- 1Department of Epidemiology and Health Statistics, School of Public Health, Capital Medical University, Beijing, China
- 2Department of Critical Care Medicine, Fuxing Hospital, Capital Medical University, Beijing, China
- 3Department of Critical Care Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
- 4Medical Intensive Care Unit, Peking Union Medical College Hospital, Beijing, China
- 5Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
- 6Department of Critical Care Medicine, Guangdong Geriatric Institute, Guangdong General Hospital, Guangdong, China
- 7Department of Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China
- 8Surgical Intensive Care Unit, Department of Anaesthesiology, ZhongShan Hospital, FuDan University, Shanghai, China
- 9Intensive Care Unit, The First Hospital of Jilin University, Changchun, China
- 10Department of Critical Care Medicine, China-Japan Friendship Hospital, Beijing, China
- 11Department of Critical Care Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 12Surgical Intensive Care Unit, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
- 13Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
- 14Department of Critical Care Medicine, General Hospital of Ningxia Medical University, Ningxia, China
- 15Department of Critical Care Medicine, Xiangya Hospital, Central South University, Changsha, China
- 16Department of Critical Care Medicine, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 17Department of Critical Care Medicine, Peking University Third Hospital, Beijing, China
- 18Surgical Intensive Care Unit, Xuanwu Hospital, Capital Medical University, Beijing, China
- 19Department of Critical Care Medicine, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Background: Sepsis is a main cause of morbidity and mortality in critically ill patients. The epidemiology of sepsis in high-income countries is well-known, but information on sepsis in middle- or low-income countries is still deficient, especially in China. The purpose of this study was to explore the prevalence, characteristics, risk factors, treatment, and outcomes of sepsis in critically ill patients in tertiary hospitals in China.
Methods: A multicenter prospective observational cohort study was performed with consecutively collected data from adults who stayed in any intensive care unit (ICU) for at least 24 h; data were collected from 1 January 2014 to 31 August 2015, and patients were followed until death or discharge from the hospital.
Results: A total of 4,910 patients were enrolled in the study. Of these, 2,086 (42.5%) presented with sepsis or septic shock on admission to the ICU or within the first 48 h after admission to the ICU. ICU mortality was higher in patients with sepsis (13.1%) and septic shock (39.0%) and varied according to geographical region. Acinetobacter, Pseudomonas, and Staphylococcus infections were associated with increased ICU mortality. In addition, age, Acute Physiology, and Chronic Health Evaluation II (APACHE II) scores, pre-existing cardiovascular diseases, malignant tumors, renal replacement therapy (RRT), and septic shock were independent risk factors for mortality in patients with sepsis. The prompt administration of antibiotics (OR 0.65, 95% CI 0.46–0.92) and 30 mL/kg of initial fluid resuscitation during the first 3 h (OR 0.43, 95% CI 0.30–0.63) improved the outcome in patients with septic shock.
Conclusions: Sepsis was common and was associated with a high mortality rate in critically ill patients in tertiary hospitals in China. The prompt administration of antibiotics and 30 mL/kg fluid resuscitation decreased the risk of mortality.
Introduction
Sepsis is a major challenge for public health; it is the main cause of morbidity and mortality in intensive care units (ICUs) and is associated with poor outcomes (1–4). In the United States, the incidence of sepsis is 535 cases per 100,000 person-years and is increasing (5). A meta-analysis of data from high-income countries predicted that 31.5 million sepsis and 19.4 million severe sepsis cases will occur annually worldwide (6). Recently, global data from the Intensive Care Over Nations (ICON) audit showed that 29.5% of patients had sepsis during their ICU stay, and the occurrence rates varied regionally from 13.6 to 39.3% (7).
Although several studies have shown that mortality due to sepsis has declined in the past two decades due to advanced supportive care and the introduction of guidelines, the exact mortality rate is still controversial (8–10). In the United States, mortality due to sepsis decreased from 27.8 to 17.9% between 1979 and 2000 (8). Similarly, in Australia and New Zealand, mortality decreased by almost half (from 35.0 to 18.4%) between 2000 and 2012 (9). However, a multicenter study showed that the in-hospital mortality rate in patients with septic shock was 50.9% in Germany and 58.6% in Italy (10, 11).
To date, there have been few large epidemiological investigations of sepsis in middle- and low-income countries. The ICON audit indicated that low income was associated with increased mortality (12). A multicenter point-prevalence study in Turkey showed that the prevalence of sepsis was 30.8%, with a 75.9% mortality rate in patients with septic shock (13). China is a middle-income country that accounts for one-fifth of the world's population. Information on the epidemiology of sepsis has thus far been limited to particular populations (14–16) or obtained with a cross-sectional study (17), which may not accurately reflect the epidemiology of sepsis in critically ill patients.
The aim of this prospective multicenter observational cohort study, which was conducted by the China Critical Care Sepsis Trial (CCCST) group, was to explore the prevalence, characteristics, risk factors, treatment, and outcomes of sepsis in critically ill patients in tertiary hospitals in China.
Methods
Study Design
The CCCST was a prospective multicenter observational cohort study designed to assess the prevalence, characteristics, risk factors, and short- and long-term outcomes in critically ill patients in 18 ICUs in 16 tertiary hospitals in 7 geographical regions from 1 January 2014 to 31 August 2015. Eligible patients who were admitted consecutively to ICUs were aged 18 years or older and stayed in the ICU for at least 24 h. If the patients were admitted to the ICU repeatedly during the same hospitalization event, only the first admission was considered.
Data Collection
The following variables were extracted from the CCCST dataset: baseline demographic data (age, sex, height, and weight), source and type of admission, main diagnosis, and comorbid conditions. The severity of illness was represented by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score (18) and the Sequential Organ Failure Assessment (SOFA) score (19), which were calculated based on clinical and laboratory values. The APACHE II score was calculated within 24 h of admission to the ICU. The SOFA score was recorded consecutively for 7 days after admission to the ICU, and each component of the SOFA score was evaluated at the onset of sepsis. The use of mechanical ventilation (MV), the use of vasopressors (including dopamine, epinephrine, norepinephrine, and dobutamine), the serum creatinine (SCr) level, and urine output were also continuously recorded for 7 days or until discharge. Other clinical variables, such as blood pressure, arterial partial oxygen pressure (PaO2), fraction of inspired oxygen (FiO2), nutritional therapy, and renal replacement therapy (RRT), were recorded.
The occurrence, severity, and diagnosis of sepsis were assessed consecutively for 28 days or until discharge. The sites and types of infection, biological samples, and culture results were also recorded. The interval between the first use of targeted antibiotics and the onset of sepsis or septic shock, other antibiotics administered before sepsis and retained pre-sepsis blood cultures were also recorded. The primary outcome was ICU mortality and in-hospital mortality; the lengths of ICU and hospital stays were secondary outcomes.
