
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 18 December 2020
Sec. Hematology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.597734
This article is part of the Research TopicThrombotic Microangiopathies, Diagnostic and Therapeutic AdvancesView all 11 articles
A major challenge encountered by clinicians is differentiating presentations characterized by significant thrombocytopenia due to overlapping clinical symptoms and signs in the setting of ambiguous laboratory results. Immature platelets represent the youngest platelets that can be measured in peripheral blood by current hematology analyzers. These young platelets are larger, with higher RNA content recently released from the bone marrow. Thrombocytopenic presentations caused directly or indirectly by immune responses can lead to compensatory bone marrow responses seeking to normalize the platelet count; thus obtaining absolute immature platelet counts may be informative while triaging patients. Over the last decade, their use has expanded beyond being an early biomarker of bone marrow reconstitution post-hematopoietic stem cell transplantation to being used to establish bone marrow responses to infection and thrombocytopenias due to immune etiologies. Its accessibility as part of more detailed platelet indices obtained with routine laboratories makes it a promising option to understand the bone marrow's real-time response to disease states characterized by thrombocytopenia. This review will look at the immature platelet count as a biomarker, while presenting current attempts trying to understand how it could be used in thrombocytopenias occurring secondary to a given immune etiology.
Immunological processes that affect platelet production and/or platelet counts represent clinical challenges not only to diagnose but also to treat. Indeed, over the years a better understanding of some of these immune processes causing thrombocytopenia has led to more timely and targeted treatment approaches resulting in better outcomes. However, difficulties still remain when overlapping clinical pictures make a definitive diagnosis challenging. This can be exemplified by microangiopathic hemolytic anemia presentations where a given etiology may not be immediately apparent (1), and even in some instances when malignant states could be misdiagnosed as immune thrombocytopenia (ITP) (2). It is with this in mind that a growing body of literature describing potential alternative biomarkers lending support to a given diagnosis that results in timelier and etiology-specific interventions can be found. One of these markers, readily obtainable in modern automated hematology analyzers with fluorescence capability, is the immature platelet fraction (%-IPF) and specifically the absolute immature platelet count (A-IPC). Regardless of how the acronyms are presented in the literature, such as designation of either immature or reticulated platelets, it has become more evident that this is an important variable when discerning processes leading to a thrombocytopenic state which has long been overlooked in clinical practice (3–8).
Immature platelets have been shown to be much larger, with higher RNA content, and more biochemically active than their mature counterparts (9). They can be affected by chemotherapy treatments and irradiation, and when immune reactions target platelets they can be elevated well above reference ranges (9, 10). They can be accurately measured in blood samples even 24 h after they have been collected (11), likely due to their increased longevity compared to mature platelets (9). Additionally, consumptive thrombocytopenic processes and those characterized by platelet hypoproduction can be readily identified looking at immature platelets (12). These counts can potentially point out if the thrombocytopenia-inducing etiology is either centrally (at bone marrow) or peripherally driven (5, 13). Likewise, immature platelets appear not to be affected by gender (14) or age since their production is preserved even in older individuals with lower platelet counts (15).
Nevertheless, tests that have the potential to be used clinically need to undergo scrutiny that at times may come into a collision course with the constrains of the technology behind them. The number of reports describing the utility of immature platelet counts has been mostly positive favoring it as a gauge to zero-in on a narrower group of potential etiologies. Yet, technological differences exemplified by changes in dyes with improved specificity for platelet elements, changes in gating, size correction, testing platform used, and wavelengths influence the analytical limits of this test (16–18); while those factors that are pre-clinical in nature such as timing of measurement with respect to specimen collection, degree of platelet activation, anticoagulant used, and quality of the sample (degree of hemolysis) may begin to explain the finite discrepancies that appear in the literature (16, 19–22).
Measurement of immature platelet counts requires establishing better defined reference intervals (16, 17). Importantly, reports describing that A-IPC changes precede those of their mature counterparts at times by 2–3 days may be an important observation when treating patients with thrombocytopenia (23–25). This is also the case in patients recovering from chemotherapy or stem cell transplantation in which immature platelets are first to return indicating that engraftment has occurred (23, 26, 27). This is undoubtedly of benefit to predict patients engrafting or for those recovering from their immune-consumptive processes. This may also help even those patients whose bone marrow fails to produce platelets in sufficient numbers to compensate for an existing thrombocytopenic state (3). Finally, research into their biology is likely to become easier thanks to the development of newer assays that immunostain, sort, and isolate immature platelets from the peripheral circulation (28, 29). Thus, this is the focus of this review to present the most current information of potential uses of immature platelets counts in clinical practice.
