: 10.1128/jcm.41.12.5645-5649.2003
7. Martin-Loeches I, Antonelli M, Cuenca-Estrella M, Dimopoulos G, Einav S, De Waele JJ, et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. (2019) 45:789–805. doi: 10.1007/s00134-019-05599-w
8. Shao TY, Ang WXG, Jiang TT, Huang FS, Andersen H, Kinder JM, et al. Commensal Candida albicans positively calibrates systemic Th17 immunological responses. Cell Host Microbe. (2019) 25:404–17 e6. doi: 10.1016/j.chom.2019.02.004
9. Meersseman W, Lagrou K, Spriet I, Maertens J, Verbeken E, Peetermans WE, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med. (2009) 35:1526–31. doi: 10.1007/s00134-009-1482-8
10. Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. (2008) 6:67–78. doi: 10.1038/nrmicro1815
11. Sedlmayer F, Hell D, Muller M, Auslander D, Fussenegger M. Designer cells programming quorum-sensing interference with microbes. Nat Commun. (2018) 9:1822. doi: 10.1038/s41467-018-04223-7
12. Perez-Rodriguez G, Dias S, Perez-Perez M, Fdez-Riverola F, Azevedo NF, Lourenco A. Agent-based model of diffusion of N-acyl homoserine lactones in a multicellular environment of Pseudomonas aeruginosa and Candida albicans. Biofouling. (2018) 34:335–45. doi: 10.1080/08927014.2018.1440392
13. Meto A, Colombari B, Sala A, Pericolini E, Meto A, Peppoloni S, et al. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent Mater J. (2019) 38:591–603. doi: 10.4012/dmj.2018-252
14. Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. (2016) 18:310–21. doi: 10.1016/j.micinf.2016.01.002
15. Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. (2018) 16:19–31. doi: 10.1038/nrmicro.2017.107
16. Ardizzoni A, Pericolini E, Paulone S, Orsi CF, Castagnoli A, Oliva I, et al. In vitro effects of commercial mouthwashes on several virulence traits of Candida albicans, viridans streptococci and Enterococcus faecalis colonizing the oral cavity. PLoS ONE. (2018) 13:e0207262. doi: 10.1371/journal.pone.0207262
17. Tan X, Chen R, Zhu S, Wang H, Yan D, Zhang X, et al. Candida albicans airway colonization facilitates subsequent Acinetobacter baumannii pneumonia in a rat model. Antimicrob Agents Chemother. (2016) 60:3348–54. doi: 10.1128/AAC.02180-15
18. Green IM, Margoni I, Nair SP, Petridis H. Adhesion of methicillin-resistant Staphylococcus aureus and Candida albicans to parylene-C-coated polymethyl methacrylate. Int J Prosthodont. (2019) 32:193–5. doi: 10.11607/ijp.5918
19. Waters NR, Samuels DJ, Behera RK, Livny J, Rhee KY, Sadykov MR, et al. A spectrum of CodY activities drives metabolic reorganization and virulence gene expression in Staphylococcus aureus. Mol Microbiol. (2016) 101:495–514. doi: 10.1111/mmi.13404
20. Padder SA, Prasad R, Shah AH. Quorum sensing: a less known mode of communication among fungi. Microbiol Res. (2018) 210:51–8. doi: 10.1016/j.micres.2018.03.007
21. Albert M, Williamson D, Muscedere J, Lauzier F, Rotstein C, Kanji S, et al. Candida in the respiratory tract secretions of critically ill patients and the impact of antifungal treatment: a randomized placebo controlled pilot trial (CANTREAT study). Intensive Care Med. (2014) 40:1313–22. doi: 10.1007/s00134-014-3352-2
22. Delisle MS, Williamson DR, Perreault MM, Albert M, Jiang X, Heyland DK. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J Crit Care. (2008) 23:11–7. doi: 10.1016/j.jcrc.2008.01.005
23. Huang Y, Jiao Y, Zhang J, Xu J, Cheng Q, Li Y, et al. Microbial etiology and prognostic factors of ventilator-associated pneumonia: a multicenter retrospective study in Shanghai. Clin Infect Dis. (2018) 67:S146–52. doi: 10.1093/cid/ciy686
24. Delisle MS, Williamson DR, Albert M, Perreault MM, Jiang X, Day AG, et al. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can Respir J. (2011) 18:131–6. doi: 10.1155/2011/827692
25. Mear JB, Kipnis E, Faure E, Dessein R, Schurtz G, Faure K, et al. Candida albicans and Pseudomonas aeruginosa interactions: more than an opportunistic criminal association? Med Mal Infect. (2013) 43:146–51. doi: 10.1016/j.medmal.2013.02.005
26. van der Geest PJ, Dieters EI, Rijnders B, Groeneveld JA. Safety and efficacy of amphotericin-B deoxycholate inhalation in critically ill patients with respiratory Candida spp. colonization: a retrospective analysis. BMC Infect Dis. (2014) 14:575. doi: 10.1186/s12879-014-0575-3
27. Dadar M, Tiwari R, Karthik K, Chakraborty S, Shahali Y, Dhama K. Candida albicans- biology, molecular characterization, pathogenicity, and advances in diagnosis and control - an update. Microb Pathog. (2018) 117:128–38. doi: 10.1016/j.micpath.2018.02.028
28. Timsit JF, Azoulay E, Schwebel C, Charles PE, Cornet M, Souweine B, et al. Empirical micafungin treatment and survival without invasive fungal infection in adults with ICU-acquired sepsis, candida colonization, and multiple organ failure: the EMPIRICUS randomized clinical trial. JAMA. (2016) 316:1555–64. doi: 10.1001/jama.2016.14655
29. Timsit JF, Schwebel C, Styfalova L, Cornet M, Poirier P, Forrestier C, et al. Impact of bronchial colonization with Candida spp. on the risk of bacterial ventilator-associated pneumonia in the ICU: the FUNGIBACT prospective cohort study. Intensive Care Med. (2019) 45:834–43. doi: 10.1007/s00134-019-05622-0
30. Yu Y, Li J, Wang S, Gao Y, Shen H, Lu L. Effect of Candida albicans bronchial colonization on hospital-acquired bacterial pneumonia in patients with systemic lupus erythematosus. Ann Transl Med. (2019) 7:673. doi: 10.21037/atm.2019.10.44
Keywords: Candida, colonization, ventilator associate pneumonia, critical ill patients, bioflim
Citation: Liu J, Yu Y-T, Xu C-H and Chen D-C (2021) Candida Colonization in the Respiratory Tract: What Is the Significance? Front. Med. 7:598037. doi: 10.3389/fmed.2020.598037
Received: 23 August 2020; Accepted: 18 December 2020;
Published: 04 February 2021.
Edited by:
Aleksandra Barac, University of Belgrade, SerbiaReviewed by:
Tarek A. Ahmad, Bibliotheca Alexandrina, EgyptCopyright © 2021 Liu, Yu, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Chang Chen, aWN1ZGNjaGVuQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.