Definitions
According to the “Surviving Sepsis Campaign (SSC): International Guidelines for the Management of Sepsis and Septic Shock: 2016” (20), sepsis was defined as life-threatening organ failure (an acute change in the SOFA score ≥2 points) caused by infection on admission to the ICU or within the first 48 h after admission to the ICU. Septic shock was defined as sepsis associated with hypotension that required the administration of vasopressors to maintain a mean arterial pressure >65 mmHg and a serum lactate level >2 mmol/L, despite adequate fluid resuscitation. Community-acquired sepsis was defined as sepsis that was present on admission or that developed within 48 h after hospital admission. Hospital-acquired sepsis was defined as sepsis that developed >48 h after hospital admission. For patients with multiple episodes of sepsis, only the first episode was recorded. Acute kidney injury (AKI) and severity were categorized according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (21). Acute respiratory distress syndrome (ARDS) was defined as a PaO2/FIO2 ratio <300 mmHg, according to the Berlin definition (22). According to the International Sepsis Forum Consensus Conference on the Definitions of Infection in the Intensive Care Unit, catheter-related sepsis was defined as at least one positive peripheral blood culture with (1) the same microorganism (the same species with the same sensitivities) isolated from the catheter segment or the periphery; (2) the same microorganism isolated from the peripheral blood; or (3) the same organism identified in paired central and peripheral blood cultures, wherein the central blood culture was positive ≥2 h earlier than the peripheral blood culture (23). An unscheduled operation without sufficient preparation within 24 h of the onset of injury was defined as emergency surgery. However, elective surgery was performed after adequate preoperative preparation.
Statistical Analysis
Continuous variables are presented as the means ± standard deviations (SDs) or medians [interquartile ranges (IQRs)], and categorical variables are presented as percentages. Differences between the groups were compared with the t-test, one-way analysis of variance or Wilcoxon rank-sum test for continuous variables and the chi-squared test for categorical variables. Multivariable stepwise logistic regression analysis was used to explore the risk factors for ICU mortality in patients with sepsis or septic shock. Variables with a p < 0.2 in the univariate analysis were entered into the multivariable logistic regression analysis. In the multivariable analysis, the centers were included as a random effect. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by logistic regression analysis. A two-sided p < 0.05 was considered statistically significant. All analyses were performed with IBM SPSS statistical software version 25.0 for Windows (IBM, Armonk, NY, USA) and R version 3.6.1 (http://www.r-project.org).
Results
Characteristics of the Participants
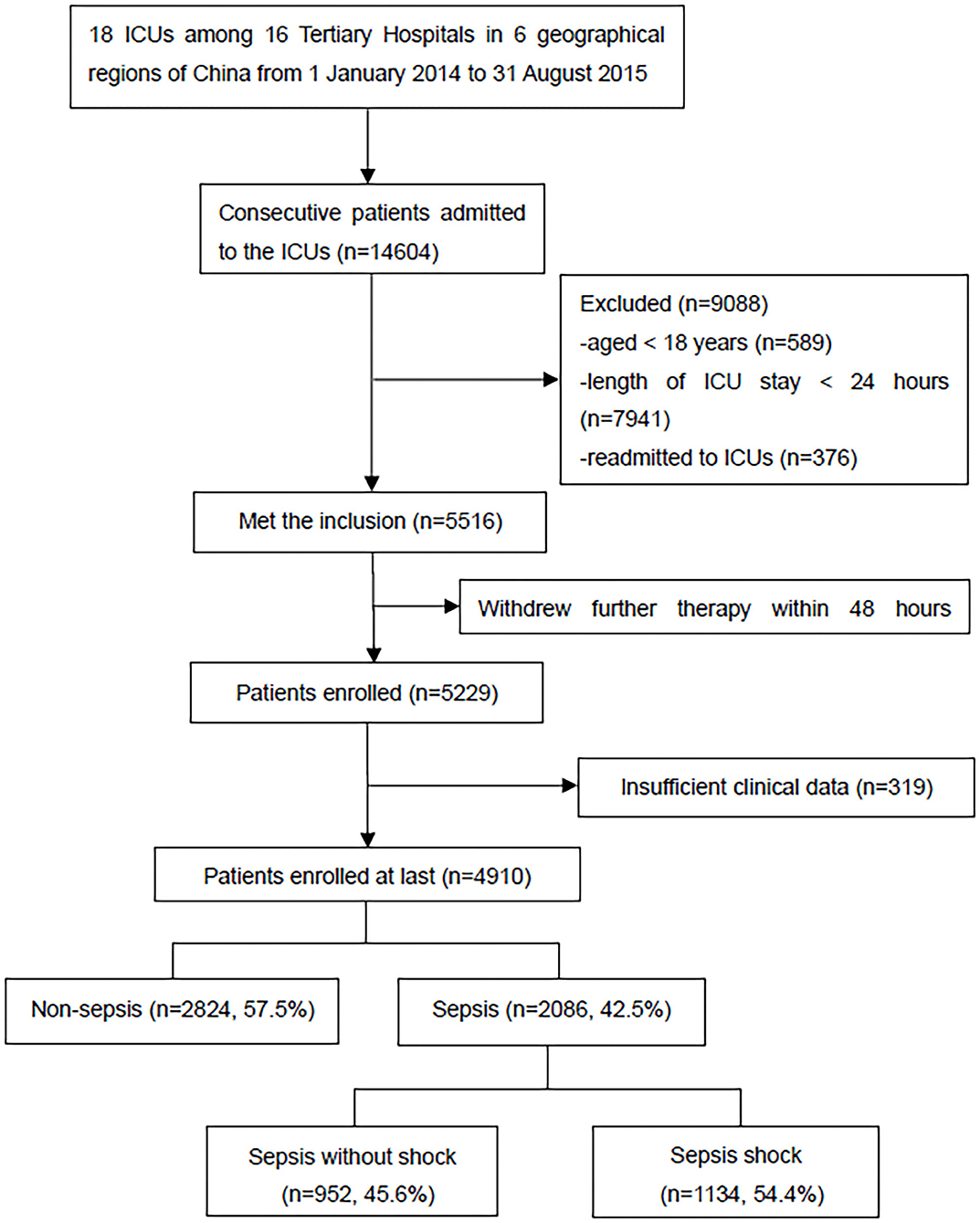
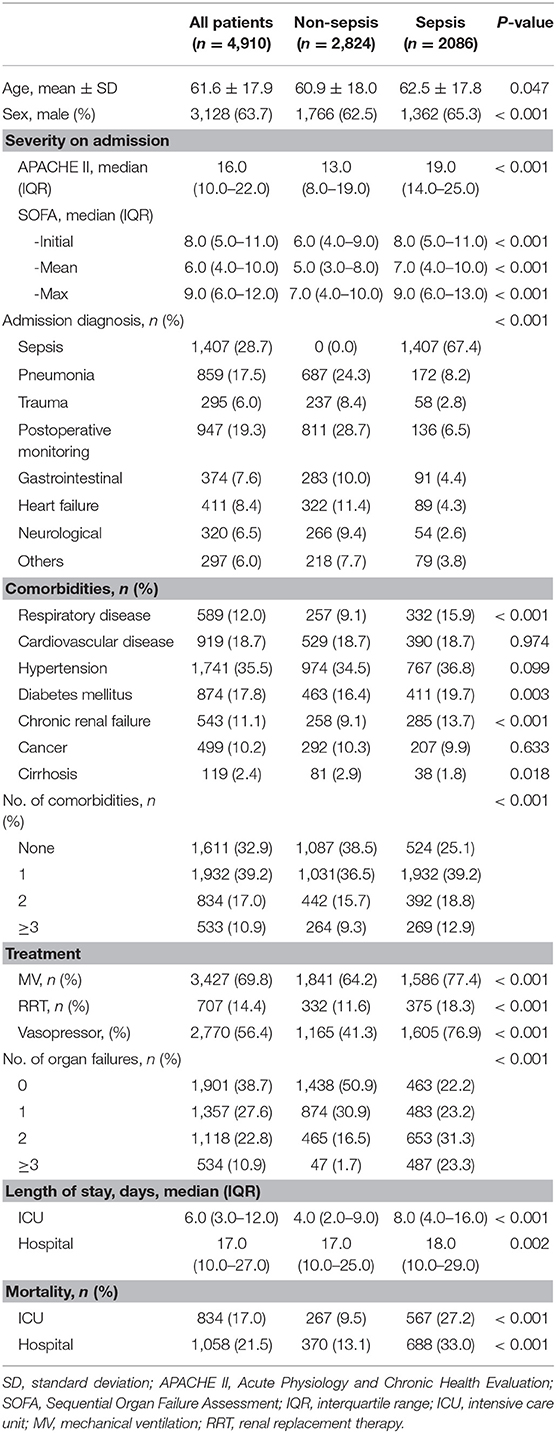
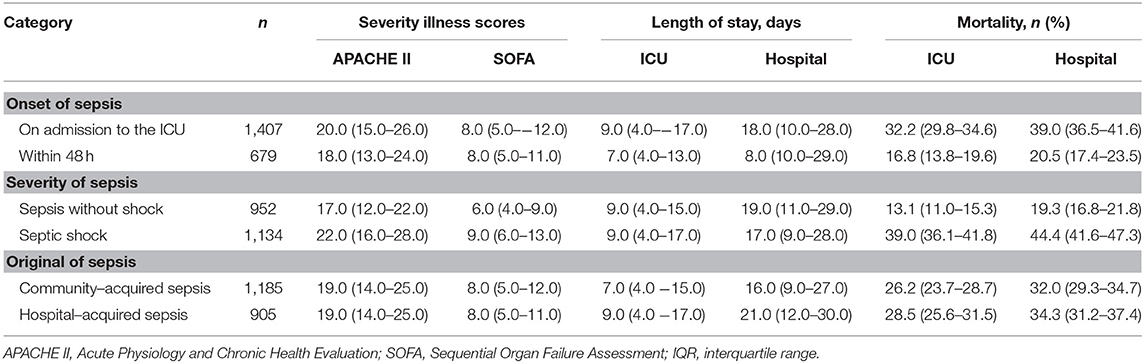
A total of 14,604 critically ill patients were consecutively admitted to the ICUs. A total of 4,910 patients were included in the CCCST. Of those, 2,086 (42.5%) had sepsis, including 1,134 (54.4%) with septic shock (Figure 1). Among all the participants, 3,009 (61.3%) patients had at least one episode of acute organ dysfunction. Patients with sepsis were more likely to be male (65.3 vs. 62.5%), have higher APACHE II (19.0 vs. 13.0) and SOFA (8.0 vs. 6.0) scores, have more comorbid conditions (no comorbidities, 25.1 vs. 38.5%) and organ dysfunction (no organ dysfunction, 22.2 vs. 50.9%), require more MV (77.4 vs. 64.2%) and RRT (18.3 vs. 11.6%), have a longer length of ICU stay (days, 8.0 vs. 4.0) and have higher ICU (27.2 vs. 9.5%) and in-hospital (33.0 vs. 13.1%) mortality rates than those who did not develop sepsis (Table 1). When sepsis was redefined based on the Sepsis-1 definition (see Supplementary Table 1), 200 (4.1%) non-sepsis patients met the definition. The characteristics and outcomes are shown in Supplementary Table 2.
Distribution of Sites and Isolated Organisms
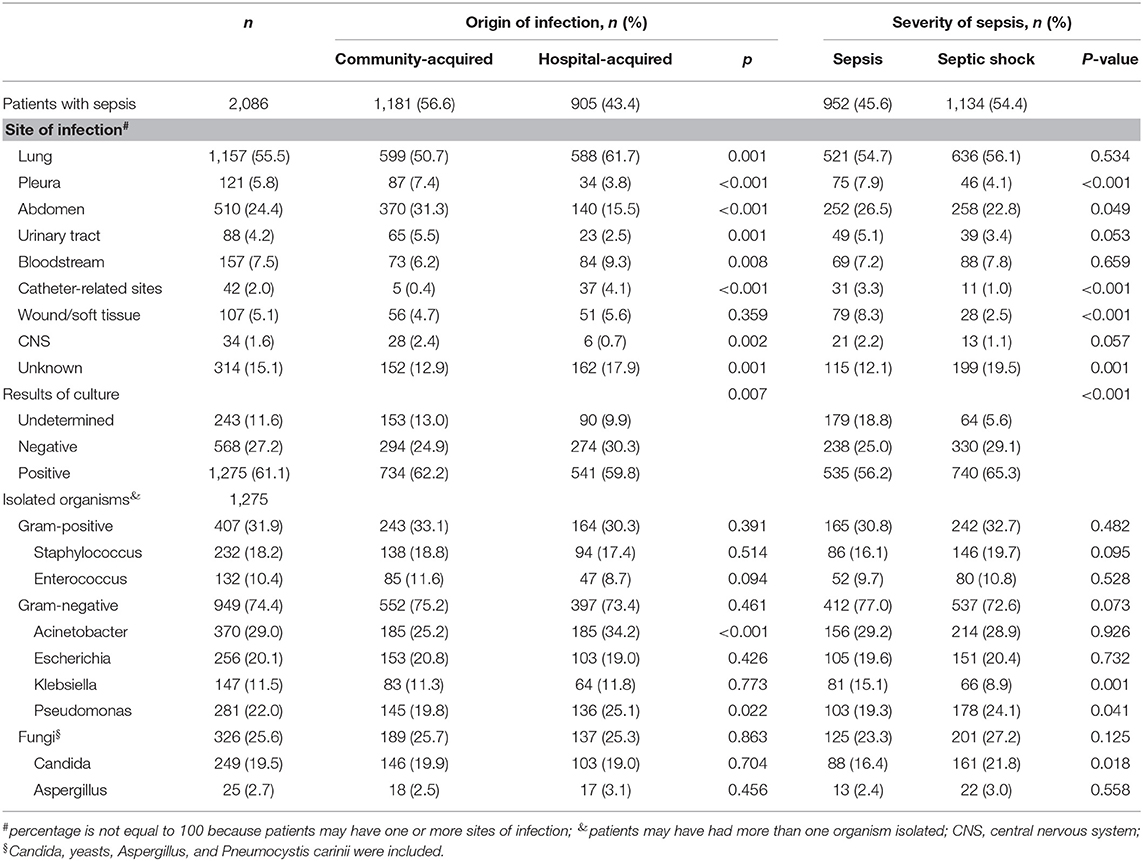
The most common site of infection in patients with sepsis was the lungs (55.5%), followed by the abdomen (24.4%). Microorganism culture was performed in 1,843 (88.4%) patients, and 1,275 (61.1%) had one or more positive cultures (Table 2). Among the patients with positive isolates, 407 (31.9%) were infected by gram-positive organisms, and 949 (74.4%) were infected by gram-negative organisms. Acinetobacter (29.0%) was the most common gram-negative organism and was usually