When the first data of changes in baseline %-IPF in the setting of ITP was reported, it raised the possibility that a real-time response by the bone marrow in this disease was possible to measure (30). Pathologically both higher platelet destruction that results in decreased platelet counts and potentially impaired thrombopoiesis influence risk of bleeding (31). Thus it should be of interest that reports appear to suggest that bone marrow attempts to compensate for platelet destruction by markedly increasing %-IPF to cope with the consumptive/destructive process (2, 8, 21, 30, 32–34); that these increases appear higher in those with chronic ITP (35); and that these dynamics may help risk stratify patients at risk of bleeding since they appear to have a higher preponderance of immature platelets (32, 36, 37). Notably, the magnitude of these compensatory increases in patients with ITP may be conveyed in a more consistent manner by looking at A-IPC at presentation (38).
However, few reports have found limited utility in using immature platelets to differentiate ITP where one hematology analyzer found some value to measuring it while the other had discrepant results (39). Regrettably, this study lacks A-IPC data analysis and determination if immature platelet counts were comparable with stratification according to platelet count (39). Despite this, recent data indicates that as ITP patients respond to treatment (40), specific changes in A-IPCs identify those with the disease (8). Based on these results, potentially helpful clinical scoring models that include immature platelet counts and favor ITP as a diagnosis have been proposed (2, 33), that take into consideration the high positive predictive value of these A-IPC changes (41). What adds to their potential usefulness is that in ITP, the higher immature platelet counts seen as patients recover from the disease (high turnover/destruction) precede by >3 days changes in mature platelet counts (25), similar to what has been described in other thrombocytopenic presentations. Thus, a model can be derived taking into account the published literature which favors a preserved and at times enhanced immature platelet/ mature platelet feedback (Figure 1A). In this model, as platelets get consumed or destroyed in the periphery, the bone marrow concomitantly increases its immature platelet output to compensate for platelet losses. Once platelet counts improve in response to disease remission and/or as a result of therapy, and platelet counts improve there is a corresponding decrease in immature platelet output as they return to baseline (10).
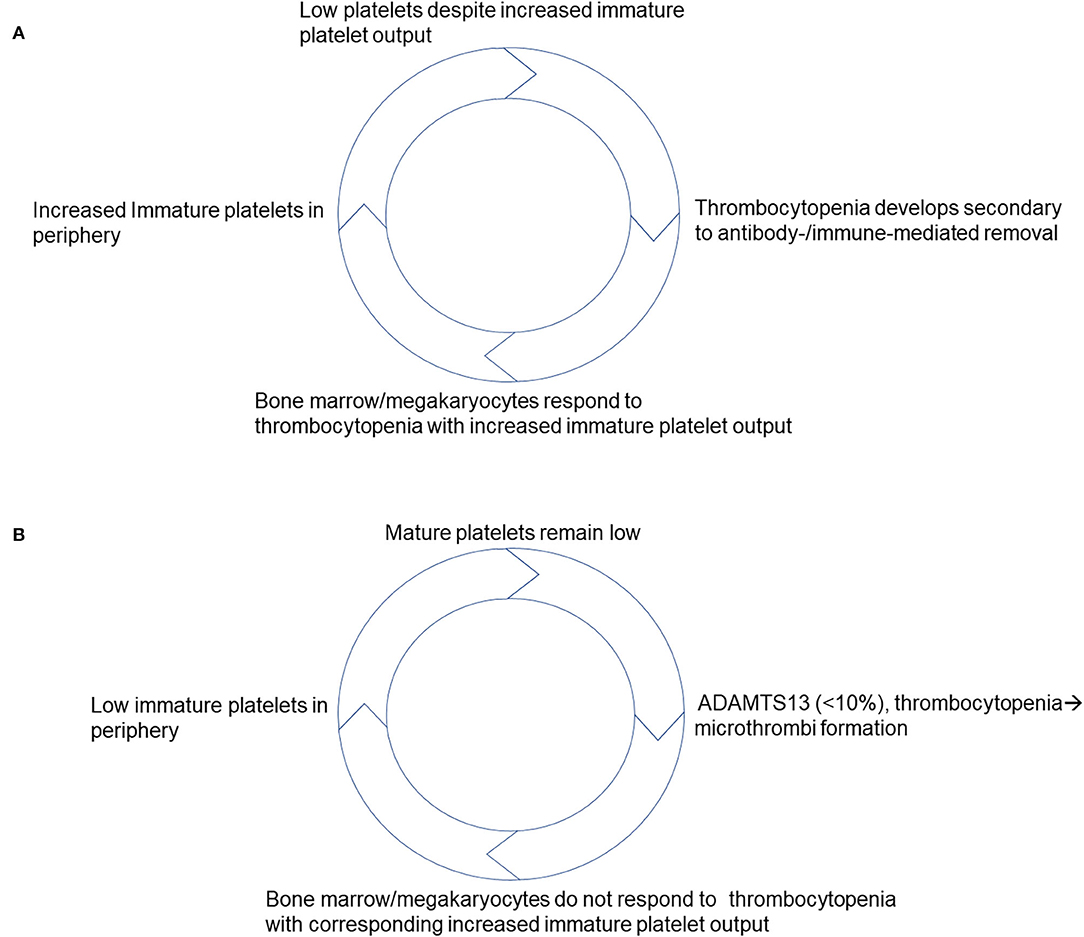
Figure 1. Schematic representation of proposed negative feedback models that take into account responses to thrombocytopenia based on immature platelet production. (A) Responses to thrombocytopenia in ITP. (B) Responses to thrombocytopenia in TTP.