isolated from the lungs (37.3%), pleura (42.9%), bloodstream (29.7%), catheter-related locations (42.9%), and central nervous system (CNS) (50.0%). Staphylococcus (18.2%) was the most common gram-positive organism and was usually isolated from patients with wound/soft tissue infections (26.8%) (Table 2, Supplementary Table 3). Patients with hospital-acquired sepsis were more likely to have positive cultures of Acinetobacter (34.2 vs. 25.2%) and Pseudomonas (25.1 vs. 19.8). In the patients with septic shock, Pseudomonas (24.1 vs. 19.3%) and Candida (21.8 vs. 19.3%) isolates were likely to be present; Klebsiella isolates were less common than Pseudomonas and Candida (8.9 vs. 15.1%) isolates (Table 2). The microorganism species were significantly different according to region (see Supplementary Table 4).
Organ Dysfunction and Elements of Sepsis
Among all patients with sepsis, approximately three-fourths (74.4%) had been given antibiotics before the onset of sepsis, and 63.2% retained bacteria in their blood culture. Patients with hospital-acquired sepsis were more likely to have higher sub-SOFA scores in any organ (except the kidneys and CNS) and more likely to have AKI (45.1 vs. 39.1%) but less likely to have ARDS (37.3 vs. 49.4%) than those with community-acquired sepsis. The patients with hospital-acquired sepsis commonly received more antibiotics pre-ICU (79.8 vs. 70.4%), antibiotics within 1 h after the recognition of sepsis (76.5 vs. 53.6%) and enteral nutritional therapy (61.8 vs. 52.3%) (Table 3).
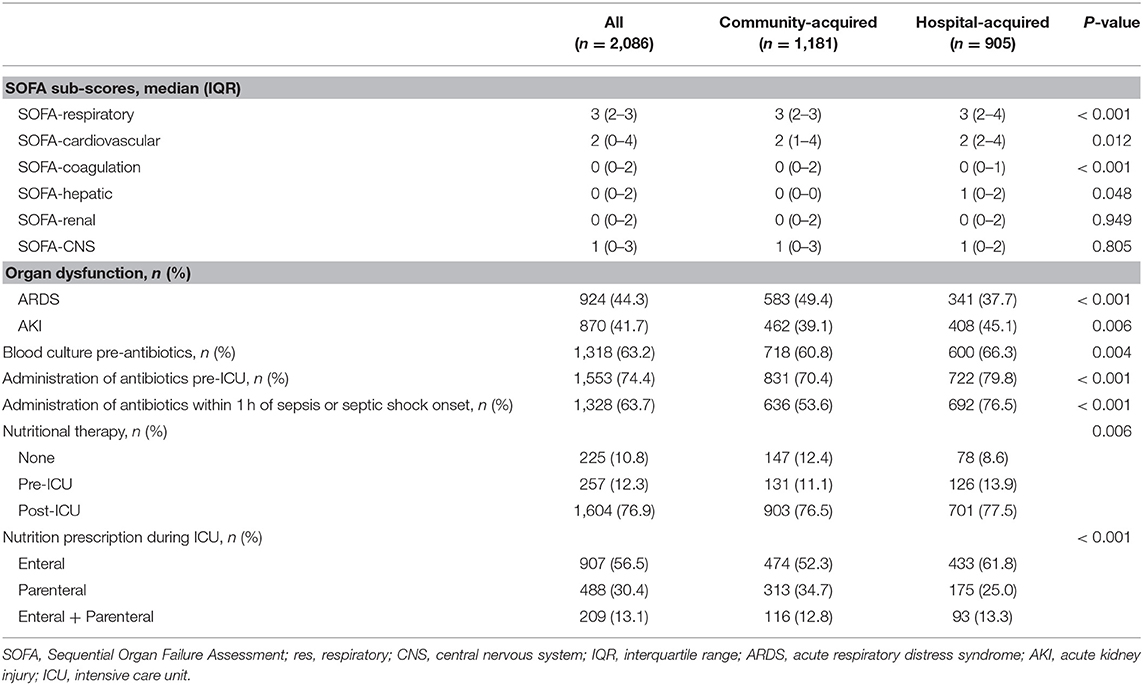
Table 3. Organ dysfunction scores, use of antibiotics, and use of nutritional therapy in patients with sepsis.
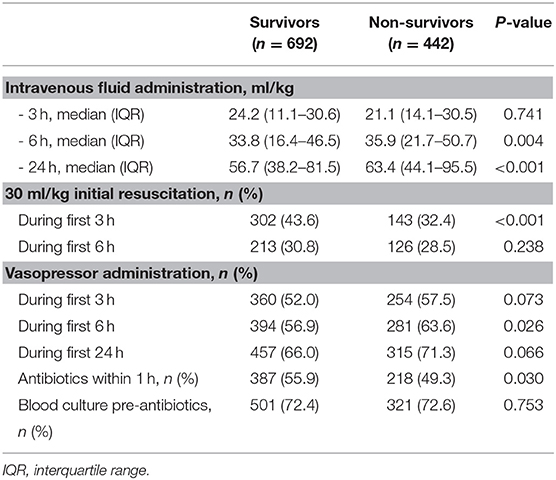
Among the patients with septic shock, the non-survivors were older, received more MV and RRT, had a longer length of hospital stay, and were more likely to have ARDS and AKI than the survivors (see Supplementary Table 5). During the first 3 h after admission to the ICU or the onset of septic shock, the survivors seemed more likely to fulfill the resuscitation goal of 30 ml/kg fluid bolus than non-survivors (43.6 vs. 32.4%), although there were no significant differences in intravenous fluid administration between the groups (24.2 vs. 21.1 ml/kg). The survivors were also commonly given antibiotics within 1 h (55.9 vs. 49.3%) of onset (Table 4).