In the TTP literature, without a doubt the discovery that deficiency either innate or immune-mediated (acquired) of the metalloprotease ADAMTS13 is at the center of its pathology has changed the way in which patients are diagnosed and triaged (42, 43). This test, however, is sent-out to reference laboratories by most institutions often leading to initiation of therapeutic plasma exchange (TPE) prior to testing results becoming available. This is an important point to consider, since TPE is associated with higher adverse reactions when plasma is used as replacement fluid as required in TTP patients, making a timely accurate diagnosis paramount (44, 45). As a result, alternatives that increase the index of suspicion for TTP while not delaying prompt initiation of therapy could prove useful in such clinical settings. Early reports indicate that the immature platelet counts of TTP patients are much lower than those of ITP patients (30). Furthermore, recent data has shown that TTP patients (ADAMTS13 <10%) have A-IPCs at presentation that are markedly decreased compared to healthy controls that differentiates this group from other thrombocytopenic patients without the enzyme deficiency (24, 46, 47), and in some, such as refractory TTP cases, it may facilitate adjustments in therapy (48, 49). In TTP patients, improvement in A-IPC precedes that of mature platelet counts by about 2 days following initiation of TPE; and counts return to baseline once mature platelet counts stabilize at a normal level (24, 46, 50).
A-IPCs have been shown to predict response to TPE in patients with high ADAMTS13 inhibitors who may require longer treatment protocols to restore platelet counts (50). However, it remains to be determined if they would be helpful to assess responses of those TTP patients who are likely to require more TPE based on their ABO blood group (51). Patients with TTP as indicated by their immature platelet counts at presentation, appear to have a suppressed negative feedback i.e., rapidly relieved by TPE initiation (24, 46, 50). Based on this, we can propose a model in which an impaired immature platelet/ mature platelet negative feedback characterizes new onset TTP. This proposed model suggests that the bone marrow appears not to respond to the existing thrombocytopenia with a corresponding increase in immature platelets until TPE is initiated (Figure 1B). Nonetheless, post TPE initiation A-IPC increases, preceding the mature platelet count changes restoring the negative feedback. Once mature platelet counts reach a normal level, A-IPC begins to decline back to baseline indicating that patients are on their way to recovery. This apparent suppressed A-IPC response implies that the feedback mechanisms at play between mature and immature platelets are disrupted in new onset TTP. This should be the focus of future research looking at the mechanisms mediating this observation to establish how disease precipitates in TTP patients.
Infectious processes may lead to decreases in mature platelets as the body fends off the infectious agent(s). Notably, even though thrombocytopenia can be seen in infections, A-IPCs are generally maintained so that at least platelet production attempts to keep up with the higher consumption (52); yet such A-IPC increases appear to correlate with higher mortality risk and disease severity in septic patients (53). These increases in immature platelets have been reported to occur earlier in patients prior to sepsis onset (54), which may be predictive of subsequent decreases in mature platelet counts once infection sets in Muronoi et al. (55). In this regard %-IPF has been reported as highly sensitive in identifying patients with sepsis regardless of extent of infection or severity (56, 57). This may relate to the significant immune hyperreactivity observed under states of severe infection leading to a disseminated platelet consumption requiring a higher immature platelet output. However, these increases may not be applicable to neonates where suppressed A-IPC characterizes those patients who did not survive disseminated infections (58). On the other hand, older children with dengue fever who recovered from the infection had increased immature platelet outputs up to 3 days prior to recovering their platelet count (59). Therefore, in an infectious presentation, the negative feedback between immature platelets and mature platelets appears generally preserved in older children and adults.
Inflammation-inducing disease processes that lead to impaired thrombopoiesis can be triaged looking at immature platelets as shown in patients with impaired liver function/cirrhosis (60). Similarly, states in which inflammation leads to platelet count changes can be ascertained looking at immature platelets. For example, higher counts of immature platelets in circulation may predict those patients at risk of subsequent inflammation post-cardiac surgery (61). Even 7 days after these surgical procedures a correlation between pro-inflammatory interleukin (IL)-6 and immature platelet counts has been reported, where the former is associated with the inflammation encountered by these patients (62). Potentially, these increases in immature platelets may be directly driven by IL-6 since this cytokine leads to thrombocytosis and platelet activation in intestinal inflammatory settings (63). However, the risk may be related to cardiovascular disease itself since human immunodeficiency virus (HIV) patients on antiretroviral therapy with cardiovascular disease have a higher number of immature platelets compared to HIV patients on therapy without cardiovascular disease (64).
Inflammation has also been shown associated with hypertension and this may lead the cardiovascular disease sequelae among other complications (65). For example, patients with malignant hypertension who present with thrombocytopenia have significantly higher immature platelet counts that are distinct from other microangiopathic hemolytic anemia processes including TTP (47, 66). Likely, sheer forces associated with hypertension lead to vascular damage and platelet consumption that result in a higher immature platelet output. Similarly, just as in other inflammatory processes it appears that the A-IPC allows for a better distinction between preeclampsia and those patients with hemolysis, elevated liver enzymes, and low platelet count syndrome (66). Along these lines, smoking causes vascular stenosis that lead to hypertensive complications and patients who are smokers have higher proportions of immature platelets (67). Paradoxically, some reports indicate that low grade inflammation may not provide enough of a stimulus to drive immature platelet production (68). Additional research is required to further characterize differences among these presentations.
Immature platelets have been used to establish when a given drug has no effect over thrombopoiesis (69). Antibody-mediated reactions to complexes that include platelet factor four are mediated by use of heparin. Since platelets are affected by the presence of antibody to the PF4-heparin complexes, this can result in changes to immature platelet output. Mild increases in %-IPF have been reported in samples tested during heparin-induced thrombocytopenia (HIT) investigations (70). However, recently it was shown that patients who test positive (HIT+) for the presence of anti-PF4-heparin antibodies have A-IPC similar to the reference range unlike patients who test negative whose immature platelets are well-below this range (71). This implies that HIT+ patients may have immature platelet responses that attempt to maintain the platelet count though not necessarily leading to an increase in net immature platelet production. Future investigation should expand upon these observations.
Expansion over the last decade of the literature showing potential uses of immature platelet measurement in a variety of thrombocytopenic clinical settings (23, 38, 52, 71, 72), represents a promising development that has evidently resulted in heightened interest on their use.
Yet, it must be acknowledged that these promising reports, favor the establishment of well-controlled clinical trials that look at how immature platelet counts could affect disease management without compromising therapy timing (73). Likewise, as mentioned earlier, there are still remaining limitations since different analyzers will require establishment of reference ranges prior to potential application, and as technology advances with newer analyzers becoming available with higher specificity and sensitivity these ranges will undoubtedly require revision. Despite these apparent shortcomings, counts below, at, or above a reference range may prove clinically informative when discerning the etiology behind a thrombocytopenic presentation.