Outcomes
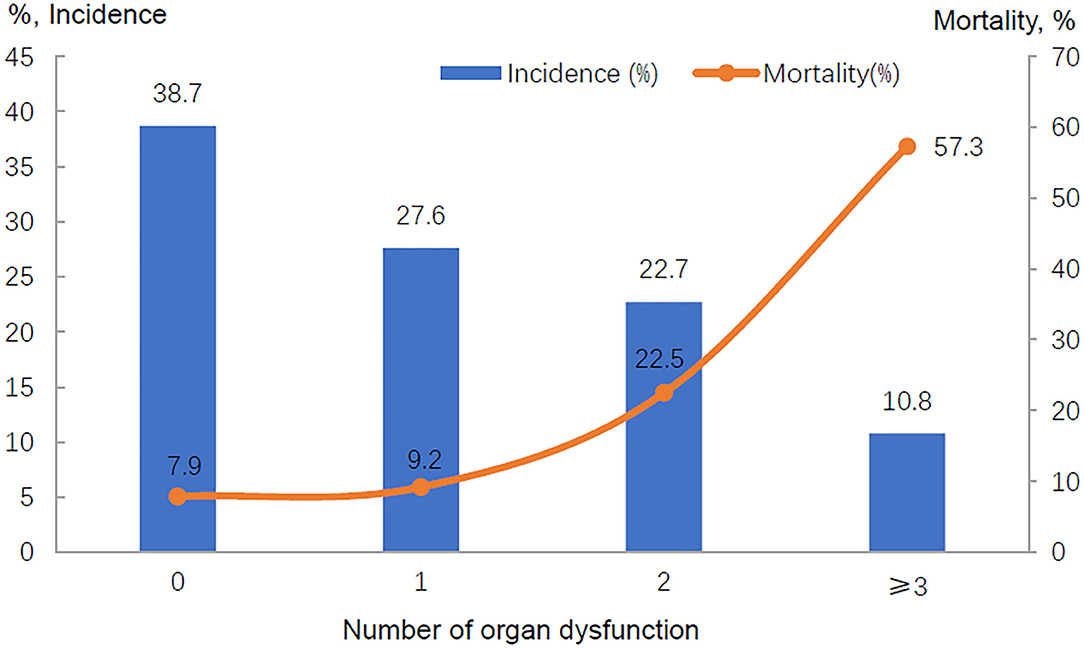
The ICU mortality rates were 27.2% in patients with sepsis and 9.5% in those without sepsis; the in-hospital mortality rates were 33.0 vs. 13.1%, respectively (Table 1). The ICU and in-hospital mortality varied by region (see Supplementary Table 6). In those patients without sepsis, the ICU mortality ranged from 0.8% (Central China) to 13.9% (North China), while in patients with septic shock, the ICU mortality ranged from 20.0% (East China) to 50.0% (Southwest China). There was also a gradual increase in ICU and in-hospital mortality with increasing severity of sepsis (Table 5). Although the patients with septic shock had a decreased length of hospital stay, they did have a significantly increased in-hospital mortality (Table 5). In addition, there was a direct relationship between the number of dysfunctional organs and ICU mortality (Figure 2, Supplementary Figure 1).
Factors Associated With Mortality in All Patients With Sepsis
According to the univariate analysis, lung infection was associated with high ICU mortality, and wound/soft tissue infection was associated with low mortality. However, patients with unknown sites of infection did not have elevated mortality rates. Gram-positive (especially Staphylococcus) and gram-negative (Acinetobacter and Pseudomonas) organisms were associated with elevated mortality in patients with positive cultures, whereas Escherichia did not increase ICU mortality (Table 6).
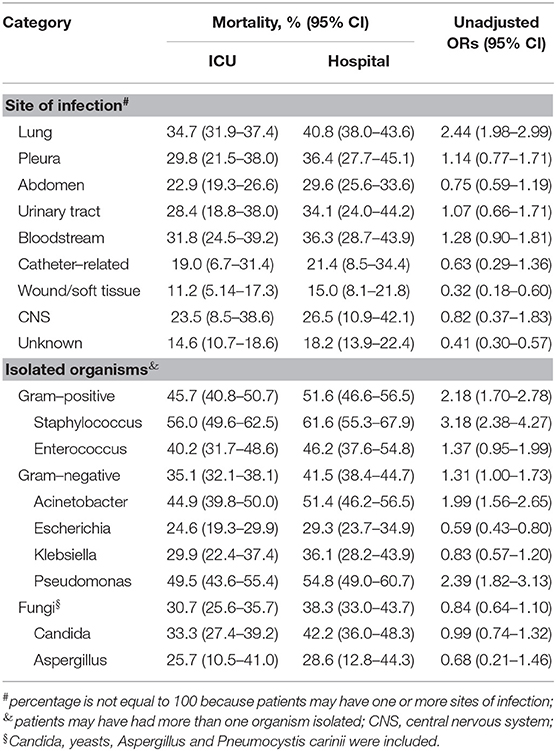
Table 6. Associations of sites of infection and isolated organisms with ICU mortality in patients with positive cultures.
After adjusting for potential confounders, having no comorbid conditions and the use of antibiotics within 1 h of sepsis onset were associated with relatively lower ICU mortality in patients with sepsis. However, advanced age; high APACHE II score; comorbid cardiovascular diseases and tumors; infection with Acinetobacter, Pseudomonas, and Staphylococcus; and the use of RRT were independent risk factors for ICU mortality in patients with sepsis. Patients with septic shock had a nearly 3-fold increased risk of mortality than those without shock (OR 2.92, 95% CI 2.04–4.17, Table 7). Similarly, the Kaplan-Meier survival curves showed a decreased probability of survival in patients with septic shock than in those without shock (see Supplementary Figure 2). Achieving the resuscitation goal of 30 ml/kg during the first 3 h (OR 0.43, 95% CI, 0.30–0.63) and administering antibiotics within 1 h (OR 0.65, 95% CI, 0.46–0.92) of the onset of septic shock decreased the odds ratio for mortality in patients with septic shock (Table 7).
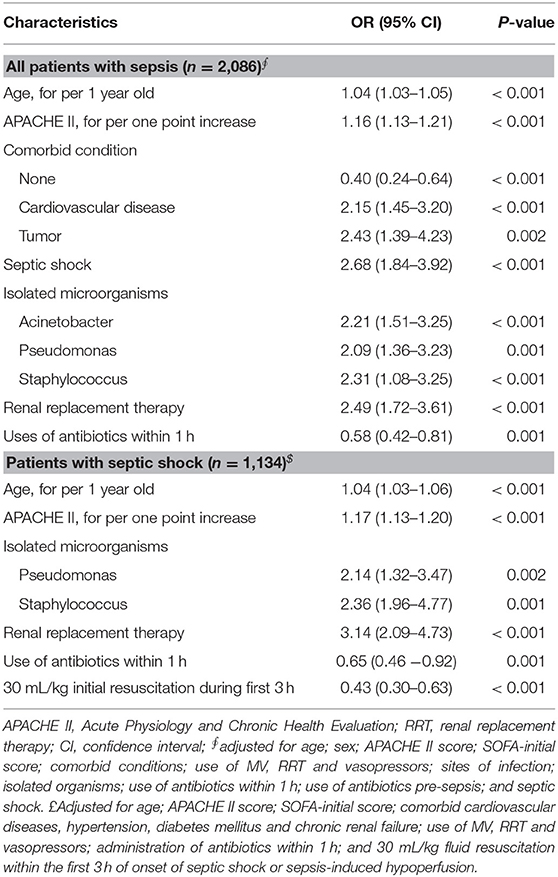
Table 7. Multivariate logistic regression analysis of ICU mortality in patients with sepsis or septic shock.
Discussion
This large-scale multicenter prospective cohort study showed a high prevalence of sepsis in Chinese ICUs in tertiary hospitals. More than two-fifths of the patients developed sepsis on admission to the ICU or within 48 h after their ICU admission, more than half was septic shock. And the in-hospital mortality in those patients with septic shock was as high as 44.4%. Furthermore, Acinetobacter and Pseudomonas were the most common isolated microorganisms, and the prompt administration of antibiotics and sufficient fluid resuscitation improved survival.
The prevalence of sepsis in Chinese ICUs was similar to that in another cross-sectional study in China (17) and higher than that in other previous studies (7, 10, 13, 24). Several reasons may explain the differences. First, we excluded those who stayed in the ICU for <24 h. Second, different definitions of sepsis and organ dysfunction might be associated with difference prevalence. Moreover, the type of ICU may affect the prevalence.
In our cohort study, the overall ICU and in-hospital mortality rates were 17.0 and 21.5%, respectively. The patients with sepsis had an elevated mortality rate: more than one-quarter of the patients with sepsis (27.2%) died in the ICU, and one-third of the patients with sepsis (33.0%) died during hospitalization. In patients with septic shock, the mortality rates increased to 39.0% in the ICU and 44.4% in the hospital. These results were similar to those reported by the ICON audit (7), the Sepsis Occurrence in Acutely Ill Patients (SOAP) study (25) and a study in Norway (26) but higher than those reported in other studies (27, 28). These discrepancies may be associated with multiple organ complications, geographical regions and the national income level. Several previous studies have reported that the mortality ranged from 7.0 to 15.0% in sepsis patients with one organ failure, while the mortality reached 45.0–88.6% in patients with four or more organ failures (14, 25, 29). Our study showed that the ICU mortality rate was 9.9% in sepsis patients with one organ failure, while it was 60.8% in those with four or more organ failures. Another finding in our study was that the mortality varied considerable among different geographical regions, which was similar to the result reported by Xie et al. (17). Patients in Southwest China had a relatively high mortality rate, which may be related to the relatively underdeveloped economy. Previous studies have found that a low gross national income was associated with a higher mortality rate (12), especially in low-income countries (30, 31). However, we did not find significantly different outcomes when sepsis was defined using the Sepsis-1 and Sepsis-3 definitions.
As in other studies, the most common site of infection was the lungs in patients with sepsis (15, 17, 25, 32). The frequency of abdominal infection was higher than that reported in previous studies (15, 33, 34). Nevertheless, several national studies reported similar rates of abdominal infection, ranging from 21.3 to 26.0% (24.4% in our study) (17, 25, 35, 36). Hospital-acquired sepsis was more likely to be associated with lung infections and less likely to be associated with abdominal infections than community-acquired sepsis. Recent studies showed that patients with hospital-acquired sepsis had an elevated mortality (37–39). However, we did not find that hospital-acquired sepsis was associated with significantly increased mortality, which may be related to our failure to include ICU-acquired sepsis.
In our study, organisms were isolated from 61.6% of the patients with sepsis; previous epidemiological studies reported both lower (11) and higher (7, 32, 40) rates of positive isolates than that in our study. Over 70% of the patients were infected by gram-negative organisms, while one-third of the patients were infected by gram-positive organisms. Interestingly, Acinetobacter was isolated from 29.0% of the sepsis patients, which was higher than the proportions reported in previous studies (7, 14, 32), possibly because of different control strategies, the use of broad-spectrum antibiotics and the multidrug resistance of Acinetobacter species, especially the high level of resistance to carbapenems. The attention paid to sanitary precautions and the quality of care have improved substantially, but nosocomial infections still occur. In addition, there were significant regional differences in the organisms isolated from the cultures, and the reasons for the variations in the distributions of microorganisms in our study are unknown; these results are probably associated with cultivation techniques and methods.
The SSC 2016 and other studies have recommended the early optimization of antibiotic therapy, and early sufficient fluid resuscitation can improve the outcomes of patients with sepsis or septic shock (20, 41, 42). Our results show that initial antibiotic administration within 1 h of the onset of sepsis decreased ICU mortality by 42% in patients with sepsis (OR 0.58, 95% CI: 0.42–0.81), and early effective fluid resuscitation decreased ICU mortality by more than half in septic shock patients (OR 0.43, 95% CI: 0.30–0.63). However, we were unable to assess the effects of the dose of antimicrobial pharmacokinetics on outcomes in those patients, which is another important factor affecting the outcomes (20, 43, 44). In addition, RRT and pre-existing cardiovascular disease and malignant tumors were also associated with mortality.
This was the first cohort study to investigate the epidemiology of sepsis in critically ill patients in tertiary hospitals in China. The sample size included in the study was very large, which was an obvious strength. Nevertheless, there were still several limitations of this study. First, we excluded patients who stayed in the ICU for <24 h and those who stopped treatment within 48 h of admission to the ICU, which may have resulted in the underestimation of the prevalence and outcomes of sepsis. Second, we recorded only the first episode of sepsis for each patient because the number of patients who had two or more episodes during their ICU stay could not be estimated. Third, some of the data that were collected at the bedside (such as Glasgow coma scale score, urine output and hourly fluid balance) may not have been entered into the medical records. Fourth, the inclusion of two centers with small sample sizes (59 eligible participants) may have affected the results, although we adjusted for center in the statistical analysis. Fifth, the data of feeding strategy were not collected and we could not investigate the association between the feeding approach and outcomes (20). In addition, due to traditional beliefs, some critically ill patients terminated therapy and returned home before improvement or death, which may have led to the underestimation of ICU or in-hospital mortality. However, the proportion of the patients who did so is unknown. Despite several limitations of our research, we report the prevalence, etiology, and prognosis of sepsis and the associated risk factors in critically ill adults in tertiary hospitals in China, providing a basis for further epidemiological and health burden studies of sepsis in China.