In summary, immature platelets are equivalent to reticulocyte counts in the setting of anemia and thus provide valuable information to the clinician when treating thrombocytopenic patients. These immature platelet counts provide the nearest to real-time information of bone marrow response to the etiology causing the thrombocytopenia, with the added benefit that once therapy is initiated it can guide a clinician to determine when therapy is causing a net shift in the production of these young platelets.
HR and RM contributed to drafting, editing, and finalizing manuscript to its current form. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Bittencourt CE, Ha JP, Maitta RW. Re-examination of 30-day survival and relapse rates in patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. PLoS ONE. (2015) 10:e0127744. doi: 10.1371/journal.pone.0127744
2. Jeon MJ, Yu ES, Kang KW, Lee BH, Park Y, Lee SR, et al. Immature platelet fraction based diagnostic predictive scoring model for immune thrombocytopenia. Korean J Intern Med. (2020) 35:970–8. doi: 10.3904/kjim.2019.093
3. Li J, Li Y, Ouyang J, Zhang F, Liang C, Ye Z, et al. Immature platelet fraction related parameters in the differential diagnosis of thrombocytopenia. Platelets. (2019) 31:771–6. doi: 10.1080/09537104.2019.1678118
4. Benlachgar N, Doghmi K, Masrar A, Mahtat EM, Harmouche H, Tazi Z. Immature platelets: a review of the available evidence. Thromb Res. (2020) 195:43–50. doi: 10.1016/j.thromres.2020.06.048
5. Van De Wyngaert Z, Fournier E, Bera E, Carrette M, Soenen V, Gauthier J, et al. Immature platelet fraction (IPF): a reliable tool to predict peripheral thrombocytopenia. Curr Res Transl Med. (2020) 68:37–42. doi: 10.1016/j.retram.2019.04.002
6. Pons I, Monteagudo M, Lucchetti G, Munoz L, Perea G, Colomina I, et al. Correlation between immature platelet fraction and reticulated platelets. Usefulness in the etiology diagnosis of thrombocytopenia. Eur J Haematol. (2010) 85:158–63. doi: 10.1111/j.1600-0609.2010.01468.x
7. Dusse LM, Freitas LG. Clinical applicability of reticulated platelets. Clin Chim Acta. (2015) 439:143–7. doi: 10.1016/j.cca.2014.10.024
8. Ali I, Graham C, Dempsey-Hibbert NC. Immature platelet fraction as a useful marker in the etiological determination of thrombocytopenia. Exp Hematol. (2019) 78:56–61. doi: 10.1016/j.exphem.2019.09.001
9. Hong H, Xiao W, Maitta RW. Steady increment of immature platelet fraction is suppressed by irradiation in single-donor platelet components during storage. PLoS ONE. (2014) 9:e85465. doi: 10.1371/journal.pone.0085465
10. Abe Y, Wada H, Tomatsu H, Sakaguchi A, Nishioka J, Yabu Y, et al. A simple technique to determine thrombopoiesis level using immature platelet fraction (IPF). Thromb Res. (2006) 118:463–9. doi: 10.1016/j.thromres.2005.09.007
11. Ruisi MM, Psaila B, Ward MJ, Villarica G, Bussel JB. Stability of measurement of the immature platelet fraction. Am J Hematol. (2010) 85:622–4. doi: 10.1002/ajh.21748
12. Barsam SJ, Psaila B, Forestier M, Page LK, Sloane PA, Geyer JT, et al. Platelet production and platelet destruction: assessing mechanisms of treatment effect in immune thrombocytopenia. Blood. (2011) 117:5723–32. doi: 10.1182/blood-2010-11-321398
13. Blandinieres A, Arlet JB, Gaussem P, Pouchot J, Darnige L. Usefulness of immature platelet fraction measurement for diagnosis and monitoring of iron deficiency associated thrombocytopenia: about two cases. Ann Biol Clin. (2020) 78:433–7. doi: 10.1684/abc.2020.1564
14. Negro F, Verdoia M, Tonon F, Nardin M, Kedhi E, De Luca G, et al. Impact of gender on immature platelet count and its relationship with coronary artery disease. J Thromb Thrombolysis. (2020) 49:511–21. doi: 10.1007/s11239-020-02080-0
15. Verdoia M, Nardin M, Negro F, Tonon F, Gioscia R, Rolla R, et al. Impact of aging on immature platelet count and its relationship with coronary artery disease. Platelets. (2020) 31:1060–8. doi: 10.1080/09537104.2020.1714572
16. Buttarello M, Mezzapelle G, Freguglia F, Plebani M. Reticulated platelets and immature platelet fraction: clinical applications and method limitations. Int J Lab Hematol. (2020) 42:363–70. doi: 10.1111/ijlh.13177
17. Wu MF, Hsieh HH, Huang WC, Lu KM, Lin JH, Lin YC, et al. Effects of analyzer detection method, ethnicity, and reference individuals on reference interval of immature platelet fraction. Clin Lab. (2018) 64:699–708. doi: 10.7754/Clin.Lab.2018.171025
18. Hoffmann JJ. Reticulated platelets: analytical aspects and clinical utility. Clin Chem Lab Med. (2014) 52:1107–17. doi: 10.1515/cclm-2014-0165
19. Wada A, Takagi Y, Kono M, Morikawa T. Accuracy of a new platelet count system (PLT-F) depends on the staining property of its reagents. PLoS ONE. (2015) 10:e0141311. doi: 10.1371/journal.pone.0141311
20. Hoffmann JJ, van den Broek NM, Curvers J. Reference intervals of reticulated platelets and other platelet parameters and their associations. Arch Pathol Lab Med. (2013) 137:1635–40. doi: 10.5858/arpa.2012-0624-OA
21. Schmoeller D, Picarelli MM, Paz Munhoz T, Poli de Figueiredo CE, Staub HL. Mean platelet volume and immature platelet fraction in autoimmune disorders. Front Med. (2017) 4:146. doi: 10.3389/fmed.2017.00146
22. Ibrahim H, Nadipalli S, Usmani S, DeLao T, Green L, Kleiman NS. Detection and quantification of circulating immature platelets: agreement between flow cytometric and automated detection. J Thromb Thrombolysis. (2016) 42:77–83. doi: 10.1007/s11239-016-1338-3
23. Have LW, Hasle H, Vestergaard EM, Kjaersgaard M. Absolute immature platelet count may predict imminent platelet recovery in thrombocytopenic children following chemotherapy. Pediatr Blood Cancer. (2013) 60:1198–203. doi: 10.1002/pbc.24484
24. Hong H, Xiao W, Stempak LM, Sandhaus LM, Maitta RW. Absolute immature platelet count dynamics in diagnosing and monitoring the clinical course of thrombotic thrombocytopenic purpura. Transfusion. (2015) 55:756–65. doi: 10.1111/trf.12912
25. Jeon K, Kim M, Lee J, Lee JS, Kim HS, Kang HJ, et al. Immature platelet fraction: a useful marker for identifying the cause of thrombocytopenia and predicting platelet recovery. Medicine. (2020) 99:e19096. doi: 10.1097/MD.0000000000019096
26. Yamaoka G, Kubota Y, Nomura T, Inage T, Arai T, Kitanaka A, et al. The immature platelet fraction is a useful marker for predicting the timing of platelet recovery in patients with cancer after chemotherapy and hematopoietic stem cell transplantation. Int J Lab Hematol. (2010) 32(6 Pt 1):e208–16. doi: 10.1111/j.1751-553X.2010.01232.x
27. Zucker ML, Murphy CA, Rachel JM, Martinez GA, Abhyankar S, McGuirk JP, et al. Immature platelet fraction as a predictor of platelet recovery following hematopoietic progenitor cell transplantation. Lab Hematol. (2006) 12:125–30. doi: 10.1532/LH96.06012
28. Bernlochner I, Klug M, Larasati D, Von Scheidt M, Santovito D, Hristov M, et al. Sorting and magnetic-based isolation of reticulated platelets from peripheral blood. Platelets. (2020) 1–7. doi: 10.1080/09537104.2020.1724923
29. Hille L, Cederqvist M, Hromek J, Stratz C, Trenk D, Nuhrenberg TG. Evaluation of an alternative staining method using SYTO 13 to determine reticulated platelets. Thromb Haemost. (2019) 119:779–85. doi: 10.1055/s-0039-1681101
30. Briggs C, Kunka S, Hart D, Oguni S, Machin SJ. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol. (2004) 126:93–9. doi: 10.1111/j.1365-2141.2004.04987.x
31. Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. (2009) 113:2386–93. doi: 10.1182/blood-2008-07-162503
32. Frelinger AL 3rd, Grace RF, Gerrits AJ, Berny-Lang MA, Brown T, Carmichael SL, et al. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood. (2015) 126:873–9. doi: 10.1182/blood-2015-02-628461
33. Kurata Y, Hayashi S, Kiyoi T, Kosugi S, Kashiwagi H, Honda S, et al. Diagnostic value of tests for reticulated platelets, plasma glycocalicin, and thrombopoietin levels for discriminating between hyperdestructive and hypoplastic thrombocytopenia. Am J Clin Pathol. (2001) 115:656–64. doi: 10.1309/RAW2-0LQW-8YTX-941V
34. Ferreira FLB, Colella MP, Medina SS, Costa-Lima C, Fiusa MML, Costa LNG, et al. Evaluation of the immature platelet fraction contribute to the differential diagnosis of hereditary, immune and other acquired thrombocytopenias. Sci Rep. (2017) 7:3355. doi: 10.1038/s41598-017-03668-y
35. Adly AA, Ragab IA, Ismail EA, Farahat MM. Evaluation of the immature platelet fraction in the diagnosis and prognosis of childhood immune thrombocytopenia. Platelets. (2015) 26:645–50. doi: 10.3109/09537104.2014.969220
36. McDonnell A, Bride KL, Lim D, Paessler M, Witmer CM, Lambert MP. Utility of the immature platelet fraction in pediatric immune thrombocytopenia: differentiating from bone marrow failure and predicting bleeding risk. Pediatr Blood Cancer. (2018) 65:e26812. doi: 10.1002/pbc.26812
37. Frelinger AL 3rd, Grace RF, Gerrits AJ, Carmichael SL, Forde EE, Michelson AD. Platelet function in ITP, independent of platelet count, is consistent over time and is associated with both current and subsequent bleeding severity. Thromb Haemost. (2018) 118:143–51. doi: 10.1160/TH17-06-0387