In conclusion, sepsis is common in critically ill patients in tertiary hospitals in China and is associated with increased mortality. Acinetobacter, Pseudomonas, and Staphylococcus infections were independent risk factors for ICU mortality. The initial administration of antibiotics within 1 h of sepsis onset and adequate fluid resuscitation decreased the risk of mortality. To reduce the burden of sepsis, national policies are urgently needed. Health-care-associated infection prevention programmes, early detection strategies, and appropriate antibiotic and fluid management strategies for the treatment of sepsis are critical.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The study protocol was approved by the ethics committees of Fuxing Hospital, Capital Medical University (approval notice number 2013FXHEC-KY018), and all other centers. Written informed consent for participation was not required for this observational survey. The patient records and information were anonymized before analysis.
Author Contributions
XX conceived of, designed, and supervised the study. MW, WenL, YK, LW, TQ, XM, DZ, YW, QZ, MD, WenxL, BS, XC, YA, TL, XZ, JJ, JZ, and the CCCST workgroup participated in the data collection. MW, LJ, BZ, and YH finalized the analysis, designed the study, and interpreted the findings. MW and LJ wrote the drafts of the manuscript. BZ, BD, and LW interpreted the findings and commented on and helped revise the drafts of the manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Science and Technology Supporting Plan of the Ministry of Science and Technology of the People's Republic of China (2012BAI11B05). The funding source had no role in the writing of the manuscript or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the National Science and Technology Supporting Plan of the Ministry of Science and Technology of the People's Republic of China, a government fund used to improve health-care quality and data collection, for its financial support. We also acknowledge all the following members of the CCCST workgroup who contributed data and samples and have made this work possible: Qi Jiang, Zhen Zhao, Hui Song, Ling Ma, Chunyang Li, Linlin Cao, Peng Wang, Department of Critical Care Medicine, Fuxing Hospital, Capital Medical University, Beijing, China; Ying Wen, Department of Critical Care Medicine, Fuxing Hospital, Capital Medical University, Beitaipingzhuang Community Health Service Center, Haidian District, Beijing, China; Yibing Zhu, Department of Statistics, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China; Shouhong Wang, Department of Critical Care Medicine, Guangdong Geriatric Institute, Guangdong General Hospital, Guangdong, China; Xuelian Liao, Department of Critical Care Medicine, West China Hospital, Sichuan University, Sichuan, China; Liang Wang and Xin Li, Department of Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China; Xiaoxia Peng, Centre for Clinical Epidemiology and Evidence-based Medicine, Beijing Children's Hospital, Capital Medical University, National Centre for Children Health, Beijing, China; Yuan Xu, Department of Critical Care Medicine, Beijing Tsinghua Changgung Hospital, Beijing, China.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.593808/full#supplementary-material
Abbreviations
ICU, intensive care unit; APACHE II, Acute Physiology and Chronic Health Evaluation II; RRT, renal replacement therapy; OR, odds ratio; ICON, Intensive Care Over Nations; SOFA, Sequential Organ Failure Assessment; MV, mechanical ventilation; SCr, serum creatinine; PaO2, arterial partial oxygen pressure; FiO2, fraction of inspired oxygen; SSC, Surviving Sepsis Campaign; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; SD, standard deviation; IQR, interquartile range; CI, confidence interval; SOAP, Sepsis Occurrence in Acutely Ill Patients; ICD, International Classification of Diseases; CRF, case report form; GCS, Glasgow coma scale.
References
1. Calsavara AJC, Costa PA, Nobre V, Teixeira AL. Factors associated with short and long term cognitive changes in patients with sepsis. Sci Rep. (2018) 8:4509. doi: 10.1038/s41598-018-22754-3
2. Francisco J, Aragao I, Cardoso T. Risk factors for long-term mortality in patients admitted with severe infection. BMC Infect Dis. (2018) 18:161. doi: 10.1186/s12879-018-3054-4
3. Syngal P, Giuliano JS. Health-related quality of life after pediatric severe sepsis. Healthcare. (2018) 6:113. doi: 10.3390/healthcare6030113
4. Killien EY, Farris RWD, Watson RS, Dervan LA, Zimmerman JJ. Health-related quality of life among survivors of pediatric sepsis. Pediatr Crit Care Med. (2019) 20:501–9. doi: 10.1097/PCC.0000000000001886
5. Nolan A, Weiden MD. Trends in sepsis and infection sources in the United States. a population-based study. Ann Am Thorac Soc. (2015) 12:784. doi: 10.1513/AnnalsATS.201501-044LE
6. Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. (2016) 193:259–72. doi: 10.1164/rccm.201504-0781OC
7. Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Namendys-Silva SA, et al. Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis. (2018) 5:ofy313. doi: 10.1093/ofid/ofy313
8. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. (2003) 348:1546–54. doi: 10.1056/NEJMoa022139
9. Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. (2014) 311:1308–16. doi: 10.1001/jama.2014.2637
10. SepNet Critical Care Trials Group. Incidence of severe sepsis and septic shock in German intensive care units: the prospective, multicenter INSEP study. Intensive Care Med. (2016). 42:1980–9. doi: 10.1007/s00134-016-4504-3
11. Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, et al. Epidemiology and outcome of sepsis syndromes in Italian ICUs: a muticenter, observational cohort study in the region of piedmont. Minerva Anestesiol. (2013) 79:993–1002
12. Vincent J-L, Marshall JC, Ñamendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the Intensive Care Over Nations (ICON) audit. Lancet Respir Med. (2014) 2:380–6. doi: 10.1016/S2213-2600(14)70061-X
13. Baykara N, Akalin H, Arslantas MK, Hanci V, Caglayan C, Kahveci F, et al. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit Care. (2018) 22:93. doi: 10.1186/s13054-018-2013-1
14. Cheng B, Xie G, Yao S, Wu X, Guo Q, Gu M, et al. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med. (2007) 35:2538–46. doi: 10.1097/01.CCM.0000284492.30800.00