38. Greene LA, Chen S, Seery C, Imahiyerobo AM, Bussel JB. Beyond the platelet count: immature platelet fraction and thromboelastometry correlate with bleeding in patients with immune thrombocytopenia. Br J Haematol. (2014) 166:592–600. doi: 10.1111/bjh.12929
39. Cybulska A, Meintker L, Ringwald J, Krause SW. Measurements of immature platelets with haematology analysers are of limited value to separate immune thrombocytopenia from bone marrow failure. Br J Haematol. (2017) 177:612–9. doi: 10.1111/bjh.14628
40. Takami A, Mizuno S, Nakamura A, Kanasugi J, Yamamoto H, Vu Quang L, et al. Pretreatment immature platelet fraction as a surrogate of reticulated platelets predicts the response to corticosteroids in adults with immune thrombocytopenia. Acta Haematol. (2020) 1–5. doi: 10.1159/000510460
41. Sachs UJ. Diagnosing immune thrombocytopenia. Hamostaseologie. (2019) 39:250–8. doi: 10.1055/s-0039-1678739
42. Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, et al. von willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. (1998) 339:1578–84. doi: 10.1056/NEJM199811263392202
43. Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. (1998) 339:1585–94. doi: 10.1056/NEJM199811263392203
44. Kundrapu S, Datla S, Griffin V, Maitta RW. Adverse events during apheresis: a 10-year experience at a tertiary academic medical center. J Clin Apher. (2019) 34:528–36. doi: 10.1002/jca.21706
45. Maitta RW. Current state of apheresis technology and its applications. Transfus Apher Sci. (2018) 57:606–13. doi: 10.1016/j.transci.2018.09.009
46. Stefaniuk CM, Reeves HM, Maitta RW. Dynamic changes in absolute immature platelet count suggest the presence of a coexisting immune process in the setting of thrombotic thrombocytopenic purpura. Transfusion. (2017) 57:913–8. doi: 10.1111/trf.13974
47. Zheng Y, Hong H, Reeves HM, Maitta RW. Absolute immature platelet count helps differentiate thrombotic thrombocytopenic purpura from hypertension-induced thrombotic microangiopathy. Transfus Apher Sci. (2014) 51:54–7. doi: 10.1016/j.transci.2014.07.004
48. Kier YE, Stempak LM, Maitta RW. Immature platelet fraction can help adjust therapy in refractory thrombotic microangiopathic hemolytic anemia cases. Transfus Apher Sci. (2013) 49:644–6. doi: 10.1016/j.transci.2013.07.005
49. Kundrapu S, Reeves HM, Maitta RW. Absolute immature platelet counts suggest platelet production suppression during complicated relapsing thrombotic thrombocytopenic purpura. Acta Haematol. (2020). doi: 10.1159/000510913. [Epub ahead of print].
50. Gokozan HN, Reeves HM, Maitta RW. Absolute immature platelet count dynamics of thrombotic thrombocytopenic purpura patients with high ADAMTS13 inhibitor. Thromb Res. (2019) 179:128–31. doi: 10.1016/j.thromres.2019.05.011
51. Behtaj M, Zhu ML, Bittencourt CE, Ha JP, Maitta RW. Non-O blood group thrombotic thrombocytopenic purpura patients take longer to recover as measured by number of therapeutic plasma exchanges needed for platelet recovery. Thromb Res. (2020) 185:78–84. doi: 10.1016/j.thromres.2019.11.022
52. Koyama K, Katayama S, Muronoi T, Tonai K, Goto Y, Koinuma T, et al. Time course of immature platelet count and its relation to thrombocytopenia and mortality in patients with sepsis. PLoS ONE. (2018) 13:e0192064. doi: 10.1371/journal.pone.0192064
53. Thorup CV, Christensen S, Hvas AM. Immature platelets as a predictor of disease severity and mortality in sepsis and septic shock: a systematic review. Semin Thromb Hemost. (2020) 46:320–7. doi: 10.1055/s-0039-3400256
54. Buoro S, Manenti B, Seghezzi M, Dominoni P, Barbui T, Ghirardi A, et al. Innovative haematological parameters for early diagnosis of sepsis in adult patients admitted in intensive care unit. J Clin Pathol. (2018) 71:330–5. doi: 10.1136/jclinpath-2017-204643
55. Muronoi T, Koyama K, Nunomiya S, Lefor AK, Wada M, Koinuma T, et al. Immature platelet fraction predicts coagulopathy-related platelet consumption and mortality in patients with sepsis. Thromb Res. (2016) 144:169–75. doi: 10.1016/j.thromres.2016.06.002
56. Park SH, Ha SO, Cho YU, Park CJ, Jang S, Hong SB. Immature platelet fraction in septic patients: clinical relevance of immature platelet fraction is limited to the sensitive and accurate discrimination of septic patients from non-septic patients, not to the discrimination of sepsis severity. Ann Lab Med. (2016) 36:1–8. doi: 10.3343/alm.2016.36.1.1
57. Enz Hubert RM, Rodrigues MV, Andreguetto BD, Santos TM, de Fatima Pereira Gilberti M, de Castro V, et al. Association of the immature platelet fraction with sepsis diagnosis and severity. Sci Rep. (2015) 5:8019. doi: 10.1038/srep08019
58. Cremer M, Weimann A, Szekessy D, Hammer H, Buhrer C, Dame C. Low immature platelet fraction suggests decreased megakaryopoiesis in neonates with sepsis or necrotizing enterocolitis. J Perinatol. (2013) 33:622–6. doi: 10.1038/jp.2013.21
59. Chuansumrit A, Apiwattanakul N, Sirachainan N, Paisooksantivatana K, Athipongarporn A, Tangbubpha N, et al. The use of immature platelet fraction to predict time to platelet recovery in patients with dengue infection. Paediatr Int Child Health. (2020) 40:124–8. doi: 10.1080/20469047.2019.1697574
60. Nomura T, Kubota Y, Kitanaka A, Kurokouchi K, Inage T, Saigo K, et al. Immature platelet fraction measurement in patients with chronic liver disease: a convenient marker for evaluating cirrhotic change. Int J Lab Hematol. (2010) 32:299–306. doi: 10.1111/j.1751-553X.2009.01184.x
61. Imperiali CE, Lopez-Delgado JC, Dastis-Arias M, Sanchez-Navarro L. Evaluation of the delta of immature platelet fraction as a predictive biomarker of inflammatory response after cardiac surgery. J Clin Pathol. (2019) 73:335–40. doi: 10.1136/jclinpath-2019-206068
62. Cavalca V, Rocca B, Veglia F, Petrucci G, Porro B, Myasoedova V, et al. On-pump cardiac surgery enhances platelet renewal and impairs aspirin pharmacodynamics: effects of improved dosing regimens. Clin Pharmacol Ther. (2017) 102:849–58. doi: 10.1002/cpt.702
63. Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Yan LS, Zhang S, et al. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol. (2013) 183:173–81. doi: 10.1016/j.ajpath.2013.03.014
64. Goedel A, Muller S, Schwerdtfeger C, Zink A, Noe S, Bongiovanni D, et al. Influence of antiretroviral therapy and cardiovascular disease on the immature platelet fraction in patients living with HIV. Platelets. (2019) 31:756–62. doi: 10.1080/09537104.2019.1678114
65. Dale BL, Madhur MS. Linking inflammation and hypertension via LNK/SH2B3. Curr Opin Nephrol Hypertens. (2016) 25:87–93. doi: 10.1097/MNH.0000000000000196
66. Bernstein U, Kaiser T, Stepan H, Jank A. The immature platelet fraction in hypertensive disease during pregnancy. Arch Gynecol Obstet. (2019) 299:1537–43. doi: 10.1007/s00404-019-05102-2
67. Nardin M, Verdoia M, Negro F, Rolla R, Tonon F, De Luca G. Impact of active smoking on the immature platelet fraction and its relationship with the extent of coronary artery disease. Eur J Clin Invest. (2020) 50:e13181. doi: 10.1111/eci.13181
68. Larsen SB, Grove EL, Hvas AM, Kristensen SD. Platelet turnover in stable coronary artery disease - influence of thrombopoietin and low-grade inflammation. PLoS ONE. (2014) 9:e85566. doi: 10.1371/journal.pone.0085566
69. Kurahashi H, Takami A, Murotani K, Numoto S, Okumura A. Decreased platelet count in children with epilepsy treated with valproateand its relationship to the immature platelet fraction. Int J Hematol. (2018) 107:105–11. doi: 10.1007/s12185-017-2323-0
70. Meintker L, Haimerl M, Ringwald J, Krause SW. Measurement of immature platelets with abbott CD-sapphire and sysmex XE-5000 in haematology and oncology patients. Clin Chem Lab Med. (2013) 51:2125–31. doi: 10.1515/cclm-2013-0252
71. Chen W, Ha JP, Hong H, Maitta RW. Absolute immature platelet counts in the setting of suspected heparin-induced thrombocytopenia may predict anti-PF4-heparin immunoassay testing results. Transfus Apher Sci. (2018) 57:507–11. doi: 10.1016/j.transci.2018.04.001
72. Johnson S, Baker B. A CBC algorithm combined with immature platelet fraction is able to identify JAK2 V617F mutation-positive polycythaemia vera patients. Int J Lab Hematol. (2019) 41:271–6. doi: 10.1111/ijlh.12967
Keywords: absolute immature platelet count, thrombotic thrombocytopenic purpura, immune thrombocytopenia, immature platelet fraction, immature platelets
Citation: Reeves HM and Maitta RW (2020) Immature Platelet Dynamics in Immune-Mediated Thrombocytopenic States. Front. Med. 7:597734. doi: 10.3389/fmed.2020.597734
Received: 21 August 2020; Accepted: 24 November 2020;
Published: 18 December 2020.
Edited by:
Alejandro Lazo-Langner, Western University, CanadaReviewed by:
Ming Hou, Shandong University, ChinaCopyright © 2020 Reeves and Maitta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert W. Maitta, cm9iZXJ0Lm1haXR0YUBjYXNlLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.