15. Zhou J, Tian H, Du X, Xi X, An Y, Duan M, et al. Population-based epidemiology of sepsis in a subdistrict of Beijing. Crit Care Med. (2017) 45:1168–76. doi: 10.1097/CCM.0000000000002414
16. Weng L, Zeng XY, Yin P, Wang LJ, Wang CY, Jiang W, et al. Sepsis-related mortality in China: a descriptive analysis. Intensive Care Med. (2018) 44:1071–80. doi: 10.1007/s00134-018-5203-z
17. Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, et al. The epidemiology of sepsis in chinese icus: a national cross-sectional survey. Crit Care Med. (2020) 48:e209–e18. doi: 10.1097/CCM.0000000000004155
18. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. (1985) 13:818–29. doi: 10.1097/00003246-198510000-00009
19. Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. (1993) 270:2957–63. doi: 10.1001/jama.270.24.2957
20. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. (2017) 43:304–77. doi: 10.1007/s00134-017-4683-6
21. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012). 120:C179–84. doi: 10.1159/000339789
22. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
23. Calandra T, Cohen J, International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. (2005). 33:1538–48. doi: 10.1097/01.CCM.0000168253.91200.83
24. Machado FR, Cavalcanti AB, Bozza FA, Ferreira EM, Carrara FSA, Sousa JL, et al. The epidemiology of sepsis in Brazilian intensive care units (the sepsis PREvalence assessment database, SPREAD): an observational study. Lancet Infect Dis. (2017) 17:1180–9. doi: 10.1016/S1473-3099(17)30322-5
25. Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. (2006) 34:344–53. doi: 10.1097/01.CCM.0000194725.48928.3A
26. Knoop ST, Skrede S, Langeland N, Flaatten HK. Epidemiology and impact on all-cause mortality of sepsis in Norwegian hospitals: a national retrospective study. PLoS ONE. (2017) 12:e0187990. doi: 10.1371/journal.pone.0187990
27. Neira RAQ, Hamacher S, Japiassu AM. Epidemiology of sepsis in Brazil: incidence, lethality, costs, and other indicators for Brazilian unified health system hospitalizations from 2006 to (2015). PLoS ONE. (2018) 13:e0195873. doi: 10.1371/journal.pone.0195873
28. Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the united states-an analysis based on timing of diagnosis and severity level. Crit Care Med. (2018) 46:1889–97. doi: 10.1097/CCM.0000000000003342
29. Blanco J, Muriel-Bombin A, Sagredo V, Taboada F, Gandia F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicenter study. Crit Care. (2008) 12:R158. doi: 10.1186/cc7157
30. Baelani I, Jochberger S, Laimer T, Otieno D, Kabutu J, Wilson I, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care. (2011) 15:R10. doi: 10.1186/cc9410
31. Hsia RY, Mbembati NA, Macfarlane S, Kruk ME. Access to emergency and surgical care in sub-Saharan Africa: the infrastructure gap. Health Policy Plan. (2012) 27:234–44. doi: 10.1093/heapol/czr023
32. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. (2009) 302:2323–9. doi: 10.1001/jama.2009.1754
33. Alvaro-Meca A, Jimenez-Sousa MA, Micheloud D, Sanchez-Lopez A, Heredia-Rodriguez M, Tamayo E, et al. Epidemiological trends of sepsis in the twenty-first century (2000-2013): an analysis of incidence, mortality, and associated costs in Spain. Popul Health Metr. (2018) 16:4. doi: 10.1186/s12963-018-0160-x
34. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. (2001) 29:1303–10. doi: 10.1097/00003246-200107000-00002
35. Ogura H, Gando S, Saitoh D, Takeyama N, Kushimoto S, Fujishima S, et al. Epidemiology of severe sepsis in Japanese intensive care units: a prospective multicenter study. J Infect Chemother. (2014) 20:157–62. doi: 10.1016/j.jiac.2013.07.006
36. Engel C, Brunkhorst FM, Bone HG, Brunkhorst R, Gerlach H, Grond S, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med. (2007) 33:606–18. doi: 10.1007/s00134-006-0517-7
37. Giannoni E, Agyeman PKA, Stocker M, Posfay-Barbe KM, Heininger U, Spycher BD, et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J Pediatr. (2018) 201:106–14.e104. doi: 10.1016/j.jpeds.2018.05.048
38. Padro T, Smotherman C, Gautam S, Gerdik C, Gray-Eurom K, Guirgis FW. Admission characteristics predictive of in-hospital death from hospital-acquired sepsis: a comparison to community-acquired sepsis. J Crit Care. (2019) 51:145–8. doi: 10.1016/j.jcrc.2019.02.023
39. Westphal GA, Pereira AB, Fachin SM, Barreto ACC, Bornschein ACGJ, Filho MC, et al. Characteristics and outcomes of patients with community-acquired and hospital-acquired sepsis. Rev Bras Ter Intensiva. (2019) 31:71–8. doi: 10.5935/0103-507X.20190013
40. Brun-Buisson C, Meshaka P, Pinton P, Vallet B, EPISEPSIS Study Group. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. (2004) 30:580–8. doi: 10.1007/s00134-003-2121-4
41. Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. (2017) 376:2235–44. doi: 10.1056/NEJMoa1703058
42. Leisman DE, Doerfler ME, Ward MF, Masick KD, Wie BJ, Gribben JL, et al. Survival benefit and cost savings from compliance with a simplified 3-hour sepsis bundle in a series of prospective, multisite, observational cohorts. Crit Care Med. (2017) 45:395–406. doi: 10.1097/CCM.0000000000002184
43. Blot S, Koulenti D, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care. (2014) 18:R99. doi: 10.1186/cc13874
Keywords: sepsis, septic shock, mortality, prevalance, risk factor
Citation: Wang M, Jiang L, Zhu B, Li W, Du B, Kang Y, Weng L, Qin T, Ma X, Zhu D, Wang Y, Zhan Q, Duan M, Li W, Sun B, Cao X, Ai Y, Li T, Zhu X, Jia J, Zhou J, He Y, Xi X and China Critical Care Sepsis Trial (CCCST) workgroup (2020) The Prevalence, Risk Factors, and Outcomes of Sepsis in Critically Ill Patients in China: A Multicenter Prospective Cohort Study. Front. Med. 7:593808. doi: 10.3389/fmed.2020.593808
Received: 11 August 2020; Accepted: 16 November 2020;
Published: 17 December 2020.
Edited by:
Marcelo Arruda Nakazone, Faculty of Medicine of São José do Rio Preto, BrazilReviewed by:
Massimo Girardis, University Hospital of Modena, ItalyChun-Ta Huang, National Taiwan University Hospital, Taiwan
Copyright © 2020 Wang, Jiang, Zhu, Li, Du, Kang, Weng, Qin, Ma, Zhu, Wang, Zhan, Duan, Li, Sun, Cao, Ai, Li, Zhu, Jia, Zhou, He, Xi and China Critical Care Sepsis Trial (CCCST) workgroup. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan He, eWFuaGUxMjIwJiN4MDAwNDA7MTI2LmNvbQ==; Xiuming Xi, eGl4aXVtaW5nMjkzNyYjeDAwMDQwO3NpbmEuY29t
†These authors have contributed equally to this work
 Meiping Wang
Meiping Wang Li Jiang3†
Li Jiang3† Wen Li
Wen Li Bing Sun
Bing Sun